Abstract
Pleiotrophin (PTN) is produced under ischemic conditions and has been shown to induce angiogenesis in vivo. We studied whether or not PTN exerts chemotaxis of pro-angiogenic early endothelial progenitor cells (EPCs), a population of circulating cells that have been reported to participate in and stimulate angiogenesis. Chemotaxis of EPCs, isolated from blood of healthy humans (n=5), was measured in transwell assays. PTN at 10–500 ng/mL elicited dose-dependent chemotaxis of both EPCs and human umbilical vein endothelial cells (HUVECs), but not of human coronary artery smooth muscle cells (CASMCs) and T98G glioblastoma cells that lack PTN receptors. The degree of chemotaxis was comparable to that induced by the angiogenic factors VEGF and SDF-1α. Chemotaxis to PTN was blocked by the NOS inhibitors L-NNA and L-NMMA, the NO scavenger PTIO, the phosphoinositide-3 kinase inhibitor wortmannin, and the guanylyl cyclase inhibitor ODQ, suggesting dependence of EPC chemotaxis on these pathways. PTN induced NOS-dependent production of NO to a similar degree as did VEGF, as indicated by the NO indicator DAF-2. PTN increased proliferation in EPCs and HUVECs to a similar extent as VEGF, but did not induce proliferation of CASMCs. While L-NNA abolished PTN-induced migration in EPCs and HUVECs, it did not inhibit PTN- and VEGF-enhanced proliferation and also caused proliferation by itself. These data suggest that PTN may mediate its pro-angiogenic effects by increasing the local number of not only endothelial cells but also early EPCs at angiogenic sites.
Keywords: pleiotrophin, endothelial nitric oxide synthase, endothelial progenitor cells, proliferation, chemotaxis
INTRODUCTION
Tissue engineering and stem cell therapy are potential approaches for the replacement and functional reconstitution of vascular and cardiac tissue (Jackson et al., 2001; Springer et al., 2001; Dimmeler et al., 2005). As these concepts evolve, it is important to increase our understanding of the effect of cytokines on progenitor cells. Several factors including vascular endothelial growth factor (VEGF) and stromal cell derived factor 1 alpha (SDF-1α) have been shown to be expressed in ischemic tissues and are able to enhance angiogenesis, vasculogenesis, and arteriogenesis (Springer et al., 2003; Christman et al., 2005; Dimmeler et al., 2005; Ruiz de Almodovar et al., 2006; Roy et al., 2006). While the discovery of these effects in animal models initially had promising prospects for therapeutic use, clinical trials of angiogenic factor delivery have been mostly disappointing, underscoring the need for studies both of progenitor cells and of an expanding arsenal of angiogenic factors (Springer, 2006).
Pleiotrophin (PTN) is a developmentally regulated 136-aa (15.3 kDa) secreted growth/differentiation cytokine that is expressed during embryogenesis and in adults. It possesses diverse functions ranging from stimulation of neurite outgrowth to tumor angiogenesis (Deuel et al., 2002). PTN was first discovered as a mitogen produced by bovine uterus and as a neurite outgrowth factor in neonatal rat brain (Milner et al., 1989; Rauvala, 1989; Li et al., 1990). Subsequently, PTN was found to be expressed during development (Li et al., 1990), after ischemic injury to the brain (Yeh et al., 1998), during wound healing in skin and bone models (Petersen and Rafii, 2001; Deuel et al., 2002), and during growth of tumor cells (Fang et al., 1992). PTN was initially suspected to be involved in angiogenesis by virtue of its expression by endothelial cells during healing from ischemic brain injury, and was found to stabilize the formation of tube structures by cultured capillary endothelial cells (Deuel et al., 2002). Subsequently, it was discovered that the Ptn gene (gene is Ptn, protein is PTN) is expressed in response to ischemic injury in the rat heart (Christman et al., 2003), and that injection of Ptn plasmid DNA induces capillary and arteriole growth in ischemic myocardium in rats (Christman et al., 2005), indicating that PTN may have a utility for therapeutic angiogenesis. The mechanisms by which PTN mediates its proangiogenic effects are not completely understood, although the binding of PTN to its receptor RPTPβ (receptor-type protein-tyrosine phosphatase β) inhibits protein tyrosine phosphatase activity (Pariser et al., 2005), and expression of PTN by monocytes causes them to exhibit endothelial phenotypes (Sharifi et al., 2006).
In recent years, it has become clear that new vessel formation not only depends on local sprouting of existing vessels with the proliferation of resident cells, but also on the recruitment of endothelial progenitor cells (EPCs) from circulating blood (Asahara et al., 1997). EPCs comprise a population of blood and bone marrow mononuclear cells that is characterized by endothelial physical characteristics and gene expression profiles, and is thought to contain at least two distinct subpopulations that are pro-angiogenic and pro-endothelial, respectively (Hur et al., 2004; Jin et al., 2006). Certain angiogenic factors like VEGF that induce “classical” sprouting angiogenesis also exert effects on EPCs (Asahara et al., 1999). In order to test whether PTN has a chemotactic and mitotic effect on pro-angiogenic early EPCs, we studied the effect of PTN on the migration and proliferation of EPCs and compared it to its effect on human umbilical vein endothelial cells (HUVECs).
We show that PTN chemotactically attracts human EPCs and acts as a mitogen. Mechanistically, we show that the chemotactic response is dependent on nitric oxide, nitric oxide synthase, phosphoinositide-3 kinase (PI3K), and guanylyl cyclase; and we demonstrate a direct induction of cellular NO production by PTN.
MATERIALS AND METHODS
Cell culture and characterization of blood-derived EPCs
Endothelial progenitor cells were differentiated ex vivo from peripheral blood mononuclear cells (PBMCs). Blood was drawn from the cubital vein of 5 healthy volunteers aged 30±3 years into vacuum tubes pre-filled with a liquid density gradient medium and PBMCs were isolated based on the Ficoll method (Vacutainer CPT, Becton Dickinson, Franklin Lakes, NJ). As cardiovascular risk factors are associated with impaired number and function of EPCs, (Vasa et al., 2001; Heiss et al., 2005) exclusion criteria consisted of hypertension, diabetes mellitus, smoking, and hypercholesterolemia. In order to remove mature endothelial cells from the harvested cell population, the cells were preplated on fibronectin-coated culture plates for 1 day in EBM-2 MV (supplemented with Singlequots (Cambrex) and 20% fetal bovine serum (HyClone, Logan, UT)). The adherent cells were discarded and the non-adherent cells were moved to a new dish and cultured for another 6 days, during which time many cells became newly adherent.
To confirm the endothelial phenotype of ex vivo differentiated EPCs at day 7, we performed fluorescent staining to detect lectin-binding, acLDL-uptake, and eNOS expression, as well as FACS analysis of endothelial markers. RPTPβ expression (PTN receptor) was also confirmed by fluorescent staining. Adherent EPCs on fibronectin coated glass slides (Nalgen NUNC, Naperville, IL) were incubated for 1 h with 2 µg/mL DiI-acLDL (Invitrogen, Carlsbad, CA) in EBM-2 MV, washed twice with PBS and fixed in 2% formaldehyde/PBS. After blocking with 2% goat serum/PBS for 1 h, cells were washed and incubated with 23 µg/mL FITC-conjugated Ulex europeus agglutinin-1 (UEA-1, Sigma, St. Louis, MO). To assay for eNOS and RPTPβ expression, fixed cells were blocked and permeablized (1h, 0.3% Triton X, 2% goat serum, 0.02% sodium azide in PBS). After washing with PBS, cells were incubated with primary monoclonal anti human eNOS or anti human RPTPβ antibodies generated in mouse (1:200, Clone NOS-E1, Sigma; 1:200, clone 12, BD Pharmingen) for 1 h in the dark, washed again, and incubated with secondary Alexa 660 conjugated anti-mouse antibodies from goat (1:200, Invitrogen). The nuclei were stained with 120 ng/mL Hoechst 33258 (Invitrogen). The slides were observed using a Nikon E800 fluorescence microscope and Openlab software (Improvision, Lexington, MA).
To further characterize EPCs, FACS analysis was performed with adherent cells. After discarding suspension cells and rinsing with PBS, cells were detached by repetitive flushing with cold 1 mM EDTA/PBS. Cells were pelleted, adjusted to 106 cells/mL in FACS buffer, and incubated for 15 min with normal human IgG (1 mg/mL, Zymed, San Francisco, CA) to block FC receptors . Staining was performed for 20 min on ice with 100 µL cell suspension and the following fluorescently labeled antibodies: CD45-PerCP (1:5), CD34-PE (1:5), CD133-PE (1:10; Miltenyi Biotech, Auburn, CA), KDR-APC (1:10), CD31-PC5 (1:5), CXCR4-APC (1:5), CD14-PerCP (1:5), and CD11b-APC (1:10; Pharmingen, San Diego, CA). After washing with FACS buffer, cells were fixed with 1% formaldehyde/PBS and stored at 4°C until flow-cytometry analysis. 10,000 events were counted (FACSCalibur, BD, San Diego, CA).
Pooled human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex, cultured in EBM-2 (supplemented with Singlequots and 5% FBS) and used no later than passage 3. Human coronary artery smooth muscle cells were purchased from Lonza (Basel, Switzerland) and cultured in smooth muscle growth medium (supplemented with Singlequots and 5% FBS). T98G glioblastoma cells, previously described to be irresponsive to PTN due to a lack of pleiotrophin receptors (Lu et al., 2005), were purchased from American Type Culture Collection (Manassas, VA) and cultured in complete growth medium (Minimum essential medium (Eagle) with 2 mM L-glutamine and Earle's BSS adjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, and 1.0 mM sodium pyruvate, 90%) supplemented with 10% FBS and 5% DMSO.
Chemotaxis assay
Cell migration was quantified by a transwell chemotaxis assay using a modified Boyden chamber (Falk et al., 1980). Briefly, chemotaxis was measured as follows: After detachment, 2×104 cells were resuspended in basal cell media (without supplements, 1% BSA) and plated in the upper of two chambers divided by a membrane with 8 µm pores (Corning Transwell). Chemoattractants specific to the experiment were added to the lower chamber. The NOS inhibitors, NO scavenger, phosphoinositide-3 kinase inhibitor, and guanylyl cyclase inhibitor were added to both upper and lower chamber (all from Sigma). L-NNA (NG-Nitro-L-arginine) and L-NMMA (NG-Methyl-L-arginine) are irreversible and reversible, respectively, inhibitors of constitutive nitric oxide synthase. PTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide) is a water-soluble and stable free radical molecule that reacts stoichiometrically with NO (Pietraforte et al., 1995). Wortmannin is a specific phosphatidylinositol 3-kinase (PI3-K) inhibitor. ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) is a highly selective, irreversible, heme-site inhibitor of soluble guanylyl cyclase. PTN was compared to vascular endothelial growth factor (VEGF, Sigma) and stromal cellderived factor (SDF-1α; Sigma) at 10–500 ng/mL. After 6 h, the membranes were washed twice in PBS and fixed in 4% formaldehyde. After wiping cells off the upper side of the membrane with a cotton swab (Q-tip), the membranes were detached and mounted on glass slides with a nuclear dye-containing mounting medium (Vectashield DAPI). Migrated cells were counted on the lower side of the membrane by fluorescence microscopy. Chemotaxis was quantified as net number of migrated cells (cell number/HPF with chemoattractant minus cell number/HPF without chemoattractant). Each experimental condition was performed in triplicate and the number of migrated cells was determined from 5 random 100x-fields (0.998 mm2) per membrane.
NO production
In order to confirm that EPCs produce NO in response to PTN and to show that 100 µM L-NNA is sufficient to block NO production, we incubated EPCs with an NO sensitive fluorescent dye 4,5-Diaminofluorescein (DAF-2) diacetate (Sigma). DAF-2 diacetate is membrane-permeable and is deacetylated by intracellular esterases to 4,5-diaminofluorescein. DAF-2, however, remains essentially nonfluorescent until it reacts with the nitrosonium cation (produced by spontaneous oxidation of nitric oxide) to form a fluorescent heterocycle, which becomes trapped in the cell's cytoplasm (Kojima et al., 1998) (excitation 492 nm, emission 515 nm). EPCs cultured on fibronectin-coated glass slides were serum starved with 0.5% BSA/EBM-2 for 2 h. Cells were pre-incubated with inhibitors (1–100 µM L-NMMA, 100 µM PTIO) and DAF-2 DA (10 µM) for 30 min. VEGF (50 ng/mL) and PTN (50 ng/mL) were added for another 20 min. Cells were washed with PBS once and fixed in 2% formaldehyde/PBS for 15 min. Cells were washed with PBS, nuclei stained with Hoechst, and cells mounted. Fluorescence images were taken using the identical exposure settings for all conditions.
Cell proliferation
BrdU incorporation assays were performed following the manufacturer’s protocol (Cell Proliferation BrdU Assay, Roche). Cells were detached, resuspended in EBM-2 supplemented with 1% BSA, and plated at 104 /well in 96-well cell culture plates (Corning, Corning, NY). The cells were preincubated with test mitogens for 48 h. BrdU was added and cells were incubated for another 24 h. BrdU incorporation was determined colorimetrically after incubation with anti-BrdU antibodies conjugated with horseradish peroxidase.
Crystal violet staining was performed by fixing cells in 2% formaldehyde for 15 min, followed by staining with crystal blue (5 min, 0.1% crystal violet, 10% methanol, 90% PBS). After rinsing and drying, the cells were destained in 10% acetic acid and optical density was determined at 600 nm by a microplate reader. Cell numbers were determined using a standard curve with 10,000–70,000 cells. Doubling time was calculated during the exponential phase of cell growth as follows:
Doubling time = t/(3.32[logNt - logN0])
(t=time between two measurements during exponential growth phase; N0=initial cell number, Nt=number of cells after t)
Statistical analyses
Data are presented as mean ± standard error of the mean. Group differences were calculated with repeated measurements ANOVA and consecutive pairwise comparisons. P-values of less than 0.05 were regarded as significant. Correlations were Pearson’s r. All experiments were performed in triplicates and volunteers gave written informed consent. The protocol was approved by the UCSF Committee on Human Research.
RESULTS
Characterization of the early pro-angiogenic EPC population
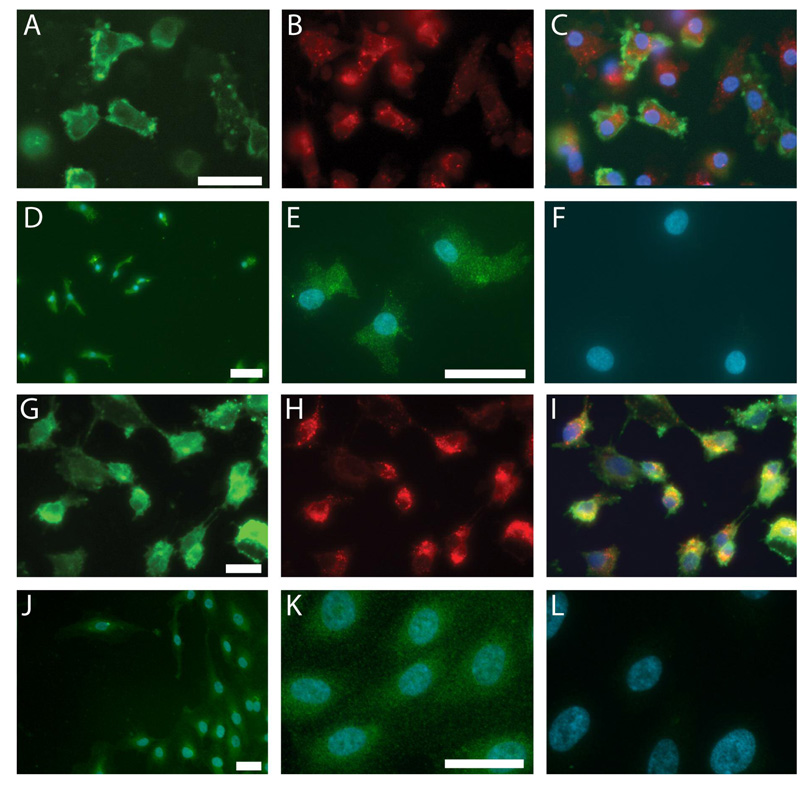
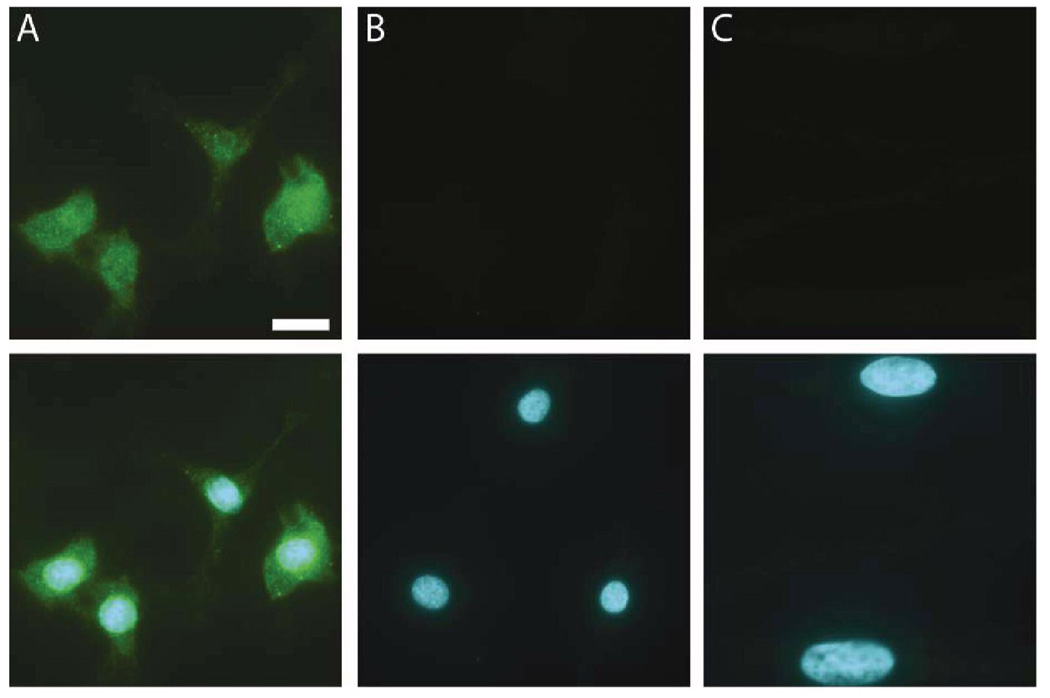
To confirm that the cells acquired endothelial phenotypes after becoming adherent, we assessed their ability to take up acLDL and bind to UEA-1 lectin. The majority of these ex vivo differentiated EPCs (Figure 1) showed endothelial characteristics similar to HUVECs as defined by positivity for acLDL-uptake, UEA-1 binding, and eNOS expression which was present on almost all cells (>95%). After detachment, the cells also expressed CD45 (93%), CD34 (1.1%), CD133 (0.9%), KDR (24%), CD31 (49%), CXCR4 (57%), CD14 (68%), and CD11b (54%) as determined by FACS. This cell population is consistent with the early pro-angiogenic EPC type described by other authors (Kalka et al., 2000; Hur et al., 2004). Furthermore, cultured EPCs expressed the pleiotrophin receptor RPTPβ (Figure 2).
Figure 1. Characterization of EPCs (A–F) and HUVECs (G–L): lectin-binding, LDL-uptake, and eNOS expression.
EPCs and HUVECs exhibit UEA-1 lectinbinding (A,G) and acLDL-uptake (B,H). Merged image of both stains with blue nuclei (C,I). eNOS expression in EPCs (D,E) and HUVECs (J,K). Merged image of eNOS with blue nuclei. No primary antibody (F,L). Scale bars=20 µm.
Figure 2. PTN receptor expression in EPCs but not CASMCs.
Immunofluorescence images of PTN receptors RPTPβ (green) in EPCs (A). (B) shows negative control lacking primary antibody, and (C) shows RPTPβ immunostaining of hCASMCs, which lack the receptor. Lower row shows upper row images merged with blue nuclei. Scale bar=10 µm.
PTN chemotactically attracts EPCs
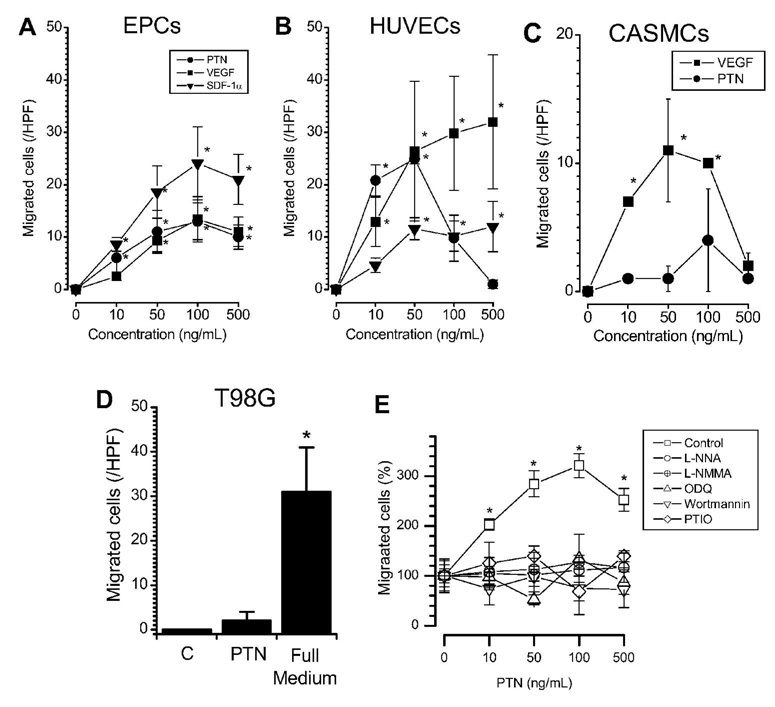
In order to determine the migratory response of EPCs to PTN, we employed the well established and commonly used transwell assay for cell migration (Vasa et al., 2001; Heiss et al., 2005). EPCs and HUVECs exhibited comparable dose-dependent chemotaxis toward PTN at 10–500 ng/mL (Figure 3A,B). In EPCs, the responses to PTN were not significantly different from the respective responses towards VEGF and SDF-1α in all measured concentrations (Figure 3A), with slightly maximal response to the 100 ng/mL condition. In HUVECs, the responses to all three chemokines were similar at 10–50 ng/mL (Figure 3B). However, unlike the dose-response curves observed for EPCs responding to all three chemokines similarly, the response of HUVECs peaked at 50 ng/mL PTN and decreased at higher doses while their response to VEGF and SDF-1α leveled off beyond that concentration. As negative controls, CASMCs and T98G cells did not migrate toward PTN, despite migrating toward VEGF and full growth medium as positive controls (Figure 3C,D), consistent with their lack of PTN receptor expression (Figure 2 and (Lu et al., 2005)).
Figure 3. Chemotaxis toward PTN in EPCs.
PTN elicits chemotaxis of EPCs (A, n=5) as compared to other chemokines (VEGF, SDF-1) and (B) to a similar extent as endothelial cells (HUVECs, n=3). In contrast, PTN does not elicit chemotaxis in (C) CASMCs or (D) T98G cells, which have the ability to migrate to VEGF or complete growth medium controls, respectively. Mechanistic inhibitor studies are shown in (E). Chemotaxis of EPCs to PTN is dependent on functional NOS, guanylyl cyclase, and phosphoinositide-3 kinase. * p<0.05 vs. control (repeated measurements ANOVA). Symbols and columns are mean values±SE.
Mechanism of PTN mediated chemotaxis
In order to gain mechanistic insight into PTN mediated chemotaxis of EPCs, we performed inhibitor studies. Chemotaxis toward PTN at 10–500 ng/mL was inhibited in the presence of NOS-inhibitors (L-NNA and L-NMMA), an NO scavenger (PTIO), a phosphoinositol 3-phosphate kinase inhibitor (wortmannin), and a guanylyl cyclase inhibitor (ODQ) (Figure 3E). Unspecific, non-directional cell movement, without addition of chemokines, remained unaffected by these inhibitors suggesting that the observed lack of chemotaxis is due to specific inhibition of pathways and cannot be explained by unspecific cell toxicity or globally disabled cell motility.
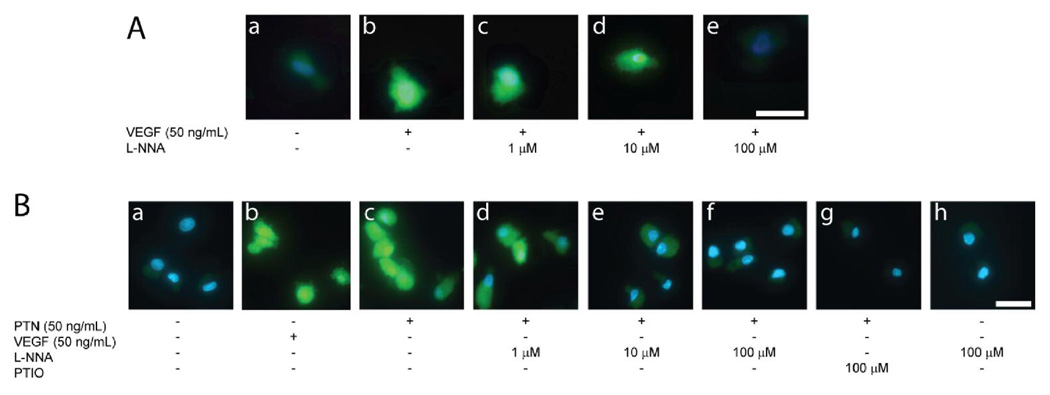
PTN induces NOS-dependent NO production
In order to confirm the presence of a functional NO synthase, we performed staining experiments with the NO-sensitive fluorescent dye DAF-2. Incubation with PTN at 50 ng/mL led to an increase in fluorescence comparable to incubation with VEGF (Figure 4). Co-incubation with L-NNA, a competitive NOS inhibitor, led to inhibition of this signal at 100 uM suggesting that this concentration is sufficient to inhibit NOS dependent NO production. Furthermore, PTIO, an NO scavenger, also abolished the signal, suggesting NO-dependence.
Figure 4. NO production by EPCs in response to PTN.
Incubation of EPCs with PTN and VEGF leads to similar increase in fluorescence of the NO sensitive fluorescent dye DAF-2 (B a and b). This is inhibited by competitive NOS inhibitor L-NNA (B c–e) and NO scavenger PTIO (B f). Scale bar=15 µm.
Enhanced NOS-independent proliferation with PTN
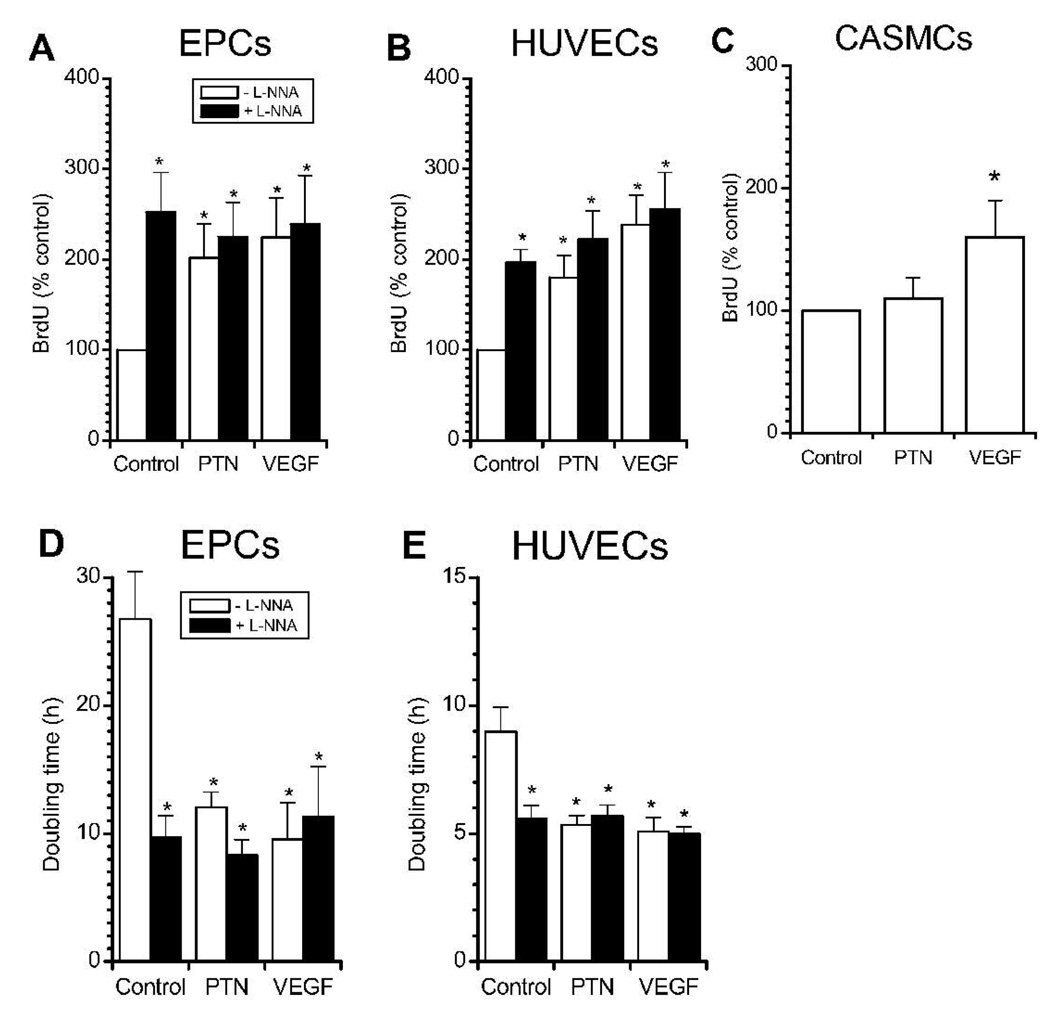
PTN significantly increased proliferation in EPCs and HUVECs as determined by higher BrdU uptake and lower doubling time. BrdU incorporation over 48 h during exposure to PTN was similar to that during VEGF exposure (Figure 5A, B). Interestingly, while L-NNA abolished PTN- and VEGF-induced migration in both cell types, it did not inhibit PTN- and VEGF-enhanced proliferation and even caused proliferation by itself. The magnitude of L-NNA-related proliferation was similar to that of PTN and VEGF. In contrast, while CASMC proliferation was induced by VEGF, PTN had no effect on these cells, consistent with their lack of PTN receptor expression (Figure 2) and absence of PTN-induced migration.
Figure 5. PTN stimulates EPC proliferation.
BrdU incorporation is increased by PTN (50 ng/mL) in EPCs (A, n=5) and HUVECs (B, n=5) but not CASMC (C, n=3). Corresponding population doubling times of EPCs and HUVECs are depicted in (D) and (E), respectively. Co-incubation with NOS-inhibitor L-NNA (100 µM) stimulated proliferation alone but did not change PTN- and VEGF-mediated proliferation. VEGF (50 ng/mL) positive control elicited responses similar to those of PTN.* p<0.05 vs. control (repeated measurements ANOVA).
In order to confirm these results and also to determine a more direct readout of proliferation, we measured doubling time under similar conditions (Figure 5D, E). In the absence of cytokines, EPCs exhibited approximately 3-fold longer doubling times compared to HUVECs (27±4 h vs. 9±1 h, p<0.01; note the different scales of the y axis of each graph). PTN significantly decreased doubling time in EPCs from 27±4 h to 12±1 h. No significant differences were observed between VEGF and PTN (12±1 h vs 10±3 h). Under all conditions, doubling times for EPCs were significantly longer than those for HUVECs. In agreement with the results of BrdU incorporation, the doubling times for both cell types were reduced by addition of NOS inhibitor L-NNA alone.
DISCUSSION
The key findings of this study are summarized as follows. PTN chemotactically attracted EPCs and stimulated NO production. Chemotaxis toward PTN was blocked by L-NNA, L-NMMA, PTIO, wortmannin, and ODQ, suggesting dependence of this process on nitric oxide synthase, nitric oxide, phosphoinositide-3 kinase, and guanylyl cyclase. Furthermore, PTN stimulated EPC proliferation. Whereas NOS inhibition abolished migration toward PTN, proliferation remained unaffected. The effects of PTN on EPCs were qualitatively similar to its effects on endothelial cells.
Role of PTN in EPC migration and proliferation
To our knowledge, this is the first study to show that PTN not only chemotactically attracts endothelial cells and stimulates their proliferation, but has similar effects on early pro-angiogenic EPCs. Interestingly, PTN induces EPC migration of a similar magnitude as that caused by VEGF, which is a very strong inducer of angiogenic activity (Springer et al., 1998). Souttou and colleagues (2001) have previously shown that PTN attracts HUVECs and causes growth of several mature endothelial cell types including HUVECs, bovine choroid endothelial cells, and rat aortic endothelial cells. Our results suggest that PTN can stimulate angiogenesis, sprouting of local vessels, and vasculogenesis in part by causing attraction and proliferation of circulating or tissue resident endothelial progenitors, which are derived from mononuclear cells. Sharifi and colleagues (2006) have recently reported that monocytes transduced to express PTN downregulated their expression of monocyte markers and upregulated the expression of endothelial markers, were able to take part in capillary tube formation in vitro and newly developed blood vessels in vivo, and increased perfusion in a mouse model of hindlimb ischemia; all properties that were not observed in monocytes transduced to express a control gene. Thus, it appears that PTN produced by monocytes induces them to take on endothelial characteristics, potentially through a progenitor cell intermediate (overlapping identities between EPCs and monocytes have been espoused by Rehman et al (2003)), while exogenous PTN presented to EPCs and ECs chemotactically attracts them and causes them to proliferate without affecting SMCs.
EPC identity
There is still considerable controversy regarding the phenotypic identity of EPCs. It should be stressed that the cell isolates referred to as EPCs are a heterogeneous population of cells rather than a single cell type, so any attempt to characterize properties of “endothelial progenitor cells” must take into account that only a particular subpopulation may be responsible for the observed effect. In many studies, there is significant overlap with other cell types, in particular monocytes and endothelial cells (Rehman et al., 2003; Nakul-Aquaronne et al., 2003), although monocytes and EPCs isolated using a similar protocol to our own behave differently upon introduction into models of ischemic disease (Urbich et al., 2003). Furthermore, we have ensured that mature endothelial cells are excluded from our population of study (see Methods). Two different types of EPCs are described in literature: the early endothelium-like angiogenic cells with low proliferative potential and the potentially distinct population of cells that give rise to highly proliferative endothelial cells (late outgrowth cells) (Hur et al., 2004). Both cell types were shown to express endothelial markers including eNOS, CD31, and KDR. Notably, marker expression studies using adherent cells suggest that this cell population is very homogenous with almost all cells expressing endothelial markers (eNOS, acLDL uptake, lectin binding, KDR). In contrast, FACS studies using detached cells suggest a greater degree of heterogeneity with endothelial marker expression on a lower percentage of the cells (KDR, CD31, VE-cadherin). This discrepancy may be explained by anchorage-dependent expression of endothelial markers. Further studies are needed to address this. Taken together, we propose that the EPCs used in the present study represent the early type with lower proliferative potential as compared to mature endothelial cells. Nevertheless, the present results further corroborate that these cells show both functional and phenotypical similarities with mature endothelial cells (HUVECs).
Role of NOS in cell migration and proliferation
We and others have shown that both nitric oxide-dependent vasodilation and circulating NO species, as well as the number and function of EPCs, are decreased in parallel in patients with higher cardiovascular risk and atherosclerotic disease (Hill et al., 2003; Heiss et al., 2005; Werner et al., 2005; Heiss et al., 2006). However, the role of NOS in migration and proliferation of endothelial cells and endothelial progenitors is controversial, as different researchers have reported contradictory results from experiments probing NOS dependence. This may be explained in part by differences in the applied methodology as well as complex interactions between the read-outs of cell migration and proliferation. As migration is commonly measured as the number of cells on one side of a membrane in transwell assays, proliferation strongly affects the read-out. This becomes more important when cells are incubated for longer periods of time approaching their doubling time. In other words, the longer a migration assay is incubated, the more the cytokine gradient decreases due to diffusion and the more the assay is confounded by possible effects of the chemokines on proliferation.
The situation gets even more complicated if an inhibitor is added to the assay that has different effects on migration and proliferation, such as the NOSinhibitor L-NNA. Nitric oxide appears to stimulate migration (Isenberg et al., 2006), but inhibit proliferation (Bussolati et al., 2001; Ozuyaman et al., 2005). In our present study, we observed NOS-dependent migration of EPCs and HUVECs towards PTN and VEGF but NOS-independent stimulation of proliferation. In other migration studies (data not shown), we have observed a complete inhibition of EPC and HUVEC migration toward VEGF with L-NNA only when incubation times are short. With longer incubation times, the number of cells on the opposite side of the membrane is not significantly different between VEGF or VEGF+L-NNA. This may explain why other groups who use long incubation time in their migration assays are not able to detect NOS dependence of migration (Souttou et al., 2001; Hoetzer et al., 2005). Hoetzer et al. did not observe NOS-dependence when allowing EPCs to migrate towards VEGF for 24 h (Hoetzer et al., 2005). This notion is further corroborated by our present results. We show that after 6 h there are already significantly more HUVECs in the VEGF+L-NNA migration as compared to control. This is potentially due to proliferation on the membrane. Souttou and colleagues (2001) have previously shown that PTN attracts and causes growth of HUVECs, in agreement with our results. However, their interpretation of NOS-dependence was different from ours, as they reported that the migration (12 h) was not inhibited by L-NAME, a different NOS inhibitor. Interestingly, the authors report that PTN-mediated migration was inhibited with wortmannin, a phosphoinositide-3 kinase (PI3K) inhibitor, suggesting different effects of PI3K and NOS on cell proliferation. Corroborating our results, this group also reported that PTN stimulated the proliferation as determined by thymidine uptake and that this was not inhibited by L-NAME. Özüyaman and colleagues (2005) also report that NO inhibits proliferation of bone-marrow derived EPCs in mice but stimulates their mobilization. We thus propose that the NOS dependence of EC/EPC migration requires relatively short incubation time to be detectable in transwell gradient migration assays.
Physiologically, it makes sense that differentiation of EPCs leads to increased NO production and decreased proliferation. This is consistent with the notion that EPCs, after migrating towards a PTN source, will be exposed to higher PTN concentrations that lead to proliferation. The differentiation that has been proposed to occur at angiogenic sites would cause them to progressively produce NO and thereby further inhibit proliferation.
In conclusion, we report here that PTN induces NOS-dependent chemoattraction of EPCs in a similar fashion as mature endothelial cells, and that PTN induces NOS-independent EPC and HUVEC proliferation. PTN may exert at least some of its pro-angiogenic activity by recruiting progenitor cells to the sites of its production.
ACKNOWLEDGEMENTS
This work was supported by awards from the American Heart Association to CH, and from the NIH (HLO86917), the American Heart Association, and the UCSF Academic Senate Committee on Research to MLS. The authors thank Shereen Saini for technical assistance.
Contract grant sponsor: AHA; Contract grant number: 0525078Y
Contract grant sponsor: AHA; Contract grant number: 0535244N
Contract grant sponsor: NIH; Contract grant number: HLO86917
Contract grant sponsor: UCSF Academic Senate Committee on Research
LITERATURE CITED
- Asahara T, Murohara T, Sullivan A, Silver M, van der ZR, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman KL, Fang Q, Kim AJ, Sievers RE, Fok HH, Candia AF, Colley KJ, Herradon G, Ezquerra L, Deuel TF, Lee RJ. Pleiotrophin induces formation of functional neovasculature in vivo. Biochem Biophys Res Commun. 2005;332:1146–1152. doi: 10.1016/j.bbrc.2005.04.174. [DOI] [PubMed] [Google Scholar]
- Christman KL, Sievers RE, Fang Q, Colley KJ, Lee RJ. Myocardial ischemia induced upregulation of pleiotrophin gene. 2003:393a. [Google Scholar]
- Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY. Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch Biochem Biophys. 2002;397:162–171. doi: 10.1006/abbi.2001.2705. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W, Goodwin RH, Jr, Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fang W, Hartmann N, Chow DT, Riegel AT, Wellstein A. Pleiotrophin stimulates fibroblasts and endothelial and epithelial cells and is expressed in human cancer. J Biol Chem. 1992;267:25889–25897. [PubMed] [Google Scholar]
- Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Heiss C, Lauer T, Dejam A, Kleinbongard P, Hamada S, Rassaf T, Matern S, Feelisch M, Kelm M. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol. 2006;47:573–579. doi: 10.1016/j.jacc.2005.06.089. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Hoetzer GL, Irmiger HM, Keith RS, Westbrook KM, DeSouza CA. Endothelial nitric oxide synthase inhibition does not alter endothelial progenitor cell colony forming capacity or migratory activity. J Cardiovasc Pharmacol. 2005;46:387–389. doi: 10.1097/01.fjc.0000175456.53869.ab. [DOI] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Ridnour LA, Thomas DD, Wink DA, Roberts DD, Espey MG. Guanylyl cyclase-dependent chemotaxis of endothelial cells in response to nitric oxide gradients. Free Radic Biol Med. 2006;40:1028–1033. doi: 10.1016/j.freeradbiomed.2005.10.053. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, Milbrandt J, Deuel TF. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science. 1990;250:1690–1694. doi: 10.1126/science.2270483. [DOI] [PubMed] [Google Scholar]
- Lu KV, Jong KA, Kim GY, Singh J, Dia EQ, Yoshimoto K, Wang MY, Cloughesy TF, Nelson SF, Mischel PS. Differential induction of glioblastoma migration and growth by two forms of pleiotrophin. J Biol Chem. 2005;280:26953–26964. doi: 10.1074/jbc.M502614200. [DOI] [PubMed] [Google Scholar]
- Milner PG, Li YS, Hoffman RM, Kodner CM, Siegel NR, Deuel TF. A novel 17 kD heparin-binding growth factor (HBGF-8) in bovine uterus: purification and N-terminal amino acid sequence. Biochem Biophys Res Commun. 1989;165:1096–1103. doi: 10.1016/0006-291x(89)92715-0. [DOI] [PubMed] [Google Scholar]
- Nakul-Aquaronne D, Bayle J, Frelin C. Coexpression of endothelial markers and CD14 by cytokine mobilized CD34+ cells under angiogenic stimulation. Cardiovasc Res. 2003;57:816–823. doi: 10.1016/s0008-6363(02)00776-9. [DOI] [PubMed] [Google Scholar]
- Ozuyaman B, Ebner P, Niesler U, Ziemann J, Kleinbongard P, Jax T, Godecke A, Kelm M, Kalka C. Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thromb Haemost. 2005;94:770–772. doi: 10.1160/TH05-01-0038. [DOI] [PubMed] [Google Scholar]
- Pariser H, Herradon G, Ezquerra L, Perez-Pinera P, Deuel TF. Pleiotrophin regulates serine phosphorylation and the cellular distribution of {beta}-adducin through activation of protein kinase C. Proceedings of the National Academy of Sciences. 2005;102:12407–12412. doi: 10.1073/pnas.0505901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen W, Rafii M. Immunolocalization of the angiogenetic factor pleiotrophin (PTN) in the growth plate of mice. Arch Orthop Trauma Surg. 2001;121:414–416. doi: 10.1007/s004020000246. [DOI] [PubMed] [Google Scholar]
- Pietraforte D, Mallozzi C, Scorza G, Minetti M. Role of thiols in the targeting of S-nitroso thiols to red blood cells. Biochemistry. 1995;34:7177–7185. doi: 10.1021/bi00021a032. [DOI] [PubMed] [Google Scholar]
- Rauvala H. An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J. 1989;8:2933–2941. doi: 10.1002/j.1460-2075.1989.tb08443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Luttun A, Carmeliet P. An SDF-1 Trap for Myeloid Cells Stimulates Angiogenesis. Cell. 2006;124:18–21. doi: 10.1016/j.cell.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Sharifi BG, Zeng Z, Wang L, Song L, Chen H, Qin M, Sierra-Honigmann MR, Wachsmann-Hogiu S, Shah PK. Pleiotrophin induces transdifferentiation of monocytes into functional endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1273–1280. doi: 10.1161/01.ATV.0000222017.05085.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souttou B, Raulais D, Vigny M. Pleiotrophin induces angiogenesis: involvement of the phosphoinositide-3 kinase but not the nitric oxide synthase pathways. J Cell Physiol. 2001;187:59–64. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1051>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Springer ML. A balancing act: therapeutic approaches for the modulation of angiogenesis. Curr Opin Investig Drugs. 2006;7:243–250. [PubMed] [Google Scholar]
- Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998;2:549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- Springer ML, Ozawa CR, Banfi A, Kraft PE, Ip TK, Brazelton TR, Blau HM. Localized arteriole formation directly adjacent to the site of VEGF-induced angiogenesis in muscle. Mol Ther. 2003;7:441–449. doi: 10.1016/s1525-0016(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Springer ML, Brazelton TR, Blau HM. Not the usual suspects: the unexpected sources of tissue regeneration. J Clin Invest. 2001;107:1355–1356. doi: 10.1172/JCI13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- Yeh HJ, He YY, Xu J, Hsu CY, Deuel TF. Upregulation of pleiotrophin gene expression in developing microvasculature, macrophages, and astrocytes after acute ischemic brain injury. J Neurosci. 1998;18:3699–3707. doi: 10.1523/JNEUROSCI.18-10-03699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]