Abstract
The main objectives of our study were to determine the bioavailability of omega-3 (ω-3) to the tumor, to understand its mechanisms, and to determine the feasibility of targeting the ω-6 polyunsaturated fatty acids (PUFAs) metabolizing 15-lipoxygenase-1 (15-LO-1) and cyclooxygenase-2 (COX-2) pathways. Nude mice injected subcutaneously with LAPC-4 prostate cancer cells were randomly divided into three different isocaloric (and same percent [%] of total fat) diet groups: high ω-6 linoleic acid (LA), high ω-3 stearidonic acid (SDA) PUFAs, and normal (control) diets. Tumor growth and apoptosis were examined as end points after administration of short-term (5 weeks) ω-3 and ω-6 fatty acid diets. Tumor tissue membranes were examined for growth, lipids, enzyme activities, apoptosis, and proliferation. Tumors from the LA diet-fed mice exhibited the most rapid growth compared with tumors from the control and SDA diet-fed mice. Moreover, a diet switch from LA to SDA caused a dramatic decrease in the growth of tumors in 5 weeks, whereas tumors grew more aggressively when mice were switched from an SDA to an LA diet. Evaluating tumor proliferation (Ki-67) and apoptosis (caspase-3) in mice fed the LA and SDA diets suggested increased percentage proliferation index from the ω-6 diet-fed mice compared with the tumors from the ω-3 SDA-fed mice. Further, increased apoptosis was observed in tumors from ω-3 SDA diet-fed mice versus tumors from ω-6 diet-fed mice. Levels of membrane phospholipids of red blood cells reflected dietary changes and correlated with the levels observed in tumors. Linoleic or arachidonic acid and metabolites (eicosanoid/prostaglandins) were analyzed for 15-LO-1 and COX-2 activities by high-performance liquid chromatography. We also examined the percent unsaturated or saturated fatty acids in the total phospholipids, PUFA ω-6/ω-3 ratios, and other major enzymes (elongase, Delta [Δ]-5-desaturase, and Δ-6-desaturase) of ω-6 catabolic pathways from the tumors. We observed a 2.7-fold increase in the ω-6/ω-3 ratio in tumors from LA diet-fed mice and a 4.2-fold decrease in the ratio in tumors from the SDA diet-fed mice. There was an increased Δ-6-desaturase and Δ-9 desaturase enzyme activities and reduced estimated Δ-5-desaturase activity in tumors from mice fed the SDA diet. Opposite effects were observed in tumors from mice fed the LA diet. Together, these observations provide mechanistic roles of ω-3 fatty acids in slowing prostate cancer growth by altering ω-6/ω-3 ratios through diet and by promoting apoptosis and inhibiting proliferation in tumors by directly competing with ω-6 fatty acids for 15-LO-1 and COX-2 activities.
Introduction
Prostate cancer (PCa) still remains one of the leading causes of cancer death among men in the United States [1]. Current therapies for PCa include radical prostatectomy, hormonal therapy, and targeted radiation. Unfortunately, all available therapies have associated risks and limitations, and new therapeutic strategies are critically needed [2,3]. One promising strategy involves the use of dietary interventions [4,5]. International incidence patterns and migration studies, epidemiological data as well as animal and in vitro studies, indicate that consuming a diet rich in fat increases the risk for developing PCa [6–8]. Dietary fat also includes ω-3 and ω-6 polyunsaturated fatty acids (PUFAs), both of which play important roles in many human biological processes including PCa [9,10]. Because humans cannot synthesize ω-3 and ω-6 PUFAs, they are considered essential fatty acids. Although all mammalian cells can interconvert the PUFAs within each series by elongation, desaturation, and retroconversion, the two series are not interchangeable owing to the lack of the Fat-1 gene [11], which encodes the ω-3 desaturase enzyme [12].
Linoleic acid (LA; 18:2ω-6) represents a ω-6 PUFA commonly found in high-fat Western diets [13]. Terrestrial plants synthesize LA, and once ingested by mammals, LA is either metabolized to 13-(S)-hydroxyoctadecadienoic acid [13-(S)-HODE] or converted further to arachidonic acid (AA; 20:4ω-6). Three fatty acids, found primarily in fish oils, comprise the ω-3 family: alpha linolenic acid (ALA or α-LNA; 18:3ω-3), eicosapentaenoic acid (EPA; 20:5ω-3), and docosahexaenoic acid (DHA; 22:6ω-3). ALA, synthesized by cold-water vegetation, is converted by fish to EPA and DHA. Of note, through the same series of enzymes used to convert LA to AA, humans can synthesize EPA and DHA from ingested ALA. The conversion of dietary ALA to EPA and DHA is also dependent on the dietary ω-6/ω-3 ratio [13]. Importantly, US diets provide excessive LA [14] that competes with ALA for the desaturase and elongase enzymes, impeding the formation of EPA and DHA. Studies have suggested that the high incidence of PCa in Americans may, in part, result from an imbalance in the ratio of ω-3 to ω-6 fatty acids because the typical American diet is low in ω-3 and high in ω-6 [9]. Recently, we demonstrated increased expression of a ω-6 LA-metabolizing enzyme, 15-lipoxygenase-1 (15-LO-1, ALOX15) in prostate tumor tissues compared with normal adjacent tissue [15–17]. Although AA can also act as a substrate for 15-LO-1, yielding the anti-inflammatory and proapoptotic metabolite 15-(S)-hydroxyeicosatetraenoic acid [15-(S)-HETE], the 15-LO-1 enzyme greatly prefers LA. 15-LO-1 metabolizes LA to 13-(S)-HODE, which can regulate cell growth, differentiation, and vascular homeostasis [15–17].
In addition, AA also acts as a substrate for cyclooxygenases 1 and 2 (COX-1 and COX-2) [18]. Most tissues constitutively express low protein levels of COX-1 and no COX-2. Growth factors or inflammatory agents rapidly induce COX-2 expression in prostate, and studies show overexpression of COX-2 in PCa [19,20]. COX-2 overexpression, leading to the production of proinflammatory prostaglandins (e.g., PGE2), possibly contributes to PCa pathobiology [21]. Recently, a study has shown that the combination of DHA and celecoxib (COX-2-specific inhibitor) prevents PCa cell growth in vitro [22]. This inhibition of disease development likely results, in part, from the ability of the ω-3 PUFA, EPA, to successfully compete with LA and AA for 15-LO-1 and COX-2, respectively. 15-LO-1 metabolizes EPA to 15-hydroxyeicosapentaenoic acid (15-HEPE) [23], a metabolite shown to have antitumorigenic properties, whereas COX-2 metabolizes EPA to the anti-inflammatory and antitumorigenic PGE3 [23,24]. The ω-3 PUFA EPA also serves as a substrate for 15-LO-1 and COX-2, but metabolism of EPA by these enzymes results in the formation of antitumorigenic products. Therefore, ω-3 fatty acids may not only decrease production of the protumorigenic metabolites derived from the ω-6 fatty acid pathway but also result in increased production of antitumorigenic metabolites.
On the basis of these and our previous observation [25], we further hypothesized that prostate tumor growth can be modulated by dietarily targeting the 15-LO-1 and cyclooxygenase (COX)-2 enzymes. This article describes experiments designed to show that dietarily targeting the 15-LO-1 and COX-2 enzymes in vivo can slow PCa progression.
Materials and Methods
Cell Culture
Los Angeles Prostate Cancer-4 (LAPC-4) PCa cells were kindly provided by Dr. Robert Reiter (University of California-Los Angeles) and maintained in phenol red-free Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) containing 5% heat-inactivated fetal calf serum (Sigma-Aldrich, St. Louis, MO) with streptomycin-penicillin antibiotics in a 5% CO2 incubator at 37°C.
15-LO-1 and COX Enzyme Activities in Tumors
The activities of 15-LO-1 and COX were estimated as described previously by us [25]. Briefly, tissue homogenates (total protein, 800 µg) were incubated with 25 µM [1-14C] AA or [1-14C] LA (1 x 106 cpm; 15 nmol). The reaction mixture (600 µl) contained 50 mM Tris-HCl (pH 7.4) and 5 mM CaCl2. In all buffers, protease inhibitors were added just before use: phenylmethylsulfonyl fluoride and benzamidine at 1 mM each, aprotinin and leupeptin at 10 µg/ml, and pepstatin A at 1 µg/ml. After the incubation, 0.3 mg of sodium borohydride was added, and the mixture was kept on ice for 15 minutes. This mixture was then acidified with HCl (pH 3.0). Appropriate unlabeled eicosanoids were used as internal standards. The sample was extracted in 2 ml of ethyl ether and solvent-evaporated, and the dried material was dissolved in 50 µl of methanol/water (3:1) solvent. Reverse-phase high-performance liquid chromatography (HPLC) analyses was conducted with a C18 Ultra sphere column (5 µm; 4.6 x 250 mm; Altex Scientific, Beckman Instruments, Fullerton, CA) equipped with a Waters Model 6000A pump and a Waters Model 717 Autosampler injector (Milford, MA). Metabolite separation was achieved using a 55% to 100% methanol stepwise gradient at 1.1 ml/min. Eluted radioactivity was monitored using a Flo-One/Beta detector (Radiomatic Instruments, Tampa, FL) linked with an IBM Pentium 4 computer for data processing. UV analysis was also performed (for nonradioactive detection of stearidonic acid (SDA), EPA, and 15-HEPE) by monitoring absorbance at 234 nm with a Waters 486 detector. [1-14C] Arachidonic acid and [1-14C] LA (40–60 mCi/mmol) were from DuPont-New England Nuclear (Boston, MA). Unlabeled eicosanoids such as 13-HODE, 15-HETE, 15-HEPE, and PGs such as PGE2 and PGE3 were from Cayman Chemical (Ann Arbor, MI). All solvents were HPLC grade and were from Baker (Phillipsburg, NJ). The 15-LO-1 and COX enzyme activities were expressed as percent conversion of substrate(s) to product(s).
Enzyme Activity Calculation of Other Enzymes of Eicosanoids Pathway
The activities of enzymes involved in fatty acid biosynthesis were estimated as the product-to-precursor ratios of the percentages of individual fatty acids [26]. The estimated enzyme activities included those of elongase, calculated as the stearic acid (18:0)-to-palmitic acid (16:0) ratio; Δ-5-desaturase, calculated as the AA [20:4(ω-6)]-to-dihomo-gamma (γ)-linolenic acid (DGLA) [20:3(ω-6)] ratio; Δ-6 desaturase, calculated as the DGLA [20:3(ω-6)]-to-LA [18:2(ω-6)] ratio (assuming that Δ-6 desaturase and not elongase is rate limiting); and Δ-9 desaturase, calculated as the oleic acid [18:1(ω-9)]-to-stearic acid [18:0(ω-9)] ratio.
Diets, Experimental Strategy, Feeding Protocol, LAPC-4 Cell Injection, and Tumor Analyses
We used three different isocaloric diets (caloric density 4.4 kcal/g) for our study (Table 1). These custom-made, semipurified diets were prepared and irradiated by Purina Test Diet, Inc. (Richmond, IN). High ω-6 LA and high ω-3 SDA fat diet groups (experimental) were identical to those that are described before [25]. Use of pure EPA is cost-prohibitive, and therefore, SDA, an immediate precursor of EPA, served as a legitimate substitute. Furthermore, because of concerns with mercury and polychlorinated biphenyl contamination of fish and fish oils, which currently provide the major sources of long-chain ω-3 in the human diet, land-based sources of functional ω-3 fatty acids such as SDA are currently being developed [27]. Normal-fat diet (control) was also isocaloric when adjusted by adding dextrin and corn oil.
Table 1.
Composition of Isocaloric Diets (g/100 g).
| Ingredients* | Normal | SDA (ω-3) | LA (ω-6) |
| Sucrose | 15.0 | 35.0 | 35.0 |
| Casein-vitamin-Free | 21.0 | 21.0 | 21.0 |
| RP mineral mix no. 10 (adds 1.29% fiber) | 5.0 | 5.0 | 5.0 |
| Powdered cellulose | 3.0 | 3.0 | 3.0 |
| RP vitamin mix (adds 1.9% sucrose) | 2.0 | 2.0 | 2.0 |
| Choline chloride | 0.2 | 0.2 | 0.2 |
| dl-Methionine | 0.2 | 0.2 | 0.2 |
| SDA | 0.0 | 15.0 | 0.0 |
| Safflower oil | 0.0 | 0.0 | 15.0 |
| Dextrin | 38.6 | 18.6 | 18.6 |
| Corn oil | 5.0 | 0.0 | 0.0 |
| Lard | 10.0 | 0.0 | 0.0 |
Caloric density is 4.4 kcal/g.
Values provided by the supplier.
The experimental strategy is illustrated in detail in Figure 2. Initially, we took a total of 101 athymic male BALB/C nude (nu/nu) (6–8 weeks old) mice obtained from Charles River (Wilmington, MA) and fed them initially a no-fat diet for 2 weeks as described before [25]. As illustrated in Figure 2, after 2 weeks, we split these mice into three groups, namely, two experimental groups (a total of n = 76 divided into 38 mice per high ω-6 LA and ω-3 SDA fat diet groups [experimental]) and a control group [n = 25 per normal dietary group]). These mice were housed in single-sterile animal cages to allow for the maintenance of isocaloric intake between the diet groups. Cages, bedding, and water were autoclaved before use. Specially designed feeding receptacles were placed in the cages so that food intake could be carefully monitored. Sterile techniques were used whenever handling the cages, mice, and food. The Pittsburgh Animal Research Committee approved the experiments, and animals were cared for in accordance with institutional guidelines.
Figure 2.
Experimental strategy.
After 2 weeks on no-fat diet, all the n = 101 mice were injected with LAPC-4 cells. This time point was counted as week 1. The mice were injected in duplicate subcutaneously in the right and left lateral flank with 1 x 106 LAPC-4 tumor cells in 0.1 ml of Matrigel (Collaborative Biomedical Products, Bedford, MA). When tumors became palpable, the tumor dimensions were measured [28]. Tumor growth and apoptosis were examined as end points after administration of different experimental and control short-term (5 week) diets. At week 23, half of the mice from each PUFA group were switched to the opposing diets (i.e., from SDA to LA diet and vice versa), whereas the other half of the mice remained on their original diet. Tumors were examined for growth, lipids, enzyme activities, apoptosis, and proliferation indices.
Red Blood Cells Membrane and Tumor Phospholipid Content Analyses
Phospholipid content from red blood cells (RBCs) and tumor tissue membranes were analyzed by temperature-programmed microcapillary gas liquid chromatography as described before [25]. The fatty acids are expressed as a percentage from total phospholipids measured (C14/C22). The interassay coefficient of variation for determining the different fatty acids by this method ranged between 2.6% and 9.1%, reflecting the high reproducibility of the assay.
Assessment of Apoptotic and Proliferation Indices by Immunohistochemistry
Sections of formalin-fixed, paraffin-embedded LAPC-4 tumor tissues (5 µm) were tested for the presence Ki-67 and caspase-3 (1:50), using an avidin-biotin complex technique and steam heat-induced antigen retrieval. Cells were defined as apoptotic if the whole nuclear area of the cell labeled positively for caspase-3. Apoptotic bodies were defined as small positively labeled globular bodies in the cytoplasm of the tumor cells (singly or in groups). To estimate the apoptotic index (the percentage of apoptotic events in a given area), apoptotic cells and bodies were counted in 10 high-power fields, and this figure was divided by the number of tumor cells in the same high-power fields. We also estimated the apoptotic index by light microscopy using hematoxylin-stained slides from the same tumor sections as the caspase-3. The intensity of staining in 10 high-power fields were scored descriptively or semiquantitatively by a pathologist as 1+ (0%–25% positive cells), 2+ (25%–50% positive cells), 3+ (50%–75% positive cells), and ≥4+ (75%–100% positive cells) in a blinded manner. Proliferation was similarly examined, and index was estimated as total Ki-67-labeled cells/total cells counted.
Statistical Analyses
Statistical analyses (SAS version 5.0) were performed by Student's t test or analysis of variance. Correlations between outcome variables were computed using the Spearman correlation coefficient. P ≤ .05 was considered significant. Data are expressed as mean ± SD.
Results
Effect of Diets on Tumor Growth
We initially examined the effects of the specific diets (i.e., normal, ω-3 SDA, and ω-6 LA) on tumor growth (n = 20 mice per diet group) during a total of a 28-week period (Table 1 and Figure 1). At week 23, 10 mice from ω-3 SDA diet-fed mice were switched to ω-6 LA diet and 10 mice from ω-6 LA diet-fed mice were switched to ω-3 SDA diet. The remaining 10 mice continued to be fed with their original diets. Twenty normal diet-fed mice served as controls. Tumors from each of these cohorts were measured/week for five more weeks (i.e., from week 24 to 28). Tumors from the LA diet-fed mice exhibited the most rapid growth compared with tumors from the control and SDA diet-fed mice. Moreover, a diet switch from LA to SDA caused a dramatic decrease in the growth of tumors in 5 weeks (from week 23 to 28), whereas tumors grew more aggressively when mice were switched from an SDA to an LA diet in the same period (Figure 1).
Figure 1.
Average tumor volume per week in experimental mice. The tumor dimensions were measured using a caliper. Tumor volumes were calculated using the formula: length x width x height x 0.5236 (correction factor). For clarity, the SD error bars from the mean of 10 values [SD was <10% of the mean] have been omitted. ○: Normal (control) diet-fed mice;Δ: LA (ω-6) diet-fed mice;●: SDA (ω-3) diet-fed mice; □: switch from LA to SDA diet; and ♦: switch from SDA to LA diet.
Effect of Diets on Proliferation and Apoptotic Indices in Tumors
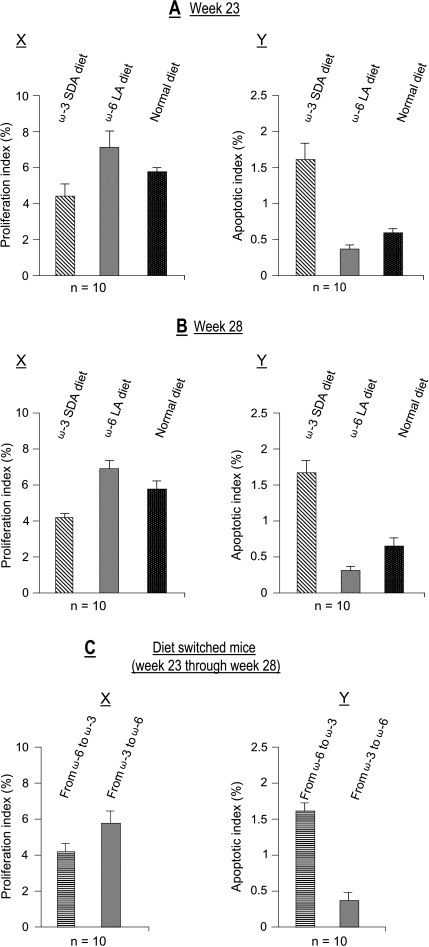
On the basis of the experimental strategy outlined in Figure 2, we used immunohistochemistry to evaluate tumor proliferation (Ki-67) and apoptosis' (caspase-3) in mice (n = 10 per diet groups) fed the normal, LA, and SDA diets at week 23 (Figure 3A) and week 28 (Figure 3B) time points. As depicted in Figure 3, AX and BX, the calculated percentage proliferation index was 4–5 ± 0.2–1 in the SDA diet-fed mice, 7 ± 1–1.5 in the LA diet-fed mice, and 5.8 ± 0.3–0.4 in the normal diet-fed mice. Simultaneously, as depicted in Figure 3, AY and BY, the calculated percentage apoptotic index was 1.6–1.7 ± 0.2–0.3 in the SDA diet-fed mice, 0.4 ± 0.1 in the LA diet-fed mice, and 0.6 ± 0.2-0.1 in the normal diet-fed mice. As expected, there were no apparent major differences in week 28 versus week 23 in either the proliferation or the apoptosis index. However, as shown in Figure 3C, we assessed proliferation (X) and apoptotic indices (Y) in tumors after the 5-week period (from week 24 to 28) from diet-switched mice (i.e., from LA to SDA and vice versa, n = 10 per diet group). We observed a dramatic decrease in the proliferation index and an increase in apoptotic index in tumors from mice that were switched from LA to SDA diet. Interestingly, these changes were reversed in tumors from mice that were switched from SDA to LA diet.
Figure 3.
Assessment in weeks 23 (A) and 28 (B) of proliferation (X) and apoptotic indices (Y) in tumors from mice fed with SDA ( ), LA (
), LA ( ), and normal diets (■). Similar assessments were performed as shown in diet-switched mice (C) (i.e., from LA to SDA [
), and normal diets (■). Similar assessments were performed as shown in diet-switched mice (C) (i.e., from LA to SDA [ ] and vice versa [
] and vice versa [ ]) after a 5-week period. Sections of formalin-fixed, paraffin-embedded tumor tissues were tested for the presence of Ki-67 (proliferation marker) and caspase-3 (apoptosis marker). Proliferation and apoptotic indices (%) were estimated as described in the Materials and Methods section. A total of n = 10 tumor tissues per group were examined.
]) after a 5-week period. Sections of formalin-fixed, paraffin-embedded tumor tissues were tested for the presence of Ki-67 (proliferation marker) and caspase-3 (apoptosis marker). Proliferation and apoptotic indices (%) were estimated as described in the Materials and Methods section. A total of n = 10 tumor tissues per group were examined.
Red Blood Cells and Tumor Phospholipid Content Analyses
As shown in Table 2, A (RBCs) and B (tumors), levels of ω-6 LA, AA, and ω-3 EPA and DHA in membrane phospholipids of RBCs reflected dietary changes in normal, LA, and SDA diet-fed mice (n = 10 per diet groups). There were no significant differences in membrane phospholipid content in both these tissues observed at week 23 versus week 28 (a 5-week period). This study also ascertained that the RBCs membrane phospholipid analysis is suitable for the accurate measurement of dietary intake of PUFAs. Interestingly, when cohorts of mice (n = 10) were switched from ω-6 diet to ω-3 diet and vice versa and allowed to feed further for a 5-week period, the levels of the ω-6 and ω-3 PUFAs in both RBCs and tumor membrane phospholipids were also modulated accordingly (Table 2, A and B).
Table 2.
Composition of LA, AA, EPA, and DHA in Phospholipids (n = 10).
| % of Total Phospholipids | Diet Group n = 10 | |||||||
| Normal | LA Diet | SDA Diet | ω-6 → ω-3 | ω-3 → ω-6 | ||||
| 23rd Week | 28th Week | 23rd Week | 28th Week | 23rd Week | 28th Week | 5th Week | 5th Week | |
| (A) RBC membranes | ||||||||
| LA | 6.2 ± 0.6 | 5.9 ± 0.8 | 12.2 ± 0.6 | 11.7 ± 0.6 | 2.2 ± 0.1 | 2.4 ± 0.1 | 3.2 ± 0.6 | 11.4 ± 0.3 |
| AA | 6.3 ± 0.3 | 6.7 ± 0.4 | 8.2 ± 0.6 | 8.4 ± 0.5 | 6.1 ± 0.1 | 6.4 ± 0.3 | 6.4 ± 0.6 | 7.1 ± 0.7 |
| EPA | ND | ND | ND | ND | 3.4 ± 0.2 | 3.2 ± 0.3 | 3.9 ± 1.2 | 0.9 ± 0.4 |
| DHA | ND | ND | ND | ND | 6.2 ± 0.8 | 5.9 ± 1.2 | 5.8 ± 1.2 | 0.4 ± 0.06 |
| (B) Tumors from mice fed with normal, LA, and SDA diets | ||||||||
| LA | 5.9 ± 0.4 | 5.8 ± 0.7 | 12.4 ± 0.4 | 12.1 ± 0.7 | 2.5 ± 0.7 | 2.3 ± 0.9 | 3.1 ± 0.5 | 11.8 ± 0.4 |
| AA | 6.1 ± 0.3 | 6.2 ± 0.3 | 7.8 ± 0.5 | 7.9 ± 0.7 | 6.4 ± 0.2 | 6.1 ± 0.5 | 6.6 ± 0.8 | 6.9 ± 0.8 |
| EPA | ND | ND | ND | ND | 3.3 ± 0.3 | 3.5 ± 0.1 | 3.4 ± 0.5 | 1.1 ± 0.2 |
| DHA | ND | ND | ND | ND | 6.4 ± 0.4 | 5.7 ± 0.6 | 5.9 ± 1.1 | 0.6 ± 0.04 |
Fatty acid methyl esters were analyzed by gas liquid chromatography as described in the Materials and Methods section. The fatty acids are expressed as percentage (%) from total phospholipids and represent the mean ± SD of 10 determinants.
Modulation of 15-LO-1, COX, and Key Enzymes of the ω-6 Pathway in Tumors
To assess whether the tumor-modulating effects of ω-3 SDA (anti-tumorigenic) and ω-6 LA (protumorigenic) diets are suggestive of their actions in diet-switching experiments, we examined tissue metabolites from different dietary cohorts by HPLC analysis (Table 3). Tumor lysates from LA diet-fed mice showed two-fold higher levels of 13-(S)-HODE versus normal diet-fed mice and remained undetectable in SDA diet-fed mice. Interestingly, when cohorts of mice were switched from ω-6 diet to ω-3 diet (from LA diet to SDA diet), there was a significant decrease in 13-HODE from 2.1 ± 0.2 to 0.2 ± 0.1 (n = 10, P < .05) and a decrease from 1.8 ± 0.06 to 0.3 ± 0.03 (n = 10, P < .02) in 15-HEPE when cohorts of mice were switched from ω-3 diet to ω-6 diet (from SDA diet to LA diet). This confirmed 15-LO-1 activity in the tumors. Although we did not observe major differences in the total PG levels in either diet fed or diet switch groups, formation of PGs confirmed COX activity. We did not find detectable levels of 15-HETE, indicating either a deficiency in 15-LO-2 enzyme or a poor 15-LO-1/AA metabolism.
Table 3.
Enzyme (15-LO-1 and COX) Activity Profiles from the Tumors of Mice Fed with LA, SDA, LA to SDA, and SDA to LA Diets Were Measured as Described in the Materials and Methods Section.
| Diet Group at Week 23 (n = 10) | Metabolic Products | |||
| 13-HODE | 15-HETE | 15-HEPE | Total PGs | |
| Normal | 0.9 ± 0.2 | 0.1* | 0 | 6.1 ± 0.33 |
| LA diet | 2.1 ± 0.2† | ND | 0 | 6.2 ± 0.46 |
| SDA diet | ND† | ND | 1.8 ± 0.06‡ | 7.1 ± 0.26 |
| LA to SDA (after a 5-week time point) | 0.2 ± 0.1† | ND | 1.4 ± 0.03‡ | 7.6 ± 0.54 |
| SDA to LA (after a 5-week time point) | 1.8§ | ND | 0.3 ± 0.03‡ | 6.4 ± 0.22 |
Percent (%) conversion of the products represents mean ± SD of 10 determinations from one experiment.
ND indicates not detectable.
Observed in two tissues.
P < .05.
P < .02.
Observed in one tissue.
In comparison with mice fed the normal diet, we observed a 2.7-fold increase in the ω-6/ω-3 ratio in tumors from LA-fed mice and a 4.2-fold decrease in the ratio in tumors from the SDA-fed mice (Table 4). We observed increased estimated activity of Δ-6-desaturase enzyme in tumors from mice fed the SDA diet and the decreased activity in tumors from mice fed the LA diet. Similarly, we observed a reduced estimated Δ-5-desaturase activity in the tumors from SDA diet cohort. Interestingly, the estimated activity of the anabolic n-9 monounsaturated fatty acid pathway enzyme, Δ-9-desaturase, increased in tumors from SDA diet-fed mice while it decreased in tumors from LA diet-fed mice (Table 4).
Table 4.
Phospholipid Analysis of Unsaturated and Saturated Fatty Acids (PUFAs), PUFA Ratios, and Activities of Key Enzymes Involved in Fatty Acid Biosynthesis.
| % Unsaturated of Total Phospholipids | % Saturated of Total Phospholipids | PUFA n-6/n-3 | Elongase | Δ-5 Desaturase | Δ-6 Desaturase | Δ-9 Desaturase | |
| Diet group at week 23 | |||||||
| Normal | 49.4 ± 1.7 | 2.7 ± 1.5 | 3.4 ± 1.1 | 84.1 ± 1.7 | 7.1 ± 0.6 | 3.1 ± 0.6 | 12.7 ± 0.6 |
| LA diet | 51.1 ± 1.2 | 44.1 ± 1.3 | 9.4 ± 1.1 | 110.1 ± 1.6 | 14.4 ± 0.3 | 7.4 ± 0.5 | 7.1 ± 0.7 |
| SDA diet | 50.2 ± 0.9 | 43.2 ± 1.1 | 0.8 ± 0.1 | 42.2 ± 0.9 | 0.78 ± 0.06 | 12.9 ± 1.2 | 28.7 ± 1.2 |
| Diet group at week 28 | |||||||
| Normal | 48.7 ± 1.1 | 43.2 ± 1.7 | 3.5 ± 1.3 | 86.1 ± 2.4 | 6.9 ± 0.8 | 3.3 ± 0.2 | 13.0 ± 0.5 |
| LA (high fat, n-6) | 52.3 ± 1.1 | 43.7 ± 1.4 | 9.2 ± 1.3 | 115.3 ± 2.3 | 16 ± 0.5 | 6.2 ± 0.7 | 6.2 ± 0.7 |
| LA to SDA (after 5 weeks of exposure) | 50.1 ± 1.3 | 42.8 ± 1.3 | 2.4 ± 0.6 | 32.3 ± 0.7 | 0.92 ± 0.04 | 15.4 ± 2.2 | 25.4 ± 2.6 |
| SDA diet | 49.5 ± 1.4 | 44.1 ± 1.5 | 0.9 ± 0.2 | 37.4 ± 1.7 | 0.59 ± 0.05 | 11.7 ± 1.7 | 27.7 ± 1.7 |
| SDA to LA (after 5 weeks of exposure) | 52.6 ± 0.7 | 43.3 ± 1.1 | 8.1 ± 0.4 | 108.1 ± 2.9 | 12.2 ± 0.01 | 6.8 ± 0.2 | 7.2 ± 1.2 |
Fatty acid methyl esters were analyzed by gas chromatography and enzyme activities were estimated as the product-to-precursor ratios of the percentages of individual fatty acids as described in the Materials and Methods section. Values represent mean ± SD of 10 determinations.
Discussion
Ideally, the ratio of ω-3 to ω-6 PUFAs in the human body should be 1:4 to 1:1. A typical US diet is low in ω-3 and high in ω-6, and many individuals contain 10 to 20 times more ω-6 PUFAs than ω-3 PUFAs and international incidence patterns and migrations studies, epidemiological data as well as animal and in vitro studies, indicate that consuming a diet rich in fat increases the risk for developing PCa [8,29–33].
Our previous in vitro and in vivo studies demonstrate the presence of ω-6 LA-metabolizing enzyme 15-LO-1 at higher levels in PCa and as a key enzyme that contributes to the initiation and development of the neoplastic phenotype in PCa [16,17,25,28,34–36]. In this study, we provide information on the mechanisms of how the metabolites of antitumorigenic ω-3 fatty acids can modulate the protumorigenic ω-6 LA and AA enzymatic pathways.
Our hypothesis was that diet high in ω-3 PUFAs competes with ω-6 LA and -AA as a substrate for the enzyme 15-LO-1, upregulated in PCa, resulting in reduced levels of the protumorigenic metabolite of LA, 13-(S)-HODE, and increased levels of the antitumorigenic metabolites EPA (SDA metabolite), 15-HEPE, and PGE3. Eicosapentaenoic acid can also compete with AA as a substrate for the COX-2 enzyme, also upregulated in PCa, resulting in reduced levels of the proinflammatory metabolite of AA, PGE2, and increased levels of the anti-inflammatory metabolites of EPA, PGE3. The absence of detectable 15-HETE indicated either a deficiency in 15-LO-2 enzyme or a poor 15-LO-1/AA metabolism [37–39]. Importantly, these results further corroborate our previous in vitro and in vivo study that SDA (and EPA) does not inhibit the activities of either 15-LO-1 or COX and that tumor growth reflects the substrate competition of ω-3 with ω-6 fatty acids [25].
The increased estimated activity of Δ-6-desaturase enzyme in tumors from mice fed the SDA diet and the decreased activity in tumors from mice fed the LA diet could be associated with the accumulation of the antiproliferative GLA and DGLA [40]. Similarly, reduced estimated Δ-5-desaturase activity in the tumors from SDA diet cohort confirms the previous observation from in vitro studies with LAPC-4 cells [25], suggesting that ω-3 SDA inhibits Δ-5-desaturase enzyme activity. This then further limits the formation of DHA and causes the accumulation of GLA and DGLA.
The increased estimated activity of the anabolic ω-9 monounsaturated fatty acid pathway enzyme, Δ-9-desaturase, in tumors from SDA diet-fed mice but decreased activity in LA diet-fed mice suggests that SDA can favor the conversion of stearic acid (18:0, n-9) to oleic acid (18:1, n-9). However, this conversion is inhibited by LA. Together, these observations provide additional support for the role of ω-3 SDA in slowing PCa growth. The observation of Δ-9-desaturase activity modulation by SDA may also suggest that the tumor inhibition is either independent of oleic acid action [41]. This is particularly an important consideration for clinical trial studies in PCa patients using oleic acid as a placebo.
In addition to 15-LO-1, evidence also suggests that other metabolic enzymes play important roles in PCa pathobiology. Arachidonic acid obtained through diet or LA metabolic conversion is the preferred substrate for 15-LO-2. 15-LO-2 converts AA to 15-(S)-HETE, a metabolite shown to both enhance apoptosis and act as a negative cell cycle regulator [42]. Furthermore, 15-(S)-HETE has anti-inflammatory properties [24,43,44], and studies support an association between inflammation and PCa [45,46]. Similarly, AA also acts as a substrate for COX-2 leading to the production of PGs, such as PGE2, that have proinflammatory effects and thus possibly contribute to PCa pathobiology. Although the present study did not specifically evaluate for both COX-1 and COX-2 levels or for PGs such as PGE2 versus PGE3, previous studies have demonstrated an overexpression of COX-2 [19,20] and an antitumorigenic role of PGE3 in PCa [23,47]. Therefore, owing to the abundance of COX-2 in PCa tissue, it was reasonable to assume that most AA in tumors cells would be converted to PGE2 if ω-3s were low or absent.
More specifically, use of dietary ω-3 PUFAs as agents for cancer prevention can prove a valuable strategy in the fight against PCa [48–57] and recurrence. Consequently, 15-LO-1 metabolizes EPA to 15-hydroxyeicosapentaenoic acid [15-HEPE] [47], a metabolite shown to have antitumorigenic properties, whereas COX-2 that metabolizes EPA to the anti-inflammatory as well as antitumorigenic PGE3 [23,45,58]. Therefore, based on the substrates, that is, dietary ω-6 or ω-3 in concert with the activities of 15-LO-1 and COXs, cells can be predisposed to either a proliferative or antiproliferative outcome. This effect seems to be independent of percent unsaturated or percent saturated fatty acids in the total phospholipids as observed in tumors and suggests that the cells are able to maintain membrane fluidity and functionality of proteins in the membrane.
This study supports our hypothesis and explains the observation that: 1) EPA and LA both compete as substrates for the enzyme 15-LO-1, which results in a decrease in the protumorigenic metabolite of LA, 13-(S)-HODE, and an increase in the antitumorigenic metabolite of EPA, 15-HEPE; and 2) EPA and AA both compete as substrates for the enzyme COX-2, which may result in the production of “good” PGs (for e.g., PGE3). Thus, SDA-derived EPA alone and EPA-derived fatty acids such as DHA or a combination in fish oil are promising dietary intervention agents against PCa aimed at 15-LO-1 and COX-2 as the molecular targets.
Abbreviations
- 15-LO-1
15-lipoxygenase-1
- AA
arachidonic acid
- COX
cyclooxygenase
- HPLC
high-performance liquid chromatography
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxyoctadecadienoic acid
- LO
lipoxygenase
- LA
linoleic acid
- PG
prostaglandin
- LAPC-4
Los Angeles Prostate Cancer-4
- PUFAs
polyunsaturated fatty acids
Footnotes
This work was supported in part by the US Army Department of Defense grant (W81XWH-07) to U.K. and in no way reflects the opinion of the US Government.
References
- 1.ACS, author. American Cancer Society: Cancer Facts and Figures. Atlanta, GA: 2008. [Google Scholar]
- 2.Marberger M, Carroll PR, Zelefsky MJ, Coleman JA, Hricak H, Scardino PT, Abenhaim LL. New treatments for localized prostate cancer. Urology. 2008;72:S36–S43. doi: 10.1016/j.urology.2008.08.506. [DOI] [PubMed] [Google Scholar]
- 3.Zerbib M, Zelefsky MJ, Higano CS, Carroll PR. Conventional treatments of localized prostate cancer. Urology. 2008;72:S25–S35. doi: 10.1016/j.urology.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Van Patten CL, de Boer JG, Tomlinson Guns ES. Diet and dietary supplement intervention trials for the prevention of prostate cancer recurrence: a review of the randomized controlled trial evidence. J Urol. 2008;180:2314–2322. doi: 10.1016/j.juro.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 5.Syed DN, Suh Y, Afaq F, Mukhtar H. Dietary agents for chemoprevention of prostate cancer. Cancer Lett. 2008;265:167–176. doi: 10.1016/j.canlet.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn JE. Cancer epidemiology in populations of the United States—with emphasis on Hawaii and California—and Japan. Cancer Res. 1975;35:3240–3245. [PubMed] [Google Scholar]
- 7.Ritch CR, Wan RL, Stephens LB, Taxy JB, Huo D, Gong EM, Zagaja GP, Brendler CB. Dietary fatty acids correlate with prostate cancer biopsy grade and volume in Jamaican men. J Urol. 2007;177:97–101. doi: 10.1016/j.juro.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 8.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 9.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K, Jakobsen MU, Johnsen NF, Tjonneland A, Linseisen J, Rohrmann S, et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;87:1405–1413. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Nie D, Witt WT, Chen Q, Shen M, Xie H, Lai L, Dai Y, Zhang J. Expression of the fat-1 gene diminishes prostate cancer growth in vivo through enhancing apoptosis and inhibiting GSK-3{beta} phosphorylation. Mol Cancer Ther. 2008;7:3203–3211. doi: 10.1158/1535-7163.MCT-08-0494. [DOI] [PubMed] [Google Scholar]
- 12.Kang JX. Fat-1 transgenic mice: a new model for omega-3 research. Prostaglandins Leukot Essent Fatty Acids. 2007;77:263–267. doi: 10.1016/j.plefa.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta. 1994;1213:277–288. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC. Specific fatty acids and risks of breast and prostate cancer: dietary intake. Am J Clin Nutr. 1997;66:1557S–1563S. doi: 10.1093/ajcn/66.6.1557S. [DOI] [PubMed] [Google Scholar]
- 15.Kelavkar U, Cohen C, Eling T, Badr K. 15-Lipoxygenase-1 overexpression in prostate adenocarcinoma. Adv Exp Med Biol. 2002;507:133–145. doi: 10.1007/978-1-4615-0193-0_22. [DOI] [PubMed] [Google Scholar]
- 16.Kelavkar U, Cohen C, Kamitani H, Eling T, Badr K. Concordant induction of 15-lipoxygenase-1 and mutant p53 expression in human prostate adenocarcinoma: correlation with Gleason staging. Carcinogenesis. 2000;21:1777–1787. doi: 10.1093/carcin/21.10.1777. [DOI] [PubMed] [Google Scholar]
- 17.Kelavkar U, Glasgow W, Eling TE. The effect of 15-lipoxygenase-1 expression on cancer cells. Curr Urol Rep. 2002;3:207–214. doi: 10.1007/s11934-002-0066-8. [DOI] [PubMed] [Google Scholar]
- 18.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993;330:156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- 20.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang P, Chan D, Felix E, Madden T, Klein RD, Shureiqi I, Chen X, Dannenberg AJ, Newman RA. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids. 2006;75:385–395. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Narayanan NK, Narayanan BA, Reddy BS. A combination of docosa-hexaenoic acid and celecoxib prevents prostate cancer cell growth in vitro and is associated with modulation of nuclear factor-kappaB, and steroid hormone receptors. Int J Oncol. 2005;26:785–792. [PubMed] [Google Scholar]
- 23.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serhan CN. Novel omega-3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther. 2005;105:7–21. doi: 10.1016/j.pharmthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Kelavkar UP, Hutzley J, Dhir R, Kim P, Allen KGD, McHugh K. Prostate tumor growth and recurrence can be modulated by the omega-6:omega-3 ratio in diet: athymic mouse xenograft model simulating radial prostatectomy. Neoplasia. 2006;8:112–124. doi: 10.1593/neo.05637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson A, Sjodin A, Hedman A, Olsson R, Vessby B. Fatty acid profile of skeletal muscle phospholipids in trained and untrained young men. Am J Physiol Endocrinol Metab. 2000;279:E744–E751. doi: 10.1152/ajpendo.2000.279.4.E744. [DOI] [PubMed] [Google Scholar]
- 27.Ursin VM. Modification of plant lipids for human health: development of functional land-based omega-3 fatty acids. J Nutr. 2003;133:4271–4274. doi: 10.1093/jn/133.12.4271. [DOI] [PubMed] [Google Scholar]
- 28.Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22:1765–1773. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- 29.Dunn AJ, Chuluyan HE. The role of cyclo-oxygenase and lipoxygenase in the interleukin-1-induced activation of the HPA axis: dependence on the route of injection. Life Sci. 1992;51:219–225. doi: 10.1016/0024-3205(92)90078-4. [DOI] [PubMed] [Google Scholar]
- 30.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 31.Allison DB, Egan SK, Barraj LM, Caughman C, Infante M, Heimbach JT. Estimated intakes of trans fatty and other fatty acids in the US population. J Am Diet Assoc. 1999;99:166–174. doi: 10.1016/S0002-8223(99)00041-3. quiz 175–176. [DOI] [PubMed] [Google Scholar]
- 32.Brawley OW, Knopf K, Thompson I. The epidemiology of prostate cancer, part II: the risk factors. Semin Urol Oncol. 1998;16:193–201. [PubMed] [Google Scholar]
- 33.Le Marchand L, Kolonel LN, Wilkens LR, Myers BC, Hirohata T. Animal fat consumption and prostate cancer: a prospective study in Hawaii. Epidemiology. 1994;5:276–282. doi: 10.1097/00001648-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Kelavkar U, Yang L, Landsittel D, Chandran U, Dhir R. The Yin and Yang of 15-lipoxygenase-1 and Delta-5-desaturase: dietary omega-6 linoleic acid metabolic pathway in prostate carcinogenesis. J Carcinog. 2006;5:9–11. doi: 10.1186/1477-3163-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelavkar UP, Cohen C. 15-Lipoxygenase-1 expression upregulates and activates insulin-like growth factor-1 receptor in prostate cancer cells. Neoplasia. 2004;6:41–52. doi: 10.1016/s1476-5586(04)80052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelavkar UP, Glasgow W, Olson SJ, Foster BA, Shappell SB. Overexpression of 12/15-lipoxygenase, an ortholog of human 15-lipoxygenase-1, in the prostate tumors of TRAMP mice. Neoplasia. 2004;6:821–830. doi: 10.1593/neo.04286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shappell SB, Boeglin WE, Olson SJ, Kasper S, Brash AR. 15-Lipoxygenase-2 (15-LOX-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. Am J Pathol. 1999;155:235–245. doi: 10.1016/S0002-9440(10)65117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shappell SB, Manning S, Boeglin WE, Guan YF, Roberts RL, Davis L, Olson SJ, Jack GS, Coffey CS, Wheeler TM, et al. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia. 2001;3:287–303. doi: 10.1038/sj.neo.7900166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack GS, Brash AR, Olson SJ, Manning S, Coffey CS, Smith JA, Jr, Shappell SB. Reduced 15-lipoxygenase-2 immunostaining in prostate adenocarcinoma: correlation with grade and expression in high-grade prostatic intraepithelial neoplasia. Hum Pathol. 2000;31:1146–1154. doi: 10.1053/hupa.2000.16670. [DOI] [PubMed] [Google Scholar]
- 40.Ziboh A, Vang K. 15-Lipoxygenase metabolites of γ-linolenic acid/eicosapentaenoic acid suppress growth and arachidonic acid metabolism in human prostatic adenocarcinoma cells: Possible implications of dietary fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2005;72:363–372. doi: 10.1016/j.plefa.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Escrich E, Mora R, Grau L, Costa I, Solanas M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol Nutr Food Res. 2007;51:1279–1292. doi: 10.1002/mnfr.200700213. [DOI] [PubMed] [Google Scholar]
- 42.Tang S, Bhatia B, Maldonado CJ, Yang P, Newman RA, Liu J, Chandra D, Traag J, Klein RD, Fischer SM, et al. Evidence that arachidonate 15-lipoxygenase 2 is a negative cell cycle regulator in normal prostate epithelial cells. J Biol Chem. 2002;277:16189–16201. doi: 10.1074/jbc.M111936200. [DOI] [PubMed] [Google Scholar]
- 43.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8:115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Nelson WG, DeWeese TL, DeMarzo AM. The diet, prostate inflammation, and the development of prostate cancer. Cancer Metastasis Rev. 2002;21:3–16. doi: 10.1023/a:1020110718701. [DOI] [PubMed] [Google Scholar]
- 46.Donnell RF. Epidemiology of inflammation and prostate cancer. Curr Urol Rep. 2004;5:297. doi: 10.1007/s11934-004-0053-3. [DOI] [PubMed] [Google Scholar]
- 47.Hughes-Fulford M, Chen Y, Tjandrawinata RR. Fatty acid regulates gene expression and growth of human prostate cancer PC-3 cells. Carcinogenesis. 2001;22:701–707. doi: 10.1093/carcin/22.5.701. [DOI] [PubMed] [Google Scholar]
- 48.Lee MM, Chang JS, Jacobs B, Wrensch MR. Complementary and alternative medicine use among men with prostate cancer in 4 ethnic populations. Am J Public Health. 2002;92:1606–1609. doi: 10.2105/ajph.92.10.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sparber A, Bauer L, Curt G, Eisenberg D, Levin T, Parks S, Steinberg SM, Wootton J. Use of complementary medicine by adult patients participating in cancer clinical trials. Oncol Nurs Forum. 1999;27:623–630. [PubMed] [Google Scholar]
- 50.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103:323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 51.Downer SM, Cody MM, McCluskey P, Wilson PD, Arnott SJ, Lister TA, Slevin ML. Pursuit and practice of complementary therapies by cancer patients receiving conventional treatment. BMJ. 1994;309:86–89. doi: 10.1136/bmj.309.6947.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wynder EL, Mabuchi K, Whitmore WF., Jr Epidemiology of cancer of the prostate. Cancer. 1971;28:344–360. doi: 10.1002/1097-0142(197108)28:2<344::aid-cncr2820280214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 53.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–1766. doi: 10.1016/S0140-6736(00)04889-3. [DOI] [PubMed] [Google Scholar]
- 54.Terry PD, Rohan TE, Wolk A. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr. 2003;77:532–543. doi: 10.1093/ajcn/77.3.532. [DOI] [PubMed] [Google Scholar]
- 55.Yang YJ, Lee SH, Hong SJ, Chung BC. Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clin Biochem. 1999;32:405–409. doi: 10.1016/s0009-9120(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 56.Freeman VL, Meydani M, Yong S, Pyle J, Flanigan RC, Waters WB, Wojcik EM. Prostatic levels of fatty acids and the histopathology of localized prostate cancer. J Urol. 2000;164:2168–2172. [PubMed] [Google Scholar]
- 57.Freeman VL, Meydani M, Hur K, Flanigan RC. Inverse association between prostatic polyunsaturated fatty acid and risk of locally advanced prostate carcinoma. Cancer. 2004;101:2744–2754. doi: 10.1002/cncr.20676. [DOI] [PubMed] [Google Scholar]
- 58.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]