Abstract
Objective
Farnesoid X Receptor (FXR) mediates important signaling functions of bile acids in diverse cell types including those residing in the vascular wall. Indeed, recent work has identified FXR as a potential regulator of vascular structure and function in part through transcriptional activation of MMP-9. However, the signal transduction pathways linking bile acids to changes in actin cytoskeleton that are responsible for bile acid–induced vascular cell migration remain unexplored.
Methods and Results
The FXR agonist and prototypical bile acid, chenodeoxycholic acid (CDCA), significantly increased endothelial cell (EC) motility, as analyzed by time lapse video microscopy, and tube formation, an in vitro correlate for angiogenesis. Increased cell motility was associated with prominent increases in focal adhesion (FA) plaques and was inhibited by FXR or MMP-9 siRNA, indicating a FXR–MMP-9–dependency of this signaling pathway. Mechanistically, incubation of cells with CDCA was associated with phosphorylation of a key FA protein, Focal Adhesion Kinase (FAK) at Y397 but not at Y576/577, or Y925. Studies using a site-specific phosphorylation mutant (phosphodeficient) of FAK revealed that FAK phosphorylation at tyrosine residue –397 was required for CDCA induced activation of the downstream FA assembly protein, paxillin. Lastly, siRNA-based silencing of FAK as well as phosphodeficient FAK mutant inhibited CDCA induced upregulation of MMP-9, cell motility, and vascular tube formation.
Conclusion
Thus, this study demonstrates a pivotal role for FAK in the process of FXR-induced and MMP-9–dependent EC motility and vascular tube formation.
Keywords: bile acids, FXR, MMP-9, FAK, angiogenesis
Farnesoid X Receptor (FXR) belongs to a nuclear receptor superfamily of transcription factors that is activated by both natural ligands including the bile acid, CDCA, and synthetic ligands, such as 6-ECDCA.1,2 Although FXR was originally identified in hepatocyte homoeostasis, it has become increasingly clear that this nuclear receptor system is important in a number of different cell types and mediates diverse functions including cholesterol homeostasis and apoptosis.1,3,4 Additionally, increasing evidence supports a role for bile acids and FXR in the process of liver repair and vascular remodeling in part through effects on endothelial cells (ECs).5–7 Indeed, angiogenesis is increasingly recognized as a facilitator of repair mechanisms in response to liver injury,8 which is often associated with an increase in bile acids such as CDCA in the liver and blood.9 In this regard, although EC motility is a fundamental step in the process of angiogenesis and vascular remodeling, the signal transduction pathways linking bile acids to changes in actin cytoskeleton that are responsible for bile acid–induced EC motility remain unexplored.
Focal Adhesion Kinase (FAK) is a nonreceptor protein tyrosine kinase that localizes to focal adhesions (FAs) in response to specific stimuli.10 Studies have shown that FAK and paxillin, another FA associated protein, are important components in signaling events that lead to cell migration, and their interplay with MMP-9 pathways, though suggested, are not fully elucidated.11,12 However, FAK and paxillin have been shown to have stimulatory as well as inhibitory roles on cell migration.11–13 Furthermore, the majority of these studies were performed in epithelial cells as well as normal fibroblasts, and thus the role of FAK and paxillin on bile acid–induced EC motility is of interest.
In the present study we identify a key role for FAK in the process by which FXR activation leads to EC motility. Our data indicates that CDCA-induced MMP-9–dependent phosphorylation of FAK at tyrosine residue −397 leads to activation of the FA protein paxillin, with ensuing formation of FAs and increased cell motility and vascular tube formation, an in vitro read out of angiogenesis. Importantly, complementary use of siRNA and dominant negative approaches establish a mechanistic role for FAK in this process. Thus, the work identifies important signaling pathways that link FXR to the actin remodeling steps that are requisite for cell motility and angiogenesis.
Methods
Cell Culture and Transfection
Human umbilical vein endothelial cells (HUVECs; P1–P4) were grown in EBM-2 medium supplemented with EGM-2, 10% fetal bovine serum, and 1% streptomycin/penicillin. Cells were incubated with CDCA or equivalent volume of vehicle (Me2SO) at a concentration of 50 μmol/L14,15 and duration of incubation of 12 hours, unless indicated otherwise. HepG2 cells were also used in luciferase assays which were not feasible in ECs.14 Cells were transfected with FXR, MMP-9, or control siRNA (Ambion) or FAK siRNA (Dharmacon) using Oligofectamine (Invitrogen) as described previously.14 Green Fluorescent Protein (Ad GFP) or Y397F-FAK (phosphodefective FAK) mutant adenoviral constructs were generated as previously described.16 Cells grown to confluence in 100-mm dishes were infected with 100 multiplicity of infection of Ad GFP or Ad Y397F-FAK (phosphodefective FAK) mutant overnight in serum containing medium, after which the media was replaced with fresh media with no virus. In all experiments cells were used between 32 and 36 hours postvirus infection.
Immunofluorescence
Cells were serum-deprived for 12 hours and then stimulated with 50 μmol/L CDCA for 12 hours, (a time point at which CDCA-induced MMP-9 protein expression is achieved) rinsed quickly with phosphate buffered saline, and fixed with 2% paraformaldehyde. Cells were then labeled with antibodies recognizing p-FAK or vinculin or stained with phalloidin as described previously.17 In some experiments ECs were transfected with either FXR siRNA or MMP-9 siRNA and then treated with either vehicle or 50 μmol/L CDCA and stained with antibodies recognizing p-FAK or vinculin. Laser scanning confocal microscopy was performed using a Zeiss LSM 510 system (Carl Zeiss Microscopy), captured red and green fluorescence images were background subtracted, and a threshold was set to restrict analysis of FAs using an overlay image, which were then analyzed using the Image Pro-Plus software (version 6.2, Media Cybernatics). The number of FA plaques per cell was calculated from a total of 30 cells, and the data generated using the software were exported to Excel and representative figure were plotted in graph. For stress fiber analysis, images were analyzed using LSM image analysis. Fluorescent intensity was measured across the cell, and graphical values were generated as a measure of intensity along the specific distance across the cell.
Quantitative RT-PCR
Real-time fluorescence monitoring was performed with the Applied Biosystems 7500 Real-Time PCR System instrument as described previously.14 FAK, FXR, or MMP-9 mRNA was normalized to GAPDH mRNA and shown as the -fold change.
Western Blot Analysis
Cell lysates of serum-deprived ECs stimulated with 50 μmol/L CDCA were used as described previously14 for Western blotting with antiphospho FAK (Tyr 397; Chemicon International), antiphospho FAK (Tyr 576/577; Upstate), antiphospho FAK (Tyr 925; Cell Signaling), FAK, antiphospho paxillin (pY118), antiphospho c-Jun (Cell Signaling), anti c-Jun, anti-HA, and MMP-9 Abs (BD Biosciences), antipaxillin (Upstate) B-actin, or vinculin (Sigma).
Time-Lapse Video Microscopy
Endogenous expression of FXR, or MMP-9, or FAK was silenced using siRNA specific for FXR, or MMP-9, or FAK, respectively, before incubation with either vehicle or 50 μmol/L CDCA. Live cell motility was tracked using time-lapse video microscopy with a Zeiss Axiovert equipped with phase-contrast and epifluorescence microscopy and a temperature-controlled stage (Medical Systems Corp) to maintain 37°C and 5% CO2 incubation. Images were collected under low-light illumination using an intensified CCD C2400 camera (Hamamatsu Photonics K.K.) at 63× magnification every 3 minutes for a total period of 3 to 6 hours. Image processing and data analysis were performed using MetaMorph (Universal Imaging/Molecular Devices) software, and the data (distance [μm] or velocity [μm/s]) were transferred to Excel (Microsoft) for analysis and representation.18
Vascular Tube Formation Assay
Cells were placed on 100 μL Matrigel after 30 minutes of preincubation at 37°C. siRNA transfections were performed to silence endogenous expression of FXR, or MMP-9, or FAK in ECs, using respective siRNA. In some experiments, ECs were transduced with Ad Y397F FAK to overexpress the phosphodeficient FAK mutant, or Ad GFP control. Cells were washed, trypsinized, and seeded at 2×104 cells per well on Matrigel Matrix (BD Biosciences)–coated chamber slides. Cells were incubated in the presence of either vehicle or 50 μmol/L CDCA for 6 to 12 hours at 37°C and 5% CO2 and imaged using 4× objective and analyzed using Image-Pro Plus software for quantification of tube formation as described previously.18
Luciferase Reporter Assay
Cells were transfected with wild-type or mutated AP-1 human MMP-9-promoter-luciferase reporter constructs19 (Dr H. Sato, Japan) with 0.01 μg Renilla luciferase reporter vector to control for transfection efficiency (pRL-TK) using Lipofectamine 2000 (Invitrogen), and luciferase assays were conducted using a dual luciferase kit (Promega) as described previously.14
Gel Shift Assays
Nuclear extract from vehicle or CDCA (1 to 100 μmol/L) stimulated ECs was incubated with 32P-labeled probe encoding the AP-1 binding site of MMP-9 promoter 5′-TGACCCCTGAGTCAGCACTT-3′ in a binding buffer and electrophoresed and autoradiographed as described previously.14
Statistical Analysis
The data in the bar graphs represent the mean±SEM of at least 3 independent experiments, each performed with duplicate samples. Blots and immunofluorescence figure represent typical experiments reproduced at least 3 times with similar results. Statistical analyses were performed using a Student t test, with a 2-tailed value of P<0.05 considered significant.
Results
CDCA Upregulates Motility and Angiogenic Capacity of ECs in an FXR-MMP-9–Dependent Manner
First, we examined the effects of bile acid stimulation on EC motility and angiogenic capacity. ECs were incubated with vehicle or CDCA, and real-time cell motility was evaluated by time-lapse video microscopy and analyzed using Metamorph software. CDCA significantly increased EC chemokinesis as assessed by total distance traveled (Figure 1A). Next, we examined the mechanistic role of FXR and MMP-9 in this process using a siRNA approach, because prior studies have identified an FXR-induced MMP-9 transcription activation process in ECs in response to bile acids.14 A concentration-dependent decrease in FXR and MMP-9 mRNA level was observed in response to FXR siRNA and MMP-9 siRNA, respectively, in cells with 30 nm siRNA, a concentration which depletes 75% of FXR mRNA levels (supplemental Figure IA, available online at http://atvb.ahajournals.org) and 80% of MMP-9 mRNA levels, respectively (supplemental Figure IB). A dose dependent increase in MMP-9 activity was also observed in presence of CDCA using gelatin zymography (supplemental Figure IC). CDCA significantly increased EC motility in control siRNA-transfected cells (Figure 1B). However, this effect was absent in cells transfected with either FXR siRNA or MMP-9 siRNA (Figure 1B).
Figure 1.
CDCA potentiates EC motility and tube formation in a FXR-MMP-9–dependent manner. A, CDCA-induced cells (n=60 cells) showed significant increase in motility. B, CDCA increased EC (n=60 cells) motility in cells transfected with control siRNA but not with FXR siRNA or MMP-9 siRNA. C, CDCA significantly increased the tube length in ECs. D, CDCA significantly increased tube length in cells transfected with control siRNA but not with FXR siRNA or MMP-9 siRNA. Rop, representative photomicrographs from 3 independent experiments bottom, graphical representation, n=3, *P<0.05.
Next, to determine whether cell motility translates into increased in vitro angiogenic capacity of ECs in response to bile acids, we performed tube formation assays. ECs were seeded on matrigel coated slides, and tube-like structures were quantified using image analysis software. In presence of CDCA, ECs formed more tubes as compared to vehicle (Figure 1C). This response occurred in a concentration-dependent manner between concentrations of CDCA of 0 to 100 μmol/L (data not shown); a 50-μmol/L dose was subsequently used for ensuing experiments based on use of this concentration in prior literature and its approximation of bile acid levels in humans under pathophysiologic states.15 Tube formation ability was also analyzed in presence of FXR or MMP-9 silencing and compared with control siRNA transfected cells. CDCA significantly increased total tube length as compared to vehicle in the control siRNA transfected group, whereas CDCA-induced increase in tube length formation was absent in EC transfected with either FXR siRNA or MMP-9 siRNA (Figure 1D). These studies firmly establish the effect of CDCA on EC motility and angiogenic capacity is through an FXR-MMP-9 pathway.
CDCA Activates FAK Through Phosphorylation of Specific Tyrosine Residues
We next sought to explore the mechanism by which CDCA stimulates EC motility and tube formation. Because changes in actin cytoskeleton are essential for cell motility, we examined actin dynamics in cells stimulated with CDCA. A prominent observation was a proliferation in FA plaques which occurred in ECs in response to bile acid stimulation as assessed by increased detection of FA marker, vinculin (Figure 2A). To explore the mechanism of increased FA formation in CDCA-stimulated cells, we next examined the role of the key FA protein, FAK. FAK is a nonreceptor tyrosine kinase, which is activated by phosphorylation20 and serves as a key molecule in FA assembly.21 To explore the role of FAK in the signal transduction pathway of CDCA-induced EC motility we used immunofluorescence analysis to study cells treated with either vehicle or CDCA. Immunofluorescence microscopy using an antibody recognizing phosphorylated FAK Y397 (p-FAK Y397) revealed increased p-FAK staining in CDCA stimulated cells as compared to cells incubated with vehicle (Figure 2B). The FA nature of this staining was further confirmed by costaining cells with F-actin which revealed numerous stress fibers which inserted into the p-FAK Y397 positive FAs in CDCA stimulated cells as compared to vehicle incubated cells (Figure 2C). This further indicates the mature nature of CDCA-induced FAs compared to the less mature FA complexes observed with vehicle conditions. Quantitative analysis of stress fibers, as represented graphically, using LSM software depicts high levels of stress fibers (red intensity profiles) in the cells treated with CDCA (Figure 2C, right graph) as compared to cells incubated with vehicle (Figure 2C, left graph).
Figure 2.
CDCA-induced FA formation occurs through FAK phosphorylation at tyrosine residue −397 but not −576/577 or −925. A, Prominent increase in vinculin-positive FA plaques was observed in cells (n=30 cells) treated with CDCA for 12 hours. B, p-FAK Y397–positive FA plaques were significantly higher in CDCA-treated groups. C, Stress fiber formation by CDCA was higher as compared to vehicle. Left, Representative micrographs and images processed using software are from 3 independent experiments; right, graphical representation, n=3, *P<0.05. D, CDCA increased FAK phosphorylation at Tyr-397 but not at Tyr-576/577 or Tyr-925 tyrosine residues. Left, Representative Western blots from 3 independent experiments which were quantified and ratios of (left graph) p-FAK Y397/FAK, (middle graph) p-FAK Y576/577/FAK, and (right graph) p-FAK Y925/FAK were calculated and plotted graphically (n=3, *P<0.05).
FAK can be tyrosine phosphorylated on a number of residues, including Tyr-397, −576/577, −925 in response to various stimuli.22 To study the CDCA responsive phosphorylation sites on FAK we used a series of phosphospecific antibodies for Western blot analysis. CDCA promoted increased phosphorylation of FAK at Tyr-397, but not at Tyr-576/577 or Tyr-925 (Figure 2D, left panel). Blots from 3 independent experiments were quantified and plotted as a change in ratio of p-FAK Y397 to FAK (Figure 2D, left graph), p-FAK Y576/577 to FAK (Figure 2D, middle graph), and p-FAK Y925 to FAK (Figure 2D, right graph) in the CDCA-stimulated cells as compared to vehicle incubated cells. These studies demonstrate a site-specific tyrosine residue phosphorylation of FAK in the process of CDCA-induced motility signaling.
CDCA-Induced FA Formation Requires FXR and MMP-9
Bile acids may signal through multiple pathways including FXR, EGF transactivation, and TGR-5.1,23–25 To study the role of FXR in CDCA-induced phosphorylation of FAK at Tyr-397 we first silenced FXR by transfecting ECs with FXR siRNA or control siRNA at a concentration that is required for 75% knock down of FXR (supplemental Figure IA). Transfected cells were then treated with either vehicle or CDCA. p-FAK Y397 staining in the FA plaques were significantly increased in the control siRNA transfected cells treated with CDCA, whereas cells transfected with FXR siRNA showed reduced number of p-FAK Y397 associated FA plaques in presence of CDCA (supplemental Figure IIA, upper panel: photomicrograph, lower panel: graphical representation). Corroborative immunoblot analysis showed decreased phosphorylation of FAK at Y397 in cells transfected with FXR siRNA (supplemental Figure IIB).
Next, we studied the effect of MMP-9 silencing on FXR agonist-induced activation of FA plaques. MMP-9 silencing using MMP-9 siRNA showed significant decrease in MMP-9 mRNA (supplemental Figure 1B). CDCA treatment increased the peripheral localization of vinculin positive FA plaques in the control siRNA transfected cells. However, this effect was lost in cells transfected with MMP-9 siRNA (supplemental Figure IIIA, upper panel). Quantitation of vinculin positive FAs per cell shows a significant increase in CDCA-treated group as compared to vehicle in the control siRNA transfected ECs but not in MMP-9 siRNA transfected group (supplemental Figure IIIA, lower panel). Corroborative immunoblot analysis of cells transfected with either control siRNA or MMP-9 siNRA showed similar levels of total vinculin and β-Actin (supplemental Figure IIIB). These studies indicate that increase in FA plaques in presence of CDCA follows an FXR–MMP-9–dependent signaling pathway.
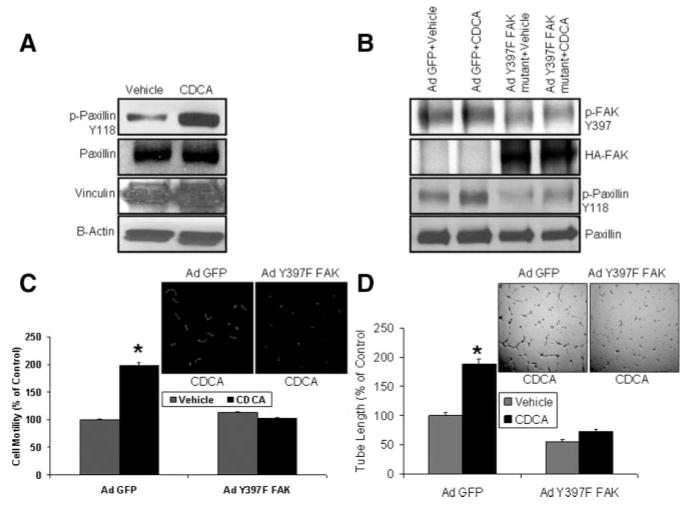
Phosphorylation at Y397 on FAK Is Required for Activation of Paxillin by CDCA and Enhanced Cell Motility and Tube Formation
We next sought to understand further the mechanism by which CDCA promotes FA formation in ECs. In this regard, CDCA also phosphorylated paxillin, a downstream signaling target of FAK at Tyr-118 on paxillin (Figure 3A). To confirm a requisite role for FAK phosphorylation at Y397 in this process, we used an HA-tagged adenoviral construct encoding a FAK with a mutation of Y397, the residue which is phosphorylated in response to CDCA (Figure 2D). Overexpression of Ad Y397F-FAK HA-tagged mutant was confirmed by immnobloting with HA antibody in Western blot analysis. FAK and paxillin activation in response to CDCA was examined by Western blot in ECs transduced with Ad GFP or Ad-Y397F-FAK mutant. CDCA-induced phosphorylation of FAK Y397 or paxillin Y118 was evident in cells transduced with Ad GFP control, but not in cells transduced with Ad-Y397F-FAK mutant (Figure 3B), indicating that phosphorylation of the tyrosine residue at 397 position on FAK is essential for activation of paxillin in the motility signaling pathway activated by CDCA.
Figure 3.
Phosphodeficient FAK mutant downregulates CDCA-induced activation of paxillin, EC motility, and tube formation. A, CDCA increased phosphorylation of paxillin at Y118, but not vinculin, paxillin, and β-Actin (loading control) in ECs. B, Cells transduced Ad Y397F-FAK mutant showed decrease in phosphorylation of FAK at Y397 and paxillin at Y118. C, CDCA increased EC motility in Ad GFP but not in Ad Y397F-FAK mutant transduced cells (n=45 cells). D, CDCA-induced increased tube formation in Ad GFP but not in Ad Y397F-FAK mutant transduced ECs. (Representative Western blots or photomicrographs are from 3 independent experiments, n=3, *P<0.05).
Next, to further corroborate a mechanistic role for FAK in CDCA-induced FA formation, FAK was silenced in ECs using FAK siRNA and cells were analyzed for motility and tube formation ability. A concentration-dependent decrease in FAK mRNA level was observed in response to FAK siRNA in cells with 30 nm FAK siRNA, effectively knocking down 75% of FAK mRNA levels (supplemental Figure IVA). CDCA significantly increased EC motility in control siRNA transfected group but not in FAK siRNA transfected groups as assessed by real-time cell motility (supplemental Figure IVB). A similar decrease in tube length formation in FAK siRNA transfected group was observed as compared to control siRNA transfected group (supplemental Figure IVC). To further confirm the role of phosphorylation of FAK at tyr-397 in EC motility and angiogenesis, we transduced ECs with either Ad GFP or Ad Y397F FAK mutant and then studied real-time cell motility and vascular tube formation assay in presence or absence of CDCA. There was a significant increase in EC motility in presence of CDCA in cells transduced with Ad GFP but not with Ad Y397F FAK mutant (Figure 3C). Similar increase in tube length formation in presence of CDCA was observed in ECs transduced with Ad GFP but not with Ad Y397F FAK mutant (Figure 3D). These studies indicate a critical role of FAK in FXR-induced EC motility and angiogenesis.
FAK Silencing Abolishes the Expression of MMP-9
After establishing a critical role of FAK in CDCA induced EC motility, we next sought to determine the site of FAK within the proposed FXR–MMP-9 signal transduction pathway. Interestingly, FAK has previously been implicated in the pathway of MMP-9 upregulation via JNK and c-Jun signaling.26,27 Additionally, FAK may also be downstream of MMP9, and activated through MMP-9–induced changes in extracellular matrix.28,29 To address these possibilities in the context of our CDCA-motility model, we silenced FAK in ECs using FAK siRNA and measured mRNA expression of MMP-9 in presence or absence of CDCA. CDCA-induced MMP-9 expression was lost in cells transfected with FAK siRNA (supplemental Figure IVD) supporting a requisite role of FAK for CDCA induction of MMP-9. Furthermore, ECs transduced with Ad Y397F FAK mutant showed a similar decrease in CDCA-induced MMP-9 mRNA levels (Figure 4A) as well as protein levels (Figure 4B) as compared to Ad GFP transduced cells, further supporting the importance of activation of FAK by site specific phosphorylation at Y397 in this pathway. Interestingly, we also observed decreased phosphorylation of FAK at Y397 residue in presence of MMP-9 silencing by Western blot analysis (Figure 4C) and by immunofluorescence analysis (Figure 4D), indicating that MMP-9 is required for FAK activation in ECs. Thus, these studies demonstrate a dynamic interplay between FAK and MMP-9 in FXR-induced motility signaling in ECs.
Figure 4.
CDCA-induced EC motility involves a reciprocal regulation of FAK and MMP-9. A and B, CDCA increased MMP-9 mRNA (A) and protein (B) levels in ECs transduced with Ad GFP but not Ad Y397F-FAK mutant. C, MMP-9 silencing decreases FAK phosphorylation at tyr 397 residue. D, CDCA-induced increase staining of p-FAK Y397–positive FA plaques in cells transfected with control siRNA but not MMP-9 siRNA (upper, representative micrographs; lower, images processed using software; right, quantification of p-FAK Y397–positive FA plaques per cell, n=3, *P<0.05, representative blots and micrographs are from 3 independent experiments).
FAK Regulates MMP-9 Gene Transcription by Activation at the AP-1 Motif
To explore the potential mechanisms of MMP-9 transcription we studied the human wild-type MMP-9 reporter luciferase construct or one with a point mutation in the AP-1 site. The AP-1 mutant construct evidenced significantly decreased MMP-9 promoter activity, suggesting that activator protein binding to the AP-1 motif of the MMP-9 promoter may regulate MMP-9 gene transcription (supplemental Figure VA). Phorbol 13-myristate 12-acetate (PMA), a previously established inducer of MMP-9 gene transcription regulates through AP-1 activation. However, the induction effect of PMA on the MMP-9 promoter activity was lost in presence of a point mutation in the AP-1 site thereby served as a positive control (supplemental Figure VA). To expand this observation, we performed EMSA using the nuclear extract prepared from ECs incubated with varying concentrations of CDCA. A concentration-dependent increase in protein binding to a radiolabeled AP-1 motif from the MMP-9 promoter was observed in response to increasing concentrations of CDCA (Figure 5A). To further explore the role of FAK in MMP-9 gene transcription we performed reporter assays in cells cotransfected with human wild-type MMP-9 promoter and either FAK siRNA or scrambled siRNA and then incubated cells with vehicle or 50 μmol/L CDCA. CDCA significantly increased MMP-9 relative luciferase promoter activity in cells cotransfected with control siRNA, as compared to those transfected with FAK siRNA (supplemental Figure VB). Next we overexpressed the Ad Y397F FAK mutant or Ad GFP in cells transfected with wild-type human MMP-9 promoter and then incubated the cells with vehicle or CDCA. Interestingly, the relative luciferase activity (RLA) was significantly increased in cells overexpressing the Ad GFP as compared with Ad Y397F FAK mutant (Figure 5B). Because c-Jun is a component of the AP-1 transcription complex of MMP-9 promoter,26 we finally performed immunoblot analysis of c-Jun which revealed CDCA-induced phosphorylation of c-Jun (p-c-Jun) in ECs transduced with Ad GFP but not in cells transduced with Ad Y397F FAK (Figure 5C). In sum, these results support an important role of FAK in FXR-induced MMP-9 gene transcription.
Figure 5.
FAK regulates MMP-9 promoter activity. A, Increase in protein binding on the AP-1 motif of MMP-9 promoter in response to increasing CDCA concentration (arrow). B, RLA of wild-type MMP-9 promoter was significantly increased in CDCA-treated cells overexpressing Ad GFP but not Ad Y397F FAK (n=3, *P<0.05). C, CDCA-induced phosphorylation of c-Jun in cells transduced with Ad GFP but not with Ad Y397F-FAK mutant. (Representative autoradiographs or Western blots are from 3 independent experiments). D, Schematic representation of the model. FXR activation upregulates MMP-9,14 which then activates FAK and other FA plaque proteins that in turn promotes EC motility and angiogenesis.
Discussion
The present studies explore the signal transduction mechanism of FXR-induced EC motility and angiogenesis. The novel observations we have generated are that FXR agonists: (1) induce cell motility and tube formation capacity of ECs in a FXR-MMP-9–dependent manner; (2) activate peripheral FA plaque formation through a FXR-MMP-9–dependent pathway; (3) phosphorylate FAK on Y397, but not on Y576/577, or Y925, with downstream activation of paxillin on Y118. Thus this work identifies important mechanisms responsible for the novel concept of bile acid regulation of vascular EC function and biology.
FXR pathway relevance to vascular biology has traditionally been thought to be via cholesterol regulation.30 However, recent studies raise the intriguing possibility that bile acids, such as CDCA, may regulate vascular function through direct modulation of EC recruitment to sites of liver injury and angiogenesis perhaps via an MMP-9–dependent mechanism.5,8,14,31–33 Data from the present study identifies a role of FXR in the process of EC motility that is achieved through MMP-9, and its cross-talk with FAK. Our data shows that FAK silencing abrogates EC motility, and tube formation capability in presence of CDCA also corroborates with prior studies showing that FAK deficient (FAK−/−) cells exhibit a rounded morphology with migration defects.11,28,29 Thus, our work adds a new role of the FXR pathway into existing angiogenesis research involving MMP-9 and FAK.
A key step in the process of EC motility is the dynamic interactions between ECs and the surrounding extra cellular matrix, which occurs at FAs. FAs are typically composed of integrins and various other proteins that link the actin cytoskeleton to the extracellular matrix. In presence of a motility stimulus, integrin-mediated cell adhesions can lead to FAK activation and autophosphorylation.34 Phosphorylation of FAK in turn triggers downstream signaling events, including phosphorylation of paxillin, which occur directly or indirectly through Src.22,35 The major site of autophosphorylation of FAK, tyrosine 397, lies to the amino-terminal side of the catalytic domain and serves as a binding site for the SH2 domain of Src.36 In this study, we observed a lack of CDCA-induced paxillin phosphorylation in cells transduced with adenoviral phosphodefective FAK (Y397F-FAK) mutant. Alternative FAK tyrosine residues are implicated in generating distinct signals as well. For example, FAK Tyr-576/577 upregulates FAK kinase activity, whereas FAK Tyr-925 activates the Ras-MAPK pathway.37 However, in the present study, we did not observe any change in phosphorylation status of FAK at tyrosine residue −576/577 and −925 in ECs in presence of FXR agonists, further establishing specificity of effect at tyr 397.
Studies in various carcinoma cells have shown a role of FAK in MMP-9 gene expression through the activation of JNK and c-Jun.26,27 Data from our present study demonstrating that MMP-9 expression by CDCA was perturbed in presence of FAK silencing support these prior observations. However, we also observed that MMP-9 silencing abrogates the activation of FAK as assessed by FAK phosphorylation at tyrosine 397 residue. Thus our findings decipher an interesting coordinated interaction between FAK and MMP-9 whereby activation of FAK and its downstream target paxillin occurs through a MMP-9–dependent pathway, whereas the upregulation of MMP-9, in turn, is regulated by FAK at the AP-1 motif of the MMP-9 promoter via c-Jun activation (Figure 5D). We anticipate that MMP-9 may degrade matrix, which is sensed by focal adhesion proteins (ie, integrins, src, etc) that signal to FAK phosphorylation. In turn, FAK remodels focal adhesions, thereby enabling ECs to move. In summary, our data demonstrate that bile acids activate FAK and its downstream signaling target paxillin, thereby increasing peripheral FA plaque formation through an MMP-9–dependent mechanism; these events promote EC motility and vascular tube formation. With increasing evidence demonstrating a key role for bile acids in liver injury and repair,5 we anticipate that better understanding of the mechanisms of FXR induced EC motility and angiogenesis may lead to the design of novel therapeutics aimed at hepatic vascular remodeling and angiogenesis.
Supplementary Material
Acknowledgments
Sources of Funding
These studies were supported by National Institutes of Health Grants R01-DK059615 and R01-HL086990 (to V.H. S.) and Pilot and Feasibility Award by Mayo Clinic Center on Cell Signaling in Gastroenterology (to A.D.).
Footnotes
Disclosures
None.
References
- 1.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 2.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 3.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci U S A. 2004;101:3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 6.He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S, Pitt B, Xie W, Li S. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res. 2006;98:192–199. doi: 10.1161/01.RES.0000200400.55539.85. [DOI] [PubMed] [Google Scholar]
- 7.Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Torimura T, Sakamoto M, Hashimoto O, Taniguchi E, Inoue K, Sakata R, Kumashiro R, Murohara T, Ueno T, Sata M. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology. 2007;133:91–107.e1. doi: 10.1053/j.gastro.2007.03.110. [DOI] [PubMed] [Google Scholar]
- 9.Fischer S, Beuers U, Spengler U, Zwiebel FM, Koebe HG. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin Chim Acta. 1996;251:173–186. doi: 10.1016/0009-8981(96)06305-x. [DOI] [PubMed] [Google Scholar]
- 10.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 12.Tumbarello DA, Brown MC, Hetey SE, Turner CE. Regulation of paxillin family members during epithelial-mesenchymal transformation: a putative role for paxillin delta. J Cell Sci. 2005;118:4849–4863. doi: 10.1242/jcs.02615. [DOI] [PubMed] [Google Scholar]
- 13.Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol. 2004;166:283–295. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A, Fernandez-Zapico ME, Cao S, Yao J, Fiorucci S, Hebbel RP, Urrutia R, Shah VH. Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J Biol Chem. 2006;281:39105–39113. doi: 10.1074/jbc.M607720200. [DOI] [PubMed] [Google Scholar]
- 15.Ostrow J. Metabolism of bile salts in cholestasis. In: Tavoloni NBPE, editor. Hepatic Transport and Bile Secretion: Physiology and Pathophysiology. Raven; New York: 1993. [Google Scholar]
- 16.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006;281:2296–2305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- 17.Langer DA, Das A, Semela D, Kang-Decker N, Hendrickson H, Bronk SF, Katusic ZS, Gores GJ, Shah VH. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology. 2008;47:1983–1993. doi: 10.1002/hep.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-B2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135:671–679. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- 20.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 21.Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, Cosgrove D, Kalluri R. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J Clin Invest. 2005;115:2801–2810. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. Faseb J. 2003;17:2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- 23.Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanay-akkara M, Apicella C, Capasso L, Paludetto R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G906–G913. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 26.Hong IK, Jin YJ, Byun HJ, Jeoung DI, Kim YM, Lee H. Homophilic interactions of Tetraspanin CD151 up-regulate motility and matrix metalloproteinase-9 expression of human melanoma cells through adhesion-dependent c-Jun activation signaling pathways. J Biol Chem. 2006;281:24279–24292. doi: 10.1074/jbc.M601209200. [DOI] [PubMed] [Google Scholar]
- 27.Rothhut B, Ghoneim C, Antonicelli F, Soula-Rothhut M. Epidermal growth factor stimulates matrix metalloproteinase-9 expression and invasion in human follicular thyroid carcinoma cells through Focal adhesion kinase. Biochimie. 2007;89:613–624. doi: 10.1016/j.biochi.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Bhoopathi P, Chetty C, Kunigal S, Vanamala SK, Rao JS, Lakka SS. Blockade of tumor growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 2008;283:1545–1552. doi: 10.1074/jbc.M707931200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Haussinger D, Kurz AK, Wettstein M, Graf D, Vom Dahl S, Schliess F. Involvement of integrins and Src in tauroursodeoxycholate-induced and swelling-induced choleresis. Gastroenterology. 2003;124:1476–1487. doi: 10.1016/s0016-5085(03)00274-9. [DOI] [PubMed] [Google Scholar]
- 30.Lambert G, Amar MJ, Guo G, Brewer HB, Jr, Gonzalez FJ, Sinal CJ. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 31.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 32.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439 –23442. [PubMed] [Google Scholar]
- 35.Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.