Abstract
Purpose
To evaluate the toxicity and response rate of bortezomib with concurrent radiotherapy and temozolomide in the treatment of patients with CNS malignancies.
Patients and Methods
This open-label, dose-escalation, phase 1 clinical study evaluated the safety of 3 dose levels of intravenously administered bortezomib (0.7, 1.0, 1.3 mg/m2/dose) on days 1, 4, 8, 11 of a 21-day cycle, in addition to concurrent radiation therapy and temozolomide at a daily dose of 75 mg/m2 starting on day 1. The primary endpoint was dose-limiting toxicity (DLT), defined as any Grade 4–5 toxicity or Grade 3 toxicity(ies) directly attributable to protocol treatment, requiring hospitalization and/or radiation interruption. Secondary endpoints included feasibility, non-dose-limiting toxicity, and response.
Results
Twenty-seven patients were enrolled, 23 of whom had a high grade glioma (ten recurrent and 13 newly diagnosed). There were no dose-limiting toxicities (DLTs) noted in any dose groups, including the highest dose level group (1.3 mg/m2/dose). The most frequent toxicities were grade 1 and 2 stomatitis, erythema, and alopecia. All 27 patients were evaluable for response. At a median follow-up of 15.0 months, 9 patients were still alive; median survival was 17.4 months for all patients and 15.0 months for patients with high-grade gliomas.
Conclusion
Bortezomib administered at its typical “systemic” dose (1.3 mg/m2) is well tolerated and safe in combination with temozolomide and radiation when used in the treatment of CNS malignancies. A phase II study to characterize efficacy is warranted.
Keywords: proteasome inhibitors, bortezomib, CNS malignancy, GBM, radiation
Introduction
High grade gliomas, including anaplastic astrocytomas (AA, WHO grade III) and glioblastoma multiforme (GBM, WHO grade IV) are the most frequent types of primary brain cancer in adults. Outcomes for these patients are poor, with most GBM patients dying within a year of diagnosis (1). The mainstay of post-surgical treatment is radiation therapy that can increase survival time for GBM from 3–4 months to 10–12 months. Attempts have been made to further improve patient outcomes by using chemotherapy. However, chemotherapy agents such as carmustine or procarbazine, lomustine, and vincristine (PCV) failed to show a survival advantage compared with radiation alone in phase III trials in GBM patients (2–6). Stupp et al (3) recently showed that the addition of temozolomide (Temodar®; Schering-Plough Corp, Kenilworth, NJ) concurrent with radiation and continued as maintenance therapy has improved survival in tested patients. Temozolomide has quickly established itself as standard of care along with radiation for the treatment of GBM. The improvement in median survival with temozolomide (12.1 months with radiation alone to 14.6 months with the combination of radiation therapy and temozolomide) however remains modest. The treatment goal remains to identify therapies in order to further improve these results.
One emerging treatment option involves novel agents such as bortezomib (Velcade®; Millennium Pharmaceuticals Inc, Cambridge, MA). Bortezomib was the first proteasome inhibitor approved for use in clinical trials. It functions via proteasome inhibition resulting in cell-cycle re-distribution and inhibition of transcription factors such as nuclear factor kappa-B (NF-kB) and Akt, both of which play a role in angiogenesis and cellular proliferation. Bortezomib has been studied in several settings. Bortezomib is FDA-approved for initial treatment of patients with multiple myeloma and in mantle cell lymphoma patients who have received at least one prior therapy (8–9). Promising results have been seen with bortezomib alone or bortezomib combinations in the treatment of small cell lung cancer (SCLC) (10) and non-small cell lung cancer (11–12) and other solid tumors (12–15). Pre-clinical investigation has shown that gliomas are responsive in vivo to bortezomib (16–17) but this response point has not yet been tested in the clinical setting for CNS malignancies.
Patients and Methods
This study was approved by the Thomas Jefferson University Cancer Center Review Committee (CCRRC) and Institutional Review Board (IRB) prior to patient recruitment and accrual. Eligible patients had a histologically or cytologically confirmed diagnosis of a CNS malignancy (either primary or metastatic) and their treatment consisted of a minimum 2-week course of radiation therapy. Other eligibility requirements included ECOG performance status 0–2, life expectancy > 3 months, adequate hematologic reserve (defined as WBC > 3 109/L, ANC > 1.5 109 L, Hgb > 9.0 g/dL, plt > 100 109/L) and age > 18. Previous radiotherapy chemotherapy or combination chemoradiotherapy was permitted. Exclusion criteria included any patient with Grade 2 peripheral neuropathy within 14 days before enrollment, New York Hospital Association Class III or IV heart failure, hypersensitivity to bortezomib, boron or mannitol, HIV positivity, pregnant or lactating females, history of medical noncompliance, or administration of other investigational drugs within 14 days prior to enrollment.
Study Design
This was designed as an open-label phase I dose escalation study, using a two step dose level approach with the experimental agent (bortezomib) to establish maximum tolerated doses (MTD) and types and degrees of toxicities associated with bortezomib in combination with radiation and chemotherapy (temozolomide). All treatments consisted of radiation therapy with fraction sizes ranging from 1.8–3.5 Gy/day for total doses not to exceed 90 Gy. For all patients, temozolomide was administered during radiotherapy at a daily dose of 75 mg/m2. When indicated, temozolomide was also used in the maintenance phase after completion of the radiation therapy. The starting dose level of bortezomib was 0.7 mg/m2 on days 1, 4, 8, 11 of a 21-day cycle for up to 2 cycles. Bortezomib was administered as an IV push given over 4–5 seconds followed by a standard saline flush. Post-radiotherapy bortezomib was not given. After completion of the radiation therapy and concurrent bortezomib, patients entered a 4-week post-treatment observation period. Dose escalation was performed only after assessment to rule out any dose limiting toxicities (defined as any Grade 4–5 toxicity or Grade 3 toxicity requiring hospitalization or radiotherapy interruption) in each dosing group following the 4-week observation period. Per protocol, three patients were to be enrolled at the initial dose (0.7 mg/m2). If no DLTs occurred in this patient cohort then three additional patients then would be enrolled at an increased dose (1.0 mg/m2) and if no DLT were found at this dose level, patients then would be enrolled at the highest dose (1.3 mg/m2). If one patient of the three evaluable patients at a given dose level had a DLT, 3 additional patients then would be enrolled at the same dose level. The maximum tolerated dose (MTD) of bortezomib was defined as the highest dose given as a single bolus administered twice per week for 2 weeks, followed by a one week rest period during radiation-therapy for a maximum of 8 weeks (three cycles), in which no more than 30% of the patient population experienced a DLT. The reason for the 30% toxicity rate is based on an estimated 10% DLT for the radiotherapy alone. We had no plans to escalate the dose beyond Dose Level 3 (1.3 mg/m2). If no DLT was observed at this dose, then the MTD was to be defined at this dose.
Toxicity and Response Evaluation
Toxicity was defined in accordance with the NCI common toxicity criteria (CTC) version 3.0. Dose Limiting Toxicity was defined as any grade 4–5 toxicity or any grade 3 toxicity directly attributable to protocol treatment requiring hospitalization and/or an interruption of radiotherapy. MTD was defined as the highest bortezomib dose at which not more than 30% of the patients would experience a DLT. Patients were evaluated for potential dose-limiting toxicity weekly during treatment and for 4 additional weeks after the completion of treatment. Tumor response was measured based on brain MRI scans obtained approximately one month after the completion of treatment and at regular follow-up appointments thereafter, varying according to tumor type. Tumor response was based on Response Evaluation Criteria in Solid Tumors (RECIST).
Results
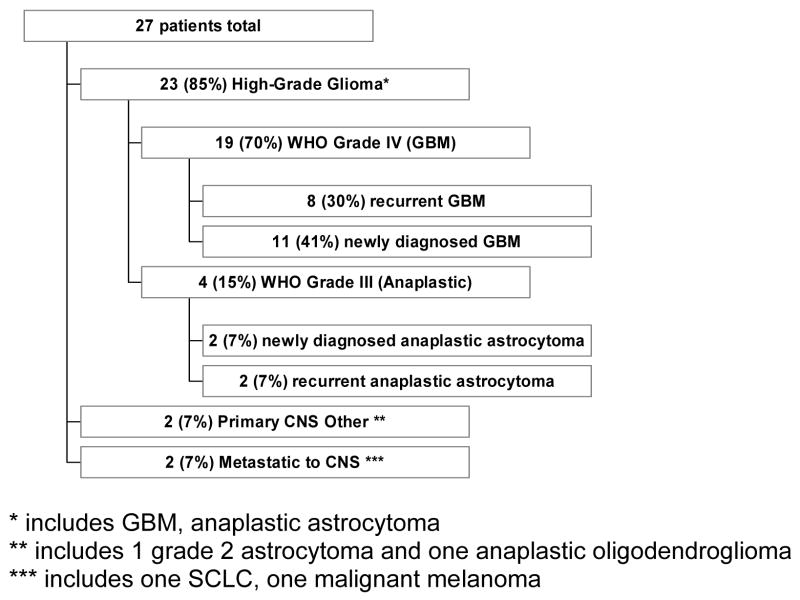
A total of 27 patients were enrolled. Patient characteristics are shown in Table I and Figure I. The median age at the start of treatment was 52 years for all patients, and most enrolled patients (85%) had Karnofsky performance scores of 80 or above. Eleven patients had newly diagnosed GBM, 8 patients had recurrent GBM, 2 patients had recurrent anaplastic astrocytoma, 2 patients had newly diagnosed anaplastic astrocytoma, 2 patients had CNS metastatic lesions (one from SCLC and one from melanoma), 1 patient had an anaplastic oligodendroglioma and 1 patient had a WHO grade II astrocytoma.
Table I.
Patient Characteristics
| All Patients | High Grade Recurrent | High Grade newly diagnosed | Other | |
|---|---|---|---|---|
| No. Patients | 27 | 10 | 13 | 4 |
| Age (Median) | 52 | 62 | 52 | 43 |
| Gender | ||||
| Male | 15 | 4 | 9 | 2 |
| Female | 12 | 6 | 4 | 2 |
| Previous RT | 12 | 9 | 0 | 3 |
| Previous Chemotherapy | 11 | 8 | 0 | 3 |
| KPS > 70 | 4 | 2 | 2 | 0 |
| KPS ≥ 80 | 23 | 8 | 11 | 4 |
Figure I.
Tumor type by histology
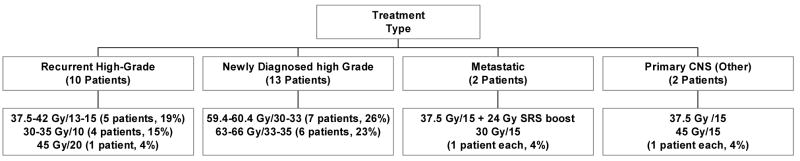
Eight patients were treated with bortezomib at 0.7 mg/m2, all of whom had high grade gliomas including two with recurrence after prior radiotherapy; 10 patients were treated with bortezomib at 1.0 mg/m2 (9 high grade gliomas, three of which were recurrent), and 9 patients were treated at the highest bortezomib dose level of 1.3 mg/m2 (6 high-grade gliomas, of which four were recurrent). The median number of bortezomib doses for all patients was 6 (4 for recurrent high-grade gliomas compared with 9 for newly diagnosed high-grade gliomas). There were no dose reductions of bortezomib in any of the dose levels. (Tables II and III and Figure II).
Table II.
Treatment Characteristics
| All Patients | High Grade Recurrent | High Grade Newly Diagnosed | Other | |
|---|---|---|---|---|
| Bortezomib 0.7 mg/m2 | 8 | 2 | 6 | 0 |
| Bortezomib 1.0 mg/m2 | 10 | 4 | 5 | 1 |
| Bortezomib 1.3 mg/m2 | 9 | 4 | 2 | 3 |
| Total Bortezomib Dose (Median, mg) | 11.88 | 8.72 | 14.56 | 10.6 |
| Number of Bortezomib doses (median) | 6 | 4 | 9 | 4 |
Table III.
Radiation Treatment Characteristics
| All Patients | High Grade Recurrent | High Grade Newly Diagnosed | Other | |
|---|---|---|---|---|
| RT Dose (Median, Gy) | 59.4 | 37.5 | 60.4 | 37.5 |
| RT Dose (Range, Gy) | 30–66 | 30–45 | 59.4–66 | 30–61.5 |
| RT dose per fraction | ||||
| 1.8–2 | 13 | 0 | 13 | 0 |
| 2.25–2.5 | 5 | 3 | 0 | 2 |
| 2.75–3 | 5 | 4 | 0 | 1 |
| 3.5–3.75 | 4 | 3 | 0 | 1 |
| Number of fractions | ||||
| 10 | 5 | 4 | 0 | 1 |
| 13–15 | 8 | 5 | 0 | 3 |
| 20 | 1 | 1 | 0 | 0 |
| 30–35 | 13 | 0 | 13 | 0 |
| RT Treatment Time (median, days) | 34 | 16 | 46 | 19 |
Figure II.
Radiation treatment dose and fractionation by histology
Toxicity/Safety
All 27 patients were evaluated for toxicity both during treatment and at a 4-week observation period after the completion of treatment. No DLTs were observed in any dose group. No Grade 4 or 5 toxicities were noted. Six grade 3 toxicities were observed in five different patients with one patient having both hyponatremia and dyspnea. None of the Grade 3 toxicities required hospitalization and/or treatment interruptions. The grade 3 toxicities included one of each of the following; headache, neuropathy, syncope, hyponatremia, dyspnea, and stupor. The grade 3 neuropathy, syncope, hyponatremia, and dyspnea occurred midway through the radiation treatments, the headache occurred during the second week of radiation therapy, and the grade 3 stupor was noted at the 4-week follow-up visit.
The most frequent Grade 1 and 2 toxicities were stomatitis (5 patients), erythema (4 patients), and alopecia (12 patients). The majority of toxicity (erythema and alopecia) was attributed to the radiotherapy and likely not associated with the bortezomib. Grade 1 and 2 toxicities (especially alopecia and erythema) were seen most frequently in the newly diagnosed high-grade glioma subset of patients, likely related to the longer course of radiation for these patients. See Table IV.
Table IV.
Toxicity
| All Patients | High Grade Recurrent | High Grade Newly Diagnosed | Other | |||||
|---|---|---|---|---|---|---|---|---|
| Toxicity Grade | 1–2 | 3 | 1–2 | 3 | 1–2 | 3 | 1–2 | 3 |
| Hematologic | ||||||||
| Neutropenia | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Febrile | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| neutropenia | ||||||||
| Infection | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Anemia | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Thrombocytopenia | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| HEENT | ||||||||
| Stomatitis | 5 | 0 | 1 | 0 | 2 | 0 | 2 | 0 |
| Alopeica* | 12 | 0 | 2 | 0 | 7 | 0 | 3 | 0 |
| Erythema* | 4 | 0 | 1 | 0 | 3 | 0 | 0 | 0 |
| CNS | ||||||||
| Headache | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 0 |
| Nausea | 4 | 0 | 1 | 0 | 2 | 0 | 1 | 0 |
| Vomiting | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Syncope | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Neuropathy | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Stupor | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Lethargy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Misc | ||||||||
| Fatigue | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| Hyponatremia | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Dyspnea | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
Likely secondary to radiation
Response
All 27 patients were evaluated according to the RECIST criteria for 1-month tumor response on post-treatment imaging; 14 of the patients were determined to have RECIST criteria target lesions and 13 patients had non-target lesions that were evaluated for response.
At the 1-month MRI scan, 20 (74%) patients had stable disease and 7 (26%) patients had disease progression. In the 23 high-grade glioma patients, 16 (70%) had stable disease and 7 (30%) had progression. Of the 13 newly diagnosed high-grade glioma patients, 9 (69%) had stable disease and 4 (31%) had progression, for the 10 recurrent high-grade glioma patients, 7 had stable disease and 3 had progression.
On further follow-up MRI examinations, with a median time of 9.4 months, 16 patients had stable disease and 11 had disease progression. For the 23 patients with high-grade gliomas, 12 had stable disease and 11 had progression. In the 13 previously untreated high-grade glioma patients, 5 had stable disease and 8 had progression. Of the 10 patients with recurrent high-grade glioma, 7 had stable disease and 3 had progression. In terms of the bortezomib dose level, 3 of 8 (38%) patients at a bortezomib dose level of 0.7 mg/m2 had stable disease; at the bortezomib dose level of 1.0 mg/m2 5 of 10 (50%) patients had stable disease; and at the highest dose level of 1.3 mg/m2 7 of 9 (78%) patients had stable disease.
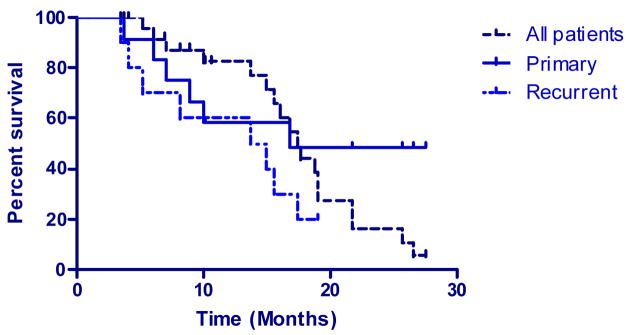
With a median overall follow-up time of 15.0 months, 9 of the 27 (33%) patients remained alive. The survival range for all patients was 3.5 to 27.6 months with a median survival of 17.4 months. Of the 23 high-grade glioma patients, 8 patients were still alive with a range of 3.5 to 27.6 months, the median survival for these patients was 15.0 months. The median survival time for newly diagnosed high-grade glioma patients was 16.9 months and 14.4 months for recurrent GBM patients. See Figure IV.
Figure IV.
Kaplan-Meier overall survival
Twenty-two patients (81%) were alive six months after treatment. Progression-free survival at six months was 56%, with fifteen patients having stable disease. Five (50%) of the ten recurrent high-grade glioma patients showed signs of progression at six months compared with 7 of 13 (53%) of newly diagnosed high-grade glioma patients. No patient had disease progression at the six-month mark in the non-high grade subset.
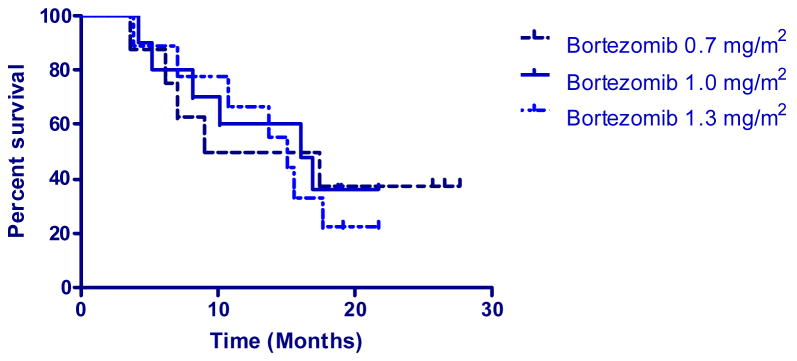
The median survival time for patients receiving bortezomib dose levels was 13.2 months at a dose level of 0.7 mg/m2, 16.1 months at a dose level of 1.0 mg/m2, and 15.0 months for the dose level of 1.3 mg/m2. See Figure V.
Figure V.
Kaplan-Meier survival by bortezomib dose
Use of cortical steroids was also analyzed. Prior to the initiation of treatment, 20 patients (74%) were on steroids and at last follow-up, 17 (63%) patients required steroids. Twelve patients were able to either decrease (9 patients) or completely discontinue steroids (3 patients), 3 patients had increased steroid requirements, and 5 patients had either unchanged steroid use or had incomplete information regarding steroid usage.
Discussion
There is a sound theoretical basis for using bortezomib in the treatment of CNS malignancies, particularly in high-grade glioma. It is well established that loss of phosphatase and tensin homolog (PTEN) and amplification of the epidermal growth factor receptor (EGFR) gene contribute to the malignant phenotype of glioma. Downstream targets of the PTEN and EGFR signaling pathways, Akt and NF-kB, have been shown to play important roles in the control of cell proliferation, apoptosis, and oncogenesis. There are significant positive correlations between the activation status of Akt and NF-kB and glioma grade (18). NF-kB is also constitutively activated in glioblastoma surgical samples, primary cultures, and cell lines and promotes their growth and survival (19). In preclinical studies, bortezomib was shown to decrease the transcription of both NF-kB and Akt through its inhibition of proteasome 26-S activity (20). Laboratory testing in the setting of melanoma has shown a synergistic effect in the combination of bortezomib and temozolomide (21).
Bortezomib has been tested in multiple cancer types including multiple myeloma (8), mantle cell lymphoma (9) small cell (10) and non-small cell lung cancer (11–12), GI malignancies (14), breast cancer (15), and prostate cancer (16). We have shown in this phase I study that bortezomib can safely be combined with radiation-therapy and temozolomide chemotherapy. This is the first known report using bortezomib in CNS malignancies and the first to combine bortezomib with temozolomide and radiation-therapy. This phase I dose escalation trial has demonstrated minimal toxicity with no DLTs and the MTD of bortezomib of 1.3 m/mg2. We found the combination of radiation-therapy, temozolomide, and bortezomib to be well tolerated. In addition to the absence of DLTs there were no Grade 4 toxicities observed and only a small number of Grade 3 toxicities.
A secondary endpoint of this study was to assess tumor response. We acknowledge that the extent of bortezomib CNS penetration in patients with newly diagnosed brain tumors is unknown, making treatment outcome an important surrogate to evaluate bortezomib CNS penetration. Using the RECIST criteria, 15 (55%) of the patients had stable disease at 6 months post-treatment. No patients in this study however, realized an objective response by RECIST criteria. It should be noted however, that the assessment of objective response immediately following radiotherapy may be problematic because of radiation-induced inflammation and contrast enhancement. Most of our patients were treated with bortezomib below the dose this study established as the MTD and this further limits the usefulness of assessing anti-tumor efficacy from our study. It is worth noting that of the 9 patients in this study treated with a bortezomib dose of 1.3 mg/m2, the median survival was 15.0 months and 8 patients had stable disease at 6-months post-treatment. In the newly diagnosed high-grade glioma patients, our median survival time of 16.9 months compares favorably with other reported data. The best results published to date in the treatment of GBM uses adjuvant radiation therapy and temozolomide with a median survival of 14.6 months (7). Median survival time for recurrent high-grade gliomas usually is poor, the median survival for recurrent glioma patients in this study was 14.4 months. Carson (21) reported a median 7 month survival for recurrent GBM patients treated in 10 phase I or II trials.
The recursive partitioning analysis (RPA) tool can also be used for patient comparison. The majority of the newly diagnosed high-grade glioma patients were RPA 4 and 5 (7 and 4 patients respectively) with respective median survival times of 10.1 and 12.9 months. In a review of newly diagnosed high-grade gliomas, Mirimanoff (22) recently reported a 15 month median survival for RPA category 4 patients and 10 months for RPA 5 for newly diagnosed GBM patients on a phase III EORTC trial involving temozolomide and radiation. Four of the 10 recurrent high-grade glioma patients in our report were in the RPA 7 and had a median survival time of 9.6 months compared to a median survival of 4.9 months reported by Carson (21) for the same patient subset.
In both recurrent and newly diagnosed high-grade gliomas, treatment with radiation-therapy, temozolomide and bortezomib appears effective when compared to historical norms. However, this phase I study was not intended to compare the addition of bortezomib with the standard therapy for high-grade gliomas. A formal phase II study is needed to evaluate the potential effect of the addition of bortezomib with the current standard of care (temozolomide and radiation therapy).
Conclusion
Bortezomib at full dose with concurrent temozolomide and radiation therapy is safe and effective in the treatment of CNS malignancies and is accompanied by a modest toxicity profile. We have been able to establish a MTD for bortezomib of 1.3 mg/m2. Further investigation in the form of a phase II trial is justified.
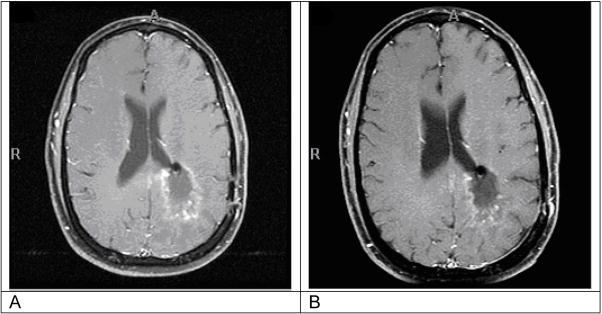
Figure III.
Example of brain MRI of patient with stable disease
Footnotes
Dr. Adam P. Dicker received an unrestricted grant from Millennium Pharmaceuticals in association with this trial. There are no other conflicts of interest for Dr. Dicker or for any of the other authors.
This information was presented as a poster at the 2007 American Society of Clinical Oncology (ASCO), annual meeting May 2007 in Chicago IL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.DeAngelis LM. Brain tumors: N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. J Neurosurg. 1978;49:333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 3.Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67:121–32. [PubMed] [Google Scholar]
- 4.Chang CH, Horton J, Schoenfeld D, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas: a joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52:997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma: Brain Tumor Cooperative Group trial 8001. J Neurosurg. 1989;71:1–9. doi: 10.3171/jns.1989.71.1.0001. [DOI] [PubMed] [Google Scholar]
- 6.Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19:509–18. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Sonneveld P, Schuster MW, et al. Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 9.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24 (30):4867–74. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 10.Lara PN, Jr, Chansky K, Davies AM, et al. Bortezomib (PS-341) in relapsed or refractory extensive stage small cell lung cancer: a Southwest Oncology Group phase II trial (S0327) J Thorac Oncol. 2006;9:996–1001. [PubMed] [Google Scholar]
- 11.Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24(31):5025–33. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 12.Lara PN, Jr, Koczywas M, Quinn DI, et al. Bortezomib plus docetaxel in advanced non-small cell lung cancer and other solid tumors: a phase I California Cancer Consortium trial. J Thorac Oncol. 2006;1(2):126–34. [PubMed] [Google Scholar]
- 13.Dreicer R, Petrylak D, Agus D, et al. Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin Cancer Res. 2007;13(4):1208–15. doi: 10.1158/1078-0432.CCR-06-2046. [DOI] [PubMed] [Google Scholar]
- 14.Ryan DP, O’Neil BH, Supko JG, Roch, et al. A Phase I study of bortezomib plus irinotecan in patients with advanced solid tumors. Cancer. 2006;107(11):2688. doi: 10.1002/cncr.22280. [DOI] [PubMed] [Google Scholar]
- 15.Yang CH, Gonzalez-Angulo AM, Reuben JM, et al. Bortezomib (VELCADE(R)) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 2006 Jan 10; doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- 16.Koschny R, Holland H, Sykora J, et al. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res. 2007;13(11):3403–12. doi: 10.1158/1078-0432.CCR-07-0251. [DOI] [PubMed] [Google Scholar]
- 17.Styczynski J, Olszewska-Slonina D, Kolodziej B, et al. Activity of bortezomib in glioblastoma. Anticancer Res. 2006;26(6B):4499–503. [PubMed] [Google Scholar]
- 18.Wang H, Wang H, Zhang W, et al. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84(8):941–51. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 19.Pierre A, Robe Mohamed Bentires-Alj, Bonif Marianne, et al. In vitro and In vivo Activity of the Nuclear Factor-B Inhibitor Sulfasalazine in Human Glioblastomas. Clin Cancer Res. 2004;10:5595–5603. doi: 10.1158/1078-0432.CCR-03-0392. [DOI] [PubMed] [Google Scholar]
- 20.Russo A, Terrasi M, Agnese V, et al. Apoptosis: a relevant tool for anticancer therapy. Ann Oncol. 2006;17(Suppl 7 )(8):115–23. doi: 10.1093/annonc/mdl963. [DOI] [PubMed] [Google Scholar]
- 21.Amiri JI, Horton LW, LaFleur BJ. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implications for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res. 2004;64(14):4912–8. doi: 10.1158/0008-5472.CAN-04-0673. [DOI] [PubMed] [Google Scholar]
- 22.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25 (18):2601–6. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive portioning analysis of the EORTC 26981/22981 –NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24 (16):2563–9. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]