Abstract
The urokinase plasminogen activator system is involved in angiogenesis and tumor growth of malignant gliomas, which are highly neovascularized and so may be amenable to antiangiogenic therapy. In this paper, we describe the activity of Å6, an octamer capped peptide derived from the non-receptor-binding region of urokinase plasminogen activator. Å6 inhibited human microvascular endothelial cell migration but had no effect on the proliferation of human microvascular endothelial cells or U87MG glioma cells in vitro. In contrast, Å6 or cisplatin (CDDP) alone suppressed subcutaneous tumor growth in vivo by 48% and 53%, respectively, and, more strikingly, the combination of Å6 plus CDDP inhibited tumor growth by 92%. Such combination treatment also greatly reduced the volume of intracranial tumor xenografts and increased survival of tumor-bearing animals when compared with CDDP or Å6 alone. Tumors from the combination treatment group had significantly reduced neovascularization, suggesting a mechanism involving Å6-mediated inhibition of endothelial cell motility, thereby eliciting vascular sensitivity to CDDP-mediated toxicity. These data suggest that the combination of an angiogenesis inhibitor that targets endothelial cells with a cytotoxic agent may be a useful therapeutic approach.
Expression of the urokinase plasminogen activator (uPA) and its receptor (uPAR) has been observed during tumor angiogenesis (1–3). Angiogenesis involves several processes, including proteolysis and remodeling of the basement membrane, endothelial cell activation, proliferation, migration, and tissue infiltration from preexisting blood vessels (4, 5). Endothelial cells can be induced to migrate in vitro by various angiogenic growth factors such as basic fibroblast growth factor and vascular endothelial growth factor. These factors up-regulate the expression of uPA (6) and uPAR (7, 8) in endothelial cells. These molecules are central to several cell-surface cascades used by endothelial cells during the formation of new vessels, and interference with the activities of the uPA system has been demonstrated in some cases to inhibit events associated with angiogenesis in vitro (1, 9, 10) and in vivo (1, 2).
We recently have identified an epitope from within the connecting peptide region of uPA that mediates cell motility and contractility (11, 12). A peptide (Å6) derived from this region of uPA inhibits tumor cell invasion and smooth muscle contraction in vitro and has antiangiogenic and antitumor activity in vivo (11, 12). Although it is not known whether this region directly modulates tumor progression when it is part of uPA, several lines of evidence implicate it in cell migration. For example, uPA phosphorylated in this region (Ser 138) is no longer chemotactic for monocytes (13). Proteolytic cleavage within this region also has been demonstrated to abrogate the stimulatory activity of uPA on vascular smooth muscle cell (SMC) proliferation (14) and the ability of uPA to promote the binding of soluble uPAR to hematopoietic cells (15). Finally, a mutant form of uPA lacking the growth factor-like domain (the uPAR binding domain of uPA) was still able to bind to SMC and stimulate migration, suggesting either a novel receptor or secondary interactions of uPA with uPAR (16).
Previous studies demonstrated the ability of Å6 monotherapy to inhibit tumor progression in several animal models of breast cancer cell growth and metastasis (11), where it was much more effective against slower-growing cell lines and for which the mechanism leading to decreased angiogenesis was not elucidated. To evaluate the antiangiogenic mechanism of Å6, we chose to study glioblastoma (GBM). GBM is an aggressively invasive and highly neovascularized tumor (17, 18) with a median survival period of approximately 12 months (19, 20) and for which the uPA system is involved in invasion and angiogenesis (21, 22). Here, we report that Å6 inhibits the migration of microvascular endothelial cells. Although this activity was insufficient to significantly inhibit GBM tumor progression in our model, its combination with an agent that inhibits endothelial cell proliferation [cisplatin (CDDP)] was very effective, even against rapidly growing GBM tumor lines in vivo.
Materials and Methods
Cells and Cell Culture.
The human GBM cell lines used in this study were described previously (23). All cell lines were cultured under previously reported conditions (24).
Migration Assay.
Transwells (Costar; 8-μm pore size) were coated with a mixture of type I collagen (50 μg/ml) and fibronectin (50 μg/ml) in PBS by adding 200 μl of the solution per transwell and allowing the membranes to air dry in a laminar flow hood overnight at room temperature. The transwells then were assembled in a 24-well plate, and the lower chambers were filled with endothelial cell basal medium-2 (800 μl; Clonetics) containing basic fibroblast growth factor (1 ng/ml) as a chemoattractant. Human lung microvessel endothelial cells (HMVECs; passage 2–5) in endothelial cell basal medium-2 (8 × 105 cells per ml) were added to the upper chamber of each transwell. Å6 was added to both chambers and the plate was placed at 37°C in 5% CO2 for 5 h. Cells that had migrated to the lower surface of the filters were stained with Giemsa (EM Science) and counted. Data are presented as the average number of migrated cells per 10 fields (×200).
Growth Inhibition Assay.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to evaluate the cytotoxicity as described (24). Briefly, cells (1 × 104 cells per well) were plated at 37°C in a 96-well microplate overnight. The cells then were treated with 200 μl of fresh medium containing 10% serum and Å6 (10 to 150 μM), CDDP (2.5 μg/ml to 10 μg/ml), or a combination of both drugs. Six wells for each treatment schedule were performed. After 24 h, 10 μl of 5 mg/ml MTT solution was added to culture media. After an additional 4-h incubation, 100 μl of DMSO was added, and the A at 570 nm was determined by using a microplate reader (Molecular Devices). The effects of treatment are expressed as percentage of growth inhibition with untreated cells as the control.
In Vivo Tumor Growth Inhibition.
U87MG cells (1 × 106 cells) were suspended in 0.1 ml of PBS and injected s.c. into the right flank of 4- to 6-week-old female nude mice of BALB/c background (Simonsen Laboratories, Gilroy, CA). For the treatment of the established xenografts, the tumors were permitted to establish and grow for 14 days until tumor volume reached 80–100 mm3. At this time, animals were randomized into 12 groups. Tumor size was measured every other day, and tumor volume was calculated from the formula (longest diameter) × (shortest diameter)2 × 0.5. For intracerebral stereotactic inoculation, 1 × 105 U87MG cells in 5 μl of PBS were inoculated into the right corpus striatum of the nude mouse brain as described (25).
Å6 or PBS was administered i.p. twice a day for 21 consecutive days. Either CDDP (3 mg/kg) or sterile normal saline was administered i.p for every other day for a total of 9 doses. All treatment protocols were approved by the animal care and use committee of the University of California San Diego.
Immunohistochemistry.
To assess angiogenesis in tumors, zinc-fixed, paraffin-embedded tumor sections were immunostained with a monoclonal rat anti-mouse CD31 antibody (1:200; BD PharMingen). Assessment of tumor cell proliferation was performed by Ki-67 immunohistochemistry on formalin-fixed paraffin-embedded tumor tissues. After deparaffinization and rehydration, the tissue sections were incubated with 3% hydrogen peroxidase in methanol to quench endogenous peroxidase. The sections were blocked for 30 min with goat serum and incubated with the primary antibody at 4°C overnight. The sections then were washed with PBS and incubated with a biotinylated secondary antibody for 30 min. After several washes with PBS, products were visualized with the streptavidin horseradish peroxidase and diaminobenzidine as chromogen. Hematoxylin was used as counterstain. As a measure of proliferation, Ki-67 labeling index was determined according to percentage of Ki-67 labeled nuclei to the total number of nuclei in high-power field (×400). Approximately 2,000 nuclei were counted in each case by a process of systematic random sampling.
Angiogenesis was quantitated by using two different methods. For this purpose, sections were immunostained with anti-CD31 but not counterstained with hematoxylin and analyzed by using computerized image analysis system. Microvessel density (MVD) and microvessel area (MVA) were determined by capturing digital images of the sections at ×200 magnification with a charge-coupled device color camera. Images then were analyzed with image pro plus version 4.0 software (Media Cybernetics, Silver Spring, MD). MVA was determined by measuring the total amount of staining in each section. Four fields were evaluated for each slide. This value was represented as a percentage of the total area in each field. For MVD, positively stained foci were counted with the software regardless of the size of the stained area. Thus, positively stained cells (punctate immunostaining) and large, positively stained vessels with lumens both counted as one focus using this method. MVD also was obtained by manually counting the positive foci for slides counterstained with hematoxylin and compared with the values obtained by using the image pro software for noncounterstained sections. Results were confirmed in each experiment by at least two observers.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Biotin Nick End-Labeling (TUNEL) Assay.
Apoptotic cells in tumor tissue were detected by using TUNEL method as described (24). TUNEL-positive cells were counted at ×400 magnification. The apoptotic index was calculated as a ratio of the apoptotic cell number to the total cell number in each field.
Statistical Analysis.
The data were analyzed for significance by Student's t test, except for the in vivo survival assays, which used Cox–Mantel and Wilcoxon analyses. Therapeutic synergism between Å6 and CDDP was assessed as previously described (26).
Results
Å6 Inhibits HMVECs' Migration but Not Their Proliferation or the Proliferation of Glioma Cells.
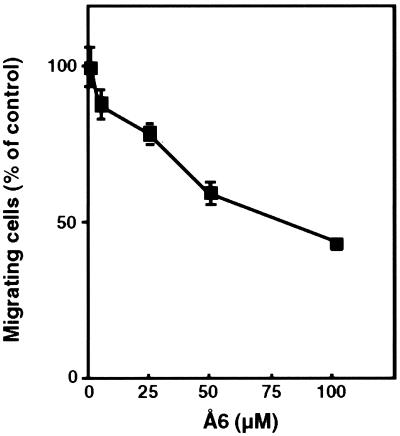
Because the uPA/uPAR system is involved in cell motility, we tested the ability of Å6 to inhibit the migration of HMVECs. The agent inhibited the migration of HMVECs (Fig. 1), although having no effect on the proliferation of HMVECs in similar concentration ranges (data not shown).
Figure 1.
Dose-dependent inhibitory effect of Å6 on the basic fibroblast growth factor-stimulated migration of HMVECs. Migration assays were performed as described in Materials and Methods. Data were calculated by taking the number of cells invading in the absence of Å6 as 100%. Results represent the mean ± SD of 10 fields. The experiment was repeated two times with similar results.
In previous studies, although Å6 worked efficiently against nonestablished tumor microfoci, it worked poorly against established, rapidly proliferating tumors (11). We therefore wanted to determine whether it was more effective in vitro if administered in combination with a cytotoxic agent that could retard the proliferation of the tumor cells. We chose to use CDDP because this compound has been used in the clinical treatment of GBM (19, 27, 28). We first tested the ability of Å6 to directly inhibit the in vitro proliferation of a panel of GBM cell lines that included U87MG, U251MG, U178MG, LN229, A1207, and LNZ308. Å6 did not inhibit the proliferation of any of the cell lines tested. CDDP inhibited the proliferation of both GBM as well as endothelial cells in vitro to variable extents, but Å6 did not potentiate this effect over a range of concentrations as high as 350 μM (data not shown).
Å6 in Combination with CDDP Efficiently Inhibits in Vivo Growth of U87MG GBM Cells.
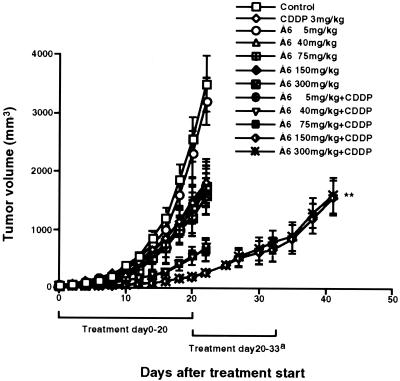
Å6 has been shown to potently inhibit the growth of MDA-MB-231 breast cancer cells only when treatment was initiated early (i.e., when the tumors had just begun to be palpable, about 10–15 mm3) (11). In that study, treatment of a fast-growing breast cancer line (Mat B-III) was only achieved when Å6 was administered prophylactically, and even then the maximal inhibition observed was only 50–60%. Here, we used a combination of Å6 and CDDP against tumors formed by aggressively growing human GBM cells. U87MG tumors established s.c. in nude mice were staged to a volume of ≈100 mm3. Treatment was initiated at this time, and Å6 was tested at several doses (5, 40, 75, 150, and 300 mg/kg per day), singly or in combination with CDDP [3 mg/kg given every other day, a dosage that previously had been shown to have some antitumor effects against GBM growth in vivo without significant generalized toxicity (24)]. Å6 and CDDP each inhibited the growth of GBM tumors by ≈40–50%. However, the combination therapy was able to inhibit the growth of these rapidly growing tumors by >90% without detectable signs of toxicity (Fig. 2). There was a greater effect at the 150 mg/kg dose of Å6 when compared with the 75 mg/kg dose, and no further enhancement of activity occurred at doses exceeding 150 mg/kg. The combination treatment was synergistic in its antitumor effects according to the criteria described by Shalinsky et al. (26). Based on these data, the 150 mg/kg dose was used for all further studies. If the tumors were allowed to grow after therapy was discontinued, they eventually resumed a rate of growth comparable to the control tumors, indicating that effect of this treatment resulted in stasis but did not lead to regression.
Figure 2.
Growth suppression of established U87MG xenografts by combination with Å6 and CDDP in vivo. Nude mice (four per group) were injected s.c. with 1 × 106 U87MG cells and were allowed to establish tumors. Tumors were staged to ≈100 mm3 before initiation of therapy. Animals were treated as described in Materials and Methods, and tumor volumes were measured serially every other day. The treatment was continued for another 14 days in combination group (a). Data are shown as the mean ± SE (bars). **, P = 0.006 (combination versus CDDP). The experiment was repeated independently two times with similar results.
Å6 Plus CDDP Treatment Leads to Tumor Growth Delay.
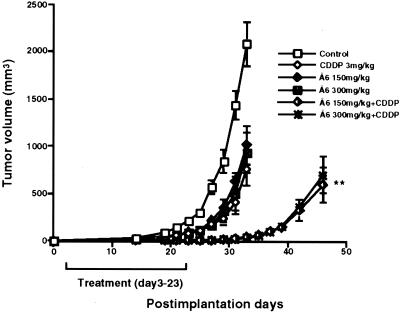
We next tested the ability of Å6 plus CDDP to inhibit the outgrowth of a small tumor by measuring the time to tumor volume when treatment was initiated 3 days after the inoculation of the tumor cells rather than when the tumors were already established. Tumors in animals treated with the combination of Å6 plus CDDP did not achieve a tumor volume of 500 mm3 until ≈20 days later than control animals (Fig. 3). Furthermore, in contrast to staged tumors, the discontinuation of treatment did not immediately result in an increased growth rate of the tumor. Rather, tumor suppression persisted for an additional 10 days after discontinuation of treatment before the growth rate appreciably increased.
Figure 3.
Supression of tumor formation by combination treatment with Å6 plus CDDP in vivo. Nude mice (four per group) were injected s.c. with 1 × 106 U87MG cells and treated with Å6 and CDDP as described in Materials and Methods. Data are shown as the mean ± SE (bars). **, P = 0.006 (combination versus CDDP). The experiment was repeated independently two times with similar results.
Combination Treatment with Å6 and CDDP Inhibits Intracranial Tumor Growth.
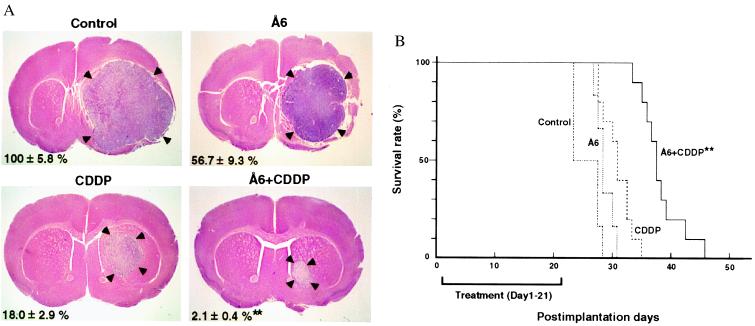
To ensure that the antitumor effect observed was not a phenomenon specific to the s.c. site, the effect of Å6 on the growth of U87MG tumor cells implanted intracranially was investigated. Mice implanted with tumor cells then were either killed on day 21 for histopathological analysis or monitored for survival. Consistent with the results observed in s.c. xenografts, the combination treatment significantly reduced tumor volumes in comparison to the other treatment groups (Fig. 4A). Moreover, combination therapy significantly increased survival compared with the groups of mice treated with either Å6 or CDDP alone (Fig. 4B). The combination treatment also was evaluated against intracranially xenografted A1207 cells with qualitatively similar results (data not shown).
Figure 4.
(A) Growth inhibition of intracranial tumors by the combination of Å6 and CDDP. U87MG cells (1 × 105) were injected intracerebrally and the animals were treated from postimplantation days 1 through 21 as described in Materials and Methods. On day 21, animals were euthanized and their brains were harvested, fixed, and sectioned. After hematoxylin/eosin staining, tumor volumes were determined by using the diameters that were measured at the maximal brain tumor dimensions in the coronal sections. Arrowheads indicate tumor tissue. Data were calculated by taking the tumor volume of control as 100%. Values are mean ± SD. **, P < 0.01 combination versus CDDP. (B) Extended survival of nude mice bearing intracerebral U87MG xenografts on treatment with Å6 and CDDP. Nude mice were treated as in A, except they were not euthanized at day 21 and were observed after discontinuation of therapy. Statistical significance was achieved by Cox–Mantel and Wilcoxon analyses of a Kaplan–Meier survival curve. **, P < 0.01 (combination versus CDDP).
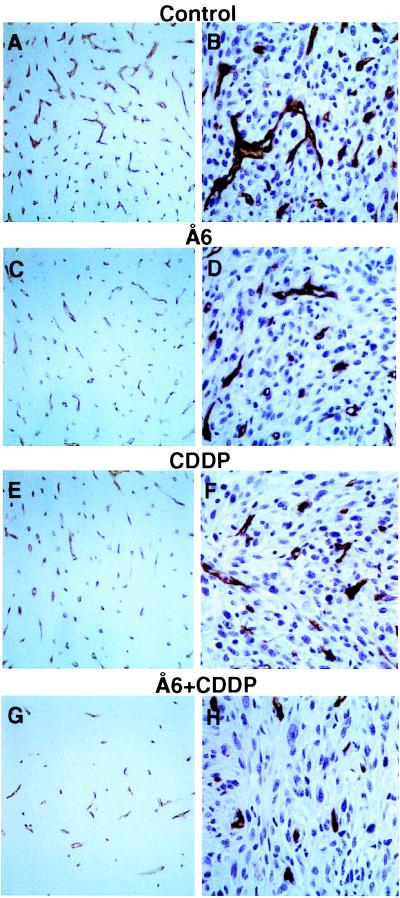
Combination Treatment with Å6 and CDDP Inhibits Tumor Angiogenesis.
To analyze tumor vascularization, intracranial tumor sections were immunostained for CD31. Each split panel in Fig. 5 depicts representative sections from each treatment group. Control tumors were highly vascularized with numerous large lumens and branches (Fig. 5 A and B). In contrast, a decrease in vessel number and prominent small vessels with fewer branches were observed in the treated groups (Fig. 5 C–H). These findings were especially evident in the combination treatment group (Fig. 5 G and H) when compared with the control groups. The effect of combination therapy on tumor vascularization was evaluated quantitatively by using two different methods. To measure the MVD, computerized image analysis was used on anti-CD31 immunostained sections that were not counterstained with hematoxylin. Å6 or CDDP moderately inhibited MVD by 25% or 39% respectively, whereas combination treatment with Å6 and CDDP markedly decreased MVD by 70% when compared with the control group (Table 1). The MVD assessed by computerized imaging analysis was consistent with that from manually counted data (data not shown). Because determination of MVD does not discriminate between single CD31-positive cells, neovessels, or larger established vessels, we also compared the total immunostained area as a representation of vessel differentiation. Quantitation of the inhibition of angiogenesis was unequivocal when the results of MVA were compared with those of MVD. Tumors obtained from animals treated with the combination of Å6 plus CDDP had 80% less MVA than did the control tumors, whereas tumors treated with Å6 or CDDP alone resulted in a reduction of 23% or 28% MVA, respectively.
Figure 5.
Immunohistological analysis of vascularization of intracranial tumors from control and combination-treated animals. Tumor sections were immunostained with anti-CD31 antibody as described in Materials and Methods with (B, D, F, and H; ×400) or without (A, C, E, and G; ×200) nuclear staining.
Table 1.
Inhibition of angiogenesis by combination treatment with Å6 and CDDP
| Treatment | MVD | MVA |
|---|---|---|
| Control | 310 ± 25 (0 ± 8) | 9 ± 0.4 (0 ± 4) |
| Å6 | 234 ± 10* (25 ± 3) | 6.9 ± 0.2† (23 ± 2) |
| CDDP | 188 ± 7‡ (39 ± 2) | 6.5 ± 0.2§ (28 ± 2) |
| Å6 + CDDP | 93 ± 6¶ (70 ± 2) | 1.8 ± 0.1∥ (80 ± 1) |
Microvessel was stained with anti-CD31 antibody. MVD and MVA were analyzed by computerized image analysis as described in Materials and Methods from four randomly selected fields (×200) in intracranial tumors from four mice of each group. Percentage reduction in MVD or MVA in treated tumors (T) versus control tumors (C) was calculated by the formula (1 − T/C) × 100% (in parentheses). Data are presented as mean ± SE. Statistical significance was determined by Student's t test. *, P = 0.01 versus control. †, P = 0.002 versus control. ‡, P < 0.001 versus control. §, P < 0.001 versus control. ¶, P < 0.001 versus CDDP. ∥, P < 0.001 versus CDDP.
TUNEL staining demonstrated a significant increase in the number of TUNEL-positive cells in the combination treatment group when compared with the other treatment groups (Table 2). Histological sections of tumors from treated and control specimens also were immunostained for Ki-67 to identify proliferating cells, and the positive foci were quantitated to determine the proliferative index. The proliferative index of the combination-treated group was significantly lower than in the controls treated with CDDP alone (Table 2). This finding was of significance because Å6 did not appear to have any direct cytotoxic or antiproliferative effects on the tumor cells themselves in vitro.
Table 2.
Proliferative and apoptotic indices in intracranial U87MG glioblastoma xenografts treated with Å6 and CDDP
| Treatment | Proliferative index, % | Apoptotic index, % |
|---|---|---|
| Control | 54.8 ± 1.1 | 1.1 ± 0.3 |
| Å6 | 50.5 ± 3.5 | 1.8 ± 0.4 |
| CDDP | 43.7 ± 1.9 | 2.0 ± 0.3 |
| Å6 + CDDP | 33.7 ± 3.0* | 4.0 ± 0.5* |
Apoptotic cells were detected by TUNEL assay. Apoptotic index was assessed by the percentage of TUNEL-positive cells to the total number of cells from four randomly selected high-power fields (×400) in intracranial tumors from four mice of each group. Cell proliferative index was assessed by the percentage of Ki-67-positive cells to the total number of cells from four randomly selected high-power fields (×400) in intracranial tumors from four mice of each group. Data are presented as mean ± SD. *, P < 0.01 combination versus CDDP.
Discussion
The identification of agents that inhibit angiogenesis represents a potential therapeutic approach for the treatment of GBM. An angiogenic switch has been hypothesized to be responsible for transition from a slow, dormant phenotype to a faster, more aggressive one (4), although recent studies have demonstrated the ability of even a few tumor cells to recruit neovessels (29). Endothelial cell migration is a critical early event during angiogenesis, and the inhibition of endothelial cell motility would be expected to affect the angiogenic process. Here, we present data showing that a peptide derived from the connecting peptide region of uPA, an enzyme that is central to angiogenesis, inhibits endothelial cell migration in vitro and tumor angiogenesis in vivo.
Å6 alone had some antitumor and antiangiogenic effects in intracranial xenografted glioma tumors. These results suggest either that Å6 can cross the blood–brain barrier (which may be altered within the tumor) to exert its effect or that it may be able to work at the tumor-vasculature interface without having to diffuse into the tumor itself. Although the inhibition of endothelial cell migration by Å6 resulted in only modest inhibition of tumor growth, coupling the antimigratory activity of Å6 with the antiproliferative activity of the alkylating agent, CDDP, led to dramatic synergistic antiangiogenic and antitumor effects. The precise definition of the molecular basis of the inhibition of tumor growth by combination therapy with Å6 and CDDP requires further study, although several possible mechanisms can be envisioned. One possible explanation for the potentiation of antineoplastic activity of CDDP is that Å6 is sensitizing the GBM cells to CDDP. However, this explanation seems unlikely because Å6 is not directly cytotoxic to GBM cells and it did not increase CDDP cytotoxicity in vitro. Another possibility is that Å6 may have increased the tumor concentration of the cytotoxic agent. Previous reports have demonstrated that the antiangiogenic agents TNP-470 and minocycline enhanced the perfusion of various cytoreductive agents (including CDDP) into the tumor, thereby increasing the level of tumor cell kill, resulting in a marked reduction in tumor growth rates. TNP-470 or minocycline did not markedly increase tumor cell kill in cell culture experiments (30, 31). It is possible that in our studies, increased vessel permeability could occur after the initial disruption of angiogenesis by Å6, leading to increased tumor cell exposure to CDDP. Although Å6 had no effect on endothelial cell monolayer permeability in vitro (our unpublished data), this finding does not rule out that this effect could occur in vivo. Studies to evaluate the ability of CDDP to perfuse into a tumor in the presence of Å6 are presently underway. Finally, it could be that A6 inhibits endothelial cell motility at angiogenic sites causing them to be sensitive or more highly exposed to CDDP. The difference in the proliferative index between control tumors and tumors treated with Å6 plus CDDP was 21%, and the number of TUNEL-positive cells was 3.6 times higher in combination-treated tumors when compared with that in control tumors. However, it is not yet clear whether these differences are sufficient to explain the differences in tumor growth and survival observed between the two groups. The decrease in tumor cell proliferation and increase in TUNEL-positive cells in combination-treated tumors may reflect the lack of new vasculature and tumor cell survival factors provided by the tumor vasculature that are necessary to supply the rapidly growing tumor mass.
In this regard, a direct effect of CDDP on tumor angiogenesis was apparent. This finding is in contrast to a previous report describing the lack of antiangiogenic activity by CDDP (32). However, in that study, CDDP was tested for its ability to inhibit the neovascularization of a Matrigel plug rather than a tumor. In the context of tumor angiogenesis, CDDP could have pleiotropic activities that could directly and indirectly affect angiogenic processes. In fact, an agent that is cytotoxic to tumor cells as well as endothelial cells could be superior to an agent that only inhibits endothelial cell proliferation. Agents that inhibit the proliferation of endothelial cells do not eliminate the positive pressure on angiogenesis that is created by angiogenic factors being secreted by tumor cells and tumor-associated macrophages. Angiogenic factor-secreting cells may have to be reduced or eliminated in the tumor, in addition to directly suppressing the growth of endothelial cells, for antiangiogenic therapy to successfully lead to antitumor effects. Thus, a concerted approach targeting multiple tumor-associated cell properties seems to be a promising strategy for significantly inhibiting tumor growth even in very aggressive, highly angiogenic tumors such as GBM. Our data support this hypothesis, because the combination of an agent that inhibits endothelial cell migration with an agent that is not only cytotoxic to endothelial cells but to tumor cells as well (perhaps leading to a reduced flux of angiogenic factors) was very effective in suppressing tumor angiogenesis and tumor growth. We do not yet know whether other types of cytotoxic therapy will work in combination with Å6 or whether the observed effects are specific to CDDP or to alkylating agents. It also should be noted that in the tumors treated with the combination of Å6 plus CDDP, the MVD in the tumor was substantially lower than that in the surrounding normal brain tissue.
The activation of endothelial cells causes them to alter cell–cell and cell–matrix contacts. If these endothelial cells then are inhibited from migrating, they may be more susceptible to a cytotoxic agent as they would lack certain adhesive contacts with the matrix and themselves. Nonadherent endothelial cells are certainly more likely to apoptose in vitro, and the addition of a cytotoxic agent in vivo may commit a higher percentage of these migratory cells to an apoptotic fate. Studies are underway to further understand the molecular mechanism leading to antitumor effects by using this combination therapy. Nevertheless, the combination of Å6, which inhibits the migration of endothelial cells, with a nonspecific cytotoxic agent seems to have potential for clinical use.
Acknowledgments
We thank Dr. Motoo Nagane for helpful advice on in vivo experiments, Dr. Padma Suvarna for image analyses, and members of the Ludwig Institute for helpful discussions. K.M. was supported in part by the Japan Brain Foundation.
Abbreviations
- CDDP
cisplatin
- uPA
urokinase plasminogen activator
- uPAR
uPA receptor
- GBM
glioblastoma
- HMVEC
human lung microvessel endothelial cell
- MVD
microvessel density
- MVA
microvessel area
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.150239497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.150239497
References
- 1.Min H Y, Doyle L V, Vitt C R, Zandonella C L, Stratton-Thomas J R, Shuman M A, Rosenberg S. Cancer Res. 1996;56:2428–2433. [PubMed] [Google Scholar]
- 2.Li H, Lu H, Griscelli F, Opolon P, Sun L-Q, Ragot T, Legrand Y, Belin D, Soria J, Soria C, Perricaudet M, Yeh P. Gene Ther. 1998;5:1105–1113. doi: 10.1038/sj.gt.3300742. [DOI] [PubMed] [Google Scholar]
- 3.Mazar A P, Henkin J, Goldfarb R H. Angiogenesis. 1999;3:15–32. doi: 10.1023/a:1009095825561. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 5.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 6.Saksela O, Moscatelli D, Rifkin D B. J Cell Biol. 1987;105:957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mignatti P, Mazzieri R, Rifkin D B. J Cell Biol. 1991;113:1193–1201. doi: 10.1083/jcb.113.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandriota S J, Seghezzi G, Vassalli J D, Ferrara N, Wasi S, Mazzieri R, Mignatti P, Pepper M S. J Biol Chem. 1995;270:9709–9716. doi: 10.1074/jbc.270.17.9709. [DOI] [PubMed] [Google Scholar]
- 9.Fibbi G, Caldini R, Chevanne M, Pucci M, Schiavone N, Morbidelli L, Parenti A, Granger H J, Del Rosso M, Ziche M. Lab Invest. 1998;78:1109–1119. [PubMed] [Google Scholar]
- 10.Yasunaga C, Nakashima Y, Sueishi K. Lab Invest. 1989;61:698–704. [PubMed] [Google Scholar]
- 11.Guo, Y. J., Higazi, A. A.-R., Arakelian, A., Sachais, B. S., Cines, D. B., Goldfarb, R. H., Jones, T. R., Kwaan, H., Mazar, A. P. & Rabbaní, S. A. (2000) FASEB J., in press. [DOI] [PubMed]
- 12.Haj-Yehia, A., Nassar, T., Sachais, B. S., Kuo, A., Bdeir, K., Al-Mehdi, A. B., Mazar, A., Cines, D. B. & Higazi, A. A.-R. (2000) FASEB J., in press. [DOI] [PubMed]
- 13.Franco P, Iaccarino C, Chiaradonna F, Brandazza A, Iavarone C, Mastronicola M R, Nolli M L, Stoppelli M P. J Cell Biol. 1997;137:779–791. doi: 10.1083/jcb.137.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanse S M, Benzakour O, Kanthou C, Kost C, Lijnen H R, Preissner K T. Arterioscler Thromb Vasc Biol. 1997;17:2848–2854. doi: 10.1161/01.atv.17.11.2848. [DOI] [PubMed] [Google Scholar]
- 15.Mizukami I F, Todd R F. J Leukocyte Biol. 1998;64:203–213. doi: 10.1002/jlb.64.2.203. [DOI] [PubMed] [Google Scholar]
- 16.Poliakov A A, Mukhina S A, Traktouev D O, Bibilashvily R S, Gursky Y G, Minashkin M M, Stepanova V V, Tkachuk V A. J Recept Signal Transduction Res. 1999;19:939–951. doi: 10.3109/10799899909038433. [DOI] [PubMed] [Google Scholar]
- 17.Bjerkvig R, Lund-Johansen M, Edvardsen K. Curr Opin Oncol. 1997;9:223–229. doi: 10.1097/00001622-199709030-00002. [DOI] [PubMed] [Google Scholar]
- 18.Wesseling P, Ruiter D J, Burger P C. J Neurooncol. 1997;32:253–265. doi: 10.1023/a:1005746320099. [DOI] [PubMed] [Google Scholar]
- 19.Huncharek M, Muscat J. Anticancer Res. 1998;18:1303–1312. [PubMed] [Google Scholar]
- 20.Salcman M, Scholtz H, Kaplan R S, Kulik S. Neurosurgery. 1994;34:213–219. doi: 10.1227/00006123-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, Sawaya R, Mohanam S, Bindal A K, Bruner J M, Oka K, Rao V H, Tomonaga M, Nicolson G L, Rao J S. Cancer Res. 1994;54:3656–3661. [PubMed] [Google Scholar]
- 22.Gladson C L, Pijuan-Thompson V, Olman M A, Gillespie G Y, Yacoub I Z. Am J Pathol. 1995;146:1150–1160. [PMC free article] [PubMed] [Google Scholar]
- 23.Furnari F B, Lin H, Huang H-J S, Cavenee W K. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagane M, Pan G, Weddle J J, Dixit V M, Cavenee W K, Huang H-J S. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]
- 25.Nishikawa R, Ji X D, Harmon R C, Lazar C S, Gill G N, Cavenee W K, Huang H-J S. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shalinsky D R, Brekken J, Zou H, Bloom L A, McDermott C D, Zook S, Varki N M, Appelt K. Clin Cancer Res. 1999;5:1905–1917. [PubMed] [Google Scholar]
- 27.Boiardi A, Silvani A, Milanesi I, Botturi M, Broggi G. J Neurooncol. 1991;11:165–170. doi: 10.1007/BF02390176. [DOI] [PubMed] [Google Scholar]
- 28.Lassen U, Kristjansen P E G, Wagner A, Kostel-Janetz M, Poulsen H S. J Neurooncol. 1999;43:161–166. doi: 10.1023/a:1006254716877. [DOI] [PubMed] [Google Scholar]
- 29.Li C-Y, Shan S, Huang Q, Braun R D, Lanzen J, Hu K, Lin P, Dewhirst M W. J Natl Cancer Inst. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- 30.Teicher B A, Dupuis N P, Robinson M F, Emi Y, Goff D A. Oncol Res. 1995;7:237–243. [PubMed] [Google Scholar]
- 31.Teicher B A, Holden S A, Ara G, Sotomayor E A, Huang Z D, Chen Y-N, Brem H. Int J Cancer. 1994;57:920–925. doi: 10.1002/ijc.2910570624. [DOI] [PubMed] [Google Scholar]
- 32.Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli M R, Viale G, Giavazzi R, Taraboletti G. Clin Cancer Res. 1996;2:1843–1849. [PubMed] [Google Scholar]