Abstract
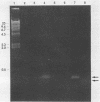
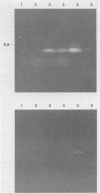
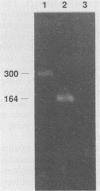
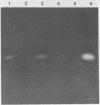
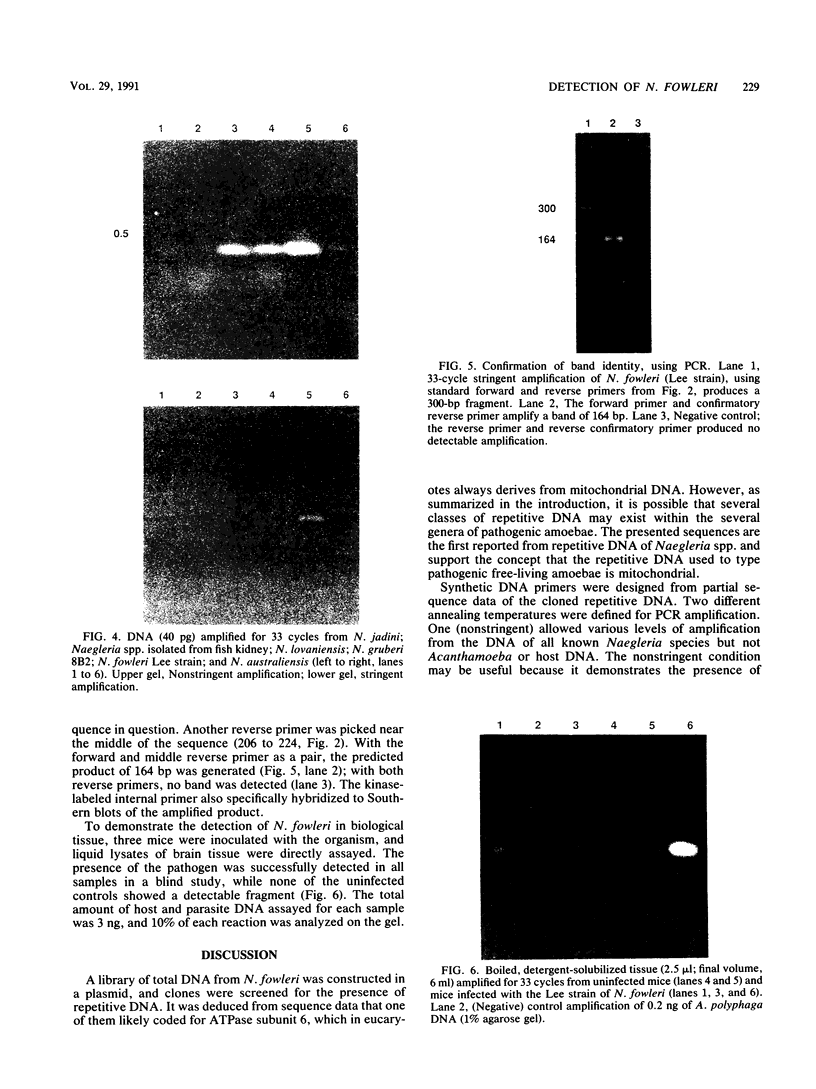
By using hybridization at low C0t values, a genomic library on Naegleria fowleri was screened for clones containing repetitive DNA. Partial sequence information from a repetitive clone, Nf9, showed sequence homologies with the mitochondrial ATPase 6 subunit from yeasts and other organisms. Synthetic DNA primers were selected and tested in amplification reactions. Nonstringent hybridization conditions were defined which allowed amplification of N. fowleri DNA and reduced amplification of DNA from nonpathogenic Naegleria species. Stringent conditions were selected which allowed detection only of N. fowleri. Identity of the amplified DNA was confirmed by using internal restriction sites and an internal primer. In a blind study, tissue from mice experimentally infected with N. fowleri was specifically detected by using stringent hybridization conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogler S. A., Zarley C. D., Burianek L. L., Fuerst P. A., Byers T. J. Interstrain mitochondrial DNA polymorphism detected in Acanthamoeba by restriction endonuclease analysis. Mol Biochem Parasitol. 1983 Jun;8(2):145–163. doi: 10.1016/0166-6851(83)90006-3. [DOI] [PubMed] [Google Scholar]
- Bohnert H. J., Herrmann R. G. The genomic complexity of Acanthamoeba castellanii mitochondrial DNA. Eur J Biochem. 1974 Dec 16;50(1):83–90. doi: 10.1111/j.1432-1033.1974.tb03874.x. [DOI] [PubMed] [Google Scholar]
- Clark C. G., Cross G. A. Circular ribosomal RNA genes are a general feature of schizopyrenid amoebae. J Protozool. 1988 May;35(2):326–329. doi: 10.1111/j.1550-7408.1988.tb04352.x. [DOI] [PubMed] [Google Scholar]
- Clark C. G., Cross G. A. rRNA genes of Naegleria gruberi are carried exclusively on a 14-kilobase-pair plasmid. Mol Cell Biol. 1987 Sep;7(9):3027–3031. doi: 10.1128/mcb.7.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio J. M., Harris G. H., Perna P. J., Paule M. R. Ribosomal ribonucleic acid repeat unit of Acanthamoeba castellanii: cloning and restriction endonuclease map. Biochemistry. 1981 Jun 23;20(13):3822–3827. doi: 10.1021/bi00516a024. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J. F. Geographic origin and spread of pathogenic Naegleria fowleri deduced from restriction enzyme patterns of repeated DNA. Biosystems. 1988;21(3-4):269–275. doi: 10.1016/0303-2647(88)90022-6. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Hill N. L., Goh D. H., Kumaratilake L. Altered neutrophils in mice immune to experimental Naegleria amoebic meningoencephalitis. Immunol Lett. 1989 Oct;22(4):301–305. doi: 10.1016/0165-2478(89)90169-7. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L. Length variation in eukaryotic rRNAs: small subunit rRNAs from the protists Acanthamoeba castellanii and Euglena gracilis. Gene. 1986;44(1):63–70. doi: 10.1016/0378-1119(86)90043-0. [DOI] [PubMed] [Google Scholar]
- Huizinga H. W., McLaughlin G. L. Thermal ecology of Naegleria fowleri from a power plant cooling reservoir. Appl Environ Microbiol. 1990 Jul;56(7):2200–2205. doi: 10.1128/aem.56.7.2200-2205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzzoco A., Colli W. Isolation of nuclei and characterization of nuclear DNA of Acanthamoeba castellanii. Biochim Biophys Acta. 1974 Dec 20;374(3):292–303. doi: 10.1016/0005-2787(74)90250-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin G. L., Brandt F. H., Visvesvara G. S. Restriction fragment length polymorphisms of the DNA of selected Naegleria and Acanthamoeba amebae. J Clin Microbiol. 1988 Sep;26(9):1655–1658. doi: 10.1128/jcm.26.9.1655-1658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin G. L., Collins W. E., Campbell G. H. Comparison of genomic, plasmid, synthetic, and combined DNA probes for detecting Plasmodium falciparum DNA. J Clin Microbiol. 1987 May;25(5):791–795. doi: 10.1128/jcm.25.5.791-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin G. L., Edlind T. D., Ihler G. M. Detection of Babesia bovis using DNA hybridization. J Protozool. 1986 Feb;33(1):125–128. doi: 10.1111/j.1550-7408.1986.tb05571.x. [DOI] [PubMed] [Google Scholar]
- Milligan S. M., Band R. N. Restriction endonuclease analysis of mitochondrial DNA as an aid in the taxonomy of Naegleria and Vahlkampfia. J Protozool. 1988 May;35(2):198–204. doi: 10.1111/j.1550-7408.1988.tb04323.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J. S., Harmatz P., Visvesvara G. S., Cohen A., Edwards J., Turner J. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982 Feb 11;306(6):346–348. doi: 10.1056/NEJM198202113060607. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M., Kjellberg F., Ayala F. J. A clonal theory of parasitic protozoa: the population structures of Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium, Trichomonas, and Trypanosoma and their medical and taxonomical consequences. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2414–2418. doi: 10.1073/pnas.87.7.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall R. L., Ironside K. S., Metler P. L., Tan E. L., Hazen T. C., Fliermans C. B. Effect of thermal additions on the density and distribution of thermophilic amoebae and pathogenic Naegleria fowleri in a newly created cooling lake. Appl Environ Microbiol. 1989 Mar;55(3):722–732. doi: 10.1128/aem.55.3.722-732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]