Abstract
Two benzodiazepine (midazolam and clorazepate) and one non-benzodiazepine (zolpidem) benzodiazepine-receptor agonists produced dose-related physical dependence, as evidenced by abstinence-induced decrease in planarian locomotor velocity (pLMV) when drug-exposed planarians were placed into drug-free water, but not when they were placed into drug-containing water (i.e., an abstinence-induced withdrawal, since the effect was only obtained in the removal of dug and not in the continued presence of drug). We have previously shown that the decrease in pLMV is associated with specific and transient withdrawal signs. In the present study, the selective benzodiazepine receptor antagonist flumazenil significantly antagonized (P < 0.05), by co-application, the ability of each agonist to produce the withdrawal. These results: (1) suggest that benzodiazepine receptor agonists, for two different chemical categories, produce dose-related physical dependence manifested as abstinence-induced withdrawal in this simple and convenient model, and (2) in the absence of cloning or radioligand binding literature, suggest a possible specific interaction site (receptor?) for these compounds in planarians.
Keywords: benzodiazepine, withdrawal, physical dependence, flumazenil, zolpidem, Planaria
1. Introduction
Previous reports of drug-induced behavioral effects in planarians (e.g., Carolei et al., 1975; Algeri et al., 1983; Venturini et al., 1989; Palladini et al., 1996) prompted us to establish a quantifiable metric that we have found useful for the demonstration and study of withdrawal in this species (Raffa and Valdez, 2001; Raffa et al., 2001). Planarians are a desirable model for these studies for several reasons, including cephalization (cephalic ganglia and bilateral spinal processes), primitive neurotransmitter systems (dopaminergic, serotonergic, opioidergic, etc.), permeability to small molecular weight drugs, and lack of a blood-brain barrier. For example, fllowing one-hour immersion in cocaine solution, planarians undergo dose-related abstinence-induced withdrawal behaviors (Raffa and Desai, 2005) and a decrease in spontaneous locomotor activity (pLMV) when placed into drug-free, but not drug-containing, water (Raffa and Valdez, 2001). In this convenient model of forced exposure, physical dependence develops within one hour and abstinence-induced withdrawal is manifested within five minutes. We previously demonstrated that the effect is not due to extraneous factors such as osmolarity or pH (Umeda et al., 2004) and the withdrawal is antagonized by selective receptor antagonists (Raffa et al., 2003a). We recently detected GABA in planarians (manuscript submitted for publication) and, hence, decided to test for benzodiazepine-induced effects (the existence of a GABAA receptor or benzodiazepine binding site in planarians has not yet, to our knowledge, been reported).
An abstinence-induced withdrawal syndrome (indicative of physical dependence) in humans has been associated with the abrupt discontinuation of benzodiazepine drugs following regular long-term use (Bateson, 2002). The phenomenon is generally mild to moderate and it develops slowly, making it difficult to emulate in acute mammalian models. Typical treatment regimens to induce physical dependence in mice, rats, cats, dogs, or baboons involve administration of a benzodiazepine for days or weeks and the withdrawal signs often do not develop until hours or even days after the discontinuation of the drug (e.g., Ryan and Boisse, 1983; McNicholas et al., 1983; Rosenberg and Chiu, 1982, 1985; Pokk and Zharhovsky, 1998; Kaminski et al., 2003; Begg et al., 2005; Listos et al., 2005).
In the present study, we report induction of physical dependence and abstinence-induced withdrawal in planarians using two benzodiazepines (midazolam and clorazepate (chlorazepate)) and a non-benzodiazepine benzodiazepine receptor agonist (zolpidem). Further, co-application of the selective benzodiazepine receptor antagonist flumazenil significantly antagonized each agonist’s ability to produce the withdrawal.
2. Materials and methods
2.1. Animals and chemicals
Planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply Co. (Burlington, NC), acclimated to temperature-controlled room conditions (21°C), and tested within 72 h of arrival. Each was used only one time. All of the drugs (midazolam maleate salt, clorazepate dipotassium salt, zolpidem, and flumazenil) and Cremophor® EL were purchased from Sigma Chemical Co. (St. Louis, MO).
2.2. Behavioral measurements
Planarian locomotor velocity (pLMV) (Raffa et al., 2001) was measured by placing individual planarians into a clear plastic petri dish (14-cm diameter) containing room-temperature (21°C) tap water treated with AmQuel® water conditioner. The dish was located over graph paper with gridlines spaced 0.5 cm apart. pLMV was quantified as the number of gridlines planarians crossed or re-crossed per minute over a 5-min observation period and is expressed as the means (± S.E.M.) of the cumulative number of gridlines crossed per minute (N = 10 planarians per group). Prior to measurement of pLMV, each planarian was placed into a 0.5 ml vial containing room-temperature vehicle or test compound(s) for 1 h. Zolpidem and flumazenil were prepared in 6% Cremophor® EL (which had no effect of its own on pLMV). Each planarian was exposed individually for 1 h to one of the following treatments: water, clorazepate (0.001, 0.01, 0.1, 1, or 10 µM), midazolam (0.001, 0.01, 0.1, 1, or 10 µM), zolpidem (0.001, 0.01, 0.1, 1, or 10 µM), flumazenil (10 µM) or agonist plus flumazenil and then tested individually for pLMV over 5 min in one of the following: water, clorazepate (10 µM), midazolam (10 µM), zolpidem (10 µM), or flumazenil (10 µM).
2.3. Statistics
Comparisons of group means were tested by one-way ANOVA and, if P < 0.05, followed by Tukey-Kramer multiple comparisons post-hoc test (significance criterion P < 0.05).
3. Results
3.1. Controls
Consistent with our previous reports (Raffa and Valdez, 2001; Raffa and Martley, 2005; Raffa and Desai, 2005; Raffa et al., 2000; 2001; 2003a,b; 2005; Umeda et al., 2004; 2005), drug-naïve planarians displayed a nearly constant locomotor velocity (pLMV) of about 10 to 13 gridlines/min when pretreated in drug-free water for 60 min and then tested in drug-free water. Likewise, when planarians were pretreated for 1 h with midazolam (10 µM), zolpidem (10 µM), or flumazenil (10 µM) and then tested in the identical concentration of the same drug, there was no significant difference in the 5-min cumulative pLMV compared to planarians pretreated in water and tested in water. Hence, exposure alone to these drugs did not produce a change in pLMV.
3.2. Midazolam (benzodiazepine)
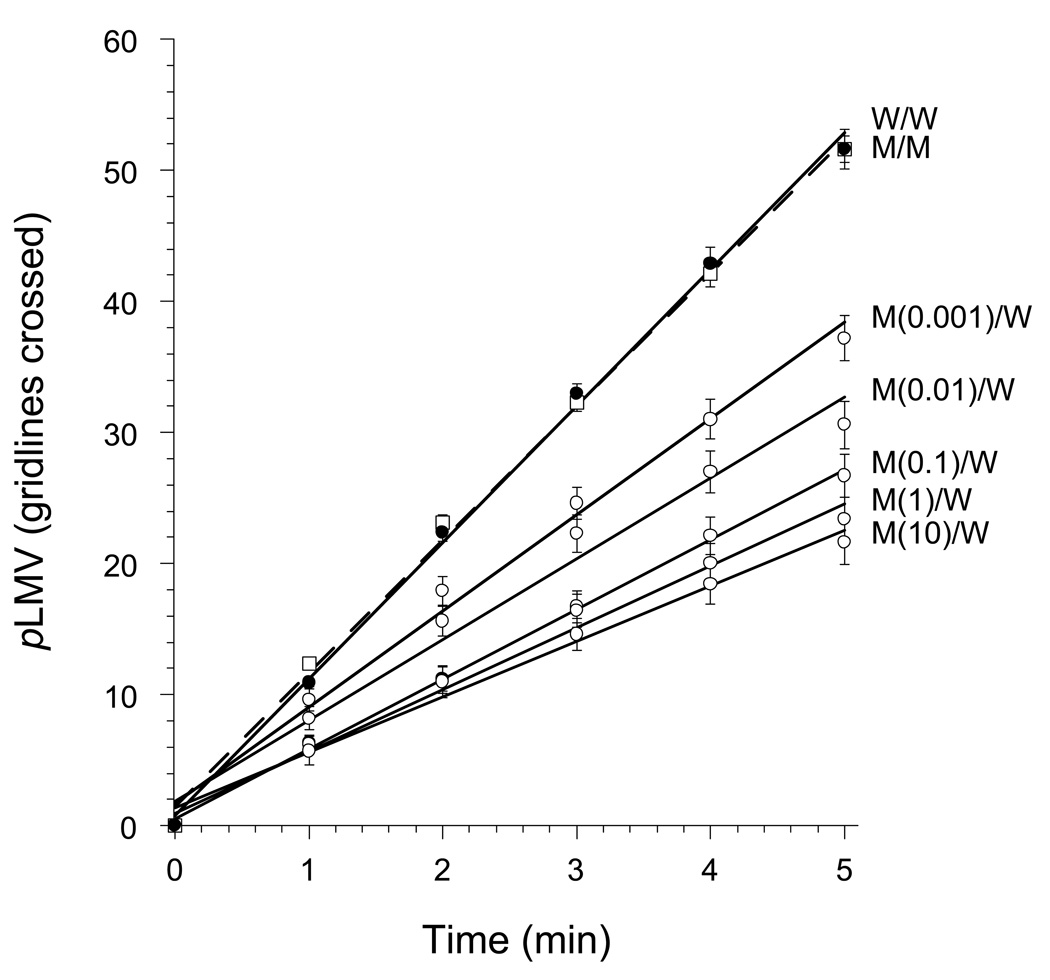
Planarians that were exposed to midazolam (0.001, 0.01, 0.1, 1, or 10 µM) for 1 h and then tested in midazolam-free water displayed a dose-related decrease in pLMV over the 5-min test period compared to midazolam-exposed planarians tested in midazolam-containing water (Fig. 1). The highest midazolam pretreatment dose (10 µM) produced approximately 60% reduction in pLMV compared to controls (ANOVA, P < 0.001, F = 63.05). This decrease in pLMV was observed only when the planarians were tested in drug-free water; it was not observed when they were tested in water containing the same, or higher, concentrations of midazolam. Of note, the pLMV, even though it was reduced, remained constant over the 5-min observation period.
Fig 1.
Dose-related decrease in pLMV, expressed as the mean ± S.E.M. cumulative number of gridlines crossed, following 1-h exposure to water (W) or midazolam (M) at 10 µM or concentration (µM) indicated, followed by testing in water (e.g., M(0.1)/W indicates 1-h immersion in 0.1 µM midazolam followed by testing in water.
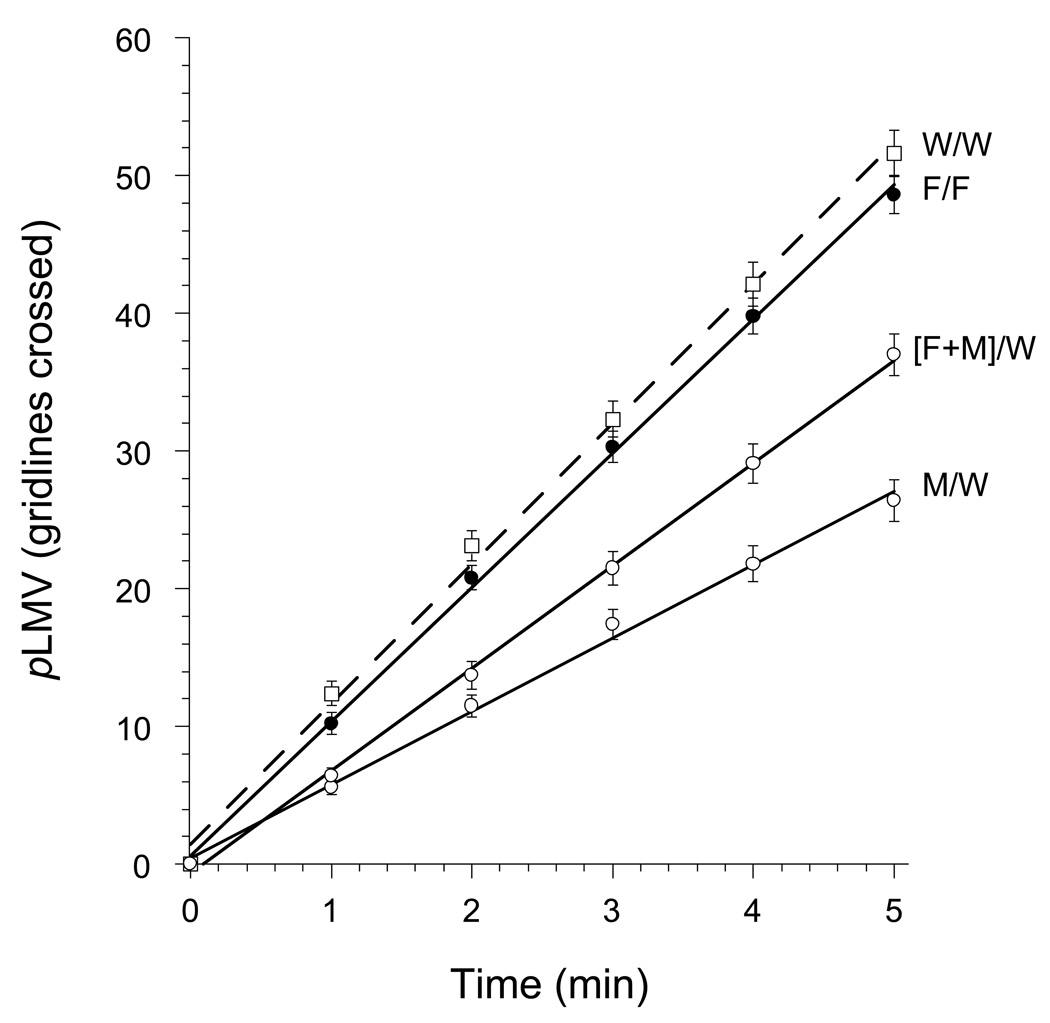
Flumazenil had no effect of its own on pLMV (P > 0.05), but it antagonized the abstinence-induced withdrawal from midazolam. That is, planarians that were co-treated with flumazenil (10 µM) along with midazolam (10 µM) during the 1 h exposure period displayed a significant prevention (ANOVA, P < 0.001, F = 56.93) of midazolam-induced reduction in pLMV (Fig. 2).
Fig 2.
Attenuation of the abstinence-induced withdrawal from midazolam (M) by co-treatment with flumazenil (F). Flumazenil alone had no effect on pLMV.
3.3. Clorazepate (benzodiazepine)
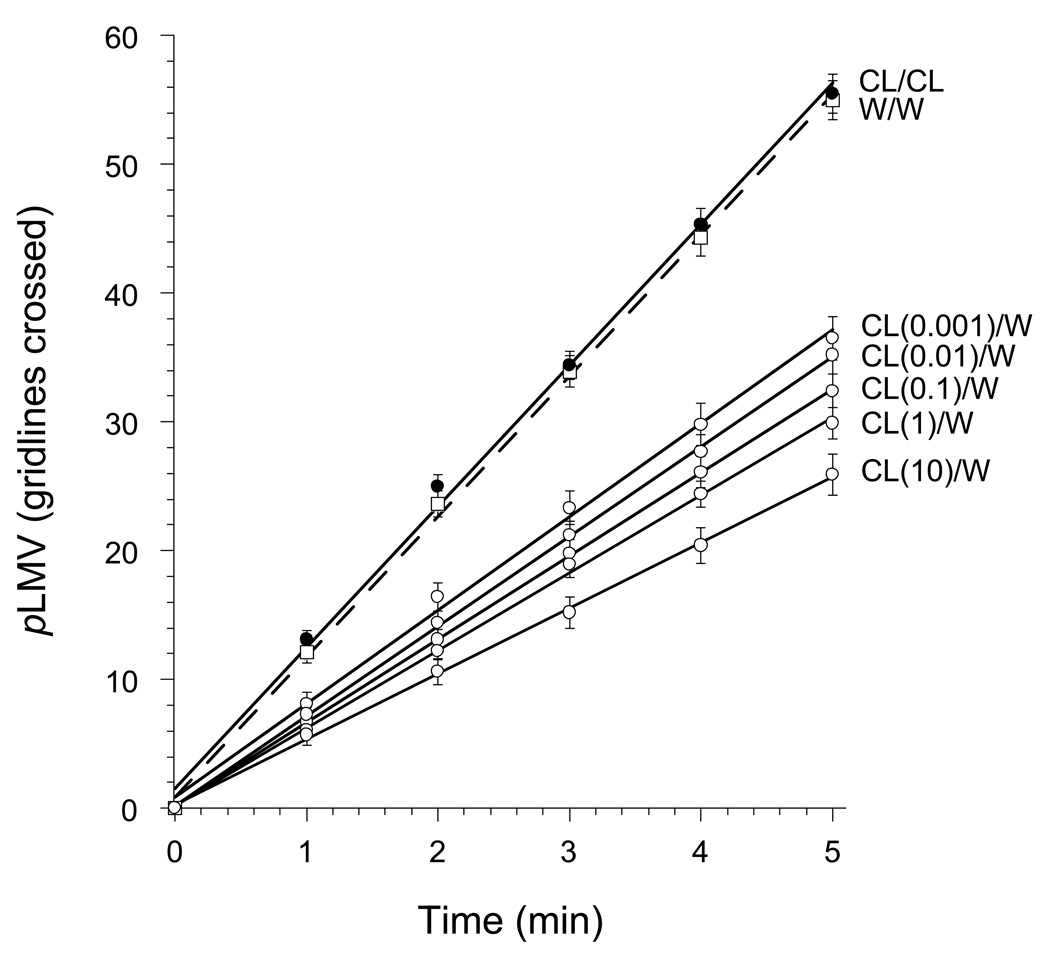
Planarians that were exposed to clorazepate (0.001, 0.01, 0.1, 1, or 10 µM) for 1 h and then tested in clorazepate-free water displayed a dose-related decrease in pLMV over the 5-min test period compared to clorazepate-exposed planarians tested in clorazepate-containing water (Fig. 3). The highest clorazepate pretreatment dose (10 µM) produced approximately 50% reduction in pLMV compared to controls (ANOVA, P < 0.001, F = 64.38). This decrease in pLMV was observed only when the planarians were tested in drug-free water; it was not observed when they were tested in water containing the same, or higher, concentrations of clorazepate. The pLMV, even though reduced, remained constant over the 5-min observation period.
Fig 3.
Dose-related decrease in pLMV (mean ± S.E.M.) cumulative number of gridlines crossed, following 1-h exposure to water (W) or clorazepate (C) at 10 µM or concentration (µM) indicated, followed by testing in water.
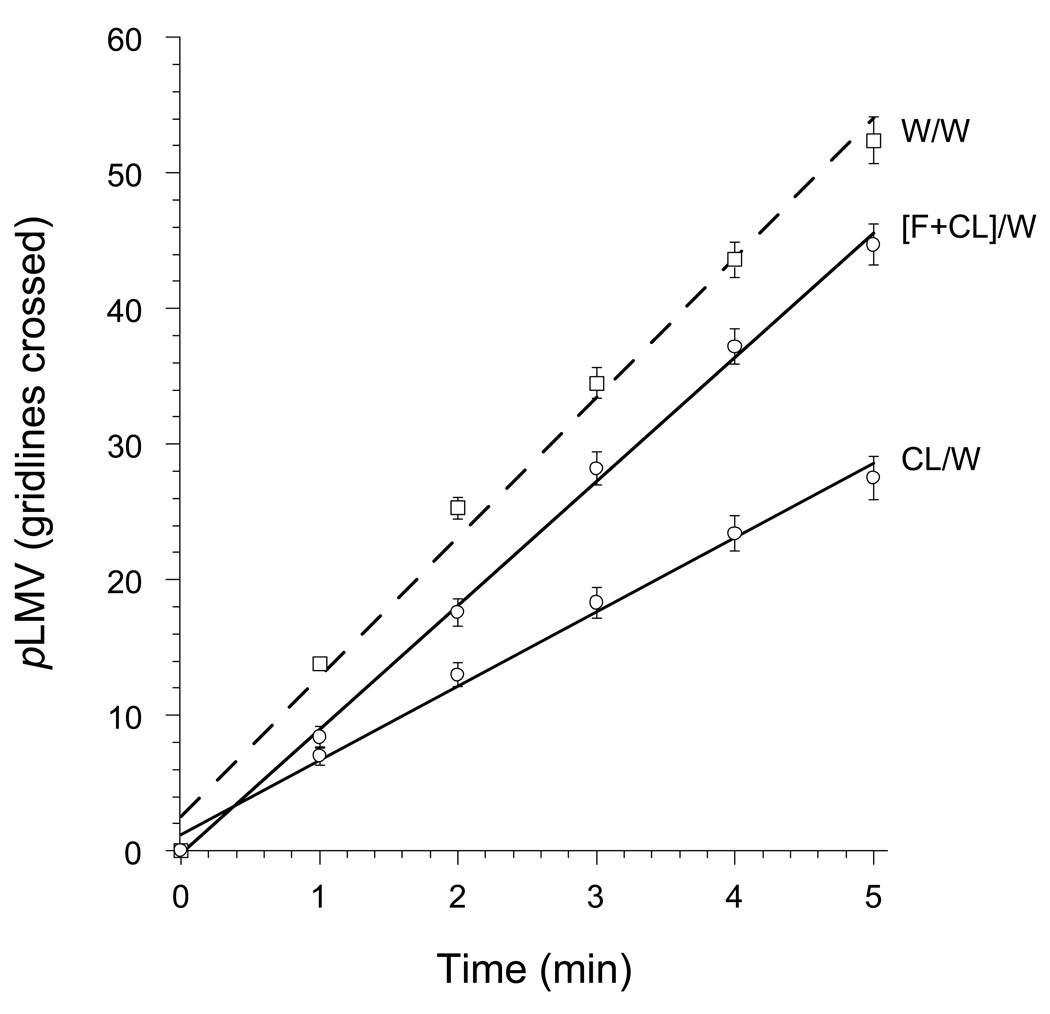
Flumazenil antagonized abstinence-induced withdrawal from clorazepate: i.e., co-treatment of planarians with flumazenil (10 µM) along with clorazepate (10 µM) during the 1 h exposure period resulted in a significant prevention (ANOVA, P < 0.001, F = 63.32) of the clorazepate-induced reduction in pLMV (Fig. 4).
Fig 4.
Attenuation of the abstinence-induced withdrawal from clorazepate (C) by co-treatment with flumazenil (F).
3.4. Zolpidem (non-benzodiazepine benzodiazepine receptor agonist)
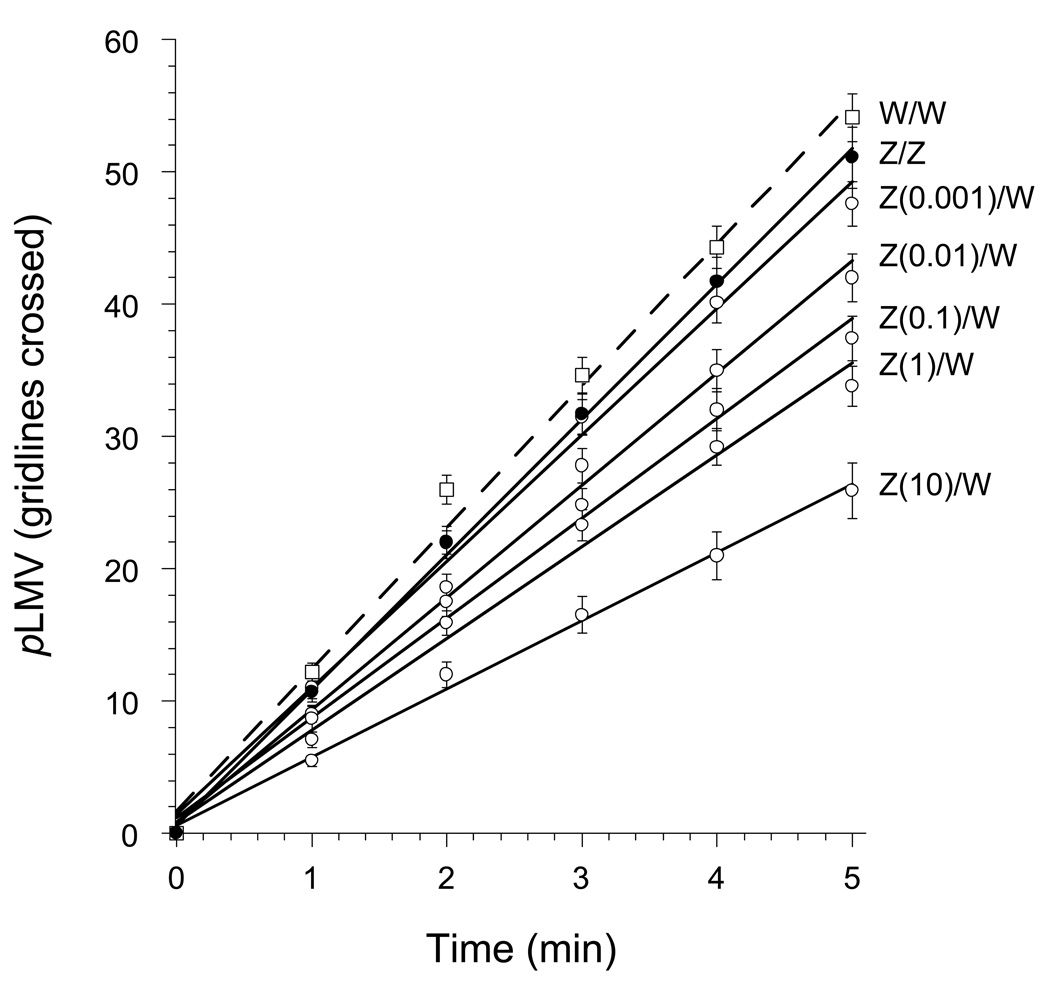
The results with zolpidem were similar to those with midazolam and clorazepate. Planarians that were exposed to zolpidem (0.001, 0.01, 0.1, 1, or 10 µM) for 1 h and then tested in zolpidem-free water displayed a dose-related decrease in pLMV over the 5-min test period in drug-free water compared to zolpidem-exposed planarians that were tested in zolpidem-containing water (Fig. 5). The highest pretreatment dose (10 µM) produced approximately 50% reduction in pLMV compared to controls (ANOVA, P < 0.001, F = 29.28). This decrease in pLMV was observed only when the planarians were tested in zolpidem-free water; it was not observed when they were tested in water containing the same, or higher, concentrations of zolpidem. The pLMV, even though reduced, remained constant over the 5-min observation period.
Fig 5.
Dose- related decrease in pLMV (mean ± S.E.M.) cumulative number of gridlines crossed, following 1-h exposure to water (W) or zolpidem (Z) at 10 µM or concentration (µM) indicated, followed by testing in water.
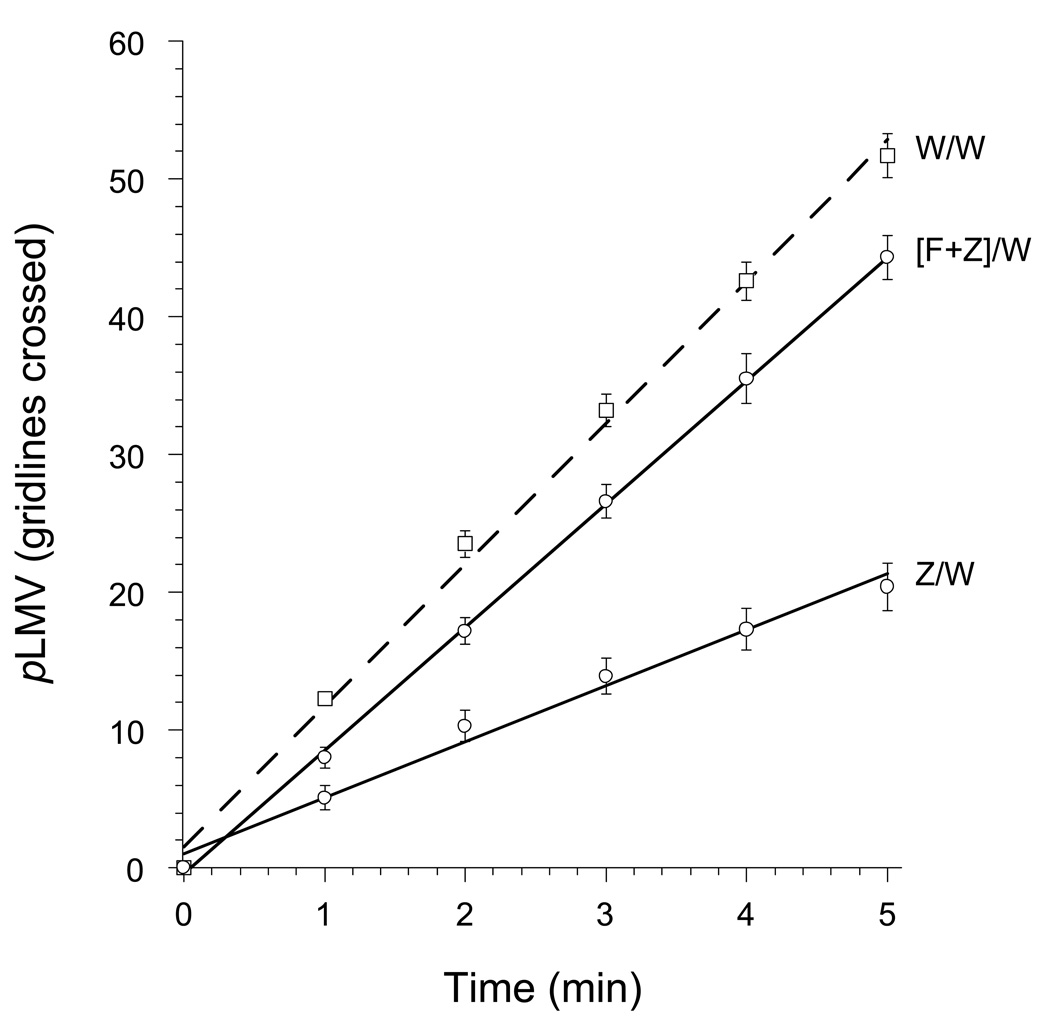
Similar to midazolam and clorazepate, flumazenil antagonized the abstinence-induced withdrawal from zolpidem: co-treatement of planarians with flumazenil (10 µM) along with zolpidem (10 µM) during the 1 h exposure period resulted in a significant prevention (ANOVA, P < 0.001, F = 100.23) of the zolpidem-induced reduction in pLMV (Fig. 6) (Table 1).
Fig 6.
Attenuation of the abstinence-induced withdrawal from zolpidem (Z) by co-treatment with flumazenil (F).
Table 1.
Attenuation of the development of physical dependence to three BZD receptor agonists by co-treatment with the BZD receptor antagonist flumazenil as manifested by an attenuated decrease in pLMV. Planarians were pretreated for 60 min and tested for 5 min under the conditions indicated. N = 10 planarians per group.
| Pretreatment | Test | pLMV | Pa |
|---|---|---|---|
| Midazolam | Water | 26.4 ± 1.5 | — |
| Midazolam + Flumazenil | Water | 37.0 ± 1.5 | < 0.001 |
| Clorazepate | Water | 27.5 ± 1.6 | — |
| Clorazepate + Flumazenil | Water | 44.7 ± 1.5 | < 0.001 |
| Zolpidem | Water | 20.4 ± 1.7 | — |
| Zolpidem + Flumazenil | Water | 44.3 ± 1.6 | < 0.001 |
Comparison of agonist to agonist + antagonist
3.5. Dose-response curves
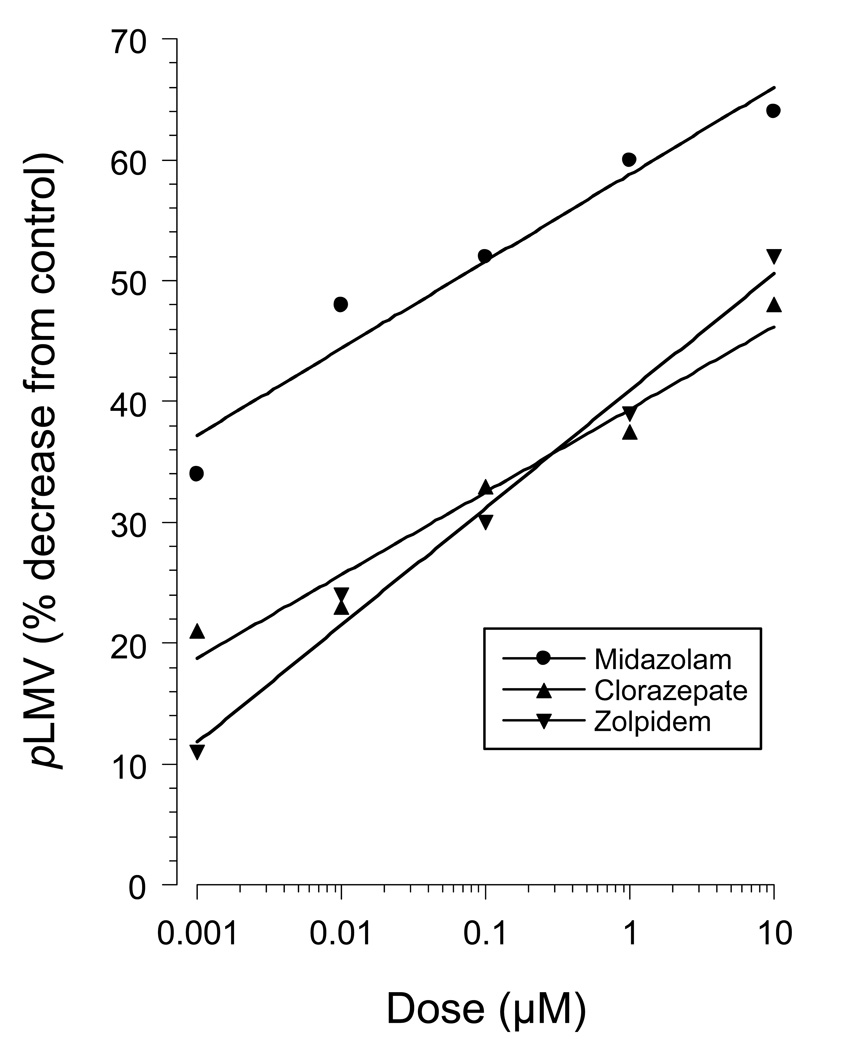
The dose-related withdrawal, expressed as the decrease in pLMV compared to controls (Fig. 7), was analyzed by nonlinear regression analysis. The results for each drug were as follows: midazolam y = 7.2log x + 58.8 (r = 0.97); clorazepate y = 6.8log x + 39.4 (r = 0.98), and zolpidem y = 9.7 log x + 40.9 (r = 0.99).
Fig 7.
Dose-response curves of abstinence-induced withdrawal from 1-h exposure to midazolam, clorazepate, or zolpidem. Values are means ± S.E.M. cumulative pLMV compared to controls (drug-exposed planarians tested in drug-free water).
4. Discussion
Measuring alterations in the nearly constant planarian spontaneous locomotor velocity over a five- or ten-minute observation period has proven to be a convenient, robust, and highly reproducible metric for quantifying the effect of drugs on planarians (Raffa and Valdez, 2001; Raffa and Martley, 2005; Raffa and Desai, 2005; Raffa et al., 2000; 2001; 2003a,b; 2005; Umeda et al., 2004; 2005). For example, planarians respond to dopaminergic ligands, including agonists, antagonists, and neuronal uptake inhibitors (e.g., Welsh and Williams, 1970; Carolei et al., 1975; Algeri et al., 1983; Venturini et al., 1989; Palladini et al., 1996) in an enantiomeric-selective and antagonist-sensitive manner (Raffa et al., 2001; 2003a). Together with changes in 2nd -messenger levels (e.g., cAMP) induced by dopaminergic agonists (Palladini et al., 1996), the collective findings suggest that the effects are mediated via dopamine receptors. An enkephalinergic system was identified in planarians by means of radioimmunological and immunocytochemical techniques (Venturini et al., 1983) and a dopamine-opioid behavioral interaction has been described (Passarelli et al., 1999). A serotonin receptor has been identified by [3H]-LSD binding and degenerate primer polymerase chain reaction (Saitoh et al., 1996) and both excitatory (aspartate and glutamate) (Rawls et al., 2006a) and inhibitory (GABA and glycine) (unpublished data) amino acids have been measured in planarians.
In previous work, we developed the pLMV behavioral metric as a means to assess and quantify the development of physical dependence in planarians as manifested in a withdrawal phenomenon (Raffa and Valdez, 2001; Raffa et al., 2001; 2003a). As brief summary of the model, planarians are exposed to drug for a period of time, usually for one hour. Following exposure to drug, planarians are placed into an observation dish and observed (usually for five or ten minutes), during which their spontaneous locomotor velocity is measured. Planarians that have been exposed to drug display a dose-related decrease in spontaneous locomotor velocity (pLMV) when subsequently tested in drug-free water, but not when tested in water containing equal concentration of drug. This is an important point, since in these cases exposure to the drug per se does not cause any decrease in pLMV. That is, planarians tested in the same concentration of drug to which they were exposed during pretreatment do not display an alteration in their pLMV. It is only those planarians that are first exposed to drug and then tested in water that display the alteration in pLMV (i.e., abstinence-induced withdrawal). Co-treatment with the appropriate receptor-selective antagonist during the 1-h exposure period attenuates the subsequent abstinence-induced withdrawal. Exposure to the same antagonist during the test period produces a dose-related decrease in pLMV (i.e., antagonist-precipitated withdrawal). Close observation of the planarians during withdrawal has revealed specific behavioral withdrawal signs that have been characterized (Raffa and Desai, 2005). We have shown that the withdrawal phenomenon is not due to adverse effects of the drugs on locomotor activity or to extraneous factors such as changes in pH, temperature, or osmolarity (Umeda et al., 2004).
In the present study, we extend our previous use of this model (Raffa and Valdez, 2001; Raffa and Desai, 2005; Raffa et al., 2003a; Umeda et al., 2004; Raffa et al., 2006; Rawls et al., 2006b) to three benzodiazepine receptor agonists. Benzodiazepine anxiolytics represent a class of therapeutic agents that also present an abuse problem. Although the development of physical dependence during chronic benzodiazepine administration and the subsequent withdrawal upon cessation of their use (abstinence) in humans is generally mild, the withdrawal dysphoria can nevertheless contribute to craving and potential relapse. Two of the agonists are benzodiazepines (midazolam and clorazepate), whereas the third (zolpidem) is a benzodiazepine receptor agonist, but is an imidazopyridine derivative structurally unrelated to benzodiazepines. The results were similar for all three drugs. Exposure for one hour in solutions of midazolam, clorazepate, or zolpidem was sufficient to induce an abstinence-induced withdrawal (manifested as a reduction in pLMV) already within one minute of immersion into drug-free water and continuing throughout the five-minute observation period. It is important to note that no such reduction in pLMV occurred if the planarians were tested in drug-containing water after exposure to the drug. Hence, drug exposure by itself had no effect on pLMV; it was only the absence of drug that elicited the reduction in pLMV.
The flumazenil-sensitive activity of clorazepate in this model deserves mention. In mammals, clorazepate is a prodrug. It is rapidly decarboxylated in gastric juice to N-desmethyl-diazepam (nordazepam, nordiazepam), which subsequently is absorbed and rapidly crosses the blood-brain barrier, whereas clorazepate does not (Frey and Scherkl, 1988). However, clorazepate possesses appreciable affinity for benzodiazepine receptors (41 nM) (Möhler and Okada, 1977). In planarians, which lack the equivalent of a blood-brain barrier, clorazepate activity would be observed even in the absence of conversion to nordazepam. The order of potency observed in this study (midazolam > zolpidem > clorazepate) is the same as the order of binding affinity of these drugs to mammalian benzodiazepine receptors (Buhr et al., 1997; Möhler and Okada, 1977).
In order to test whether the observed effect was related to a selective binding site in planarians, a selective benzodiazepine receptor antagonist was tested against the three agonists. In each case, co-treatment of the planarians with the antagonist (flumazenil) along with the agonist attenuated the subsequent abstinence-induced withdrawal and, hence, the development of the underlying physical dependence. An additional line of evidence pointing to a selective binding site is the fact that the withdrawal was observed for structurally diverse compounds (two benzodiazepines and one non-benzodiazepine) – and that both chemical classes of benzodiazepine receptor agonists were antagonized by the selective benzodiazepine receptor antagonist flumazenil. Although we have measured GABA in planarians by HPLC (submitted for publication), there is to our knowledge no report to date of a cloned planarian GABA receptor or benzodiazepine radioligand binding study. Some lower animals, e.g., C. elegans, have GABAA receptors insensitive to benzodiazepines (Holden-Dye et al., 1989; Bamber et al., 2003), but where the transition occurs is not known. Thus, it is premature to speculate about a mechanism for the present flumazenil-sensitive benzodiazepine response. Attribution of the effects to a planarian equivalent of a benzodiazepine receptor (whether or not associated with a GABA receptor) await the results of ongoing investigation.
In summary: (1) each of the three benzodiazepine receptor agonists produced an abstinence-induced withdrawal in planarians, (2) the withdrawal was elicited by two structural categories of benzodiazepine receptor agonist, benzodiazepine (midazolam and clorazepate) and imidazopyridine derivative (zolpidem), and (3) the withdrawal from all three agonists was attenuated by the benzodiazepine receptor antagonist flumazenil. In the absence of a cloned GABA receptor or benzodiazepine binding study, the present pharmacologic data tentatively suggest the existence of a benzodiazepine-like response element (receptor?) in planarians.
Acknowledgements
The authors thank Timothy Shickley, Ph.D. for suggesting the use of planarians as a model, Michael R. Jacobs, Pharm.D. for suggesting adding benzodiazepines to the list of planned studies, and Kyle Jacobs for pilot studies. This work was supported by Grant R01-DA15378 (to R.B.R.) from the NIH (NIDA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algeri S, Carolei A, Ferretti P, Gallone C, Palladini G, Venturini G. Effects of dopaminergic agents on monoamine levels and motor behaviour in Planaria. Comp. Biochem. Physiol. 1983;74C:27–29. doi: 10.1016/0742-8413(83)90142-1. [DOI] [PubMed] [Google Scholar]
- Bamber BA, Twyman RE, Jorgensen EM. Pharmacological characterization of the homomeric and heteromeric UNC-49 GABA receptors in C. elegans. Brit J. Pharmacol. 2003;138:883–893. doi: 10.1038/sj.bjp.0705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr A, Baur R, Sigel E. Subtle changes in residue 77 of the gamma subunit of α1β2γ2 GABAA receptors drastically alter the affinity for ligands of the benzodiazepine binding site. J. Biol. Chem. 1997;272:11799–11804. doi: 10.1074/jbc.272.18.11799. [DOI] [PubMed] [Google Scholar]
- Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr. Pharmaceut. Design. 2002;8:5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- Begg DP, Hallam KT, Norman TR. Attenuation of benzodiazepine withdrawal anxiety in the rat by serotonin antagonists. Behav. Brain Res. 2005;161:286–290. doi: 10.1016/j.bbr.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Carolei A, Margotta V, Palladini G. Proposal of a new model with dopaminergic-cholinergic interactions for neuropharmacological investigations. Neuropsychobiol. 1975;1:355–364. doi: 10.1159/000117512. [DOI] [PubMed] [Google Scholar]
- Frey HH, Scherkl R. Clorazepate, correlation between metabolism and anticonvulsant activity. Eur. J. Pharmacol. 1988;158:213–216. doi: 10.1016/0014-2999(88)90069-6. [DOI] [PubMed] [Google Scholar]
- Holden-Dye L, Krogsgaard-Larsen P, Nielsen L, Walker RJ. GABA receptors on the somatic muscle cells of the parasitic nematode, Ascaris suum: stereoselectivity indicates similarity to a GABAA-type agonist regonition site. Brit. J. Pharmacol. 1989;98:841–850. doi: 10.1111/j.1476-5381.1989.tb14613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Sannerud CA, Weerts EM, Lamb RJ, Griffiths RR. Physical dependence in baboons chronically treated with low and high doses of diazepam. Behav. Pharmacol. 2003;14:331–342. doi: 10.1097/01.fbp.0000082131.08343.0e. [DOI] [PubMed] [Google Scholar]
- Listos J, Malec D, Fidecka S. Influence of adenosine receptor agonists on benzodiazepine withdrawal signs in mice. Eur. J. Pharmacol. 2005;523:71–78. doi: 10.1016/j.ejphar.2005.07.025. [DOI] [PubMed] [Google Scholar]
- McNicholas LF, Martin WR, Cherian S. Physical dependence on diazepam and lorazepam in the dog. J. Pharmacol. Exp. Ther. 1983;226:783–789. [PubMed] [Google Scholar]
- Möhler H, Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977;198:849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- Palladini G, Ruggieri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp. Biochem. Physiol. C. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Passarelli F, Merante A, Pontieri FE, Margotta V, Venturini G, Palladini G. Opioid-dopamine interaction in planaria: a behavioral study. Comp. Biochem. Physiol. C. 1999;124:51–55. doi: 10.1016/s0742-8413(99)00048-1. [DOI] [PubMed] [Google Scholar]
- Pokk P, Zharkovsky A. Small platform stress attenuates the anxiogenic effect of diazepam withdrawal in the plus-maze test. Behav. Brain Res. 1998;97:153–157. doi: 10.1016/s0166-4328(98)00036-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Desai P. Description and quantification of cocaine withdrawal signs in Planaria. Brain Res. 2005;1032:200–202. doi: 10.1016/j.brainres.2004.10.052. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Martley AF. Amphetamine-induced increase in planarian locomotor activity and block by UV light. Brain Res. 2005;1031:138–140. doi: 10.1016/j.brainres.2004.10.051. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Valdez JM. Cocaine withdrawal in Planaria. Eur. J. Pharmacol. 2001;430:143–145. doi: 10.1016/s0014-2999(01)01358-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Valdez JM, Holland LJ, Schulingkamp RJ. Energy-dependent UV light-induced disruption of (–)sulpiride antagonism of dopamine. Eur. J. Pharmacol. 2000;406:R11–R12. doi: 10.1016/s0014-2999(00)00730-5. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Holland LJ, Schulingkamp RJ. Quantitative assessment of dopamine D2 antagonist activity using invertebrate (Planaria) locomotion as a functional endpoint. J. Pharmacol. Toxicol. Meth. 2001;45:223–226. doi: 10.1016/s1056-8719(01)00152-6. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Stagliano GW, Umeda S. κ-Opioid withdrawal in Planaria. Neurosci. Letts. 2003a;349:139–142. doi: 10.1016/s0304-3940(03)00814-0. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Dasrath CS, Brown DR. Disruption of a drug-induced choice behavior by UV light. Behav. Pharmacol. 2003b;14:569–571. doi: 10.1097/00008877-200311000-00010. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Brown DR, Dasrath CS. Cocaine effect on light/dark choice in Planaria: withdrawal. Pharmacologyonline. 2005;1:67–77. [Google Scholar]
- Raffa RB, Stagliano GW, Tallarida RJ. Subadditive withdrawal from cocaine/κ-opioid agonist combinations in Planaria. Brain Res. 2006;1114:31–35. doi: 10.1016/j.brainres.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Gomez T, Stagliano GW, Raffa RB. Measurement of glutamate and aspartate in Planaria. J. Pharmacol. Toxicol. Meth. 2006a;53:291–295. doi: 10.1016/j.vascn.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Rodriguez T, Baron DA, Raffa RB. A nitric oxide synthase inhibitor (L-NAME) attenuates abstinence-induced withdrawal from both cocaine and a cannabinoid agonist (WIN 55212–2) in Planaria. Brain Res. 2006b;1099:82–87. doi: 10.1016/j.brainres.2006.04.103. [DOI] [PubMed] [Google Scholar]
- Rosenberg HC, Chiu TH. An antagonist-induced benzodiazepine abstinence syndrome. Eur. J. Pharmacol. 1982;81:153–157. doi: 10.1016/0014-2999(82)90616-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg HC, Chiu TH. Time course for development of benzodiazepine tolerance and physical dependence. Neurosci. Biobehav. Revs. 1985;9:123–131. doi: 10.1016/0149-7634(85)90038-7. [DOI] [PubMed] [Google Scholar]
- Ryan GP, Boisse NR. Experimental induction of benzodiazepine tolerance and physical dependence. J. Pharmacol. Exp. Ther. 1983;226:100–107. [PubMed] [Google Scholar]
- Saitoh O, Yuruzume E, Nakata H. Identification of planarian serotonin receptor by ligand binding and PCR studies. NeuroReport. 1996;8:173–178. doi: 10.1097/00001756-199612200-00035. [DOI] [PubMed] [Google Scholar]
- Umeda S, Stagliano GW, Raffa RB. Cocaine and κ-opioid withdrawal in Planaria blocked by d-, but not l-, glucose. Brain Res. 2004;1018:181–185. doi: 10.1016/j.brainres.2004.05.057. [DOI] [PubMed] [Google Scholar]
- Umeda S, Stagliano GW, Borenstein MR, Raffa RB. A reverse-phase HPLC and fluorescence detection method for measurement of 5-hydroxytryptamine (serotonin) in Planaria. J. Pharmacol. Toxicol. Meth. 2005;51:73–76. doi: 10.1016/j.vascn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Venturini G, Carolei A, Palladini G, Margotta V, Lauro MG. Radioimmunological and immunocytochemical demonstration of Met-enkephalin in planaria. Comp. Biochem. Physiol. 1983;74:23–25. doi: 10.1016/0742-8413(83)90141-x. [DOI] [PubMed] [Google Scholar]
- Venturini G, Stocchi F, Margotta V, Ruggieri S, Bravi D, Bellantuono P, Palladini G. A pharmacological study of dopaminergic receptor in Planaria. Neuropharmacology. 1989;28:1377–1382. doi: 10.1016/0028-3908(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Welsh JH, Williams LD. Monoamine containing neurons in Planaria. J. Comp. Neurol. 1970;138:103–116. doi: 10.1002/cne.901380108. [DOI] [PubMed] [Google Scholar]