Abstract
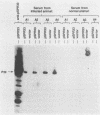
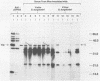
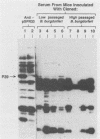
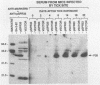
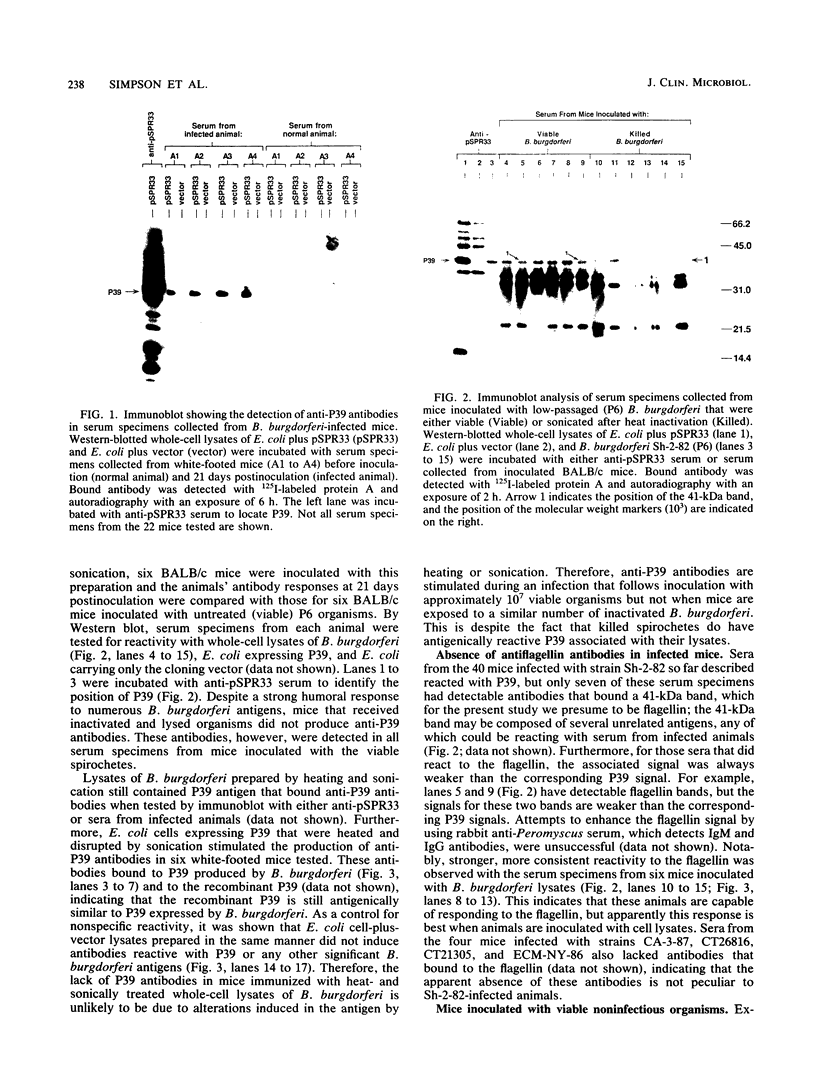
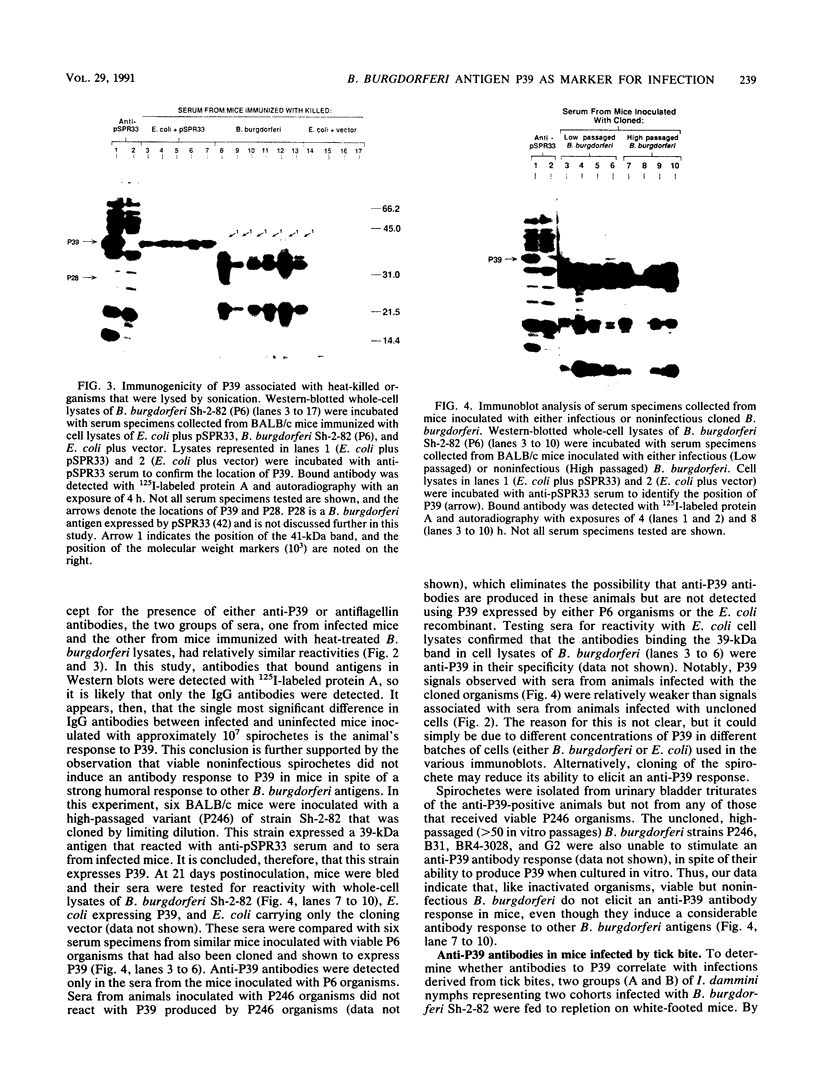
Borrelia burgdorferi expresses a conserved, species-specific 39-kDa protein (P39) that can stimulate antibodies during human infection. To confirm that anti-P39 antibodies are produced consistently in animals exposed to infectious spirochetes, white-footed mice, Peromyscus leucopus, and laboratory white mice, Mus musculus (strain BALB/c), were experimentally inoculated with either infectious or noninfectious B. burgdorferi and the antibody response to P39 was determined by immunoblot at 21 days postinoculation. All mice inoculated with approximately 10(7) infectious B. burgdorferi produced anti-P39 antibodies and were cultured positive for this spirochete. Mice inoculated with similar numbers of inactivated or viable noninfectious B. burgdorferi still producing P39 did not induce anti-P39 antibodies. By contrast, putative antiflagellin antibodies were detected in less than 18% of the infected animals, which supports the notion that antibody reactive with flagellin may not be reliable as a marker for B. burgdorferi exposure as was originally thought. Mice infected with B. burgdorferi following exposure to ticks (Ixodes dammini) produced anti-P39 antibodies no later than 7 days postinfection, indicating that P39 is an effective immunogen in natural infections. Notably, anti-P39 antibodies were the predominant B. burgdorferi reactive antibodies detected early in the infection. Our results indicate that anti-P39 antibodies are produced in response to an active infection and are therefore reliable markers for infection in experimentally and naturally inoculated animals.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ai C. X., Wen Y. X., Zhang Y. G., Wang S. S., Qiu Q. C., Shi Z. X., Li D. Y., Chen D. Q., Liu X. D., Zhao J. H. Clinical manifestations and epidemiological characteristics of Lyme disease in Hailin county, Heilongjiang Province, China. Ann N Y Acad Sci. 1988;539:302–313. doi: 10.1111/j.1749-6632.1988.tb31864.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Garcia-Monco J. C., Deponte P. C. Biological activity of Borrelia burgdorferi antigens. Ann N Y Acad Sci. 1988;539:115–125. doi: 10.1111/j.1749-6632.1988.tb31845.x. [DOI] [PubMed] [Google Scholar]
- Bosler E. M., Ormiston B. G., Coleman J. L., Hanrahan J. P., Benach J. L. Prevalence of the Lyme disease spirochete in populations of white-tailed deer and white-footed mice. Yale J Biol Med. 1984 Jul-Aug;57(4):651–659. [PMC free article] [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgess E. C., Windberg L. A. Borrelia sp. infection in coyotes, black-tailed jack rabbits and desert cottontails in southern Texas. J Wildl Dis. 1989 Jan;25(1):47–51. doi: 10.7589/0090-3558-25.1.47. [DOI] [PubMed] [Google Scholar]
- Cluss R. G., Boothby J. T. Thermoregulation of protein synthesis in Borrelia burgdorferi. Infect Immun. 1990 Apr;58(4):1038–1042. doi: 10.1128/iai.58.4.1038-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Dekonenko E. J., Steere A. C., Berardi V. P., Kravchuk L. N. Lyme borreliosis in the Soviet Union: a cooperative US-USSR report. J Infect Dis. 1988 Oct;158(4):748–753. doi: 10.1093/infdis/158.4.748. [DOI] [PubMed] [Google Scholar]
- Dupuis M. J. Les multiples manifestations neurologiques des infections à Borrelia burgdorferi. Rev Neurol (Paris) 1988;144(12):765–775. [PubMed] [Google Scholar]
- Eng T. R., Wilson M. L., Spielman A., Lastavica C. C. Greater risk of Borrelia burgdorferi infection in dogs than in people. J Infect Dis. 1988 Dec;158(6):1410–1411. doi: 10.1093/infdis/158.6.1410. [DOI] [PubMed] [Google Scholar]
- Greene R. T., Walker R. L., Nicholson W. L., Heidner H. W., Levine J. F., Burgess E. C., Wyand M., Breitschwerdt E. B., Berkhoff H. A. Immunoblot analysis of immunoglobulin G response to the Lyme disease agent (Borrelia burgdorferi) in experimentally and naturally exposed dogs. J Clin Microbiol. 1988 Apr;26(4):648–653. doi: 10.1128/jcm.26.4.648-653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicki R. L., Steere A. C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis. 1988 Apr;157(4):790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- Habicht G. S., Beck G., Benach J. L. Lyme disease. Sci Am. 1987 Jul;257(1):78–83. doi: 10.1038/scientificamerican0787-78. [DOI] [PubMed] [Google Scholar]
- Kazmierczak J. J., Burgess E. C. Antibodies to Borrelia sp. in wild foxes and coyotes from Wisconsin and Minnesota. J Wildl Dis. 1989 Jan;25(1):108–111. doi: 10.7589/0090-3558-25.1.108. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Regnery D. C. Lagomorphs as sentinels for surveillance of borreliosis in the far western United States. J Wildl Dis. 1989 Apr;25(2):189–193. doi: 10.7589/0090-3558-25.2.189. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Barbour A. G. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J Infect Dis. 1989 Jan;159(1):43–49. doi: 10.1093/infdis/159.1.43. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F. Enzyme-linked immunosorbent assays for the detection of class-specific immunoglobulins to Borrelia burgdorferi. Am J Epidemiol. 1988 Apr;127(4):818–825. doi: 10.1093/oxfordjournals.aje.a114864. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Hyland K. E., Fish D., Mcaninch J. B. Serologic analyses of Peromyscus leucopus, a rodent reservoir for Borrelia burgdorferi, in northeastern United States. J Clin Microbiol. 1988 Jun;26(6):1138–1141. doi: 10.1128/jcm.26.6.1138-1141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Miller J. N., Anderson J. F., Riviere G. R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990 Jun;28(6):1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal D., Taverna C., Hitzig W. H. Immunoblot analysis of antibody binding to polypeptides of Borrelia burgdorferi in children with different clinical manifestations of Lyme disease. Pediatr Res. 1989 Oct;26(4):377–382. doi: 10.1203/00006450-198910000-00019. [DOI] [PubMed] [Google Scholar]
- Pennell D. R., Wand P. J., Schell R. F. Evaluation of a quantitative fluorescence immunoassay (FIAX) for detection of serum antibody to Borrelia burgdorferi. J Clin Microbiol. 1987 Nov;25(11):2218–2220. doi: 10.1128/jcm.25.11.2218-2220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L. R., Sweeney A. H., Checko P. J., Magnarelli L. A., Mshar P. A., Gunn R. A., Hadler J. L. Epidemiological and clinical features of 1,149 persons with Lyme disease identified by laboratory-based surveillance in Connecticut. Yale J Biol Med. 1989 May-Jun;62(3):253–262. [PMC free article] [PubMed] [Google Scholar]
- Piesman J., Oliver J. R., Sinsky R. J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am J Trop Med Hyg. 1990 Apr;42(4):352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- Pizzarello L. D., MacDonald A. B., Semlear R., DiLeo F., Berger B. Temporal arteritis associated with Borrelia infection. A case report. J Clin Neuroophthalmol. 1989 Mar;9(1):3–6. doi: 10.3109/01658108909019502. [DOI] [PubMed] [Google Scholar]
- Rank E. L., Dias S. M., Hasson J., Duray P. H., Johnson R. C., Magnarelli L. A., Fister R. D. Human necrotizing splenitis caused by Borrelia burgdorferi. Am J Clin Pathol. 1989 Apr;91(4):493–498. doi: 10.1093/ajcp/91.4.493. [DOI] [PubMed] [Google Scholar]
- Reimers C. D., Pongratz D. E., Neubert U., Pilz A., Hübner G., Naegele M., Wilske B., Duray P. H., de Koning J. Myositis caused by Borrelia burgdorferi: report of four cases. J Neurol Sci. 1989 Jun;91(1-2):215–226. doi: 10.1016/0022-510x(89)90089-0. [DOI] [PubMed] [Google Scholar]
- Russell H., Sampson J. S., Schmid G. P., Wilkinson H. W., Plikaytis B. Enzyme-linked immunosorbent assay and indirect immunofluorescence assay for Lyme disease. J Infect Dis. 1984 Mar;149(3):465–470. doi: 10.1093/infdis/149.3.465. [DOI] [PubMed] [Google Scholar]
- Schmid G. P. The global distribution of Lyme disease. Rev Infect Dis. 1985 Jan-Feb;7(1):41–50. doi: 10.1093/clinids/7.1.41. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Kime K. K., Schrumpf M. E., Coe J. E., Simpson W. J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect Immun. 1989 Nov;57(11):3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal L. H. Lyme disease, 1988: immunologic manifestations and possible immunopathogenetic mechanisms. Semin Arthritis Rheum. 1989 Feb;18(3):151–167. doi: 10.1016/0049-0172(89)90058-9. [DOI] [PubMed] [Google Scholar]
- Simpson W. J., Garon C. F., Schwan T. G. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb Pathog. 1990 Feb;8(2):109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- Simpson W. J., Schrumpf M. E., Schwan T. G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990 Jun;28(6):1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]