Abstract
Primary gastric choriocarcinoma (PGC) is a rare tumor, and its pathogenesis is still uncertain. Most PGCs have been reported to possess an adenocarcinoma component of variable extent, and pure PGC is especially rare. The diagnosis of PGC is confirmed by exhibition of choriocarcinomatous components on biopsy and exhibition of β-hCG positive cell on immunohistochemical stain and elevation of the serum β-hCG. Moreover it must be confirmed that no other site including gonads displays any tumor masses. The PGC tends to be more invasive and to have early metastasis. The median survival is known to be less than several months. We report two cases. The first case was a 62 year-old man who was diagnosed as advanced gastric cancer (AGC) by endoscopic biopsy with hepatic metasasis and received palliative chemotherapy with modified FOLFOX regimen and Genexol plus cisplatin regimen. He underwent subtotal gastrectomy due to perforation of the stomach during chemotherapy. On post-operative biopsy, He was re-diagnosed as PGC and received another palliative chemotherapy modified FOLFIRI, BEP, EMACO, VIP. However, multiple liver metastases were aggravated, and also serum AFP level increased. Ultimately, the paient died 10 months after initial diagnosis. Another case was a 45 year-old man. On endoscopic biopsy, he was diagnosed as AGC of adenocarcinoma. On Chest and Abdomen CT, multiple pulmonary and hepatic metastasis were also confirmed. On liver biopsy, He was diagnosed as PGC. The immunohistochemical stains were performed and the results were cytokeratin positive, EMA negative and β-hCG weak positive. The serum β-hCG level was highly elevated. BEP, VIP and EMA/CO combination therapy were administered, but he died at 12th months after the initial diagnosis.
Keywords: Choriocarcinoma, Stomach
INTRODUCTION
Choriocarcinoma is a neoplasm secreting β-hCG pertinent to the uterus and pregnancy in most cases, and it is a malignant tumor that grows rapidly and metastasizes in a wide area. Choriocarcinoma occurs in the ovary or the testis primarily, and primary gastric choriocarcinoma cases have been reported to be very rare. Concerning the diagnosis of primary gastric choriocarcinoma, cases definitely diagnosed by endoscopic biopsy are small numbers, and most cases are diagnosed after surgery. In addition, in comparison with gastric adenocarcinoma, it shows very poor prognosis (1,2). On the other hand, most studies reported until now are the case report style and thus sufficient proofs on therapeutic modalities are not sufficient. We experienced 2 cases of such rare primary gastric choriocarcinoma (PGC) and thus report the cases here. The first case was diagnosed as advanced gastric cancer initially, and diagnosed as PGC by surgery during chemotherapy, and the second case is the case diagnosed as PGC from the beginning by endoscopic gastric biopsy and ultrasono-guided biopsy on metastatic hepatic lesion.
CASE REPORT
Case 1
A 62 years old male patient visited another hospital for the chief complaint of the discomfort of the upper abdomen persistent for 4 months, diagnosed as gastric adenocarcinoma by endoscopic biopsy, abdominal computed tomography and the upper gastrointestinal imaging were performed, and admitted to our hospital as inoperable gastric cancer accompanying hepatic metastasis.
At the time of admission, in physical examination, in the entire body, lymph nodes could not be palpated, chest examination was normal findings, the abdomen was distended slightly, and mild pain was accompanied. The spleen could not be palpated, edema in four limbs was not detected, and the enlargement of the testis, nodules, and tenderness were not detected. In blood test, leukocyte was 11,050/mm3, hemoglobin was 11.3 g/dl, and in other tests, abnormal findings were not detected.
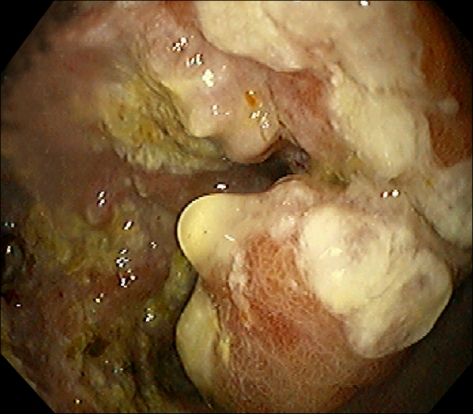
In gastrofiberscopy, infiltrating ulcer approximately 7 cm in size was shown in the Pylorus, and the lumen was narrowed (Fig. 1). It was diagnosed as adenocarcinoma of the stomach (Fig. 2). On abdominal CT, hepatic metastasis and abdominal lymph node metastasis were observed (Fig. 3).
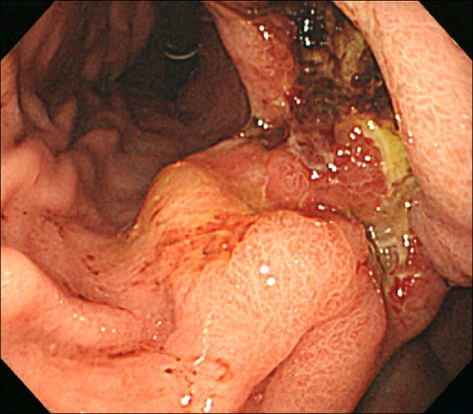
Fig. 1.
Endoscopic finding is shows a huge ulceroinfiltrative mucosal lesion on antrum of stomach.
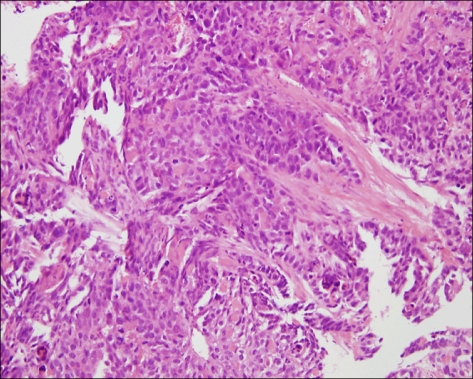
Fig. 2.
Stomach. Microscopic finding of stomach shows moderately differentiated adenocarcinoma. (H&E, ×200).
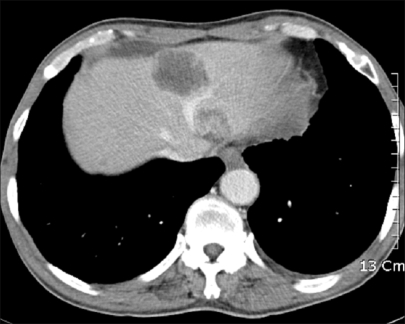
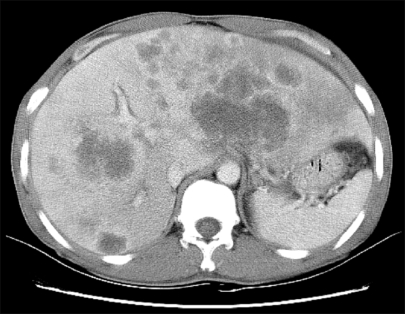
Fig. 3.
Abdominal CT. Abdominal computed tomographic finding shows low attenuating mass and nodules in both hepatic lobes with metastatic lymphadenopathies.
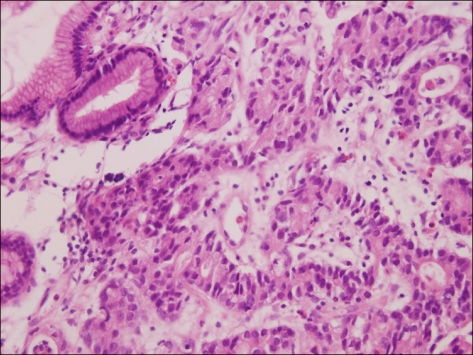
The patient was assessed as metastatic gastric cancer, and a stent was inserted to the stenosis in the pylorus area, and the first chemotherapy engaging 5-FU/Leucovorin/Oxaliplatin (Modified FOLFOX) was initiated. In the subsequent follow up examination, it was determined to be the progression of disease, and thus the second chemotherapy engaging Genexol/Cisplatin was administered. After one cycle of the second chemotherapy, because of gastric perforation, subtotal resection was performed, and in pathological findings, the result of the mixture of adenocarcinoma and choriocarcinoma within tumors was shown (Fig. 4). For the definite diagnosis of choriocarcinoma, immunohistochemical test was performed, β-hCG was positive (Fig. 5), AFP was negative, and HEPA-1 was negative, and it was diagnosed as primary gastric choriocarcinoma. Serum β-hCG measured 5 days after surgery was 532 mIU/mL (normal range, <0.5 mIU/ml). Based on the deterioration of gastric adenocarcinoma, the 3rd systemic chemotherapy engaging 5-FU/LV/Irinotecan (modified FOLFIRI) was performed. Nevertheless, in the follow up examination, hepatic metastasis was deteriorated, and serum β-hCG was elevated to 1,759 mIU/ml, and thus it was thought that primarily choriocarcinoma was deteriorated, and to confirm this, liver biopsy was performed (Fig. 6) and choriocarcinoma was detected primarily. Afterward, chemotherapy was switched for metastatic choriocarcinoma, Bleomycin/Etoposide/Cisplatin (BEP), Etoposide/Methotrexate/Actinomycin/Vincristine/Cyclophosphamide (EMACO), Vinblastine/Ifosfamide/Cisplatin (VIP) regimen was performed, nonetheless, serum β-hCG was elevated continuously, and liver metastasis lesions were deteriorated and thus chemotherapy was terminated, and the patient expired 16 months after the diagnosis of gastric lesions.
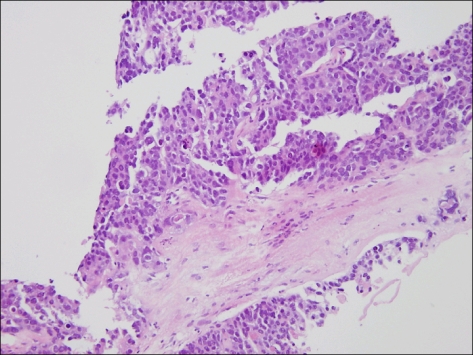
Fig. 4.
Stomach. Microscopic finding of stomach shows choriocarcinoma, characterized by dimorhpic plexiform pattern (H&E, ×400).
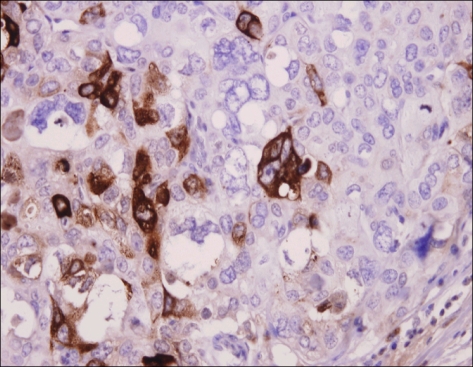
Fig. 5.
Stomach. β-hCG stain. Immunohistochemical staining for β-hCG of stomach shows a positive reaction (peroxidase-HRP, ×400).
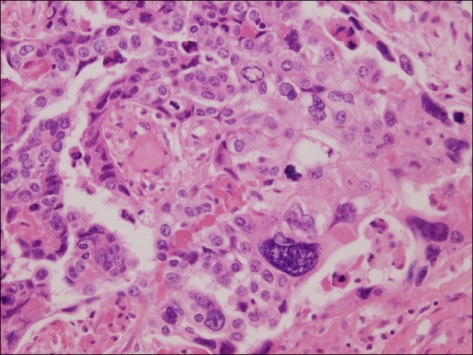
Fig. 6.
Liver. Microscopic finding of liver shows the hepatic infiltration of choriocarcinoma (H&E, ×100)
Case 2
A 45 years old male patient visited another hospital for the chief complaint of the pain in the left upper abdomen persistent for one month, and by gastric endoscopy and histological test, it was diagnosed as gastric cancer, and transferred to our hospital. In general blood test, leukocyte was 10,700/mm3, hemoglobin was 9.7 g/dl, AST/ALT was 102/103 u/l, ALP was 504 U/L, and LDH was 6024 U/L.
In gastrofiberscopy, Borrmann type II lesion in the lesser curvature of stomach angle was observed (Fig. 7), in chest simple radiological test, multicentric lung metastasis was confirmed, and on abdominal CT, multicentric metastasis findings were shown (Fig. 8), and it was confirmed to be adenocarcinoma by endoscopic biopsy (Fig. 9). However, by percutaneous liver biopsy on metastatic hepatic lesions, metastatic choriocarcinoma was suspected (Fig. 10) and thus serum β-hCG test was performed. Serum β-hCG was 437,600 mIU/ml, which was very high, and thus it was determined to correspond to choriocarcinoma. For the differentiation from the primary choriocarcinoma in the testis, testicular ultrasonography was performed, and in the test, abnormal findings were not detected.
Fig. 7.
Gastric endoscopic feature shows a huge ulceroinfiltrative mucosal lesion on lesser curvature of the body.
Fig. 8.
Abdominal CT. Two huge and several smaller variable sized low density lesions are seen in the liver.
Fig. 9.
Stomach. Microscopic finding of stomach shows moderately differentiated adenocarcinoma (H&E, ×400).
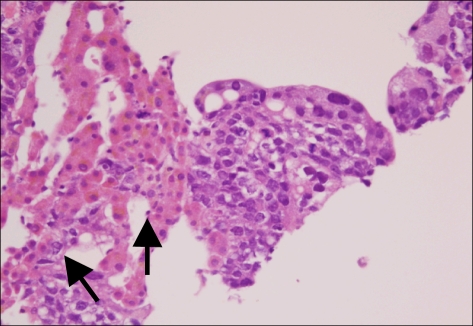
Fig. 10.
Liver. Microscopic finding of liver shows the hepatic infiltration of choriocarcinoma. Some of the multinucleated giant cells (arrows), consistent with syncytiorophoblast (H&E, ×400).
It was diagnosed as PGC, the first systemic chemotherapy engaging BEP was performed, and by the follow up examination, although on abdominal CT, it was not changed, serum β-hCG was elevated and thus it was determined to be progressed, and thus the second chemotherapy engaging VIP was performed, and in the subsequent follow up examination, it progressed, and the 3rd chemotherapy engaging EMACO was performed. Nevertheless, in the subsequent follow up observation, serum β-hCG was increased and thus it was determined to be progressed, hence, the 4th chemotherapy engaging FOLFIRI was performed. Nonetheless, the patient expired 12 months after the diagnosis of PGC due to pulmonary and hepatic metastasis.
DISCUSSION
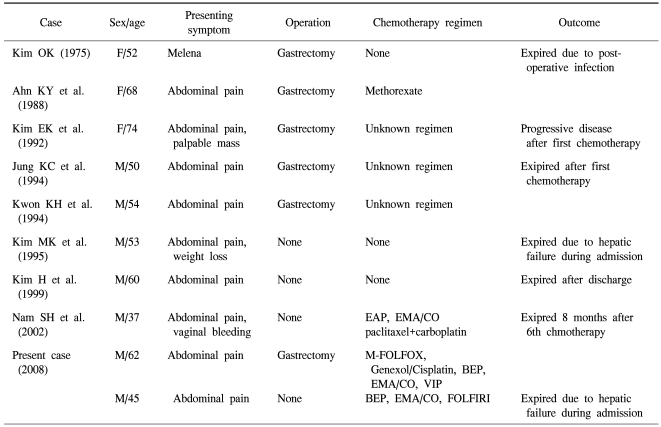
Choriocarcinoma is a germ cell tumor including syncytio-trophoblastic cells, and it secretes β-hCG. In female cases, it occurs as the pregnancy choriocarcinoma pattern, non-pregnancy choriocarcinoma occurs frequently in the mediastinum, ovary, etc., and in male cases, it occurs in the testis although rare. Extragonadal nongestational choriocarcinoma is developed rarely in the mediastinum, retroperitonium, pineal gland, liver, gallbladder, urinary tract system, etc., and the development in the stomach as primary has been known to be very rare. Until now, most cases are reported as the case report style, and the situation is that the development mechanism of disease, prognostic factors and treatment modalities are hardly known. Among them, Noguchi et al. collected 19 cases and reported, and Kobayashi et al. analyzed 53 cases of primary gastric choriocarcinoam retrospectively and reported (1~3). In Korea, primary gastric choriocarcinoma (PGC) was reported in 1975 for the first time, and until now, total 8 cases have been reported (Table 1) (4).
Table 1.
Clinical charcteristics of primary gastric choriocarcinoma from ten case reports in Korea
According to Kobayashi et al., the mean age of the onset of PGC in the male is 62.4 years, the female is 54.8 years, the ratio of the male and the female was 2.3:1, and as the development site, it was developed most frequently in the lower 1/3 of the stomach (41%). This is the result concurring to the preferential area developing gastric adenocarcinoma. In addition, tumor size was average 7 cm, and in endoscocpic results, hemorrhage or necrosis was accompanied in most cases. In histological findings, adenocarcinoma was accompanied in 70%, and at the time of surgery, most patients had metastasis. As metastasis area, lymph nodes were most common (87%) followed by the order of the liver (45%), peritoneum (23%), and lung (8%) (5).
As the mechanism of the development of PGC, several hypotheses have been proposed. First, the hypothesis that it is developed from putative displaced gonadal anlage (6), Second, by the delayed metastasis of unidentified primary lesion in the uterus (7), third, the hypothesis of the retrodifferentiation of gastric adenocarcinoma tissues (8), and fourth, the hypothesis that it is developed from teratoma (9). Among them, although it is still largely controversial, generally, the retrodifferentiation theory from gastric adenocarcinoma suggested by Pick has been accepted (10). This is that malignant gastric adenocarcinoma tissues retrodifferentiate to the embryonal ectoderm level and acquire the ability to produce trophoblasts, and Liu et al. have reported that PGC has the genetic characteristic of adenocarcinoma and pregnancy choriocarcinoma (1,9). Proofs supporting the retrodifferentiation theory are that 71% of primary choriocarcinoam is associated with adenocarcinoma, the mean onset age of these primary choriocarcinoma is 56 years, and it is comparable to the mean onset age of gastric adenocarcinoma, 62 years. On the other hand, choriocarcinoma occurred after pregnancy is developed in the female primarily. In addition, the point is that primary gastric choriocarcinoma was reported abundantly in the area where the incidence of gastric adenocarcinoma is high, particularly, Japan, etc (8).
In such manners, in PGC cases, choriocarcinoma and adenocarcinoma are present concurrently in many cases, and it was difficult to obtain sufficient specimen only by endoscopic biopsy, and thus it was diagnosed as adenocarcinoma in many cases. According to Kobayashi et al., cases diagnosed accurately by endoscopic biopsy was only 8%. Therefore, in endoscopic finding, for cases showing hemorrhage or necrotic big tumors, it is important to obtain sufficient tissues during biopsy. Kobayashi et al. have observed that prognostic factors showing short survival period were the presence of liver metastasis simultaneously, systemic chemotherapy was not administered, etc., and particularly, in cases with concurrent liver metastasis, most patients died within one month. Therefore, the authors recommended not to perform conservative gastric resection for cases with liver metastasis concurrently (4).
Regarding the diagnosis of PGC, it is ideal to diagnose by performing clinical symptoms, the quantitative test of serum chorionic gonadotropin, radiological finding, and histological test in combination, nevertheless, as in our cases, in primary gastric case, it is not easy to differentiate from advanced gastric adenocarcinoma. Therefore, a method is to rule out other possible primary lesions, and after the resection of primary lesion, to confirm that β-hCG becomes normal. In male cases, the primary lesion may be in the testis, nevertheless, it may be fibrous or calcified, and thus comprehensive tests are required. Pathological definite diagnosis is prerequisite, and for the diagnosis, after the assessment of cytotrophoblast and syncytiotrophoblast, to confirm β-hCG positve cells by immunohistochemical test, and to confirm the high elevation of β-hCG in the blood. In addition, to prove that the stomach is the primary site, the absence of choriocarcinoma in the ovary, testis and retroperitoneum should be proven.
For the treatment of gastric choriocarcinoma, chemotherapy engaging methotrexate, actinomycin D, etoposide, folinic acid, vincristin, cyclophosphoamide, etc. is the main therapy, and as supplement, surgery or radiation therapy is administered. For choriocarcinoma and adenocarcinoma, their chemotherapy modality is different, and thus for patients diagnosed to be adenocarcinoma showing elevated β-hCG, it is required to assess the concurrent presence of choriocarcinoma. As the treatment for gastrointestinal choriocarcinoma, due to the hemorrhagic characteristic of choriocarcinoma, hemorrhage is associated in most cases, and thus its treatment should be considered. For cases with liver metastasis, to prevent the death caused by hepatic hemorrhage, the ligation of hepatic artery or hepatic lobectomy may be considered. The mean survival time of patients with choriocarcinoma is less than 2 months and it is very poor. The survival rate could not be improved even by chemotherapy consisting of several chemotherapeutic agents, and radiation therapy also could not be of help to prognosis (12). According to the study reported by Kawashima et al., hepatic failure caused by liver metastasis was common cause of death, and cancerous hemorrhage was the next common cause of death. In addition, other causes of death were DIC, dyspnea, etc.
References
- 1.Liu Z, Mira JL, Cruz-Caudillo JC. Primary gastric chorio carcinoma: a case report and review of the literature. Arch Pathol Lab Med. 2001;125:1601–1604. doi: 10.5858/2001-125-1601-PGC. [DOI] [PubMed] [Google Scholar]
- 2.Kameya T, Kuramoto H, Suzuki K, Kenjo T, Oshikiri T, Hayashi H, et al. A human gastric choriocarcinoma cell line with human chorionic gonadotropin and placental alkaline phosphatase production. Cancer Res. 1975;35:2025–2032. [PubMed] [Google Scholar]
- 3.Kobayashi A, Hasebe T, Endo Y, Sasaki S, Konishi M, Sugito M, et al. Primary gastric choriocarcinoma: two case reports and a pooled analysis of 53 cases. Gastric Cancer. 2005;8:178–185. doi: 10.1007/s10120-005-0332-9. [DOI] [PubMed] [Google Scholar]
- 4.Nam SH, Im SA, Bae KS, Kang KS, Kang IS, Kwon JM, et al. A case of primary gastric choriocarcinoma presenting with amenorrhea. Cancer Res Treat. 2002;34:457–460. doi: 10.4143/crt.2002.34.6.457. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi T, Takeno S, Sato T, Takahashi Y, Uchida Y, Yokoyama S. A patient with primary gastric choriocarcinoma who received a correct preoperative diagnosis and achieved prolonged survival. Gastric Cancer. 2002;5:112–117. doi: 10.1007/s101200200019. [DOI] [PubMed] [Google Scholar]
- 6.Ozaki H, Ito I, Sano R, Hirota T, Shimosato Y. A case of choriocarcinoma of the stomach. Jpn J Clin Oncol. 1971;1:83. [Google Scholar]
- 7.Koritschoner R. Uber ein chorioepithelium ohne primartumor mit abnormalanger Latenzzeit. Beitr Z Path Anat. 1920;66:501. [Google Scholar]
- 8.Pick L. Uber die chorioepthelahnlich metastasierende from des magencarcinomas. Klin Wochenscher. 1926;5:1728. [Google Scholar]
- 9.Hartz PH, Ramirez CA. Coexistence of carcinoma and chorioepithelioma in the stomach of young man. Cancer. 1953;6:319–326. doi: 10.1002/1097-0142(195303)6:2<319::aid-cncr2820060215>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Liu AY, Chan WY, Ng EK, Zhang X, Li BC, Chow JH, et al. Gastric choriocarcinoma shows characteristics of adenocarcinoma and gestational choriocarcinoma: a comparative genomic hybridization and fluorescence in situ hybridization study. Diagn Mol Pathol. 2001;10:161–165. doi: 10.1097/00019606-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Jung KC, Kim WH, Kim YI, Choe KJ. Gastric adenocarcinoma with choriocarcinomatous and hepatoid differentiation: report of a case. Korean J Pathol. 1994;28:409–413. [Google Scholar]
- 12.Kawashima Y, Ishikawa H, Hada M, Sakata K, Hirai T, Asaumi S, et al. A case of primary gastric choriocarcinoma. Gan No Rinsho. 1989;35:1466–1472. [PubMed] [Google Scholar]