Abstract
Purpose
Lymphatic spread of tumor is an important prognostic factor for patients with non-small cell lung carcinoma (NSCLC). Vascular endothelial growth factor-C (VEGF-C) and VEGF-D play important roles in lymphangiogenesis via the VEGF receptor 3 (VEGFR-3). We sought to determine whether VEGF-C, VEGF-D and VEGFR-3 are involved in the clinical outcomes of patients with resected NSCLC.
Materials and Methods
Using immunohistochemical staining, we investigated the protein expressions of VEGF-C, VEGF-D and VEGFR-3 in the tissue array specimens from patients who underwent resection for NSCLC. The immunoreactivity for p53 was also examined. The clinicopathological implications of these molecules were statistically analyzed.
Results
Analysis of a total of 118 specimens showed that VEGF-C, VEGF-D and their co-expression were significantly associated with more advanced regional lymph node metastasis (p=0.019, p=0.044 and p=0.026, respectively, N2 versus N0 and N1). A VEGFR-3 expression had a strong correlation with peritumoral lymphatic invasion (p=0.047). On the multivariate analysis for survival and recurrence, pathologic N2 lymph node metastasis was the only independent prognostic factor, but none of the investigated molecules showed any statistical correlation with recurrence and survival.
Conclusions
The present study revealed that high expressions of VEGF-C and VEGF-D were strongly associated with more advanced regional lymph node metastasis in patients with resected NSCLC.
Keywords: Carcinoma, Non-small-cell lung, Vascular endothelial growth factor, Lymphangiogenesis, Neoplasm metastasis
INTRODUCTION
Despite the improvements for the diagnosis and management of lung cancer, this malady is still a major cause of cancer-related death worldwide, including South Korea (1). Non-small-cell lung cancer (NSCLC) represents approximately 80% of all the lung cancer cases and the majority of these patients present with advanced or metastatic disease (1). If these patients are diagnosed at an early stage, then the survival rates approach about 70%. However, when these patients are diagnosed at advanced stages II or III, the survival rates fall dramatically to about 30 or 15%, respectively (2). Despite the improvements of the staging accuracy and surgical techniques, the postoperative NSCLC recurrence rate is also too high. The most reliable prognostic factor for patients with NSCLC is the cancer stage, including the lymph node status (3). Thus, the correct determination of tumor spreading to the lymph nodes is important for making proper decisions about the optimal therapeutic strategy and precisely assessing a patient's prognosis.
The lymphatic system is an important route of metastatic spread of cancer to secondary organs. Recent studies have demonstrated that the lymphangiogenesis associated with tumors promotes metastasis via the lymphatics (4). Several molecular markers are involved in lymphangiogenesis such as vascular endothelial growth factor-C (VEGF-C), VEGF-D and the VEGF receptor 3 (VEGFR-3), and these are known to be important and specific regulatory factors for lymph node metastasis and the progression of different human cancers (5~10). VEGF-C and VEGF-D exert their effects on endothelial cells via the activation of VEGFR-2 and VEGFR-3 (also known as fms-like tyrosine kinase 4 [flt4]) (4). In adults, the VEGFR-3 expression in the endothelium is largely restricted to lymphatics, but VEGFR-3 has also been detected in blood vessels of malignant tumors and during wound healing (12). In several clinical studies, correlations of VEGF-C and VEGF-D with lymphatic spread, adjacent tissue invasion and/or a poor prognosis have been observed in colorectal (5), ovarian (6), gastric (7) and breast cancers (8). Similarly, a high density of VEGFR-3-positive vessels was found to be correlated with a poor prognosis for patients with breast cancer (9). Additionally, VEGF-C and VEGFR-3 has been found to be correlated with a poor prognosis for patients with NSCLC (10). However, to the best of our knowledge, the association between the expression of VEGF-D and the clinical outcome for NSCLC patients has not been examined.
The p53 protein stimulates the transcription of several genes that mediate cell-cycle arrest and this protein initiates apoptosis in response to DNA damage. While the wild-type p53 protein makes tumor differentiation possible, mutant p53 proteins block it (12). The p53 protein may also play an important role in the growth and differentiation of the fetal bronchial epithelium (13). It has been recently reported that mutant p53 may play a role in controlling the cell cycle, along with VEGF-C, in breast cancer tissue (14).
Thus, we sought to investigate the expressions of VEGF-D, VEGF-C and VEGFR-3 in the surgical specimens from NSCLC patients, and we wanted to determine any correlation of the VEGF-D, VEGF-C and VEGFR-3 expressions with the clinicopathological parameters and clinical outcomes.
MATERIALS AND METHODS
1) Patients and tissue specimens
The study was approved by the St. Mary's Hospital institutional review board. All the tissues investigated in this study were obtained from a series of consecutive NSCLC patients who underwent curative (R0) resection (lobectomy or pneumonectomy) between April 1997 and March 2003 at Kangnam St. Mary's Hospital, the Catholic University of Korea. Paraffin blocks with the tumor samples were available from 118 patients. We examined two histological tumor subtypes, adenocarcinoma (AC) and squamous cell carcinoma (SCC), to compare the role of the investigated markers. The clinicopathological data was obtained from the medical records, including the patients' ages and gender, the histopathological diagnosis, the pathological tumor stage and the date of the initial diagnosis and the date of relapse, death or the last follow-up. Histological classification was performed according to the WHO criteria and the postoperative pathological staging was performed according to the American Joint Committee on Cancer (AJCC) staging criteria, 6th edition.
2) Construction of the tissue microarray
All the archival tissue samples were routinely fixed in formalin and embedded in paraffin wax. Representative tissue areas were marked on standard hematoxylin and eosin stained sections that were cut from the blocks; these corresponding areas were then punched out of the paraffin block using a 2.0-mm punch, and the cores were inserted into a recipient paraffin block. To decrease any error introduced by sampling and to minimize the impact of tissue loss during processing, duplicate tissue cores per specimen were arrayed on a second recipient paraffin block. Sections (4µm) were cut from the completed array block and they were transferred to silanized glass slides.
3) Immunohistochemistry
The tissue sections were dewaxed by incubation in xylene and they were rehydrated using a graded series of ethanol solutions. The endogenous peroxidase was blocked with 3% H2O2 in methanol. Antigen retrieval was performed with citrate buffer (pH 6.0) by heating the tissue sections in a microwave vacuum histoprocessor (RHS-1; Milestone, Bergamo, Italy) at a controlled final temperature of 121℃ for 15 min, and then they were cooled to room temperature for 15 min.
Goat VEGF-C polyclonal antibody (C-20, catalog no. sc-1881; Santa Cruz Biotechnology, Santa Cruz, CA) and goat VEGF-D polyclonal antibody (R&D Systems, Minneapolis, MN) were diluted 1 : 100 and 1 : 10, respectively, with using dilution buffer. The rabbit VEGFR-3 polyclonal antibody (Zymed, San Francisco, CA) was diluted 1 : 100. The mouse monoclonal anti-p53 antibody (clone DO-1; Immunotech, Marseille, France) was diluted 1 : 100. The primary antibodies were incubated with the slides at room temperature for 1 h. Detection of the antibody reaction was accomplished using the conventional-labeled streptavidin-biotin (LSAB2 System-HRP; Dako) method. The color reaction was developed in 3,3'diaminobenzidine for 5 min and the slides were then hematoxylin-counterstained. The VEGF-C, VEGF-D, VEGFR-3 and p-53 immunostaining was independently examined by two pathologists.
For assessment of the VEGF-C and VEGF-D expressions, the staining intensity was scored as 0 (negative), 1 (weak), 2 (medium) or 3 (strong). The extent of staining was scored as 0 (0%), 1 (1~25%), 2 (26~50%), 3 (51~75%) or 4 (76~100%), according to the percentage of the positive staining areas. The sum of the intensity and extent scores was used as the final staining score (0~7). For statistical analyses, the tumors having a final staining score of ≥4 were considered to have a high expression. The staining for VEGFR-3 was classified as 0 (<10% positive staining of the tumor cells or stroma cells), 1+ (11~25%), 2+ (26~50%), 3+ (51~75%) or 4+ (>76%). Staining for p53 was also graded as 0 (<10% positive staining of tumor cells), 1+ (11~25%), 2+ (26~50%), 3+ (51~75%) or 4+ (>76%). For statistical analyses, the staining of VEGFR-3 and p53 was classified as a high expression if ≥10% of the cells showed immunoreactivity.
4) Statistical analyses
Statistical analyses were performed using the statistical software package SPSS (version 13.0; SPSS, Chicago, IL). Survival was determined from the date of surgery to the time of an event (recurrence, death) by using the Kaplan-Meier method. Following an intent-to-treat approach, the non-cancer-related deaths as well as patients lost to follow-up were included in the survival analyses, but this was considered as censored data. The relationships between the expressions of VEGF-C, VEGF-D, VEGFR-3 and p53 and the clinicopathological features were evaluated using Spearman's coefficient of rank correlation or Fisher's exact probability test. The statistical significance of the differences in the cumulative survival curves was evaluated using the log-rank test. Multivariate survival analysis was performed on all the parameters that were found to be significant on univariate analysis with using the Cox proportional hazard model. The survival rates and odds ratios are presented with their 95% confidence intervals. The statistical tests were two-sided at a 5% level of significance.
RESULTS
1) The patients' clinical characteristics
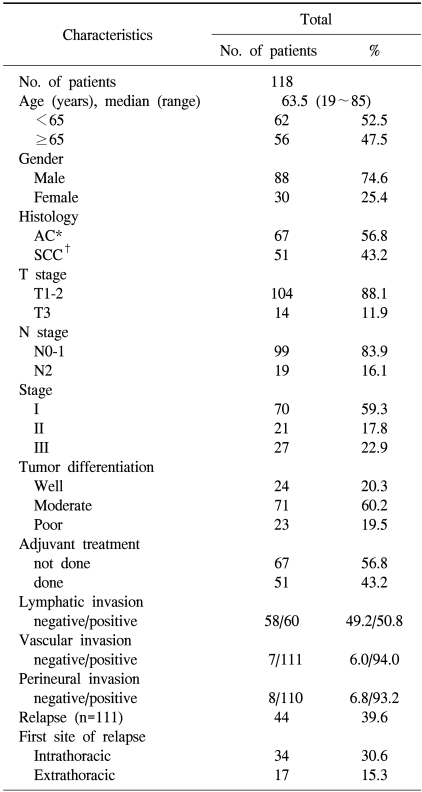
Paraffin blocks with tumor samples were available from 118 patients who had undergone surgery. With regard to treatment, none of the patients had received radiation or chemotherapy preoperatively. The median follow up duration was 50.5 months (range: 0.6~148.3 months) after the initial pathological diagnosis. The clinical and pathological characteristics of the patients are shown in Table 1. The patients consisted of 88 males and 30 females, with a median age of 63.5 years (range: 19~85 years). Histologically, 67 patients (56.8%) had AC and 51 (43.2%) had SCC; of the 118 patients, 24 (20.3%) had well differentiated carcinomas, 71 (60.2%) had moderately differentiated carcinomas and 23 (19.5%) had poorly differentiated carcinomas. A total of 51 patients (43.2%) received adjuvant treatment; of those, 45 received platinum-based chemotherapy, 4 received radiotherapy and 2 received concurrent chemoradio-therapy.
Table 1.
Clinical and pathologic characteristics of the patients
*adenocarcinoma, †squamous cell carcinoma.
2) Immunohistochemical staining
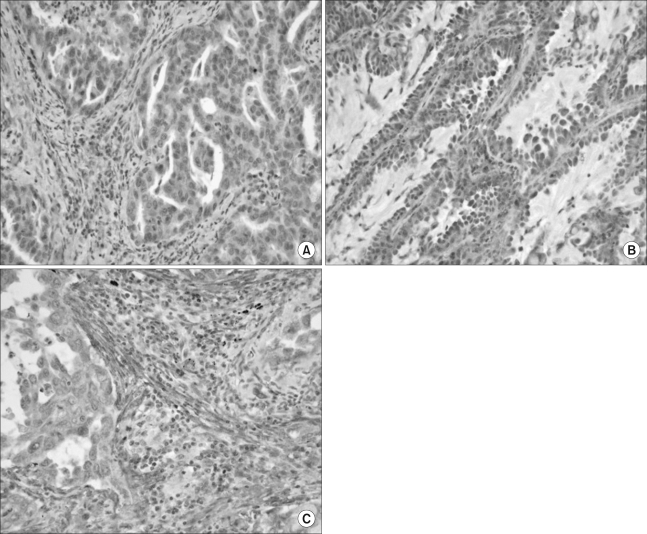
Of the 118 specimens, the VEGF-C- and VEGF-D-positive stainings were predominantly observed in the cytoplasm of the cancer cells (Fig. 1) in 71 (60.2%) and 62 (52.5%) patients, respectively (Table 2). A strong expression of VEGFR-3 was observed in 49 specimens. VEGFR-3 was highly expressed in the stroma cells and in the cytoplasm and membranes of the tumor cells, with the positive staining rate being 41.5%. We observed a VEGFR-3 expression in the endothelial cells in the stroma surrounding the cancer cells (Fig. 1). Of the 118 specimens, strong p53 nuclear immunostaining was observed in 64 (54.2%) tumors (Table 2).
Fig. 1.
Immunohistochemistry for vascular endothelial growth factor C (VEGF-C) and -D and VEGF receptor 3. (A and B) Immuno-reactivity for VEGF-C and -D was detected predominantly in the cytoplasm of the tumor cells; typical examples of VEGF-C (A) and VEGF-D (B) immunopositivity in adenocarcinoma, respectively (×400). (C) Immunoreactivity for the VEGF receptor 3 was detected in the cytoplasm of the stoma cells and tumor cells (×400).
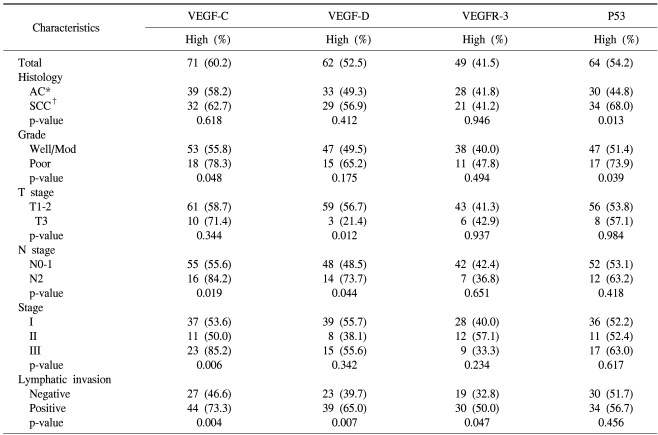
Table 2.
Clinicopathological factors and their relationship to the expression of the proteins assessed (n=118)
*adenocarcinoma, †squamous cell carcinoma.
3) Correlation between the expression of the lymphangiogenic factors and the clinicopathological features
The associations between the VEGF-C, VEGF-D and VEGFR-3 expressions and the clinicopathological features are shown in Table 2. Of the 118 primary tumor specimens, we found significant correlations for the high expression of VEGF-C with peritumoral lymphatic invasion, a more advanced pathologic stage, more advanced regional lymph node metastasis and poor tumor differentiation (p=0.004, p=0.006, p=0.019 and p=0.048, respectively). A high expression of VEGF-D was significantly correlated with lymphatic invasion, an early T-stage and pathologic N2 lymph node metastasis (p=0.007, p=0.012 and p=0.044, respectively). Moreover, the co-expression of VEGF-C and -D was found to have a significant correlation with more advanced regional lymph node metastasis (p=0.026). An increased expression of VEGFR-3 was significantly related to only peritumoral lymphatic invasion (p=0.047).
4) Correlation between p53 and the clinicopathological features
A high expression of p53 was found more frequently in the SCC histological subtype (p=0.013) than in the AC subtype or the poorly differentiated carcinomas (p=0.039; Table 2).
5) Relationships for the expression patterns among VEGF-C, VEGF-D, VEGFR-3 and p53
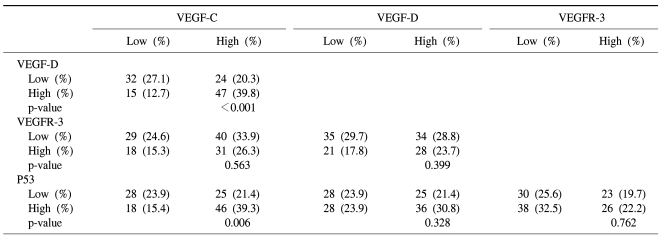
The expression patterns of VEGF-C, VEGF-D and VEGFR-3 are shown in Table 3. The correlation between the expressions of VEGF-C and VEGF-D or p53 was statistically significant (p<0.001 and p=0.006, respectively). However, the expression of VEGFR-3 was not associated with that of any other marker.
Table 3.
Relationship among expression pattern of markers (n=118)
6) Disease relapse with respect to the clinicopathological factors
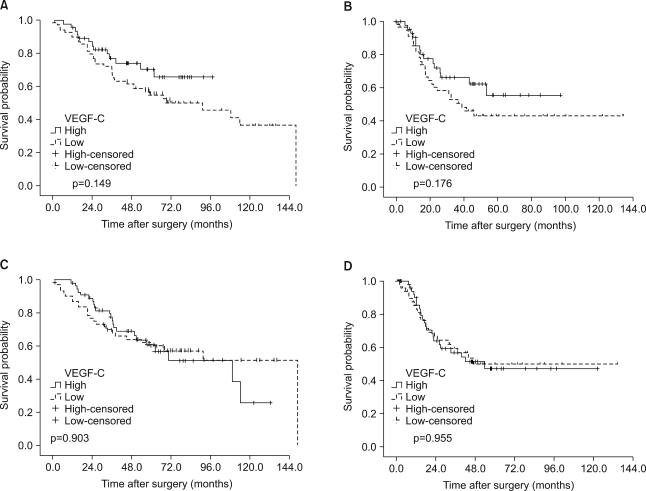
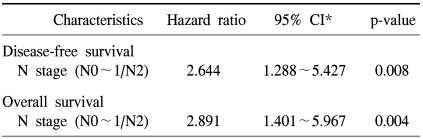
The 5-year disease-free rate for resected NSCLC was 48.3%. Recurrent tumors were noted in 44 patients (39.6%) at the end of follow-up. The data on the first site of relapse was collected for all the patients who could be evaluated (Table 1). Of those 44 patients, the most common site of the first relapse was the intrathoracic area (34 patients, 77.3%); of those 34 patients, 30 had relapses with lung metastases, 3 had mediastinal lymph node metastases and 1 had chest wall metastases. Only 17 patients (38.6%) had relapses in the extrathoracic area; of the 17, 9 had relapses with bone metastasis, 6 had brain metastases and 1 had liver metastasis. No significant association was found between the first relapse site and the expression of the molecular markers we tested for (data not shown). Univariate analysis for disease relapse showed that a more advanced regional lymph node metastasis status (pathologic N2 versus N0 and N1) and a more advanced TNM stage (stage III versus stages I and II) were significantly correlated with disease relapse (p<0.001, and p<0.001, respectively; data not shown). However, none of the expressions of the molecular markers we examined was related to relapse (Fig. 2). On the Cox multivariate analysis of all cases (n=118), only a pathologic N2 lymph node metastasis status had prognostic value for disease-free survival (hazard ratio, 2.644; 95% CI, 1.288~5.427; p=0.008; Table 4).
Fig. 2.
Survival curve for patients with non-small cell lung cancer (NSCLC), as stratified by the expression of vascular endothelial growth factor C (VEGF-C; A: overall survival, B: disease-free survival), and the expression of VEGF-D (C: overall survival, D: disease-free survival).
Table 4.
Multivariate analysis of clinicopathological characteristics and five biological factors by disease-free survival rate and overall survival rate
*confidence interval.
7) Overall survival with respect to the clinicopathological factors
The overall 5-year survival rate for resected NSCLC was 60.5%. Of the 118 patients, 69 (58.5%) were alive and 49 (41.5%) died during their follow-up. However, for 7 of those patients who died, it was difficult to determine whether the death was cancer-related due to the unavailability of a patient registry. Univariate analysis of the clinicopathological factors that were relevant to patient survival showed that the following factors were statistically significant for the overall survival of patients: a more advanced T stage (p=0.022), an advanced regional lymph node metastasis status (p<0.001) and an advanced TNM stage (p<0.001; data not shown). On the multivariate analysis for overall survival, the only significant predictor was a pathologic N2 lymph node metastasis status (hazard ratio, 2.891; 95% CI, 1.401~5.967; p=0.004; Table 4). The expressions of the molecular markers we examined were not of any statistical significance as independent prognostic factors for overall survival (Fig. 2).
DISCUSSION
In the present study, the expression of VEGF-D, which was highly associated with the expression of VEGF-C, was significantly correlated with pathological N2 lymph node metastasis in the patients who underwent resection for NSCLC. To the best of our knowledge, this study is the first to demonstrate a significant correlation between a VEGF-D expression and lymph node metastasis in resected NSCLC tissue.
In our study, the frequency of VEGF-C and VEGF-D expressions was 60.2% and 52.5%, respectively. The expression of VEGF-C was similar to that reported by a previous study (10). However, in the case of VEGF-D, its expression was slightly more frequent than that reported by Adachi et al. (15). Little data exists on the VEGF-D expression in NSCLC tissue and Adachi et al.'s report was limited to the adenocarcinoma subtype (17). The expressions of VEGF-C and VEGF-D were detected predominantly in the cytoplasm of the tumor cells, rather than in the nuclei. A cytoplasmic localization of this protein has been most often reported (7,8), but nuclear immuno-detection has also been described in breast cancer (14).
Analysis of VEGF-C/D and the clinicopathological factors showed a significant association between the tumor tissues' expression of VEGF-C and/or VEGF-D and mediastinal lymph node metastasis. Additionally, the expression of VEGF-C showed a significant relationship with that of VEGF-D (p<0.001). The findings reported by various previous studies are consistent with the hypothesis that VEGF-C and VEGF-D are stimulators of lymphangiogenesis and lymph node metastasis in human cancers (4). A positive correlation between lymph node metastasis or lymphatic vessel invasion and a VEGF-C expression has been reported in patients with NSCLC and in esophagus, breast, stomach and prostate cancers (8,10,16~18). Similarly, the VEGF-D expression is also up-regulated in several other types of tumors. An increased VEGF-D expression may be linked to lymph node metastasis in colorectal (5), ovarian cancers (6), breast (8) and gastric cancer (20). The high frequency of the expressions of both VEGF-C and VEGF-D in tumor tissues suggests the cooperative promotion of cancer invasion to the lymphatic system by these molecules. Recent studies have shown that the simultaneous expressions of VEGF-C and VEGF-D trigger lymphangiogenesis in breast cancer (8). Furthermore, Ishii et al. reported that VEGF-C-expressing clones metastasized to the same side regional lymph nodes, whereas the VEGF-D expressing clones metastasized to the mediastinal and distant lymph nodes in a mouse model (19). In contrast, according to our data, the expression of VEGF-C or VEGF-D could not distinguish between regional and distant metastasis. A recent report from Kopfstein et al. showed that unlike VEGF-C, VEGF-D induces lymphangiogenesis and it promotes metastasis to the lymph nodes and lungs in the Rip1Tag2 transgenic mouse model of pancreatic cell carcinogenesis (20). Further studies on the mechanism of action of VEGF-C and VEGF-D are warranted to examine the functional differences between them. Interestingly, of the 19 patients with N2 lymph node metastasis, 16 patients (84.2%) and 14 patients (73.7%) had a statistically significantly high expression of VEGF-C and VEGF-D, respectively, compared to the patients with N0 or N1 metastasis. The current procedures for detecting mediastinal lymph node involvement in NSCLC patients include computed tomography, positron emission tomography scanning and mediastinoscopy. Although they are clinically well established, these methods also have clear limitations for detecting micrometastasis in lymph nodes. Consequently, it would be ideal to identify molecular markers for the assessment of the nodal status in NSCLC patients. Tomita et al. did not find a significant correlation between a VEGF expression and the clinicopathological parameters in NSCLC patients with pathologic N2 lymph node metastasis (21). However, they used a monoclonal antibody to VEGF and not to VEGF-C/D. Our data suggest that the VEGF-C/D expression in the primary tumor tissue may predict mediastinal lymph node metastases in patients with equivocal N2 disease.
Regarding the relationships between the biological and clinicopathological parameters, we found that poor tumor differentiation was significantly related to high expressions of VEGF-C and p53. Liao et al. reported a meaningful relationship between VEGF and p53 in resected NSCLC (22). Mutant p53 may be correlated with the VEGF-C expression and the inability of malignant cells to control their cell cycle, and especially in the early stages of tumor development. This finding has also been demonstrated in breast cancer tissue (8). In addition, it was shown that p53 was related to cyclooxygenase (COX)-2, which promotes proliferation, inhibition of apoptosis and angiogenesis. Moreover, the co-expression of both markers had prognostic value in NSCLC patients (23). Other studies have revealed that VEGF-C also induced the up-regulation of COX-2 (4). Additionally, Katsuda et al. reported that the expressions of VEGF-C and hypoxia-inducible factor (HIF)-1α, which is a target of p53, were correlated with lymphatic invasion in patients with esophageal cancer (24). Thus, these molecules also might be associated with VEGF-C and nuclear p53 in NSCLC tumor tissues.
In this study, the frequency of a VEGFR-3 expression was 41.5%, and this was higher than that of a previous report (10). Like VEGF-C and VEGF-D, VEGFR-3 was immunodetected in the cytoplasm of malignant cells. We also found positive staining for VEGFR-3 in the endothelial cells in the stroma surrounding the cancer cells (Fig. 1). VEGFR-3 positivity in the tumor stroma or the tumor cell itself may be closely related to the grade of lymphatic invasion or lymph node metastases of cancer (6), and the expressions of both VEGF-C or -D and VEGFR-3 have been associated with enhanced lymph node metastases in various cancers (10,14,18). However, in this study, VEGFR-3 was only significantly positively correlated with lymphatic vessel invasion (p=0.047) and no significant correlation was detected between VEGFR-3 and VEGF-C/D. We observed that the expression of VEGFR-3 in tumor cells was weaker than that in the stromal cells. As a result, we included the VEGFR-3 expression not only in cancer cells, but also in endothelial cells and we used the scoring method to assess the extent of this expression (14). VEGFR-3 is primarily expressed by the endothelial cells of the lymphatics. However, several reports have shown that VEGFR-3 is also expressed by non-endothelial cell types, including several cancer cells (6) and the myoepithelial cells that surround the ducts in breast cancer (25), which may indicate a lack of lymphatic vessel specificity for VEGFR-3. Moreover, a VEGFR-3 expression in endothelial cells has less clinical significance than that in cancer cells (9).
The prognostic value of VEGF-C, VEGF-D and VEGFR-3 is controversial. In clinical studies, correlations of VEGF-C, VEGF-D and/or VEGFR-3 with an unfavorable outcome have been observed in colorectal (5), ovarian (6), gastric (7,17) and breast cancers (8), while other studies have shown no such relationship (21,22). Similarly, a high density of VEGFR-3-positive vessels has been found to be correlated with a poor prognosis for patients with breast cancer (9). However, with regard to disease relapse or survival, we failed to demonstrate any prognostic significance of the molecular markers we examined. This may reflect the limitations of our study. First, the information on the actual causes of death was incomplete. Additional information concerning the causes of death was unavailable for 14.3% of the patients who died, which limited our interpreting the prognostic importance of the investigated molecules. Second, cancer progression and metastasis are multifactorial processes. For that reason, high expressions of lymphangiogenic factors may be necessary, but not sufficient to produce tumor progression and metastasis. A third possible explanation for our results is that the primary antibody used in this study was different from those used in other studies, which may also explain the differing results.
CONCLUSION
We demonstrated that high expressions of VEGF-C and VEGF-D were strongly associated with more advanced regional lymph node metastasis in patients with resected NSCLC. The expression of these markers may provide accurate assessment of the mediastinal lymph node involvement. Further investigations are also required to clarify the functional differences between VEGF-C and VEGF-D in lymph node metastasis.
References
- 1.Park K. Second-line chemotherapy for advanced non-small cell lung cancer: past, present, and hope for the future. Cancer Res Treat. 2003;35:279–280. doi: 10.4143/crt.2003.35.4.279. [DOI] [PubMed] [Google Scholar]
- 2.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Nagai K, Yoshida J, Nishimura M, Takahashi K, Yokose T, et al. Conventional clinicopathologic prognostic factors in surgically resected nonsmall cell lung carcinoma. A comparison of prognostic factors for each pathologic TNM stage based on multivariate analyses. Cancer. 1999;86:1976–1984. doi: 10.1002/(sici)1097-0142(19991115)86:10<1976::aid-cncr14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 5.White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, et al. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002;62:1669–1675. [PubMed] [Google Scholar]
- 6.Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, et al. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer. 2003;88:237–244. doi: 10.1038/sj.bjc.6600701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juttner S, Wissmann C, Jons T, Vieth M, Hertel J, Gretschel S, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 8.Okada K, Osaki M, Araki K, Ishiguro K, Ito H, Ohgi S. Expression of hypoxia-inducible factor (HIF-1alpha), VEGF-C and VEGF-D in non-invasive and invasive breast ductal carcinomas. Anticancer Res. 2005;25:3003–3009. [PubMed] [Google Scholar]
- 9.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, et al. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154:1381–1390. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003;97:457–464. doi: 10.1002/cncr.11073. [DOI] [PubMed] [Google Scholar]
- 11.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- 12.Shaulsky G, Goldfinger N, Rotter V. Alterations in tumor development in vivo mediated by expression of wild type or mutant p53 proteins. Cancer Res. 1991;51:5232–5237. [PubMed] [Google Scholar]
- 13.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 14.Mylona E, Alexandrou P, Mpakali A, Giannopoulou I, Liapis G, Markaki S, et al. Clinicopathological and prognostic significance of vascular endothelial growth factors (VEGF)-C and -D and VEGF receptor 3 in invasive breast carcinoma. Eur J Surg Oncol. 2007;33:294–300. doi: 10.1016/j.ejso.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Adachi Y, Nakamura H, Kitamura Y, Taniguchi Y, Araki K, Shomori K, et al. Lymphatic vessel density in pulmonary adenocarcinoma immunohistochemically evaluated with anti-podoplanin or anti-D2-40 antibody is correlated with lymphatic invasion or lymph node metastases. Pathol Int. 2007;57:171–177. doi: 10.1111/j.1440-1827.2007.02077.x. [DOI] [PubMed] [Google Scholar]
- 16.Kleespies A, Bruns CJ, Jauch KW. Clinical significance of VEGF-A, -C and -D expression in esophageal malignancies. Onkologie. 2005;28:281–288. doi: 10.1159/000085198. [DOI] [PubMed] [Google Scholar]
- 17.Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, Bandou E, et al. Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int J Clin Oncol. 2005;10:318–327. doi: 10.1007/s10147-005-0508-7. [DOI] [PubMed] [Google Scholar]
- 18.Jennbacken K, Vallbo C, Wang W, Damber JE. Expression of vascular endothelial growth factor C (VEGF-C) and VEGF receptor-3 in human prostate cancer is associated with regional lymph node metastasis. Prostate. 2005;65:110–116. doi: 10.1002/pros.20276. [DOI] [PubMed] [Google Scholar]
- 19.Ishii H, Yazawa T, Sato H, Suzuki T, Ikeda M, Hayashi Y, et al. Enhancement of pleural dissemination and lymph node metastasis of intrathoracic lung cancer cells by vascular endothelial growth factors (VEGFs) Lung Cancer. 2004;45:325–337. doi: 10.1016/j.lungcan.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Kopfstein L, Veikkola T, Djonov VG, Baeriswyl V, Schomber T, Strittmatter K, et al. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am J Pathol. 2007;170:1348–1361. doi: 10.2353/ajpath.2007.060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita M, Matsuzaki Y, Shimizu T, Hara M, Ayabe T, Onitsuka T. Vascular endothelial growth factor expression in pN2 non-small cell lung cancer: lack of prognostic value. Respirology. 2005;10:31–35. doi: 10.1111/j.1440-1843.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 22.Liao M, Wang H, Lin Z, Feng J, Zhu D. Vascular endothelial growth factor and other biological predictors related to the postoperative survival rate on non-small cell lung cancer. Lung Cancer. 2001;33:125–132. doi: 10.1016/s0169-5002(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 23.Tsubochi H, Sato N, Hiyama M, Kaimori M, Endo S, Sohara Y, et al. Combined analysis of cyclooxygenase-2 expression with p53 and Ki-67 in nonsmall cell lung cancer. Ann Thorac Surg. 2006;82:1198–1204. doi: 10.1016/j.athoracsur.2006.04.069. [DOI] [PubMed] [Google Scholar]
- 24.Katsuta M, Miyashita M, Makino H, Nomura T, Shinji S, Yamashita K, et al. Correlation of hypoxia inducible factor-1alpha with lymphatic metastasis via vascular endothelial growth factor-C in human esophageal cancer. Exp Mol Pathol. 2005;78:123–130. doi: 10.1016/j.yexmp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Longatto Filho A, Martins A, Costa SM, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Pract. 2005;201:93–99. doi: 10.1016/j.prp.2004.11.008. [DOI] [PubMed] [Google Scholar]