Abstract
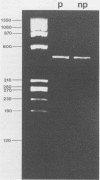
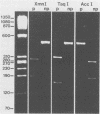
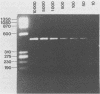
We previously reported the identification of homologous cDNA clones derived from a pathogenic isolate and a nonpathogenic isolate of Entamoeba histolytica, which had been designated cEh-P1 and cEh-NP1, respectively. Sequence analysis of both clones had revealed 10% nucleic acid substitutions, which were dispersed over the entire sequence. This genetic difference had been found to be conserved between all four pathogenic and all five nonpathogenic laboratory strains of E. histolytica tested. On the basis of nucleic acid substitutions, we have now developed a sensitive assay to distinguish pathogenic from nonpathogenic forms of E. histolytica by using fresh clinical isolates. Comparing the sequence of cEh-P1 and cEh-NP1, we identified a 482-bp segment that contained identical 5' and 3' ends but differed in internal cleavage sites for restriction endonucleases. By using oligonucleotide primers corresponding to the 5' and 3' ends of this segment, the corresponding gene was amplified by the polymerase chain reaction. Endonuclease digestion of the amplified DNA yielded restriction fragments that are characteristic for pathogenic and nonpathogenic forms. This assay allows the detection and classification of fewer than 10 amoebae within a few hours. The differentiation of 48 isolates into pathogenic and nonpathogenic strains by using this method corresponded to the clinical status of the infected individuals and to the classification obtained by isoenzyme determination. The results further support the concept that pathogenic and nonpathogenic strains of E. histolytica constitute distinct subspecies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allason-Jones E., Mindel A., Sargeaunt P., Williams P. Entamoeba histolytica as a commensal intestinal parasite in homosexual men. N Engl J Med. 1986 Aug 7;315(6):353–356. doi: 10.1056/NEJM198608073150603. [DOI] [PubMed] [Google Scholar]
- Bartlett A., Bidwell D. E. Enzyme immunoassays for parasitic diseases. Trans R Soc Trop Med Hyg. 1976;70(2):98–106. doi: 10.1016/0035-9203(76)90163-2. [DOI] [PubMed] [Google Scholar]
- Burg J. L., Grover C. M., Pouletty P., Boothroyd J. C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab F. F., Doherty M., Cai S. P., Kan Y. W., Cooper S., Rubin E. M. Detection of sickle cell anaemia and thalassaemias. Nature. 1987 Sep 24;329(6137):293–294. doi: 10.1038/329293b0. [DOI] [PubMed] [Google Scholar]
- Diamond L. S. A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen-dwelling protozoa. J Parasitol. 1982 Oct;68(5):958–959. [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Embury S. H., Scharf S. J., Saiki R. K., Gholson M. A., Golbus M., Arnheim N., Erlich H. A. Rapid prenatal diagnosis of sickle cell anemia by a new method of DNA analysis. N Engl J Med. 1987 Mar 12;316(11):656–661. doi: 10.1056/NEJM198703123161103. [DOI] [PubMed] [Google Scholar]
- Funke M., Felgner P., Geister R. Quantification of amebae specific antibodies as "Multiple of normal activity (MONA)" with a standardized enzyme immunoassay (EIA). Zentralbl Bakteriol Mikrobiol Hyg A. 1981 Dec;251(1):126–133. [PubMed] [Google Scholar]
- Garfinkel L. I., Giladi M., Huber M., Gitler C., Mirelman D., Revel M., Rozenblatt S. DNA probes specific for Entamoeba histolytica possessing pathogenic and nonpathogenic zymodemes. Infect Immun. 1989 Mar;57(3):926–931. doi: 10.1128/iai.57.3.926-931.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch J., Mannweiler E. Development and persistence of antibodies to Entamoeba histolytica in patients with amebic liver abscess. Analysis of 216 cases. Am J Trop Med Hyg. 1983 Jul;32(4):727–732. doi: 10.4269/ajtmh.1983.32.727. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Druckman D. A., Vernon A., Quinn T. C., Polk B. F., Modlin J. F., Yolken R. H., Bartlett J. G. Prevalence of enteric pathogens in homosexual men with and without acquired immunodeficiency syndrome. Gastroenterology. 1988 Apr;94(4):984–993. doi: 10.1016/0016-5085(88)90557-4. [DOI] [PubMed] [Google Scholar]
- Lench N., Stanier P., Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988 Jun 18;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Mathews H. M., Moss D. M., Healy G. R., Mildvan D. Isoenzyme analysis of Entamoeba histolytica isolated from homosexual men. J Infect Dis. 1986 Apr;153(4):793–795. doi: 10.1093/infdis/153.4.793. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Keister D. B., Caspar P. A. Experimental amebiasis. III. A rapid in vitro assay for virulence of Entamoeba histolytica. Am J Trop Med Hyg. 1978 Sep;27(5):882–887. [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Wexler A., Chayen A. Changes in isoenzyme patterns of a cloned culture of nonpathogenic Entamoeba histolytica during axenization. Infect Immun. 1986 Dec;54(3):827–832. doi: 10.1128/iai.54.3.827-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D. Effect of culture conditions and bacterial associates on the zymodemes of Entamoeba histolytica. Parasitol Today. 1987 Feb;3(2):37–37. doi: 10.1016/0169-4758(87)90210-9. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sargeaunt P. G. The reliability of Entamoeba histolytica zymodemes in clinical diagnosis. Parasitol Today. 1987 Feb;3(2):40–37. doi: 10.1016/0169-4758(87)90211-0. [DOI] [PubMed] [Google Scholar]
- Sargeaunt P. G., Williams J. E., Grene J. D. The differentiation of invasive and non-invasive Entamoeba histolytica by isoenzyme electrophoresis. Trans R Soc Trop Med Hyg. 1978;72(5):519–521. doi: 10.1016/0035-9203(78)90174-8. [DOI] [PubMed] [Google Scholar]
- Strachan W. D., Chiodini P. L., Spice W. M., Moody A. H., Ackers J. P. Immunological differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Lancet. 1988 Mar 12;1(8585):561–563. doi: 10.1016/s0140-6736(88)91355-4. [DOI] [PubMed] [Google Scholar]
- Ulrich P. P., Bhat R. A., Seto B., Mack D., Sninsky J., Vyas G. N. Enzymatic amplification of hepatitis B virus DNA in serum compared with infectivity testing in chimpanzees. J Infect Dis. 1989 Jul;160(1):37–43. doi: 10.1093/infdis/160.1.37. [DOI] [PubMed] [Google Scholar]