Abstract
Glucuronidation studies using microsomes and recombinant UDP-glucuronosyltransferases (rUGTs) can be complicated by the presence of endogenous β-glucuronidases leading to underestimation of glucuronide formation rates. Saccharolactone is the most frequently used β-glucuronidase inhibitor, although as of yet it is not clear whether this reagent should be routinely added to glucuronidation incubations. Here we determined the effect of saccharolactone on eight different UGT probe activities using pooled human liver microsomes (pHLMs) and rUGTs. Despite the use of buffered incubation solutions it was necessary to adjust the pH of saccharolactone solutions to avoid effects (enhancement or inhibition) of lowered pH on UGT activity. Saccharolactone at concentrations ranging from 1 to 20 mM failed to show enhancement of any of the glucuronidation activities evaluated that could be considered consistent with inhibition of β-glucuronidase. However, for most activities, higher saccharolactone concentrations resulted in a modest degree of inhibition. The greatest inhibitory effect was observed for 5-hydroxytryptamine and estradiol glucuronidation by pHLMs with 35% decrease at 20 mM saccharolactone concentration. Endogenous β-glucuronidase activities were also measured using various human tissue microsomes and rUGTs with estradiol-3-glucuronide and estradiol-17-glucuronide as substrates. Glucuronide hydrolysis was observed for pHLMs, lung microsomes, and insect-cell expressed rUGTs, but not for kidney or intestinal microsomes, or HEK293 microsomes. However, the extent of hydrolysis was relatively small representing only 9 to 19% of the glucuronide formation rate measured in the same preparations. Consequently, these data do not support the routine inclusion of saccharolactone in glucuronidation incubations and, if used, saccharolactone concentrations should be titrated to achieve activity enhancement without inhibition.
Introduction
Glucuronidation is one of the main conjugation reactions responsible for converting lipophilic xenobiotics and endogenous compounds into metabolites that are more water soluble, and thus, more readily excreted in the urine or bile. Conjugation with glucuronic acid is catalyzed by UDP-glucuronosyltransferases (UGTs) (Miners & Mackenzie 1991). To date, at least sixteen different UGT human isoforms have been identified, each with different and overlapping substrate specificies (Miners et al 2002). Tissue-dependent expression of various UGT isoforms has been found in humans, namely the expression of UGT1A1, 1A3, 1A4, 1A6, 1A9, 2B4, 2B7, 2B15, 2B17 are primarily expressed in the liver, whereas UGT1A7, 1A8, and 1A10 are extrahepatic isoforms (Tukey & Strassburg 2000). Conjugation with glucuronic acid results in the inactivation of many compounds and is not limited to drugs, but also to environmentally toxic chemicals, carcinogens, steroid hormones, bile acids, and bilirubin (Miners & Mackenzie 1991). UGTs are predominately localized in the smooth endoplasmic reticulum of liver cells, but have also been found in a variety of other organs including the lung, kidney, and intestinal tract (Mulder 1992).
Microsomal glucuronidation studies have exhibited a wide range in variability among different laboratories. One possible explanation for such variation is latency in UGT activity, which is seen in in vitro assays. Because the active site of the UGT is located in the lumen of the endoplasmic reticulum, a rate-limiting step in the in vitro glucuronidation reaction is the transport of substrates, cofactors, and products through the intact membrane of the liver microsome (Meech & Mackenzie 1997). In order to achieve maximum enzymatic activity, the membrane barrier must be disrupted in some fashion. In the past, detergents and sonication have been used to disrupt the integrity of the membrane, but more recently the pore-forming peptide, alamethicin, has been used (Fisher et al 2000; Soars et al 2003). Fisher et al. (2000) found that microsomes in the presence of 50 μg alamethicin per mg microsomal protein yielded a 2 to 3 times faster conjugation rate than that observed in absence of alamethicin (Fisher et al 2000).
In addition to latency, the enzyme-catalyzed hydrolysis of the newly formed glucuronide by β-glucuronidase might also affect UGT activity in microsomal incubations. Human β-glucuronidase has been found in all mammalian tissues and body fluids, with the highest activity in kidney, spleen, epididymis, liver, cancer tissue, and the gastrointestinal tract, which is distinct from the β-glucuronidase produced by gastrointestinal tract microorganisms (Marsh et al 1952; Levvy 1960; Wakabayashi 1970). One third of the β-glucuronidase found in liver cells is localized in the endoplasmic reticulum, whereas the remaining two thirds is found in the lysosome (Swank et al 1986). The close proximity of the enzymes responsible for the formation (UGT) and degradation (β-glucuronidase) of the glucuronide has the potential to result in a futile cycle, which would greatly affect the apparent glucuronidation rate of the UGT.
In order to evaluate the effect of β-glucuronidase on glucuronide formation rates, researchers have utilized different β-glucuronidase inhibitors. The primary β-glucuronidase inhibitor used for in vitro assays is D-saccharic acid 1,4-lactone (saccharolactone), which was discovered by Levvy in 1952 to competitively inhibit the enzyme (Levvy 1952). Several other β-glucuronidase inhibitors have been identified including polymeric phosphates of diethystibestrol, dienestrol, hexesterol, and benzeterol and some heavy metals (Cu+2, Ag+2, Hg+2) (Wakabayashi 1970). However, these inhibitors are not selective for β-glucuronidase, and are not used in routine microsomal glucuronidation assays.
Some investigators routinely add saccharolactone to incubation mixtures presumably because of previous reports showing enhanced activities with certain substrates. Brunelle and Verbeeck (1993) investigated the effect of saccharolactone on the formation rate of diflunisal acyl glucuronidation in rat liver microsomes. They found that the Vmax of diflunisal acyl glucuronide formation increased 2-fold with the addition of 4 mM saccharolactone (Brunelle & Verbeeck 1993). Additional follow-up experiments showed similar results both in other microsomal systems (human) (Brunelle & Verbeeck 1996), and in vivo (rat) (Brunelle & Verbeeck 1997). Other investigators have found similar results supporting the use of saccharolactone in in vitro glucuronidation experiments (Gigon & Bickel 1979; Haaz et al 1997; Kemper & Nabb 2005). One study, however, exhibited opposite results, in that 5 mM of saccharolactone decreased the formation of acetaminophen glucuronide (Alkharfy & Frye 2001). Consequently, it is unclear whether saccharolactone is routinely needed in microsomal experiments, or whether inclusion of this compound in incubations could adversely affect UGT function. Therefore in this study we investigated the effect of increasing concentrations of saccharolactone on eight different glucuronidation activities representing the majority of human hepatic UGTs using liver microsomes and recombinant enzymes. We also quantitated endogenous β-glucuronidase activities in microsomes prepared from various human tissues and recombinant enzyme preparations.
Materials and Methods
2.1 Chemicals and Reagents
Unless otherwise noted, most reagents including alamethicin, UDP-glucuronic acid (UDPGA), D-saccharic acid 1,4-lactone (saccharolactone), β-glucuronidase (Helix pomatia), acetaminophen (APAP), phenacetin, 3′-azido-3′-deoxythymidine (AZT), AZT glucuronide, codeine, codeine glucuronide, estradiol, estradiol-3-glucuronide, estradiol-17-glucuronide, trifluoperazine, salicylic acid, salicylic acid phenol glucuronide, e-4-hydroxy-tamoxifen, and 5-hydroxytryptamine were purchased from Sigma-Aldrich (St. Louis, MO). Analytical grade acetonitrile was purchased from Fisher Scientific (Fairlawn, NJ).
2.2 Liver, Kidney, Intestinal, and Lung Microsomes and Recombinant Enzymes
Pooled livers microsomes from 54 individuals were obtained from a frozen bank maintained by the Department of Pharmacology and Experimental Therapeutics, Tufts University School of Medicine, Boston MA. The microsomes were prepared from the frozen livers using a centrifugation process previously described (Court & Greenblatt 1997). The resultant pellet was then reconstituted in 20% glycerol/100 mM phosphate buffer pH 7.5, aliquoted, and stored at −80 °C in a final concentration of 4 mg/ml. Human kidney (pooled, 6 donors) and intestinal (pooled, 10 donors) microsomes were purchased from In Vitro Technologies Inc. (Baltimore, MD). Human lung microsomes (pooled, 6 donors) were obtained from Human Biologics International (Scottsdale, AZ). HEK293 cells expressing UGT1A6 were prepared in our laboratory as previously described (Krishnaswamy et al 2005). Microsomes from insect cells infected with baculovirus containing cDNA of human UGT isoforms 1A1, 1A3, 1A4, 1A6, 1A9, 2B4, 2B7, and 2B15 were purchased from BD Biosciences (Woburn, MA).
2.3 Glucuronidation Assays
Glucuronidation of seven different substrates including estradiol, trifluoperazine, 5-hydroxytryptamine, e-4-hydroxy-tamoxifen, AZT, codeine, and salicylic acid were measured to assess the effect of saccharolactone on UGT activities by human tissue microsomes and recombinant enzymes. The estradiol, trifluoperazine, 5-hydroxytryptamine, AZT, and codeine glucuronidation assays have been previously described and were performed with minor modifications (Court et al 2002; Court et al 2003; Krishnaswamy et al 2003; Court 2005). The salicylic acid glucuronidation assay had a substrate concentration of 5 mM, a protein concentration of 0.5 mg/mL, incubation time of 6 hours, and acetaminophen as the internal standard. Although salicylic acid formed both phenol and acyl glucuronides and could be detected by HPLC, we only measured phenol glucuronide formation since the acyl glucuronide peak was relatively small, and a quantitation standard was not commercially available. The e-4-hydroxy-tamoxifen glucuronidation assay had a substrate concentration of 25 μM, protein concentrations of 0.04 mg/mL for pHLMs and 0.5 mg/ml of UGT2B15, an incubation time of 30 minutes, and phenacetin as the internal standard. For each glucuronidation assay, preliminary experiments were conducted to confirm proportional glucuronide formation rate with respect to protein concentration and incubation time under the conditions that were used to obtain final data.
The basic incubation mixture consisted of the dried down substrate, pooled human liver microsomes (pHLMs) or recombinant UGT, and various concentrations of saccharolactone dissolved in 50 mM phosphate buffer (pH 7.5). Saccharolactone solutions at final concentrations of 0, 1, 2, 5, 10, and 20 mM were prepared in 50 mM phosphate buffer that was pH-adjusted to 7.5 with potassium hydroxide. The pore forming antibiotic, alamethicin, was also added to incubations at a final concentration of 50 μg/mg of protein to eliminate latency. Although in previous work (Fisher et al 2000) alamethicin and microsomes were incubated on ice for 15 minutes with prior to the 37°C incubation, preliminary experiments both with and without this additional incubation period determined that this was not necessary. Immediately prior to incubation, a UDPGA solution was added to the incubation mixture, consisting of 5 mM UDPGA, 5 mM MgCl2, and 50 mM pH 7.5 phosphate buffer to yield a final incubation volume of 100 μl. Samples were then incubated in a 37 °C water bath and the reaction stopped with 100 μl of acetonitrile containing the appropriate internal standard. After centrifugation at 13,000g for 10 minutes, the supernatants were dried down in a vacuum oven at 45 °C, reconstituted with 100 μl deionized water, and analyzed by HPLC with UV absorbance or fluorescence detection as indicated below.
A model 1100 system (Agilent, Pal Alto, CA) was used for HPLC consisting of an autoinjector, binary pump, column, and serially connected UV absorbance and fluorescence detectors. The samples were pumped at a flow rate of 1 ml/min through a 4.6 mm × 25 cm 10-μm C18 column (Synergi Hydro-RP; Phenomenex, Torrance, CA). Unless otherwise noted, the mobile phase consisted of 20 mM potassium phosphate buffer in water (pH 2.2) (solution A) and acetonitrile (solution B). The solvent gradient for salicylic acid consisted of 10 to 50% solution B over 30 minutes and was detected by UV absorbance at a wavelength of 237 nm. The solvent gradient for e-4-hydroxy-tamoxifen consisted of 35 to 45% solution B over 15 minutes and the glucuronide was detected by UV absorbance at a wavelength of 280 nm. Samples were run in triplicate. Glucuronide formation was quantified by calculating the peak height ratio of the glucuronide to the internal standard. E-4-hydroxy-tamoxifen glucuronide was identified by showing sensitivity to inclusion of cofactor, inactivation of enzyme, and treatment by β-glucuronidase. E-4-hydroxy-tamoxifen glucuronide was quantified from a standard curve using various concentrations of substrate assuming similar UV absorbance of parent and glucuronide.
2.4 β-glucuronidase activity assay
The effect of saccharolactone on β-glucuronidase activity was assayed using estradiol-3 and estradiol-17-glucuronides (100 μM and 200 μM, respectively) as substrates. The incubation mixture consisted of various tissue microsomes or recombinant enzymes, 5 mM MgCl2, alamethicin, and 50 mM phosphate buffer (pH 7.5) without UDP-glucuronic acid. The effects of saccharolactone were determined by comparing the endogenous β-glucuronidase activity of the protein source in the presence or absence of pH-adjusted 10 mM saccharolactone. The protein concentrations of the UGT2B7, UGT1A1, HEK293 vector control, and the insect vector control cells were 0.25 mg/ml. The protein concentrations of the human liver, intestine, kidney and lung microsomes were 0.5 mg/ml. The incubation mixture was placed in a water bath at 37 °C and the reaction was stopped by the addition of 50 μl of an acetonitrile and phenacetin solution at 0 and 6 hours. HPLC samples were prepared as previously described and run in triplicate using the estradiol glucuronidation HPLC assay.
2.5 Statistical Analysis
Results are expressed as the mean ± standard deviation. Statistical analysis was performed using the SigmaStat 3.0 software (Systat, San Jose, CA). A p value of less than 0.05 was considered statistically significant using an unpaired t-test.
Results
3.1 Effect of saccharolactone and incubation pH on e-4-hydroxy-tamoxifen glucuronidation
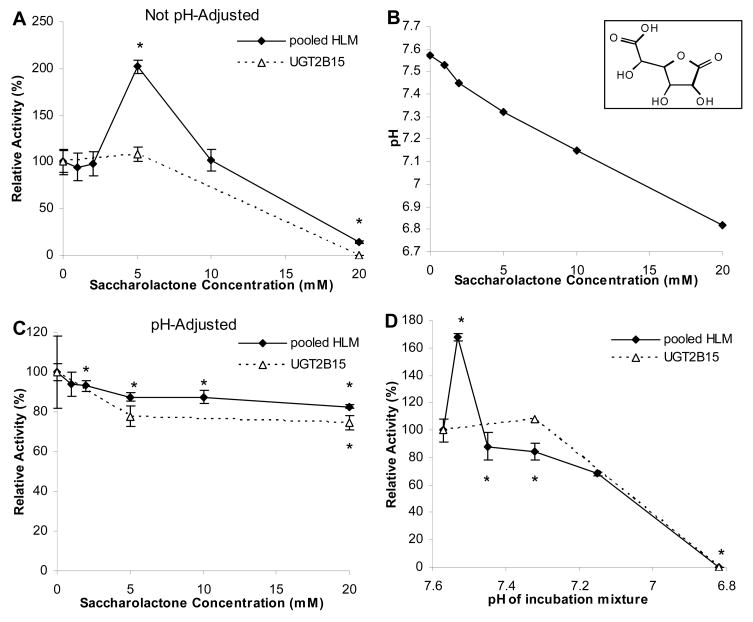
In preliminary studies the effect of increasing concentrations of saccharolactone on e-4-hydroxy-tamoxifen glucuronidation by pHLMs or UGT2B15 were evaluated using saccharolactone initially dissolved directly into incubation buffer (50 mM potassium phosphate, pH 7.5). As shown in Figure 1A, there was a significant increase (by 100%, p<0.05) in glucuronidation activity of pHLMs at the 5 mM saccharolactone concentration followed by a concentration-dependent decrease in activity such that at the 20 mM saccharolactone concentration the pHLMs activity was only 15% that of the control (i.e., without added saccharolactone) activity (p<0.05). The effect on UGT2B15 glucuronidation was somewhat different in that activities were unchanged up to the 5 mM saccharolactone concentration, but were essentially abolished at the 20 mM concentration (Figure 1A).
Figure 1.
Effect of saccharolactone and incubation pH on e-4-hydroxy-tamoxifen glucuronidation. E-4-hydroxy-tamoxifen glucuronidation by pHLMs and insect cell-expressed UGT2B15 were measured in the presence of increasing concentrations of pH-unadjusted (A) or pH-adjusted (to pH 7.5) saccharolactone (C). Shown in (B) is the effect of adding increasing amounts of saccharolactone on the pH of 50 mM phosphate buffer (initially at pH 7.5 without added saccharolactone); while (D) shows the direct effect of decreasing incubate pH (to match pH values determined in (B)) on E-4-hydroxy-tamoxifen glucuronidation. Glucuronide formation rates were expressed relative to control values that were determined without added saccharolactone (or values obtained at pH 7.5 for data shown in panel (B)). Individual data points represent the mean (SD) of triplicate measurements. *P<0.05 versus control values obtained without added saccharolactone (or values obtained at pH 7.5 for (B)) by unpaired t-test. Inset: Saccharolactone chemical structure.
Since saccharolactone is a carboxylic acid derivative, we next examined the effect of this compound on the pH of the incubation buffer system (i.e., 50 mM potassium phosphate pH 7.5). As shown in Figure 1B, there was minimal effect on pH until saccharolactone concentrations exceeded 5 mM, at which point there was a concentration-dependent decrease in pH to that of 6.8 at the 20 mM concentration. Stock solutions of saccharolactone were subsequently adjusted with potassium hydroxide to the pH of the buffer system (7.5) and glucuronidation experiments were repeated (Figure 1C). Unlike the previous results, both pHLMs and UGT2B15 did not show enhancement in activities with increasing saccharolactone concentrations, and instead a modest inhibition was observed (17 and 25% decrease, respectively) at the 20 mM saccharolactone concentration.
The direct effect of pH on e-4-hydroxy-tamoxifen glucuronidation activities was then evaluated by pH adjustment of the incubation buffer using phosphoric acid to the levels observed with the different saccharolactone concentrations (up to 20 mM), but without adding any saccharolactone. As shown in Figure 1D, the effect of decreasing pH on glucuronidation activity by both pHLMs and UGT2B15 was similar to that observed for increasing concentrations of unbuffered saccharolactone (Figure 1A), since there was a small degree of enhanced glucuronidation activity for UGT2B15. In contrast, the pHLMs exhibited a substantial increase in activity at the pH of the lower saccharolactone concentrations followed by the essential abolishment of activity at the lowest pH evaluated (equivalent to 20 mM saccharolactone). Consequently, the remaining studies were conducted using pH-adjusted saccharolactone solutions to evaluate the effect of saccharolactone independent of pH.
3.2 Effect of saccharolactone on seven other glucuronidation activities
We also investigated the effect of saccharolactone on seven other glucuronidation activities reflecting the majority of UGT isoforms expressed in liver using pHLMs and recombinant UGTs. We first compared the effects of saccharolactone on 5-hydroxytryptamine glucuronidation by pHLMs and recombinant UGT1A6 expressed in both insect cells and human embryonic kidney (HEK293) cells to determine whether there might be differences in effect related to enzyme source (Table 1). The presence of saccharolactone resulted in a concentration-dependent decrease in pHLMs glucuronidation activity. This effect first became apparent at the 5 mM concentration and exhibited its greatest effect at the 20 mM (33% decrease in activity). In contrast, UGT1A6 expressed either in insect or mammalian cells showed no effect of saccharolactone at concentrations up to 20 mM (Table 1).
Table 1.
Effect of saccharolactone on various UGT activities measured using pooled human liver microsomes (pHLMs) and recombinant UGTs. A substrate concentration of 4 mM was used for the 5-HT assay, 200 μM for the trifluoperazine, 1 mM for codeine, 500 μM for AZT, 5 mM for salicylic acid, and 100 μM for the estradiol assay.
| Saccharolactone Concentration (mM) | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 5 | 10 | 20 | |
| 5-HT glucuronidation | ||||||
| pHLM | 100±3 | 104±8 | 93±5 | 86±4a | 87±4a | 63±5a |
| UGT1A6 (insect) | 100±2 | 88±7 | 96±0 | |||
| UGT1A6 (HEK293) | 100±4 | 91±6 | 90±7 | |||
| Trifluoperazine glucuronidation | ||||||
| pHLM | 100±5 | 98±5 | 101±9 | 95±3 | 93±4 | 97±5 |
| UGT1A4 | 100±10 | 90±4 | 82±5a | |||
| Codeine glucuronidation | ||||||
| pHLM | 100±2 | 98±7 | 97±4 | 91±2a | 95±5 | 88±8 |
| UGT2B4 | 100±11 | 91±4 | 88±2 | |||
| UGT2B7 | 100±9.04 | 90±7 | 90±3 | |||
| AZT glucuronidation | ||||||
| pHLM | 100±11 | 96±2 | 98±2 | 94±2 | 94±3 | 94±1 |
| UGT2B7 | 100±3 | 102±2 | 102±8 | |||
| Salicylic Acid glucuronidation | ||||||
| pHLM | 100±8 | 101±5 | 111±5 | 83±3a | 90±1 | 85±9a |
| UGT1A9 | 100±13 | 96±7 | 99±7 | |||
| Estradiol-3 glucuronidation | ||||||
| pHLM | 100±18 | 68±5 | 84±1 | 76±9a | 67±5a | 67±7a |
| UGT1A1 | 100±12 | 92±2 | 87±5 | |||
| UGT1A3 | 100±25 | 92±18 | 97±10 | |||
| Estradiol-17 glucuronidation | ||||||
| pHLM | 100±17 | 71±4a | 81±8 | 78±7a | 68±2 | 66±6a |
| UGT1A3 | 100±12 | 90±14 | 98±10 | |||
| UGT2B7 | 100±7 | 97±2 | 87±7a | |||
Data are presented as mean±s.d. % of the control activity (measured at 0 mM), n=3.
P<0.05 points versus control activity by unpaired t-test.
Glucuronidation of other substrates including trifluoperazine, salicylic acid, codeine, and AZT were similar to one another, in that there was essentially no effect of saccharolactone on glucuronidation by both pHLMs and recombinant UGTs (Table 1). Exceptions to this included trifluoperazine glucuronidation by recombinant UGT1A4, which was inhibited at the 20 mM concentration by 18% (p<0.05); codeine glucuronidation by pHLMs was inhibited at the 5 mM concentration by 9% (p<0.05); and salicylic acid glucuronidation by pHLMs that was also inhibited at the 5 mM concentration by 25% (p<0.05) (Table 1).
The use of estradiol as a substrate enabled the simultaneous investigation of the effects of saccharolactone on 2 different glucuronidation activities at the 3- and 17-hydroxy positions. Both the estradiol-3 and estradiol-17 glucuronidation activities by pHLMs were significantly inhibited at 1, 10, and 20 mM saccharolactone concentrations, with the greatest effect observed at the 20 mM concentration (33% and 35% decrease in activity, respectively; p<0.05) (Table 1). In contrast, there was no effect of saccharolactone on estradiol glucuronidation by the recombinant UGTs 1A1 and 1A3. Recombinant UGT2B7, however, exhibited a small (13%; p<0.05), but significant reduction in activity in estradiol-17 glucuronidation at the 20 mM concentration.
3.3 Quantitation of endogenous β-glucuronidase activity in different enzyme systems
Since we failed to observe any enhancement of the different glucuronidation activities with saccharolactone, it is possible that the endogenous β-glucuronidase activity is minimal in the in vitro systems used for glucuronidation assays. We, therefore, quantified the endogenous β-glucuronidase activity in microsomes from human liver, lung, kidney, intestine tissues, as well as, recombinant enzyme preparations. Both estradiol-3-glucuronide (100 μM) and estradiol-17-glucuronide (200 μM) were used as substrates in separate incubations and the loss of glucuronide was measured by HPLC. Phosphate buffer (50 mM, pH 7.5) was used as a negative control, while bovine liver β-glucuronidase (Sigma Aldrich G-0376) was used as a positive control which resulted in complete hydrolysis of both glucuronides in less than 6 hours. The effect of adding 10 mM saccharolactone was also evaluated.
As shown in Table 2, both glucuronides exhibited similar results with low but variable β-glucuronidase activity dependent on the enzyme source. The greatest hydrolysis (about 25% degradation over 6 hours) was observed with recombinant UGT2B7 and UGT1A1, followed by human liver microsomes (15–20% degradation over 6 hr), control SF9 insect cells and lung microsomes (10–15% degradation each). The remaining tissues (intestine and kidney) and HEK293 control cell microsomes did not exhibit glucuronide degradation. Saccharolactone at 10 mM concentration only had a minimal effect in preventing degradation by the tissue microsomes, but was effective in the inhibition of hydrolysis in recombinant UGT1A1 and 2B7.
Table 2.
Effect of 10 mM saccharolactone on the hydrolysis of estradiol 3-glucuronide (E-3-G, 100 μM) and estradiol 17-glucuronide (E-17-G, 200 μM). Estradiol glucuronide concentrations were measured by HPLC before and after 6 hour incubation with human liver, intestine, kidney, and lung microsomes, insect cell expressed UGTs, and control cells.
| Glucuronide | Saccharolactone | pHLM | Intestine | Kidney | Lung | UGT2B7 | UGT1A1 | Insect control | HEK293control | Buffer |
|---|---|---|---|---|---|---|---|---|---|---|
| E-3-G | − | 82±1a,b | 104±3 | 104±1a,b | 85±6 | 77±9 | 77±2 | 90±5 | 102±4 | 101±5 |
| + | 84±2a | 101±3 | 110±3 | 80.9±5a | 91±5 | 87±7 | 89±7 | 98±10 | 95±4 | |
| E-17-G | − | 86±2b | 110±4b | 104±3 | 92±6 | 75±5 | 71±4 | 88±3 | 104±3 | 102±13 |
| + | 84±3 | 106±2 | 116±2 | 86±3 | 89±5 | 85±3 | 88±4 | 101±8 | 95±3 |
Data are presented as mean±s.d. of percent glucuronide remaining after 6 hours, n=3.
P<0.01 comparing 0 hr timepoint vs. 6 hr timepoint.
P<0.05 comparing 6 hour timepoint with and without saccharolactone by unpaired t-test.
3.4 Apparent formation rate versus degradation rate of estradiol glucuronides
Apparent formation rates of estradiol-3 and estradiol-17 glucuronides (nmoles formed in 6 hours with 0.5 mg/ml protein and 100 μM estradiol) for pooled intestinal, kidney, and liver microsomes were also measured under incubation conditions similar to the β-glucuronidase assay. Both assays were performed under conditions, resulting in the maximal formation and degradation rates. Since these values represent the net of the amount of glucuronide formed minus the amount degraded, a predicted actual formation rate was calculated by adding the amount of glucuronide determined in the previous experiments to be hydrolyzed over the same time (Table 3). All tissues evaluated were capable of forming both estradiol glucuronides with apparent formation rates for extrahepatic tissues that were close to or, in the case of 3-hydroxy glucuronidation by intestinal microsomes, substantially exceeded rates measured in hepatic microsomes. Despite these similarities, only hepatic microsomes were capable of degrading estradiol glucuronides, with the amounts degraded relative to amounts formed ranging from 9% to 19% for the 3-hydroxy and 17-hydroxyglucuronides, respectively (Table 3).
Table 3.
Rate of formation and hydrolysis of estradiol glucuronides. pHLMs, intestinal, and kidney microsomes (0.5 mg protein/ml) were incubated in the presence of β-estradiol (100 μM) and UDPGA (apparent formation rate) or estradiol glucuronide without UDPGA (hydrolysis rate) for 6 hours. The gross amount of glucuronide formed was estimated by adding the amount of glucuronide present after a 6 hour incubation (formed minus degraded) to the amount that was determined to be degraded from hydrolytic activity after 6 hours. Data represent the mean of triplicate measurements.
| Apparent nmoles formed in 6 hours | Nmoles degraded in 6 hours | Predicted actual nmoles formed in 6 hours | Nmoles degraded as a % of nmoles formed | |
|---|---|---|---|---|
| Estradiol-3-Glucuronide |
||||
| pHLMs | 3.51 | 0.36 | 3.87 | 9.30% |
| Intestine | 16.7 | 0 | 16.7 | 0% |
| Kidney | 2.99 | 0 | 2.99 | 0% |
| Estradiol-17-Glucuronide |
||||
| pHLMs | 1.23 | 0.29 | 1.52 | 19% |
| Intestine | 0.58 | 0 | 0.58 | 0% |
| Kidney | 1.11 | 0 | 1.11 | 0% |
Discussion
Saccharolactone is added to glucuronidation reaction mixtures to minimize glucuronide product hydrolysis by endogenous β-glucuronidases. Resultant activity measurements should therefore directly reflect UGT function rather than being a balance between glucuronide formation and hydrolysis associated with futile enzymatic cycling. In the present study we systematically investigated the effect of saccharolactone on eight different glucuronidation activities known to be mediated by distinct UGT isoenzymes (i.e. UGT probe activities) using both human tissue microsomes and recombinant enzyme preparations. We had expected that inhibition of endogenous β-glucuronidase activity would result in a saccharolactone concentration-dependent increase in glucuronidation activity. Our preliminary experiments with e-4-hydroxy-tamoxifen as a substrate appeared to fit this hypothesis, since there was a two-fold increase in glucuronidation activity at the 5 mM saccharolactone concentration, followed by inhibition at higher concentrations (Figure 1A). Upon closer inspection, however, this result is most likely attributable to an indirect, nonspecific effect of saccharolactone on incubate pH thereby affecting UGT activity (increased activity with slight pH decrease; decreased activity with greater pH decrease). Upon pH-adjusting the stock saccharolactone solutions, the enhancement in activity disappeared and instead resulted in modest inhibition up to the 20 mM concentration (Figure 1C). Various studies have established that UGTs have distinct pH optima for glucuronidation activity dependent on substrate and UGT isoform with values ranging from as low as 5.5 to as high as 8.5 (Basu et al 2004). However for most reported studies, incubation pH is fixed at a near neutral pH (~7.5) since this is close to that found in liver tissue, the most common tissue used in glucuronidation studies.
Relatively few studies have specifically investigated effects of saccharolactone on glucuronidation. Brunelle and Verbeeck (1993) investigated the effects of saccharolactone on phenolic and acyl glucuronidation of diflunisal by rat liver microsomes (Brunelle & Verbeeck 1993). Using 4 mM saccharolactone, they found a 2-fold higher acyl glucuronide formation, but no change in the phenol glucuronide formation suggesting perhaps different susceptibilities of the acyl and phenol glucuronides to β-glucuronidase. Brunelle and Verbeeck (1997) also investigated the effect of saccharolactone on diflunisal acyl and phenol glucuronidation in vivo in rats. Again, they saw an increase in partial clearance of the acyl glucuronide but little effect on the phenol. Of note, they also observed a pH-dependent stabilization effect of the acyl glucuronide in urine (i.e., the lower the pH the lower the acyl degradation). However, it was not appreciated in either report that acyl glucuronides can undergo spontaneous non-enzymatic hydrolysis particularly at higher pH, and that the pH lowering effect of saccharolactone may have been responsible for the apparent effect through stabilizing the acyl glucuronide rather than by inhibition of β-glucuronidase. It should be noted that our study focused on phenolic glucuronides and did not include any acyl glucuronides. Other than high pH and β-glucuronidase, acyl glucuronides can also be degraded by esterases found in blood and tissues.
Several other investigators have reported that saccharolactone either has no effect or may even inhibit glucuronidation in vitro. Boase and Miners (2002) found that 8.5 mM saccharolactone had no effect on zidovudine glucuronidation by HLMs (Boase & Miners 2002). Alkharfy and Frye (2001), however, observed that 5 mM saccharolactone decreased acetaminophen glucuronidation by HLMs by 45% (Alkharfy & Frye 2001), which was further supported by Kemp et al.’s (2002) study where 5 mM saccharolactone inhibited raloxifene glucuronidation by HLMs (Kemp et al 2002).
In the present study, saccharolactone solutions were pH-adjusted to near neutrality by the addition of potassium hydroxide. Higher incubation buffer strength (i.e., more than the 50 mM used here) could instead have been used to minimize incubate pH alterations. Brunelle and Verbeeck (1993) found that saccharolactone lowered the pH of the incubation medium and corrected for this in part by using higher Tris buffer concentrations (100 to 300 mM) (Brunelle & Verbeeck 1993). However they noted that such additional buffering capacity was insufficient for saccharolactone concentrations above 8 mM. It is should also be pointed out that high buffer concentrations have the potential to adversely affect glucuronidation in that Boase and Miners (2002) determined that increasing phosphate buffer concentrations from 50 to 200 mM decreased zidovudine glucuronidation in HLMs by about 25%.
Since we did not see any enhancement of UGT activity by saccharolactone, we decided to investigate the amount of glucuronide hydrolysis in relation to glucuronide formation using a variety of different enzyme sources and estradiol glucuronides as substrates (Table 3). These studies confirmed that estradiol glucuronide hydrolysis does occur in both tissue and recombinant enzyme systems, but at most would only decrease apparent estradiol glucuronidation rates by 9 to 19%. Confirming previous observations (Bracey & Paigen 1987; Paigen 1989), β-glucuronidase activity was primarily observed in hepatic microsomes, but also in lung microsomes and insect cell preparations. We found no evidence for β-glucuronidase activity in our intestinal epithelial cell microsomes. Although the intestine is the main site of β-glucuronidase-mediated hydrolysis of the glucuronides of drugs that undergo enterohepatic recycling, the β-glucuronidase originates from intestinal bacteria rather than the intestinal epithelial cells. This appears to be the first report of β-glucuronidase activity in recombinant enzymes expressed in insect cells. Since these preparations are generally crude cell homogenates, such activity may represent combined microsomal and lysosomal enzymes. Saccharolactone was somewhat effective in inhibiting this activity in insect cells but not in liver microsomes. The reason for this difference is not immediately apparent. It is likely that different glucuronides will differ in susceptibility to hydrolysis by tissue β-glucuronidase although this has not been systematically studied. Consequently our findings with estradiol glucuronides will need to be confirmed and extended by study of other glucuronide substrates.
Conclusions
These results of this study indicate that endogenous β-glucuronidase activity can have a measurable, albeit, relatively small influence on a range of glucuronidation activities measured in human tissues microsomes and recombinant enzymes. It is recommended that investigators should conduct preliminary experiments to assess the effect (either enhancement or inhibition) of saccharolactone on measured glucuronidation activities with different substrates and enzyme sources. Moreover, aqueous saccharolactone solutions should be pH-adjusted to that of the desired incubation pH prior to addition in order to minimize the potentially substantial effects of pH variance on UGT activity.
Acknowledgments
This publication was made possible by Grant Number F31DA023861 from the National Institute on Drug Abuse (NIDA), National Institutes of Health (Bethesda, MD) to L.O. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIDA, or the National Institutes of Health. Other support was also provided by grants R01GM061834 and R21GM074369 from the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (Bethesda, MD) to M.H.C.
References
- Alkharfy KM, Frye RF. High-performance liquid chromatographic assay for acetaminophen glucuronide in human liver microsomes. J Chromatogr B Biomed Sci Appl. 2001;753:303–8. doi: 10.1016/s0378-4347(00)00566-1. [DOI] [PubMed] [Google Scholar]
- Basu NK, Ciotti M, Hwang MS, Kole L, Mitra PS, Cho JW, Owens IS. Differential and special properties of the major human UGT1-encoded gastrointestinal UDP-glucuronosyltransferases enhance potential to control chemical uptake. J Biol Chem. 2004;279:1429–41. doi: 10.1074/jbc.M306439200. [DOI] [PubMed] [Google Scholar]
- Boase S, Miners JO. In vitro-in vivo correlations for drugs eliminated by glucuronidation: investigations with the model substrate zidovudine. Br J Clin Pharmacol. 2002;54:493–503. doi: 10.1046/j.1365-2125.2002.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracey LT, Paigen K. Changes in translational yield regulate tissue-specific expression of beta-glucuronidase. Proc Natl Acad Sci U S A. 1987;84:9020–4. doi: 10.1073/pnas.84.24.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle FM, Verbeeck RK. Glucuronidation of diflunisal by rat liver microsomes. Effect of microsomal beta-glucuronidase activity. Biochem Pharmacol. 1993;46:1953–8. doi: 10.1016/0006-2952(93)90636-b. [DOI] [PubMed] [Google Scholar]
- Brunelle FM, Verbeeck RK. Glucuronidation of diflunisal in liver and kidney microsomes of rat and man. Xenobiotica. 1996;26:123–31. doi: 10.3109/00498259609046694. [DOI] [PubMed] [Google Scholar]
- Brunelle FM, Verbeeck RK. Conjugation-deconjugation cycling of diflunisal via beta-glucuronidase catalyzed hydrolysis of its acyl glucuronide in the rat. Life Sci. 1997;60:2013–21. doi: 10.1016/s0024-3205(97)00166-5. [DOI] [PubMed] [Google Scholar]
- Court MH. Isoform-selective probe substrates for in vitro studies of human UDP-glucuronosyltransferases. Methods Enzymol. 2005;400:104–16. doi: 10.1016/S0076-6879(05)00007-8. [DOI] [PubMed] [Google Scholar]
- Court MH, Greenblatt DJ. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver microsomes. Biochem Pharmacol. 1997;53:1041–7. doi: 10.1016/s0006-2952(97)00072-5. [DOI] [PubMed] [Google Scholar]
- Court MH, Duan SX, Guillemette C, Journault K, Krishnaswamy S, Von Moltke LL, Greenblatt DJ. Stereoselective conjugation of oxazepam by human UDP-glucuronosyltransferases (UGTs): S-oxazepam is glucuronidated by UGT2B15, while R-oxazepam is glucuronidated by UGT2B7 and UGT1A9. Drug Metab Dispos. 2002;30:1257–65. doi: 10.1124/dmd.30.11.1257. [DOI] [PubMed] [Google Scholar]
- Court MH, Krishnaswamy S, Hao Q, Duan SX, Patten CJ, Von Moltke LL, Greenblatt DJ. Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab Dispos. 2003;31:1125–33. doi: 10.1124/dmd.31.9.1125. [DOI] [PubMed] [Google Scholar]
- Fisher MB, Campanale K, Ackermann BL, VandenBranden M, Wrighton SA. In vitro glucuronidation using human liver microsomes and the pore-forming peptide alamethicin. Drug Metab Dispos. 2000;28:560–6. [PubMed] [Google Scholar]
- Gigon PL, Bickel MH. Interference of UDP-glucuronyltransferase and beta-glucuronidase activity in rat liver microsomes at pH 7.5 with p-nitrophenol and p-nitrophenylglucuronide as substrates. Enzyme. 1979;24:230–8. doi: 10.1159/000458664. [DOI] [PubMed] [Google Scholar]
- Haaz MC, Rivory L, Jantet S, Ratanasavanh D, Robert J. Glucuronidation of SN-38, the active metabolite of irinotecan, by human hepatic microsomes. Pharmacol Toxicol. 1997;80:91–6. doi: 10.1111/j.1600-0773.1997.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Kemp DC, Fan PW, Stevens JC. Characterization of raloxifene glucuronidation in vitro: contribution of intestinal metabolism to presystemic clearance. Drug Metab Dispos. 2002;30:694–700. doi: 10.1124/dmd.30.6.694. [DOI] [PubMed] [Google Scholar]
- Kemper RA, Nabb DL. In vitro studies in microsomes from rat and human liver, kidney, and intestine suggest that perfluorooctanoic acid is not a substrate for microsomal UDP-glucuronosyltransferases. Drug Chem Toxicol. 2005;28:281–7. doi: 10.1081/dct-200064468. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Duan SX, Von Moltke LL, Greenblatt DJ, Court MH. Validation of serotonin (5-hydroxtryptamine) as an in vitro substrate probe for human UDP-glucuronosyltransferase (UGT) 1A6. Drug Metab Dispos. 2003;31:133–9. doi: 10.1124/dmd.31.1.133. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Hao Q, Al-Rohaimi A, Hesse LM, von Moltke LL, Greenblatt DJ, Court MH. UDP glucuronosyltransferase (UGT) 1A6 pharmacogenetics: I. Identification of polymorphisms in the 5′-regulatory and exon 1 regions, and association with human liver UGT1A6 gene expression and glucuronidation. J Pharmacol Exp Ther. 2005;313:1331–9. doi: 10.1124/jpet.104.081950. [DOI] [PubMed] [Google Scholar]
- Levvy G, Marsh CA. β-glucuronidase. In: Boyer PDLH, Myrbäck K, editors. The Enzymes. Academic Press; New York: 1960. pp. 397–407. [Google Scholar]
- Levvy GA. The preparation and properties of beta-glucuronidase. IV. Inhibition by sugar acids and their lactones. Biochem J. 1952;52:464–72. doi: 10.1042/bj0520464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh CA, Alexander F, Levvy GA. Glucuronide decomposition in the digestive tract. Nature. 1952;170:163–4. doi: 10.1038/170163a0. [DOI] [PubMed] [Google Scholar]
- Meech R, Mackenzie PI. Structure and function of uridine diphosphate glucuronosyltransferases. Clin Exp Pharmacol Physiol. 1997;24:907–15. doi: 10.1111/j.1440-1681.1997.tb02718.x. [DOI] [PubMed] [Google Scholar]
- Miners JO, Mackenzie PI. Drug glucuronidation in humans. Pharmacol Ther. 1991;51:347–69. doi: 10.1016/0163-7258(91)90065-t. [DOI] [PubMed] [Google Scholar]
- Miners JO, McKinnon RA, Mackenzie PI. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology. 2002;181–182:453–6. doi: 10.1016/s0300-483x(02)00449-3. [DOI] [PubMed] [Google Scholar]
- Mulder GJ. Glucuronidation and its role in regulation of biological activity of drugs. Annu Rev Pharmacol Toxicol. 1992;32:25–49. doi: 10.1146/annurev.pa.32.040192.000325. [DOI] [PubMed] [Google Scholar]
- Paigen K. Mammalian beta-glucuronidase: genetics, molecular biology, and cell biology. Prog Nucleic Acid Res Mol Biol. 1989;37:155–205. doi: 10.1016/s0079-6603(08)60698-4. [DOI] [PubMed] [Google Scholar]
- Soars MG, Ring BJ, Wrighton SA. The effect of incubation conditions on the enzyme kinetics of udp-glucuronosyltransferases. Drug Metab Dispos. 2003;31:762–7. doi: 10.1124/dmd.31.6.762. [DOI] [PubMed] [Google Scholar]
- Swank RT, Pfister K, Miller D, Chapman V. The egasyn gene affects the processing of oligosaccharides of lysosomal beta-glucuronidase in liver. Biochem J. 1986;240:445–54. doi: 10.1042/bj2400445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- Wakabayashi M. β-Glucuronidases in metabolic hydrolysis. In: Fishman W, editor. Metabolic Conjucation and Metabolic Hydrolysis. Academic Press; New York: 1970. pp. 519–602. [Google Scholar]