Abstract
It is now well known that most malignant tumours contain a significant amount of leucocytic infiltrates the presence of which has, on many occasions, been linked to poor patient prognosis. These leucocyte populations are recruited to tumours by chemotactic factors released by either viable or necrotic tumour cells, or by cells within the tumour stroma. In recent times, most studies have analysed the role that tumour-associated macrophages (TAM) have on tumour progression. However, there is now increasing evidence to show that neutrophils also actively participate in this process. Whilst there are some data to suggest that neutrophil-derived factors can promote genetic mutations leading to tumourigenesis, or secrete factors that promote tumour cell proliferation; there is now substantial evidence to show that neutrophils, like TAM, significantly affect tumour angiogenesis. In this review, we discuss the likely mechanisms by which neutrophils are recruited into the tumour and then elaborate on how these cells may induce tumour vascularization by the secretion of powerful pro-angiogenic factors. We also discuss possible future chemotherapeutic strategies that are aimed at limiting tumour angiogenesis by inhibiting neutrophil recruitment.
Keywords: angiogenesis, chemokine, neutrophil, therapy, tumour
Introduction
Most tumours arise from one or more often several sequential genetic mutations in the DNA that give rise to proteins with altered tertiary structure leading to lack of or altered biological functions. Uncorrected mutations in genes regulating the cell cycle lead to dysregulated cell division that can cause these cells to proliferate uncontrollably. Eventually, these mutated cells expand to become an avascular cell mass that is unable to grow more than 2–3 mm3 in size because of the lack of nutrient and oxygen delivery and the removal of waste products (Folkman 2002). For tumours to grow beyond this size, they must redirect the host vasculature and co-opt it to deliver nutrients and oxygen required for further cell proliferation. The production of new blood vessels from an existing vascular bed is known as angiogenesis and research over the last few decades has revealed a number of mechanisms by which tumours initiate and direct angiogenesis (Bergers & Benjamin 2003). Obviously, tumour cells themselves can secrete cytokines and growth factors that can stimulate tumour angiogenesis. However, recent attention has focussed on tumour-associated leucocytes that are enticed into tumours by secretion of tumour-derived chemoattractants. An increase in the number of several leucocyte sub-populations within tumours have been associated with increased tumour angiogenesis, which is often associated with poor prognosis and patient outcome (Murdoch et al. 2008). The most prominent of these leucocyte populations is the tumour-associated macrophage (TAM) and the role that this cell type plays in tumour angiogenesis has been extensively studied (Dirkx et al. 2006; Lewis & Pollard 2006; Mantovani et al. 2006). However, not all of the leucocyte-derived angiogenic factors may be attributable to TAM; indeed there is evidence to suggest that other innate immune cells may play a role in initiating tumour angiogenesis. This review will discuss the role that tumour-associated neutrophils are thought to play in directing tumour angiogenesis.
Neutrophil origin and homeostasis
Neutrophils are the most abundant leucocyte in the circulation and arise from a process known as myelopoiesis. They initially originate in the bone marrow from haematopoietic pluripotent stem cells which in turn differentiate into myeloid progenitor cells (often termed myelomonocytic stem cells). These cells can further differentiate along several pathways to form the various myeloid cell populations. In the case of neutrophils, they differentiate into CFU-granulocyte (CFU-G) cells, which further differentiate into myeloblasts, promyelocytes and then myelocytes (immature neutrophils), by which time cell proliferation stops and secondary granules begin to appear. Several factors are known to increase the proliferation and survival of differentiating neutrophils, the most prominent of which is granulocyte colony stimulating factor (G-CSF) (Barreda et al. 2004). As the myelocytes mature in the bone marrow they become more deformable, increase their motility and eventually migrate through trans-endothelial pores in the bone marrow into the bloodstream. The mechanism by which neutrophils are released from the bone marrow have been extensively studied (reviewed by von Vietinghoff & Ley 2008). The chemokine, CXCL12 (formally SDF-1α) has been found to be important in regulating neutrophil retention and release from the bone marrow and thus the number of neutrophils in the circulation at any given time. CXCL12 is abundantly expressed in the bone marrow milieu and retains neutrophils there by binding to its cell surface receptor, CXCR4, expressed by neutrophils. G-CSF down-regulates both CXCR4 and CXCL12 expression and CXCR4-mediated cell signalling is also blocked leading to release of neutrophils from the bone marrow. (Suratt et al. 2004).
Two different pools of neutrophils have been described in the circulation; the circulating pool, consisting of neutrophils that are freely circulating and the marginated pool, consisting of neutrophils that are bound to the endothelium of small vessels. The marginated pool constitutes a neutrophil reserve that can be mobilized to the circulating pool upon acute inflammation by such cytokines as interleukin (IL) 6 (Steele et al. 1987; Suwa et al. 2000). It is from the circulating pool that neutrophils are recruited to sites of inflammation and tumours (Kanwar & Cairo 1993; Friedman 2002).
Within tissues, human neutrophils are usually identified on the basis of their nuclear morphology coupled with their positive staining for myeloperoxidase. In murine tissues, neutrophils are usually identified by expression of the cell surface marker Gr-1 and lack of F4/80 (monocyte/macrophage marker) expression.
Evidence for tumour-associated neutrophils and mechanisms regulating their recruitment into tumours
A number of immunohistochemical-based studies have shown increased numbers of neutrophils in various human tumours compared with healthy tissues. Elevated levels of neutrophils were observed in biopsies from patients with colon adenocarcinoma compared with the surrounding non-diseased tissue. In these biopsies, neutrophils were found throughout the tumour but their numbers increased further in invasive and ulcerated areas (Nielsen et al. 1996). Similarly, elevated neutrophil numbers were observed in bronchioloalveolar lavage fluid and in biopsies from patients with bronchioloalveolar carcinoma compared with control subjects. In addition, univariate analysis showed that increased numbers of neutrophils in these patients correlated significantly with poor prognosis (Bellocq et al. 1998). Increased numbers of neutrophils have also been observed in patients with myxofibrosarcoma, gastric carcinoma and melanoma (Mentzel et al. 2001; Eck et al. 2003; Mhawech-Fauceglia et al. 2006).
To be recruited into tumours, neutrophils must first leave the general circulation. Neutrophil extravasation is a tightly orchestrated process that involves the rolling of neutrophils on activated endothelium followed by firm adhesion, haptotaxis along a chemotactic gradient and directed transmigration across the vasculature and into the tissue (Springer 1994). There are several potential chemotactic factors that may stimulate the migration of neutrophils across the tumour vasculature but by far the most potent, and most studied, are those belonging to the CXCL chemokine sub-family that possess the ELR (Glu–Leu–Arg) tri-peptide motif. These bind to and activate CXCR1 and/or CXCR2; seven-transmembrane G-protein-coupled receptors that are expressed on the neutrophil cell surface (Holmes et al. 1991; Murphy & Tiffany 1991; Ahuja & Murphy 1996; Wolf et al. 1998). CXCL8 (formally IL-8) is one of the best studied neutrophil chemoattractants with respect to tumour biology and is over-expressed in a multitude of different human carcinomas and tumour cell lines including breast, colon, cervical, lung, brain, prostate, ovarian and renal cell carcinomas, acute myelogenous and B-cell lymphocytic leukemia, melanoma and Hodgkin’s disease (reviewed by Xie 2001). Within tumours, both stromal and tumour cells are a rich source of CXCL8. Although high levels of CXCL8 is constitutively expressed by many tumour cells, its expression can be up-regulated further by a variety of pro-inflammatory cytokines, nitric oxide and such tumour-associated environmental conditions as hypoxia, acidosis and hyperglycaemia (Kennedy et al. 1997; Xie 2001; Sakamoto et al. 2005). It is highly likely that elevated levels of CXCL8 within the microenvironment of some tumours lead to neutrophil recruitment into the tumour. Indeed, Bellocq et al., showed a positive correlation between levels of CXCL8 in patients with bronchioloalveolar carcinoma and numbers of tumour-infiltrating neutrophils, suggesting that CXCL8 is a major force in recruiting neutrophils to these tumour sites (Bellocq et al. 1998). Furthermore, mice with subcutaneous tumours generated from human ovarian cancer cells engineered to over-express human CXCL8 (mice do not have the analogous gene for CXCL8), were found to have dramatically elevated neutrophil infiltration at the tumour site when compared with tumours grown with cells containing control vectors (Gaudry et al. 1997). Apart from CXCL8, other potent neutrophil chemotactic CXCL chemokines are capable of stimulating neutrophil chemotaxis and extravasation and these have also been found to be expressed by many human tumours. For example, elevated levels of CXCL1 (formally GRO-α) has been detected in gastric and colon carcinomas (Cuenca et al. 1992; Eck et al. 2003), and malignant melanoma (Vaupel et al. 1991). Elevated levels of CXCL5 (formally ENA-78) have been detected in non-small cell lung cancer (Wislez et al. 2004), and CXCL6 (formally GCP-2) has been observed in gastrointestinal tumours (Proost et al. 1993; Gijsbers et al. 2005) where it was associated with marked leucocyte infiltration as assessed by CD45 staining. Although CXCL6 is considered to be principally a neutrophil chemokine, a specific association between elevated levels of this chemokine with neutrophil recruitment was not examined in this study. However, not all studies have shown an association of increased tumour-derived CXCL chemokine expression with increased neutrophil recruitment. Keane et al. examined the levels of neutrophils, as well as other leucocyte populations, by flow cytometric analysis of single cells suspensions from disaggregated Lewis lung cancer tumours implanted into CXCR2−/− or CXCR2+/+ mice. Interestingly, they found no significant difference in the levels of neutrophil recruitment or infiltration of any other leucocyte population into CXCR2−/− compared with CXCR2+/+ mice, even though these tumours secreted elevated levels of CXCR2-specific ligands, CXCL1-3 (Keane et al. 2004). In contrast to other in vivo data, this suggests that murine neutrophil recruitment in to these human xenograft tumours is not influenced by the tumour-derived expression of human CXCL ELR+ chemokines. However, it must be noted that there are significant differences in chemokine receptor expression by human and murine neutrophils. Until recently, it was thought that murine neutrophils only express CXCR2. However, a murine homolog to human CXCR1 has now been identified and cloned (Moepps et al. 2006). Transfection of murine CXCR1 into murine Ba/F3 cells renders this cell line responsive to human and murine CXCL6 and CXCL8 suggesting receptor-mediated activation (Fan et al. 2007). Thus, it is possible that murine neutrophil recruitment into human tumours is directed through CXCR1 activation by human CXCL8. It may also be possible that such human CXC chemokines as CXCL5, which appears to be highly expressed predominantly in lung carcinomas (Wislez et al. 2004) do not activate murine CXCR2. The redundancy of the CXCL chemokine system is such that it is quite plausible for neutrophil recruitment into tumours to be mediated by different CXCL chemokines and via different CXC receptors depending on the tumour type.
Role of neutrophils in other aspects of tumour progression
Although this review will focus primarily on angiogenesis, it should be noted that neutrophils have also been implicated in other aspects of tumour development. There is increasing evidence to suggest that neutrophils contribute to tumourogenesis, tumour cell growth and metastasis in some tumours. For instance, Sandhu et al. showed that CXCL8-mediated neutrophil recruitment in mice positively correlated with an increase in tumour cell mutation frequency leading to tumourogenesis (Sandhu et al. 2000). Activated neutrophils up-regulate iNOS leading to the release of potent reactive oxygen species that damages DNA. This damage is usually repaired but in some cells the repair is inefficient and this can lead to genetic mutations in tumour-suppressor genes leading to dysregulated cell proliferation (Coleman & Tsongalis 1995). Neutrophils can also secrete factors that affect tumour cell proliferation. Aarbiou et al. showed increased proliferation of A549 alveolar carcinoma cells by neutrophil α-defensins in a MAP kinase-dependent manner, and this was abolished using a neutralizing anti-defensin antibody (Aarbiou et al. 2002). Neutrophils have also been linked with tumour cell metastasis. Invasion of tumour cells through a reconstituted basement membrane was increased by addition of neutrophils (Welch et al. 1989). As neutrophils transmigrate across the endothelium on their way to the tumour they release proteases that degrade the basement membrane. It is thought that the holes created in the extracellular matrix (ECM) and endothelium by these extravasating neutrophils are exploited by metastasizing tumour cells moving in the opposite direction into the circulation.
Along with having pro-tumour roles, neutrophils also have anti-tumour properties. As early as 1975, in vitro experiments demonstrated that neutrophils could kill tumour cells directly (Clark & Klebanoff 1975). Originally, these effects were thought to be mediated exclusively by myeloperoxidase, as neutrophils from patients deficient in myeloperoxidase did not show the same cytotoxic effects against tumour cells (Clark & Klebanoff 1975). However, it is now known that other factors secreted by neutrophils can kill tumour cells directly. These include release of reactive oxygen species, proteases, membrane-perforating agents, or such cytokines as TNFα and interleukin-1β (IL-1β) (Di Carlo et al. 2001). Although these data demonstrate that neutrophils have the capacity to have anti-tumour properties, there is compelling data that they also have significant pro-tumour actions, in particular their role in promoting tumour angiogenesis.
Neutrophil-induced tumour angiogenesis
Neutrophils are thought to play an important role in normal physiological angiogenesis. During the menstrual cycle, angiogenesis occurs in order to support the proliferation and growth of the endometrial tissue. The source of the potent pro-angiogenic factor vascular endothelial growth factor (VEGF) in these tissues was found to be from neutrophils. In fact, neutrophils expressing VEGF were found in the microvessels of the endometrium during the proliferative stage of the cycle when the endometrium can quadruple in thickness (Mueller et al. 2000). Moreover, in a murine study of endometrial angiogenesis, the proliferation of endothelial cells in neutrophil-depleted mice using anti-Gr-1 antibodies was significantly reduced compared with control mice, suggesting that neutrophils affect normal physiological angiogenesis (Heryanto et al. 2004). Further experimental in vivo models of angiogenesis have clearly demonstrated that neutrophils affect neo-vascularization in other tissues. For example, Benelli et al. showed that Gr-1-mediated neutrophil depletion significantly reduced angiogenesis in C57 black mice implanted with Matrigel containing CXCL1 or CXCL8 when compared with controls (Benelli et al. 2002). Similarly, Shaw et al. found that neutrophil depletion in Swiss Webster mice inhibited, but did not prevent, angiogenesis into slow-release pellets containing the pro-angiogenic factor Fibroblast Growth Factor (FGF)-2 when implanted into the corneas (Shaw et al. 2003). Further studies using the chick embryo chorioallantoic membrane (CAM) assay also showed that presence of heterophils (the chicken analogue of neutrophils) promotes angiogenesis. Here on-plants containing FGF2, VEGF or HT1080 tumour cells were placed on top of CAM from 10-day old embryos and angiogenesis quantified. Results showed a significant increase in the number of heterophils in on-plants containing angiogenic factors and HT1080 cells. At the same time, on-plants containing HT1080 cells induced a fourfold increase in angiogenesis when compared with controls, suggesting that tumour cells could stimulate neutrophil recruitment leading to tumour angiogenesis. Disruption of inflammation by treating on-plants with the anti-inflammatory agent cortisone significantly reduced heterophil numbers, which resulted in an 80% reduction in angiogenesis (Zijlstra et al. 2005).
Initial evidence to suggest that neutrophils can affect tumour angiogenesis have come from association studies using human biopsies. In patients with myxofibrosarcoma, elevated numbers of neutrophils were observed in high-grade malignant tumours and this correlated positively with increased intra-tumoural microvessel density (Mentzel et al. 2001). More convincing evidence for a role of neutrophils in tumour angiogenesis has come from animal tumour models. For instance, nude mice injected subcutaneously with human Bowes melanoma cells transfected to over-express CXCL6, induced 10 times more neutrophil infiltration than controls and this was accompanied by increased tumour angiogenesis (Van et al. 2001). Nozawa et al. used the RIP1-Tag2 transgenic mouse model to study the role of neutrophils in in vivo angiogenesis further. The RIP1-Tag2 transgenic mouse model is a well-characterized in vivo model of multi-step pancreatic carcinogenesis in which insulin-induced expression of the simian SV40 large T antigen oncogene leads to dysplastic pancreatic islets, some of which become angiogenic and later form malignant tumours. The authors found that angiogenic islets within lesions contained neutrophils. Depletion of neutrophils in these animals by anti-GR-1 therapy at early stages of tumour development significantly reduced the number of dysplastic islets that were undergoing angiogenesis (Nozawa et al. 2006). Taken together, these data strongly suggest that neutrophils play a role in tumour angiogenesis.
The mechanisms by which tumour-associated neutrophils mediate or modulate tumour angiogenesis has not been fully elucidated. Activated neutrophils can release a variety of proteases that can degrade and remodel the ECM, a process that is crucial for angiogenesis. Of the proteases released by neutrophils, matrix metalloproteinases (MMPs), in particular MMP-9, have been shown to have the most profound effects in mediating tumour angiogenesis. Proteolysis of the ECM by this MMP releases such potent angiogenic factors as VEGF and FGF2 that are usually sequestered in an inactivated form to the ECM (Coussens & Werb 1996; Bergers & Benjamin 2003). Once released, these soluble factors act upon nearby endothelial cells to prompt re-vascularization. Huang et al. showed that human ovarian tumours grown in MMP9-deficient mice displayed significantly reduced levels of tumour microvessel density when compared with wild-type mice (Huang et al. 2002). Tumour-associated neutrophil-derived MMP-9 has also been shown to contribute to tumour angiogenesis and progression associated with squamous cell carcinoma of the skin (Coussens et al. 2000). Similar findings have also been observed in the RIP1-Tag2 mouse model of pancreatic carcinoma, where neutrophils were found to be the main source of MMP9 in the angiogenic islet dysplasias and tumours. In fact, although scarce within the tumour compared with infiltrating macrophages, these neutrophils produced enough MMP-9 to drive tumour angiogenesis, suggesting that neutrophil-derived MMP-9 is crucial for catalyzing the angiogenic switch in this model (Nozawa et al. 2006). The activity of MMP-9 is regulated by tissue inhibitors of metalloproteinases (TIMPs), in particular TIMP-1. This inhibitor is often released in conjunction with MMP9 to form a complex thereby preventing its proteolytic activity. Interestingly, neutrophils do not express TIMP-1 and consequently do not produce MMP-9/TIMP-1 complexes (Masure et al. 1991). Thus, neutrophil-derived TIMP-free MMP-9 is rapidly and freely available to immediately release sequestered growth factors and remodel the ECM to promote angiogenesis (Ardi et al. 2007). This may be why only few tumour-associated neutrophils are required to promote tumour angiogenesis in the RIP1-Tag2 mouse model.
Activated neutrophils can also directly secrete a multitude of soluble pro-angiogenic factors that may also influence the angiogenic switch within tumours. For example, stimulation of neutrophils with TNFα, a cytokine highly expressed in many tumours, induces neutrophil de-granulation releasing VEGF from intracellular stores, which induces microvascular endothelial cell proliferation and tubule formation in vitro and thus, as already mentioned, may stimulate angiogenesis in vivo (McCourt et al. 1999). Furthermore, upon contact with MDA-MB-231 and T47D breast carcinoma cell lines in vitro, purified neutrophils secrete elevated levels of oncostatin M. This pleiotropic cytokine then induces these breast cancer cells to up-regulate the secretion of VEGF (Queen et al. 2005). If this happens in vivo, it is likely that oncostatin M-induced VEGF secretion will act in concert with VEGF and other growth factors released from tumour-associated neutrophils, TAM and the ECM to promote angiogenesis.
TNFα, GM-CSF and PAF-activated neutrophils also release the pro-angiogenic ELR+ chemokines CXCL8 and CXCL1 (Cassatella 1999). As well as being neutrophil chemoattractants, these chemokines have been shown to induce endothelial cell proliferation and differentiation in vitro and angiogenesis in vivo (Koch et al. 1992; Schonbeck et al. 1995; Strieter et al. 1995; Arenberg et al. 1996). The release of these pro-angiogenic CXC chemokines by neutrophils within the tumour microenvironment is likely to act in concert with CXC chemokines released by tumour cells themselves and other cells of the stoma to increase the pro-angiogenic burden within tumours. Interestingly, binding of CXCL8 stimulates fast de-granulation of MMP-9 from neutrophils. The proteolytic activity of this TIMP-free MMP-9 truncates the amino terminus of CXCL8 to produce a tenfold more potent chemokine (Van den steen et al. 2000). This could conceivably result in a positive feedback loop to recruit more neutrophils into the tumour and so stimulating further angiogenesis.
As well as producing pro-angiogenic factors that may directly stimulate endothelial cell migration, proliferation and differentiation, neutrophils have also been shown to stimulate angiogenesis through intimate cell-to-cell interactions with endothelial cells. During extravasation, neutrophils initially bind to the activated endothelium by rolling on E-selectin expressed on the surface of endothelial cells; an event that is mediated by neutrophil expressed sialyl Lewis X. This is followed by adhesion via interaction of intercellular adhesion molecule-1 (ICAM-1) on the endothelium with integrin αMβ2 (CD11b/CD18) on the neutrophil cell surface (Lawrence & Springer 1993; Springer 1994). Ligation of ICAM-1 or E-selectin can initiate intracellular signalling in endothelial cells (Durieu-Trautmann et al. 1994; Lorenzon et al. 1998). In an in vitro model of angiogenesis, co-culture of bovine aortic endothelial cells with neutrophils increased in vitro angiogenesis compared with endothelial cells alone, and this increase was inhibited by addition of blocking anti-ICAM-1 or anti-E-selectin antibodies (Yasuda et al. 2000). It appears that ligation of ICAM-1 is important in neutrophil stimulation of endothelial cells, whereas ligation of E-selectin is more important in endothelial cell-cell interaction and morphological changes (Yasuda et al. 2002). Neutrophil-induced angiogenesis was inhibited by the addition of catalase, an enzyme that catalyzes the decomposition of hydrogen peroxide (H2O2), providing evidence that H2O2 may also play a role in neutrophil-induced angiogenesis (Yasuda et al. 2000). Interestingly, H2O2 activates Ets-1, a transcription factor that regulates expression of MMPs 1, 3 and 9, and urokinase-type plasminogen activator (u-PA) in endothelial cells (Yasuda et al. 2002), all of which have been implicated in regulating angiogenesis. Both neutrophils and endothelial cells can secrete H2O2, so further studies are required to clarify the source of the H2O2 that is released when neutrophils adhere to endothelial cells. Furthermore, there is currently no evidence to show that this phenomenon occurs in vivo.
Along with the ability to release factors that stimulate angiogenesis, neutrophils have also been shown to secrete or increase the levels of anti-angiogenic factors. For instance, neutrophil elastase, a serine proteinase released from the azurophil granules of activated neutrophils, can cleave plasminogen to liberate the anti-angiogenic factor, angiostatin (kingle one to three fragments) (Scapini et al. 2002). Angiostatin generated by neutrophils can inhibit VEGF and FGF2-mediated endothelial cell proliferation in vitro and in Matrigel and CAM assays in vivo (Scapini et al. 2002). Interestingly, angiostatin also directly affects neutrophil migration towards CXCL1 and CXCL8. Treatment of mice with angiostatin significantly reduced vessel formation in CXCL1 and CXCL8 containing Matrigel sponge assays, which was associated with reduced neutrophil recruitment (Benelli et al. 2002). In addition, neutrophil elastase can also degrade VEGF and FGF2, diminishing their angiogenic activity both in vitro and in ex-vivo angiogenesis assays (Ai et al. 2007). Taken together, this suggests that neutrophils may also secrete products that try to switch off or regulate neutrophil-induced angiogenesis. It is clear that neutrophils have the capacity to secrete both pro- and anti-angiogenic factors. However, given the pro-tumour microenvironment found within most tumours, it is likely that the phenotype of tumour-associated neutrophils is skewed to that of a pro-angiogenic mediator.
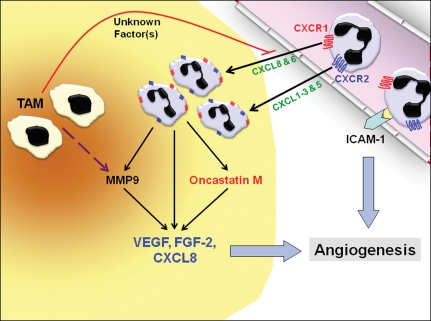
Given the evidence that neutrophils display pro- and anti-angiogenic characteristics, there is currently some debate as to the extent by which neutrophils influence tumour angiogenesis, especially as there is overwhelming evidence to show that tumour-associated macrophages strongly influence this event (Dirkx et al. 2006; Mantovani et al. 2006; Lewis et al. 2007). However, a recent study by Pahler et al. has shed light on the importance of tumour-associated neutrophils. Their study examined tumour angiogenesis (as measured by vessel density) in CCR2−/− mice with HPV-driven cervical carcinomas. Unsurprisingly, the levels of TAM in tumours grown in these mice were significantly lower than control mice, suggesting that the CCL2/CCR2 ligand-receptor axis is important in TAM recruitment in these tumours. However, surprisingly, there was only a modest delay in time to progression from dysplasia to carcinoma in the CCR2-deficient mice, and the MMP-9 expression levels and microvessel density in the tumours of these mice remained unchanged compared with wild-type tumour-bearing mice. Further investigation of the tumours from CCR2-deficient mice revealed a dramatic increase in neutrophil numbers in these tumours, which was associated with the elevated MMP-9 expression, suggesting that neutrophils were providing a secondary source of MMP-9 in the absence of TAM, thereby rescuing tumour angiogenesis. Even more strikingly, in vitro bioassays showed that macrophages produce soluble factors that can inhibit neutrophil chemotaxis and their recruitment into tumour spheroids. These data suggest a mechanism whereby CCL2 attracts pro-angiogenic CCR2+ TAM that has the ability to limit tumour infiltration by neutrophils. If such tumor-promoting macrophages are suppressed, MMP-9+ neutrophils are then recruited to the tumour to provide alternative paracrine support for tumor angiogenesis and progression (Pahler et al. 2008). Clearly, more studies are warranted on the role of neutrophils in tumour angiogenesis and the interaction these cells have with other tumour-associated leucocyte populations. The current understandings on neutrophil recruitment and roles in angiogenesis that have been discussed in this review are summarized diagrammatically in figure 1.
Figure 1.
Neutrophil-induced tumour angiogenesis. Neutrophils are recruited into the tumour from the circulation via the production of chemokines for CXCR1 (CXCL8 & 6) and/or CXCR2 (CXCL1–3 & 5). Once inside the tumour, neutrophils can secrete factors such as oncostatin M, which stimulates tumour cells to increase their vascular endothelial growth factor (VEGF) production. Neutrophils also secrete tissue inhibitors of metalloproteinase (TIMP)-free matrix metalloproteinase (MMP)9 that can act in concert with MMP9 released by tumour-associated macrophages (TAMs) to liberate pro-angiogenic growth factors (VEGF & FGF-2) that are sequestered to the extracellular matrix (ECM). Cytokine-activated neutrophils also secrete VEGF and CXCL8 by de-granulation. These potent pro-angiogenic factors then act directly on the nearby vasculature to promote tumour angiogenesis. In addition, the interaction of neutrophils with adhesion molecules (ICAM-1) on the endothelial cell surface may also stimulate angiogenesis. Interestingly, in an experimental tumour model, TAMs have been found to inhibit the infiltration of neutrophils by an as yet unidentified mechanism.
Therapeutic implications and future research directions
The increasing evidence for a role of neutrophils in tumour angiogenesis suggests that agents designed to reduce either their numbers or biological activity within the tumour environment may be useful for anti-tumour therapy. Indeed, administration of cytotoxic antibodies to reduce the number of neutrophils in murine models of tumour angiogenesis has provided convincing evidence to show that such a strategy would be beneficial in the human setting (Nozawa et al. 2006). However, as neutrophils are a key component of the innate immune response to invading pathogens, complete ablation of neutrophils from a human cancer patient (who are already immunocompromised to a certain extent) would obviously not be beneficial. An alternative strategy could be to target the molecules that recruit neutrophils to these tumours. As outlined previously, most neutrophils are likely to be recruited to the tumour by members of the CXCL chemokine family. Interestingly, Wente et al. demonstrated that administration of a CXCR2-blocking antibody reduced angiogenesis in the rat corneal micro-pocket assay in response to tumour conditioned medium (Wente et al. 2006). Although, in their experiments the number of infiltrating neutrophils into the cornea was not investigated; given other data, it is likely that this antibody prevented neutrophil infiltration that mediated angiogenesis. In recent years, various pharmaceutical companies have developed small molecule antagonists that specifically bind to and inhibit CXCR2 signalling. One such group of molecules that have been found to inhibit CXCR2 with high specificity is based on the compound thiazolo[4,5-d]pyrimidine (Hunt et al. 2007). These molecules not only bind CXCR2 with high specificity, they also inhibit neutrophil chemotaxis specifically toward CXCR2 but not CXCR1 ligands. We have recently found that pretreatment of neutrophils with this antagonist inhibits recruitment of neutrophils into A549 adenocarcinoma tumour spheroids that secrete abundant CXCR2 ligands. Furthermore, when administered into mice bearing A549 xenograft tumours, the antagonist inhibited neutrophil recruitment into these tumours, which resulted in a significant reduction in tumour growth (Tazzyman, Barry, Ashton, Lewis, Murdoch, unpublished observation). Such experiments may show the usefulness of future neutrophil-targeted cancer therapy.
Targeting tumour-associated leucocytes as a potential cancer therapy is not new. Indeed, there are many current treatment strategies that are targeted toward reducing or eradicating the recruitment of tumour-associated macrophages in the tumours. These therapies are based on reducing TAM recruitment by blocking the actions of such molecules as CSF-1, PlGF and CCL2 using therapeutic blocking antibodies or antisense probes (Aharinejad et al. 2002; Gazzaniga et al. 2007; Loberg et al. 2007). These treatments show some potential, however, the recent work of Pahler et al., which showed that neutrophils can compensate for the reduction of TAM in tumours, suggests that elimination of TAM alone may be insufficient (Pahler et al. 2008). A combined or sequential therapeutic approach to inhibit macrophage and then neutrophil infiltration may have to be considered.
Summary
Neutrophils were once an ignored cell type in the field of tumour biology. However, there is now an increasing body of evidence to show that these cells are capable of directly affecting tumour progression. There is some data to suggest that neutrophils or neutrophil-derived factors participate in tumourigenesis, tumour cell proliferation and metastasis. However, most evidence points to a role of these cells in mediating tumour angiogenesis. At present there is some uncertainty as to how important neutrophils are in contributing to leucocyte-mediated tumour angiogenesis and more research in this area is required. Nevertheless, targeting neutrophil recruitment into tumours may prove a beneficial and feasible therapeutic strategy to complement more traditional anti-cancer therapies.
Acknowledgments
The authors gratefully acknowledge the support of Simon Barry and Susan Ashton of AstraZeneca, UK and Yorkshire Cancer Research for their funding and work in this area of research. We apologize to any investigators whose papers could not be cited because of space limitations.
References
- Aarbiou J, Ertmann M, van W, et al. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J. Leukoc. Biol. 2002;72:167–174. [PubMed] [Google Scholar]
- Aharinejad S, Abraham D, Paulus P, et al. Colony-stimulating factor-1 antisense treatment suppresses growth of human tumor xenografts in mice. Cancer Res. 2002;62:5317–5324. [PubMed] [Google Scholar]
- Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- Ai S, Cheng XW, Inoue A, et al. Angiogenic activity of bFGF and VEGF suppressed by proteolytic cleavage by neutrophil elastase. Biochem. Biophys. Res. Commun. 2007;364:395–401. doi: 10.1016/j.bbrc.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J. Clin. Invest. 1996;97:2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev. Comp. Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Bellocq A, Antoine M, Flahault A, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am. J. Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- Benelli R, Morini M, Carrozzino F, et al. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267–269. doi: 10.1096/fj.01-0651fje. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Angiogenesis: tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- Clark RA, Klebanoff SJ. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J. Exp. Med. 1975;141:1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman WB, Tsongalis GJ. Multiple mechanisms account for genomic instability and molecular mutation in neoplastic transformation. Clin. Chem. 1995;41:644–657. [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem. Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca RE, Azizkhan RG, Haskill S. Characterization of GRO alpha, beta and gamma expression in human colonic tumours: potential significance of cytokine involvement. Surg. Oncol. 1992;1:323–329. doi: 10.1016/0960-7404(92)90094-2. [DOI] [PubMed] [Google Scholar]
- Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J. Leukoc. Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J. Biol. Chem. 1994;269:12536–12540. [PubMed] [Google Scholar]
- Eck M, Schmausser B, Scheller K, BRANdlein S, Muller H. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin. Exp. Immunol. 2003;134:508–515. doi: 10.1111/j.1365-2249.2003.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Patera AC, Pong-Kennedy A, et al. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J. Biol. Chem. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002;29(Suppl. 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Friedman AD. Transcriptional regulation of granulocyte and monocyte development. Oncogene. 2002;21:3377–3390. doi: 10.1038/sj.onc.1205324. [DOI] [PubMed] [Google Scholar]
- Gaudry M, Bregerie O, Andrieu V, El B, Pocidalo MA, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90:4153–4161. [PubMed] [Google Scholar]
- Gazzaniga S, Bravo AI, Guglielmotti A, et al. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J. Invest. Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- Gijsbers K, Gouwy M, Struyf S, et al. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp. Cell Res. 2005;303:331–342. doi: 10.1016/j.yexcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Heryanto B, Girling JE, Rogers PA. Intravascular neutrophils partially mediate the endometrial endothelial cell proliferative response to oestrogen in ovariectomised mice. Reproduction. 2004;127:613–620. doi: 10.1530/rep.1.00161. [DOI] [PubMed] [Google Scholar]
- Holmes WE, LEE J, Kuang WJ, Rice GC, WOOD WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- Huang S, Van A, Tedjarati S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J. Natl. Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- Hunt F, Austin C, Austin R, et al. SAR studies on thiazolo[4,5-d]pyrimidine based CXCR2 antagonists involving a novel tandem displacement reaction. Bioorg. Med. Chem. Lett. 2007;17:2731–2734. doi: 10.1016/j.bmcl.2007.02.080. [DOI] [PubMed] [Google Scholar]
- Kanwar VS, Cairo MS. Neonatal neutrophil maturation, kinetics, and function. In: Abramson JS, Wheeler JG, editors. The Neutrophil. New York: Oxford University Press; 1993. pp. 1–16. [Google Scholar]
- Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J. Immunol. 2004;172:2853–2860. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- Kennedy AS, Raleigh JA, Perez GM, et al. Proliferation and hypoxia in human squamous cell carcinoma of the cervix: first report of combined immunohistochemical assays. Int. J. Radiat. Oncol. Biol. Phys. 1997;37:897–905. doi: 10.1016/s0360-3016(96)00539-1. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J. Immunol. 1993;151:6338–6346. [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- Lorenzon P, Vecile E, Nardon E, et al. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J. Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- Masure S, Proost P, Van DJ, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur. J. Biochem. 1991;198:391–398. doi: 10.1111/j.1432-1033.1991.tb16027.x. [DOI] [PubMed] [Google Scholar]
- McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch. Surg. 1999;134:1325–1331. doi: 10.1001/archsurg.134.12.1325. [DOI] [PubMed] [Google Scholar]
- Mentzel T, Brown LF, Dvorak HF, et al. The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Arch. 2001;438:13–22. doi: 10.1007/s004280000327. [DOI] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Kaya G, Sauter G, et al. The source of APRIL up-regulation in human solid tumor lesions. J. Leukoc. Biol. 2006;80:697–704. doi: 10.1189/jlb.1105655. [DOI] [PubMed] [Google Scholar]
- Moepps B, Nuesseler E, Braun M, Gierschik P. A homolog of the human chemokine receptor CXCR1 is expressed in the mouse. Mol. Immunol. 2006;43:897–914. doi: 10.1016/j.molimm.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Mueller MD, Lebovic DI, Garrett E, Taylor RN. Neutrophils infiltrating the endometrium express vascular endothelial growth factor: potential role in endometrial angiogenesis. Fertil. Steril. 2000;74:107–112. doi: 10.1016/s0015-0282(00)00555-0. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- Nielsen BS, Timshel S, Kjeldsen L, et al. 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int. J. Cancer. 1996;65:57–62. doi: 10.1002/(SICI)1097-0215(19960103)65:1<57::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahler JC, Tazzyman S, Erez N, et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost P, De Wolf-Peeters C, Conings R, Opdenakker G, Billiau A, Van DJ. Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO, IP-10, and IL-8. J. Immunol. 1993;150:1000–1010. [PubMed] [Google Scholar]
- Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Mukae H, Fujii T, et al. Differential effects of alpha- and beta-defensin on cytokine production by cultured human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L508–L513. doi: 10.1152/ajplung.00076.2004. [DOI] [PubMed] [Google Scholar]
- Sandhu JK, Privora HF, Wenckebach G, Birnboim HC. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. Am. J. Pathol. 2000;156:509–518. doi: 10.1016/S0002-9440(10)64755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapini P, Nesi L, Morini M, et al. Generation of biologically active angiostatin kringle 1-3 by activated human neutrophils. J. Immunol. 2002;168:5798–5804. doi: 10.4049/jimmunol.168.11.5798. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Brandt E, Petersen F, Flad HD, Loppnow H. IL-8 specifically binds to endothelial but not to smooth muscle cells. J. Immunol. 1995;154:2375–2383. [PubMed] [Google Scholar]
- Shaw JP, Chuang N, Yee H, Shamamian P. Polymorphonuclear neutrophils promote rFGF-2-induced angiogenesis in vivo. J. Surg. Res. 2003;109:37–42. doi: 10.1016/s0022-4804(02)00020-3. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Steele RW, Steele CR, Pilkington NS, Jr, Charlton RK. Functional capacity of marginated and bone marrow reserve granulocytes. Infect. Immun. 1987;55:2359–2363. doi: 10.1128/iai.55.10.2359-2363.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- Suratt BT, Petty JM, Young SK, et al. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2954–H2960. doi: 10.1152/ajpheart.2000.279.6.H2954. [DOI] [PubMed] [Google Scholar]
- Van den steen PE, Proost P, Wuyts A, Van DJ, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- Van C, Van A, Wuyts A, et al. Tumor angiogenesis induced by granulocyte chemotactic protein-2 as a countercurrent principle. Am. J. Pathol. 2001;159:1405–1414. doi: 10.1016/S0002-9440(10)62527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J. Immunol. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch DR, Schissel DJ, Howrey RP, Aeed PA. Tumor-elicited polymorphonuclear cells, in contrast to “normal” circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5859–5863. doi: 10.1073/pnas.86.15.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente MN, Keane MP, Burdick MD, et al. Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Lett. 2006;241:221–227. doi: 10.1016/j.canlet.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Wislez M, Philippe C, Antoine M, et al. Upregulation of bronchioloalveolar carcinoma-derived C-X-C chemokines by tumor infiltrating inflammatory cells. Inflamm. Res. 2004;53:4–12. doi: 10.1007/s00011-003-1215-3. [DOI] [PubMed] [Google Scholar]
- Wolf M, Delgado MB, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur. J. Immunol. 1998;28:164–170. doi: 10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Shimizu S, Tokuyama S, Watanabe T, Kiuchi Y, Yamamoto T. A novel effect of polymorphonuclear leukocytes in the facilitation of angiogenesis. Life Sci. 2000;66:2113–2121. doi: 10.1016/s0024-3205(00)00537-3. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Shimizu S, Ohhinata K, et al. Differential roles of ICAM-1 and E-selectin in polymorphonuclear leukocyte-induced angiogenesis. Am. J. Physiol., Cell Physiol. 2002;282:C917–C925. doi: 10.1152/ajpcell.00223.2001. [DOI] [PubMed] [Google Scholar]
- Zijlstra A, Seandel M, Kupriyanova TA, et al. Pro-angiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood. 2005;107:317–327. doi: 10.1182/blood-2005-04-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]