Abstract
The expansion of the synovial lining of joints in rheumatoid arthritis (RA) necessitates an increase in the vascular supply to the synovium, to cope with the increased requirement for oxygen and nutrients. New blood vessel formation –‘angiogenesis’– is recognized as a key event in the formation and maintenance of the pannus in RA, suggesting that targeting blood vessels in RA may be an effective future therapeutic strategy. Although many pro-angiogenic factors have been demonstrated to be expressed in RA synovium, vascular endothelial growth factor (VEGF) has been demonstrated to a have a central involvement in the angiogenic process in RA. Nevertheless, it is unclear whether angiogenesis – whether driven by VEGF and/or other factors – should be considered as a ‘cause’ or ‘consequence’ of disease. This ongoing ‘chicken vs. egg’ debate is difficult, as even the success of angiogenesis inhibition in models of RA does not provide a direct answer to the question. This review will focus on the role of the vasculature in RA, and the contribution of different angiogenic factors in promoting disease. Although no data regarding the effectiveness of anti-angiogenic therapy in RA have been reported to date, the blockade of angiogenesis nevertheless looks to be a promising therapeutic avenue.
Keywords: arthritis, angiogenesis, VEGF
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease, with a prevalence of about 1% in most parts of the world. Patients present with pain and stiffness in multiple joints, although one-third of patients initially experience symptoms at just one location or at a few scattered sites. In the majority of patients, symptoms appear over weeks to months, starting in one joint and often accompanied by prodromal symptoms including anorexia, weakness, or fatigue. The disease course of RA may range from a brief, self-limiting oligoarticular illness with minimal joint damage, to a sustained polyarticular, synovial inflammation resulting in progressive cartilage destruction and erosion of bone. As the loss of bone and cartilage progresses, the joint surface becomes destroyed, impairing range of movement and leading to deformity. In terms of visible effects on hand appearance, the fingers in RA are typically deviated towards the little finger (ulnar deviation). Other common features in RA are Boutonniere deformity (the joint nearest the knuckle is permanently bent toward the palm while the furthest joint is bent away), swan neck deformity (in which the joint farthest from the knuckle is permanently bent toward the palm while the nearest joint is bent away from it), and the so-called ‘Z-thumb’ fixed flexion and subluxation at the metacarpophalangeal joint.
The onset age of RA is variable, ranging from children to individuals in their 90s, but the most common onset age is 40–50 years. In a proportion of RA patients, systemic and extra-articular features may be observed in addition to the characteristic joint changes, including anaemia, vasculitis, nodules in subcutaneous, pulmonary and sclera tissues, and interstitial inflammation in lungs as well as in exocrine salivary and lachrymal tissue. Furthermore, approximately half of RA patients have tendon involvement, and dorsal wrist swelling as a result of tenosynovitis can be the first presentation of the disease (Williamson & Feldon 1995). Proliferation of the synovial lining of tendons causes scarring and adhesion formation, and 50% of patients with tendon disease will also show synovial invasion into the tendon substance itself. This invasion is associated with multiple tendon ruptures and a poorer prognosis for long-term hand function (Ertel et al. 1988). Importantly, RA is associated with increased mortality, most probably because of the high frequency of cardiovascular disease (Gabriel et al. 2003; Kaplan 2006; Van Doornum et al. 2006). Recently, it was reported that the odds ratio for the risk of all-category stroke in RA was 1.64, and for the risk of ischaemic stroke was 2.66 (Nadareishvili et al. 2008). A study from Finland also reported that RA patients were at increased risk of dying of malignancies, as well as urogenital, gastrointestinal and respiratory diseases (Sihvonen et al. 2004). Depressive symptoms are highly associated with RA and may occur in nearly half of patients (Bruce 2008). It is generally considered that interactions between genetic factors, sex hormones, and possibly an infectious agent or other factor, are involved in initiating the autoimmune mechanism in RA. In 8–15% of patients, symptoms commence within a few days of a specific event, such as an infectious illness (Harris 1992). In England and Wales, there are between 250,000 and 500,000 RA patients, and hence the economic burden of musculoskeletal diseases such as RA and osteoarthritis (OA) is quite significant. For example, RA patients are more likely to stop working on health grounds than matched controls.
At the cellular level, RA is characterized by inflammation of the synovial tissue which lines joints and tendons. Normally the synovium is made up of a well-organized matrix containing proteoglycan aggregates. Within this structure are found the synovial cells (fibroblast- and macrophage-like), as well as a network of capillaries and lymphatic vessels. Between the cartilage and synovium is the synovial fluid, which nourishes and lubricates the joint. However, in RA the synovium becomes infiltrated by cells of lympho-haematopoietic origin, chiefly T-helper cells, B cells and macrophages. The synovial fluid increases in volume because of oedema, leading to joint swelling and pain. In addition, the synovium becomes thickened, from a layer of 1–2 cells to approximately 6–8 cells, and becomes locally invasive at the interface with cartilage and bone or tendon.
In the past, the traditional treatment of RA was represented by a pyramidal approach starting with non-steroidal anti-inflammatory drugs at the base of the pyramid and progressing to disease-modifying anti-rheumatic drugs (DMARD) such as gold, sulphasalazine and methotrexate (MTX). Despite such pharmacological interventions, up to 90% of patients with aggressive synovitis exhibited radiological evidence of bone erosion within 2 years of diagnosis, despite treatment. However, over the last 20 years, major advances in the understanding of the pathogenesis of RA, based on bench-bedside studies of human tissue and animal models of disease, have led to the identification of a number of new molecular targets for intervention. The first of these was tumour necrosis factor-α (TNF-α), which mediates many inflammatory and immunoregulatory activities relevant to the development of RA. TNF-α is present in synovial fluid, and in RA synovial membrane (Brennan et al. 1989). The observation of TNF-α expression in RA, inhibition by TNF-α antibody of cytokine production by ex vivo synovial cell cultures, and effectiveness of TNF-α blockade in murine arthritis, formed the basis of the hypothesis that TNF-α was a possible therapeutic target in RA (Feldmann & Maini 2002).
To date, three biological inhibitors of this cytokine have been approved for clinical use, and more than 1,500,000 patients have been treated with these inhibitors. Monoclonal antibodies such as infliximab and adalimumab bind to TNF-α with high affinity, while etanercept is a fusion protein containing soluble TNF receptor type II fragments linked to an immunoglobulin fragment. Infliximab was the first biological developed, and from the earliest trials has shown remarkable therapeutic efficacy (Maini et al. 1999; Maini & Taylor 2000). Infliximab was first approved in 1999 to be used with MTX for patients with RA who had inadequate response to MTX alone. Subsequently, the US Federal Drugs Administration (FDA) approved an expanded label for infliximab in combination with MTX as a first line regimen to treat patients with moderate to severe RA. This recommendation was based on the ASPIRE (Active Controlled Study of Patients Receiving Infliximab for Treatment of Rheumatoid Arthritis of Early Onset) study, which involved patients with RA of less than 3 years duration. In this study, patients in the infliximab groups had significantly fewer joints with new erosions after 1 year compared with controls, highlighting the impact of early intervention (Smolen et al. 2004).
Other newer generation TNF-α inhibitors are currently under trials. These include certolizumab (CDP870), a PEG-linked Fab fragment of a humanized TNF inhibitor monoclonal antibody, and golimumab (CNTO148), also a fully human anti-TNF-α monoclonal antibody. Golimumab with MTX was recently shown to reduce signs and symptoms of RA, with 61% of patients receiving golimumab plus MTX achieving an American College of Rheumatology (ACR) 20 response (defined as at least 20% improvement in tender joint count and 20% improvement in swollen joint count and at least a 20% improvement in three out of following five endpoints: patient pain assessment, patient global assessment, physician global assessment, patient self-addressed disability, erythrocyte sedimentation rate and C-reactive protein) at week 16 compared with 37% of patients in the placebo plus MTX group (Kay et al. 2008). Other cytokine targets include interleukin (IL)-2, with a humanized monoclonal antibody (daclizumab) targeted against the α-chain of the IL-2 receptor (CD25), which reduced disease severity in animal models of arthritis, and IL-6 (Williams et al. 2007). A humanized anti-IL-6 receptor antibody, tocilizumab (MRA), was tested in patients with RA, and was shown to reduce disease activity in a dose-dependent manner, also in combination with DMARD (Nishimoto et al. 2004; Genovese et al. 2008). An alternative approach involves blockade of T cell co-stimulatory pathways using cytotoxic T lymphocyte-associated antigen 4 (CTLA4)-based molecules such as abatacept. Patients with active RA and an inadequate response to TNF-α inhibitors who received abatacept plus at least one DMARD showed a better clinical improvement (ACR 20: 50.4%) compared with a placebo cohort (19.5%) at 6 months, with an improvement in physical function (Genovese et al. 2005). Abatacept was approved at the end of 2005 by the FDA for use in adult patients with moderately to severely active RA who have had an inadequate response to one or more DMARD, or TNF-α antagonists.
The advent of biological therapies, selectively targeting specific molecules, such as TNF-α, IL-6 or CTLA-4, has been a major advance in the treatment of RA. However, whereas TNF-α inhibitors such as infliximab, etanercept and adalimumab have demonstrated an acceptable safety profile, there is nonetheless an increased risk of minor infection and, more rarely, of serious infection such as tuberculosis (Keane et al. 2001; Bongartz et al. 2006). Thus, despite the clinical success of anti-cytokine biologicals, further initiatives in drug discovery are highly desirable with a view to achieve further improvements in the pharmacological management of RA. The proliferative and invasive nature of arthritic synovium has frequently led to comparisons with tumour development. Both the arthritic synovium and the growing tumour exhibit the apparently paradoxical features of hypoxia and angiogenesis, and both disease processes revolve around metabolically active cells undergoing uncontrolled proliferation and invasion within an altered pro-inflammatory micro-environment. The aggressive invasion of proliferating synovium causes joint destruction and deformities in RA, and in cancer, results in local spread and distant metastasis. This paradigm shift towards the concept of a ‘tumour-like phenotype’ in RA has made this disease – just like cancer – a potential target for anti-angiogenic therapy.
Angiogenesis: a central role in RA
Formation of new blood vessels from pre-existing vasculature (‘angiogenesis’) is important from the earliest stages of embryo development, right through to the adult stage, playing a key role in physiological processes such as healing of wounds or fractures and in the female reproductive cycle. Organization of blood vessels, in terms of time, space, and composition, needs to take into account the requirements of a particular organ or tissue, and appropriately adapt the type of mural cells and composition of sub-endothelial matrix. A range of different factors can promote angiogenesis, including fibroblast growth factor (FGF)-1 and FGF-2, angiopoietins, platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) (Ferrara et al. 2003; Roy et al. 2006).
Probably the first evidence of a pro-angiogenic phenotype in RA was the report in 1980 that synovial fluids from patients with RA contained a low molecular weight angiogenesis factor (Brown et al. 1980). This factor was most likely what is now described as endothelial cell-stimulating angiogenesis factor (ESAF), a low molecular weight non-peptide molecule with angiogenic activity. A subsequent review in 1982 suggested that in RA ‘microcirculatory compromise, concomitant with an increase in metabolic needs of synovial tissue, may initiate tissue injury via anoxia and acidosis, resulting in hydrolytic enzyme release, increased vascular permeability and acceleration of inflammatory processes’ (Rothschild & Masi 1982).
RA synovial fluids were subsequently shown to induce morphological changes in human vascular endothelial cells, with formation of tubule-like structures and induction of angiogenesis in an in vitro assay (Kumar et al. 1985; Semble et al. 1985). Changes in synovial blood vessel density and alterations in endothelial proliferative responses in RA have been demonstrated. For example, the number of synovial blood vessels has been found to correlate with synovial cell hyperplasia, mononuclear cell infiltration, and indices of joint tenderness (Rooney et al. 1988). A morphometric study has suggested that capillaries are distributed more deeply in RA synovium, compared with normal tissue, although the blood volume fraction was greater in normal knees relative to knees affected with RA (Stevens et al. 1991a,b). Another group noted that although perivascular mononuclear cell infiltration and increased thickness of the synovial lining layer were observed in tissue from both inflamed and non-inflamed joints of RA patients, vascular proliferation was seen only in tissues from inflamed joints (FitzGerald et al. 1991). Endothelial cells lining blood vessels within RA synovium have been shown to express cell cycle-associated antigens such as proliferating cell nuclear antigen (PCNA) and Ki67, and integrin αvβ3, which is associated with vascular proliferation (Ceponis et al. 1998). Endothelial proliferation and cell death indices were shown to be increased in synovium from patients with RA compared with controls or individuals with OA (Walsh et al. 1998).
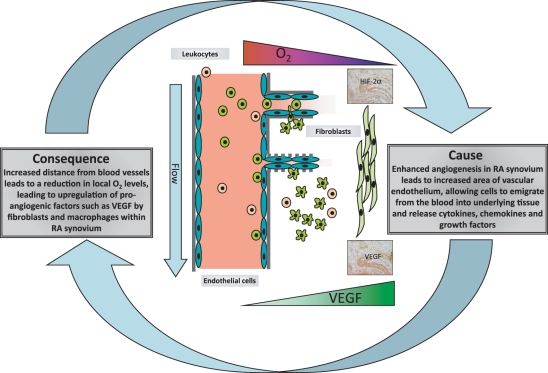
Taken together, these data suggested that alterations in the density and/or function of the blood vessels present in RA synovial tissue are a cause – or maybe consequence – of RA (Figure 1).
Figure 1.
The role of blood vessels in RA pathogenesis: cause and/or consequence. Figure shows infiltration of RA synovium by blood-derived cells, and the relationship between oxygen tension and VEGF release (by macrophage- and fibroblast like synovial cells). Typical expression patterns of VEGF and HIF-Zalpha (by immunohistochemistry) in RA synovium are illustrated.
Role of VEGF and other growth factors in regulating angiogenesis in rheumatic arthritis
The molecular characterization of key pro-angiogenic factors such as VEGF heralded the description of this and many other angiogenic growth factors in RA. The dual activities of VEGF – first as an endothelial cell mitogen and second, as a modulator of changes in vascular permeability – are both of relevance in the pathogenesis of RA. More than 10 years ago, the groups of Koch and Fava almost simultaneously reported VEGF expression in RA synovial fluids and tissue (Fava et al. 1994; Koch et al. 1994). In addition to synovial expression of VEGF, circulating (serum) levels of VEGF are increased, and correlate with inflammatory response markers such as C-reactive protein and swollen joint counts (Harada et al. 1998; Kikuchi et al. 1998; Paleolog et al. 1998; Lee et al. 2001; Sone et al. 2001b). VEGF levels are increased even in RA patients with disease duration of less than 2 years, and predict subsequent joint destruction, suggesting that angiogenesis may be an early event in RA progression (Ballara et al. 2001). We have reported a significant correlation between serum VEGF levels at presentation in untreated RA patients, and the change in hand and feet radiographs, taken at initial presentation and at 1 year follow-up and scored according to the van der Heijde modification of Sharp’s method (Ballara et al. 2001). Treatment of RA with TNF-α inhibitors (alone or with MTX), DMARD or anti-IL-6 receptor antibody significantly reduced serum VEGF concentrations (Paleolog et al. 1998; Nagashima et al. 2000; Ballara et al. 2001; Nakahara et al. 2003; Aggarwal et al. 2004; Macias et al. 2005).
It is especially relevant in the context of RA that VEGF is so powerfully up-regulated by hypoxia. In the context of tumours, hypoxia is a well-described phenomenon, arising from a hyperplastic response by the tumour cells, leading to an increased distance from pre-existing blood vessels. As arthritic synovium is also characterized by an altered proliferative response, it is not surprising that hypoxia is also thought to contribute to RA development. Around 30 years ago, Lund-Olesen demonstrated that mean synovial fluid oxygen tension in RA knee joints was as low as 27 mmHg, compared with 43 mmHg in osteoarthritis and 63 mmHg in traumatic effusions of otherwise healthy individuals (Lund-Olesen 1970). Subsequent studies supported these findings and even recorded oxygen tension values below 15 mmHg in humans (Treuhaft & McCarty 1971). Using silver microelectrodes, we recorded mean intra-articular oxygen tension values of 13 mmHg in mice with established arthritis (Etherington et al. 2002). More recently, our group has confirmed using a sensitive microelectrode technique that synovium in RA patients is more hypoxic than normal synovium (Sivakumar et al. 2008). We observed that median synovial oxygen tension in patients with RA was 6% (46 mmHg), compared with 10% (74 mmHg) in patients without RA. Furthermore, we studied patients with RA hand disease, and documented that invasive tenosynovium was significantly more hypoxic (median oxygen tension 3%, 26 mmHg) than either non-invasive tenosynovium or joint synovium in the same RA patients, suggesting that hypoxia might be driving invasion of tendon by the synovial tissue, and hence potentially promoting tendon rupture (Sivakumar et al. 2008). Contributory factors to hypoxia in RA joints include the high metabolic demands of inflamed synovial tissue and the rapid rate of synovial proliferation, so that cells become more distant from the closest blood vessels, compounding the hypoxic state. Other factors promoting raised intra-articular pressures as high as 300 mmHg include movement and accumulation of synovial fluid in involved joints (Jawed et al. 1997). This further compromises the vasculature and thus exacerbates hypoxia in an already ischaemic environment.
Tissue hypoxia has marked effects on genes involved in angiogenesis, apoptosis, vasomotor control, erythropoiesis and energy metabolism. A key regulator of the cellular response to oxygen is a transcription factor family known as hypoxia-inducible factor (HIF) (Semenza 2001). Approximately 1% of all human genes are regulated by HIF, including genes involved in angiogenesis, in VEGF, as well as apoptosis, vasomotor control, erythropoiesis and energy metabolism. HIF is a heterodimeric transcription factor, composed of two different subunits, HIF-α, which is oxygen-regulated, and HIF-β, which is expressed constitutively in the nucleus (Wang et al. 1995). There at least 2 α-sub-units, termed HIF-1α and HIF-2α. Regulation of HIF-dependent gene expression requires α-subunit accumulation in the cytoplasm and translocation into the nucleus, which enables it to dimerize with β-subunits of HIF. HIF heterodimers are then recognized by co-activators and bind to the hypoxia-response elements in the target gene to initiate transcription. A growing body of evidence implicates aberrant HIF regulation in angiogenesis as part of rheumatoid joint disease (Gaber et al. 2005), and indeed VEGF is a well-documented HIF-regulated gene. Several studies have shown that hypoxia is a potent stimulus for VEGF induction in ex vivo cultures of RA synovial membrane cells (Paleolog et al. 1998; Jain et al. 2006; Sivakumar et al. 2008). Two, closely related HIF-α isoforms (HIF-1α, HIF-2α) as well as VEGF, are expressed in human RA synovium (Hollander et al. 2001; Hitchon et al. 2002; Giatromanolaki et al. 2003), and also in experimental rat arthritis (Peters et al. 2004), suggesting that synovial hypoxia leads to up-regulation of HIF in the joint, accumulation of VEGF and induction of synovial angiogenesis. Targeted deletion of HIF-1α in myeloid cells impairs arthritis in an in vivo model of the disease (Cramer et al. 2003), further underlining the links between hypoxia and angiogenesis in RA. Hypoxia has been reported to cause a general down-regulation of gene expression in micro-array studies in murine fibroblasts. In human hepatoma cells, a significant up- or down regulation of 159 genes by hypoxia has been reported; of these 45 were upregulated and 112 were downregulated. Using HIF-1a null mouse fibroblasts, Greijer et al. were able to establish that of the genes that were upregulated in their study, 89% were dependent on HIF-1, as opposed to only 17% of the downregulated genes, supporting a role for HIF-1 in upregulating genes necessary for cell survival and adaptation to stress. On a larger scale using mouse fibroblasts and micro-array analysis, Greijer et al. were able to group hypoxia up-regulated genes into genes involved in glycolysis, and other metabolic genes, genes involved in cell mobility, and genes involved in apoptosis (Greijer et al. 2005). Recently, it has become apparent that HIF-1 and HIF-2 may exert different effects on cellular function. Despite high similarity with respect to protein sequence and activation pathway, a growing number of physiological and mechanistic differences between HIF-1 and HIF-2 are being reported. It has been shown that HIF-2, but not HIF-1, was responsible for renal cell carcinoma (RCC) growth in animals (Maranchie et al. 2002; Kondo et al. 2003). Blocking HIF-1 did not shrink tumours in that model. A panel of hypoxia-inducible genes is dependent on HIF-1α but not HIF-2α in endothelial and breast cancer cells (Sowter et al. 2003). It has been shown that in some cases RCC over-expression of HIF-2α accelerated tumour growth, whereas HIF-1α reduced tumour growth, suggesting that HIF-1α acts as a tumour suppressor, with HIF-2α being a tumour promoter (Raval et al. 2005). Similarly, hypoxia has been reported to promote cartilage matrix synthesis through HIF-2α-mediated induction of the transcription factor SOX9 and hence of cartilage genes (COL2A1 and COL9A1) (Lafont et al. 2007). It is thus clear that by having contrasting effects on regulation of HIF-target genes, HIF-1α and HIF-2α may differently contribute to RA.
In addition to VEGF, expression of angiopoietin (Ang)-1 and Ang-2 (Scott et al. 2002; Gravallese et al. 2003) and cognate receptors Tie-1 and Tie-2 (Uchida et al. 2000; Shahrara et al. 2002; DeBusk et al. 2003) in RA synovial tissue has been described. An interesting study documented that expression of Ang-2 and VEGF was higher in synovium of patients with psoriatic arthritis, relative to RA, whereas Ang-1 levels were more comparable. Psoriatic arthritis and RA exhibit different features in terms of vascular morphology, in that blood vessels in psoriatic synovium were highly tortuous in appearance, compared with the predominantly straight and branching vessels seen in RA, suggesting that the balance between Ang-1, Ang-2 and VEGF may affect vessel growth and maturation in arthritic synovium (Fearon et al. 2003). Fibroblasts from late stage RA have been reported to express elevated levels of Ang-1 and Ang-2 (Scott et al. 2002). Ang-1 is chemotactic and weakly mitogenic for endothelial cells (Davis et al. 1996; Witzenbichler et al. 1998), promotes formation of endothelial sprouts (DeBusk et al. 2004) and has been proposed to act in concert with VEGF to promote vascular network maturation (Asahara et al. 1998; Koblizek et al. 1998). Furthermore, Ang-1 was found to be a survival factor for endothelium, protecting endothelial cells from apoptosis induced by serum withdrawal, whereas Ang-2 is thought to result in vessel destabilization and regression (Papapetropoulos et al. 1999), suggesting that the Ang-Tie axis may play a multifaceted role in regulating angiogenesis in RA. Additionally, FGF-1 and FGF-2 have been detected in RA synovial tissue (Sano et al. 1990; Nakashima et al. 1994), together with PDGF (Remmers et al. 1991; Sano et al. 1993) and hepatocyte growth factor (HGF) (Koch et al. 1996). HGF may contribute to endothelial migration and angiogenesis in RA, as anti-HGF partially neutralized the chemotactic activity for endothelial cells found in RA synovial fluids (Koch et al. 1996). Patients with RA have elevated levels of HGF in the synovial fluid and serum, and these correlate with disease activity (Yukioka et al. 1994; Feuerherm et al. 2001).
Taken together, these observations led to the hypothesis that the vasculature may be a therapeutic target in RA.
Lessons from angiogenesis inhibition in cancer for future treatment of RA
The involvement of angiogenesis has made both cancer and RA a potential target for anti-angiogenic therapy. In particular, studies on the molecular and cellular mechanisms underlying cancer of the colon and rectum have made this type of cancer the first to be treated with angiogenesis inhibitors.
In 1971, Judah Folkman described the critical role of tumour angiogenesis to potentiate tumour growth and metastasis, and as a result angiogenesis has been a putative target for anti-cancer therapy since the 1970s (Folkman 1971). In particular, expression of VEGF is up-regulated in numerous solid malignancies, and interrupting the VEGF pathway has become a major focus of oncological research (Ferrara et al. 2003). The most successful anti-angiogenic approach is bevacizumab, a humanized IgG1 monoclonal anti-VEGF antibody, approved in 2004 as a combination with intra-venous 5-fluorouracil-based (5-FU) chemotherapy as a treatment for patients with previously untreated metastatic cancer of the colon or rectum. Colorectal cancer is the third most common cancer worldwide, with 307,432 new cases diagnosed in 2006 in the European Union alone. In the UK, colorectal cancer is the second leading cause of all cancer related deaths. In the original phase III clinical trial of 813 patients with untreated metastatic colorectal cancer, patients were randomized to receive irinotecan, 5-FU and leucovorin (IFL) alone, or in combination with bevacizumab at 5 mg/kg every 2 weeks. The group receiving additional bevacizumab had a longer median duration of survival, namely 20.3 months vs. 15.6 months i.e. a median survival benefit of 4.7 months, which while at first glance unremarkable, was in fact as large as or larger than that observed in any other phase III trial for the treatment of (metastatic) colorectal cancer. The progression-free survival was also increased (10.6 months vs. 6.2 months) (Hurwitz et al. 2004; Kabbinavar et al. 2005). More recently, bevacizumab was approved in combination with FOLFOX4 (5-FU, leucovorin, and oxaliplatin) for the second-line treatment of metastatic carcinoma of the colon or rectum. This was the result of a phase III trial using newer chemotherapeutic regimes, which confirmed improved overall survival in patients receiving FOLFOX-4 in combination with bevacizumab (12.5 months), relative to FOLFOX-4 alone (10.7 months) (Cohen et al. 2007).
Bevacizumab is now also approved in combination with carboplatin and paclitaxel, for first-line treatment of patients with unresectable, locally advanced, recurrent, or metastatic non-squamous non-small cell lung cancer (NSCLC). Moreover, in February 2008, accelerated approval was granted for bevacizumab in combination with paclitaxel, for the treatment of patients with metastatic HER2-negative breast cancer who have not received chemotherapy. The approval for breast cancer came after a phase III study that showed that bevacizumab in combination with paclitaxel chemotherapy resulted in a 52% reduction in the risk of disease progression or death compared with those treated with paclitaxel alone and a doubling in progression-free survival. Results from ongoing trials in patients with previously untreated (RiBBON 1) and treated (RiBBON 2) metastatic breast cancer are awaited for the accelerated approval to be converted into full approval.
While bevacizumab has been a major step forward in the treatment of some cancers, VEGF has a critical physiological role in vivo. This is highlighted by the heterozygous lethality of VEGF knock-out mice (Carmeliet et al. 1996; Ferrara et al. 1996). In colorectal patients receiving bevacizumab, out of the 402 patients assigned to receive IFL and bevacizumab, six patients (1.5%) developed perforation of the gastrointestinal tract as opposed to none in the IFL-alone control arm. Similar results were also observed in the First BEAT trial (Berry et al. 2006), established to evaluate the safety profile of bevacizumab, in which gastrointestinal perforations were reported in 1.2% of patients receiving bevacizumab. These events are for the most part mild-moderate in severity and clinically manageable. Increased thromboembolic events have been reported, as has hypertension, although the latter is generally manageable using standard anti-hypertensive approaches, as have. For example, in a clinical trial in patients with NSCLC, comparison of bevacizumab plus carboplatin and paclitaxel vs. carboplatin and paclitaxel alone, severe and life-threatening adverse events occurred more frequently in patients receiving bevacizumab, namely, hypertension (8%vs. 0.7%) and thrombosis/embolism (5%vs. 3%). Fatal, treatment-related adverse events in patients receiving bevacizumab included pulmonary haemorrhage, gastrointestinal haemorrhage and myocardial infarction (Cohen et al. 2007). The induction by VEGF of the vasodilatior nitric oxide (NO), through the activation of endothelial NO synthase, may underlie the apparent hypertension and increased risk of thromboembolic events associated with bevacizumab. Nevertheless, even patients over 65 years of age with a high likelihood of thromboembolic events may benefit from bevacizumab, and clearly in such situations the patient risk-benefit ratio needs to be weighed up. The importance of angiogenesis in wound-healing suggests that bevacizumab might impair this process, which would have significant implications for patients undergoing surgery. While slower wound-healing is listed as a possible side-effect, a study found that bevacizumab administered in combination with 5-FU/leucovorin-based chemotherapy 28–60 days after primary cancer surgery caused no increased risk of wound-healing complications compared with chemotherapy alone. Nonetheless, wound-healing complications were increased in patients who had major surgery during bevacizumab therapy (13% bevacizumab-treated patients vs. 3.4% control patients) (Scappaticci et al. 2007). Proteinuria has also been reported, suggesting possible renal side-effects, and discontinuation of bevacizumab is recommended if marked proteinuria persists. Finally, VEGF has important neuroprotective effects, which were highlighted by a study showing that a targeted reduction in VEGF expression in the mouse spinal cord was associated with adult-onset progressive motor neuron degeneration, reminiscent of amyotrophic lateral sclerosis (Oosthuyse et al. 2001).
The first generation of angiogenesis inhibitors has clearly revolutionized our approach in the management of advanced colorectal cancer. The success story of bevacizumab provides direction and encouragement for the potential use of angiogenesis blockade in other diseases.
Potential for anti-angiogenic therapy in RA
The relative success of VEGF blockade in cancer prompted much speculation that RA might also be a potential target for angiogenesis inhibitors. Rodent models have been used extensively to study the mechanisms underlying the angiogenic process in arthritic diseases and to develop new therapeutic interventions, including those based on inhibition of VEGF. Arthritis can be induced in genetic susceptible mouse strains by immunization with type II collagen, resulting in an autoimmune response against autoantigens in the articular cartilage and eventually leading to a destructive polyarthritis. Heterologous collagen-induced arthritis (CIA) in mice shares many features with RA, including linkage to the major histocompatibility region, infiltration of synovium by blood-derived cells, synovial hyperplasia, pannus formation, angiogenesis, as well as destruction of cartilage and bone. This model has been widely used to study mechanisms involved in the arthritic process and to identify new strategies for RA treatment, such as TNF-α inhibitors. While no animal model of disease is ideal, heterologous CIA has been used extensively to investigate new therapeutic targets, in part attributable to the success of this model in predicting the success of TNF-α blockade (Williams et al. 1994, 1995, 2000). Moreover, we and others have shown that inhibition of angiogenesis ameliorates disease (Kim et al. 2002; Sumariwalla et al. 2003; Bainbridge et al. 2007).
In terms of specific targeting VEGF in disease models, Lu et al. showed that VEGF and its receptors are expressed during the development of murine CIA, and the level of VEGF expression correlated with disease severity and the degree of neovascularization. Neutralization of VEGF activity by administration of anti-VEGF antibody delayed disease onset, but appeared less effective when administered during the chronic phase of disease (Lu et al. 2000). In another study, application of anti-VEGF treatment in established bovine CIA inhibited synovitis, as indicated by a reduction in clinical scores and paw-swelling relative to untreated mice (Sone et al. 2001a). A soluble form of VEGF receptor (VEGFR)-1, a naturally occurring antagonist of VEGF, has been shown to significantly suppress established arthritis. Mice that received adenoviral vectors expressing human soluble VEGFR-1 after the onset of arthritis showed reductions in the extent and severity of disease (assessed as the clinical score), and decreased paw-swelling and joint destruction, when compared with control animals. Furthermore, decreased levels of VEGF were observed in ankle lysates of these animals (Miotla et al. 2000; Afuwape et al. 2003; Jin et al. 2008). A different strategy to limit angiogenesis via the VEGF pathway was to directly target VEGFR. In a spontaneous model of arthritis in KRN/NOD mice, De Bandt et al. observed that treatment with anti-VEGFR-1 (but not anti-VEGFR-2) antibody abrogated bone and cartilage destruction. The antibody delayed the onset of arthritis and attenuated the severity of disease (de Bandt et al. 2003). The group of Carmeliet also compared different approaches targeting VEGF (using anti-VEGFR-1 and anti-VEGFR-2 antibodies) in chicken CIA in mice. Treatment with anti-VEGFR-1 reduced the incidence of joint disease by 60%, and suppressed the development of clinical symptoms by 85%, whereas anti-VEGFR-2 appeared ineffective (Luttun et al. 2002). The reason underlying the effectiveness of VEGFR-1 blockade as compared with VEGFR-2-targeted approaches is unclear, although one possible explanation put forward is that VEGF, via VEGFR-1, also promotes monocyte- and neutrophil trafficking (Clauss et al. 1990, 1996). Thus, inhibition of VEGFR-1 would, in addition to affecting vessel growth, reduce cell trafficking to the RA synovium. The VEGFR tyrosine kinase inhibitor PTK787/ZK222584 has also been shown to be effective in arthritis models (Grosios et al. 2004). Knockout of the VEGF-B gene also reduced angiogenesis in Vegf-B−/− mice, and attenuated both collagen-induced and adjuvant-induced arthritis, suggesting that VEGF-B contributes to the angiogenic process in RA (Mould et al. 2003). However, while targeting VEGF in models of disease has been effective, the side-effects of therapies such as bevacizumab include increased risk of thromboembolic events. It has already been highlighted that RA is associated with increased frequency of cardiovascular disease (Gabriel et al. 2003; Kaplan 2006; Van Doornum et al. 2006). The complex nature of RA makes it difficult to predict the consequences of VEGF inhibition in vivo, although in the future combining animal models such as CIA with atherosclerosis models such as apoE−/− mice should yield some information on whether this might be a useful therapeutic approach in human disease.
In terms of other angiogenic targets for RA therapy, we have recently reported that a splice variant of Tie-1 markedly reduced disease severity in murine CIA. Angiopoietin signalling was until recently considered to be mediated via Tie-2. However, the embryonic lethality of Tie-1 knockout mice suggested that Tie-1 signalling is important in vascular network formation. It is now thought that Tie-1 may modulate signalling through Tie-2 (Kontos et al. 2002; Saharinen et al. 2005; Yuan et al. 2007). It was reported that activation of Tie-1 ectodomain cleavage increased activation of Tie-2, which could potentially control signalling via Tie-2 (Marron et al. 2007). It is therefore of interest that Tie-1 may modulate CIA, and we believe that ours is the first demonstration that inhibition of the Ang-Tie axis can markedly reduce arthritis severity. (Jin et al. 2008). In addition, a competitive HGF antagonist, NK4, which binds to the c-Met receptor, but does not induce tyrosine phosphorylation of c-Met, has been described (Date et al. 1997). Interestingly, NK4 is able to inhibit angiogenesis not only induced by HGF, but also by other pro-angiogenic factors like FGF-2 and VEGF (Kuba et al. 2000; Nakabayashi et al. 2003; Matsumoto & Nakamura 2005), but its efficacy in RA has not been assessed. However, the role of HGF in RA may be complex, as evidenced by the report that HGF suppresses CIA in mice (Okunishi et al. 2007).
Conclusions
There has been a phenomenal interest in the development of inhibitors of angiogenesis, which represent a major success in the fight against cancer. However while the spotlight has been on malignancies, the many parallels between tumour growth and RA reinforce the hypothesis that inhibition of angiogenesis might also potentially be effective in RA. The question of ‘cause or consequence’– that is, whether angiogenesis drives RA or whether the enhanced synovial proliferation promotes angiogenesis – is a difficult one, to say the least, as angiogenesis, hypoxia and synovial expansion are so intricately linked (Figure 1). As briefly mentioned, the thromboembolic complications of bevacizumab in cancer patients raise the question of the side-effects of VEGF inhibition in RA, and more sophisticated animal model studies are needed before such concerns are addressed. Anti-angiogenic therapy is not likely to ever be a replacement for treatments such as TNF inhibitors, rituximab, abatacept, tocilizumab, daclizumab or others. Instead, use of anti-angiogenic therapy is likely as an adjunct, thus allowing concomitant targeting of the inflammatory cascade (using cytokine- or T-cell/B-cell-targeted approaches) and blood vessel formation, hence ‘starving’ the synovium of nutrients and oxygen. Unfortunately, only one clinical trial has been initiated so far in RA using involving anti-αvβ3 antibody, which closed early because of lack of benefit. In terms of RA patients, patients with RA of less than 3-year duration show marked synovial thickening and vascular signal, suggesting these may go hand-in-hand (Taylor et al. 2006). It is possible to use in vivo models to address this question, in that animals can be followed from the induction of disease. In an interesting study in murine CIA, in vivo fluorescence microscopy of knee using labelled-dextran joints revealed marked leucocyte activation and interaction with synovial microvessels before onset of clinical symptoms of arthritis (Gierer et al. 2005). We and others are currently using more sophisticated and specific in vivo imaging approaches to determine whether the alterations in the vasculature precede synovial thickening in CIA. Either way, however, the unique role of angiogenesis in RA supports the premise that this process is an exciting target for therapy in RA, whether using bevacizumab, or newer generation angiogenesis inhibitors.
References
- Afuwape AO, Feldmann M, Paleolog EM. Adenoviral delivery of soluble VEGF receptor 1 (sFlt-1) abrogates disease activity in murine collagen-induced arthritis. Gene Ther. 2003;10:1950–1960. doi: 10.1038/sj.gt.3302104. [DOI] [PubMed] [Google Scholar]
- Aggarwal A, Panda S, Misra R. Effect of etanercept on matrix metalloproteinases and angiogenic vascular endothelial growth factor: a time kinetic study. Ann. Rheum. Dis. 2004;63:891–892. doi: 10.1136/ard.2003.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ. Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Bainbridge J, Madden L, Essex D, Binks M, Malhotra R, Paleolog EM. Methionine aminopeptidase-2 blockade reduces chronic collagen-induced arthritis: potential role for angiogenesis inhibition. Arthritis Res. Ther. 2007;9:R127. doi: 10.1186/ar2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballara SC, Taylor PC, Reusch P, et al. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 2001;44:2055–2064. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- de Bandt M, Ben Mahdi MH, Ollivier V, et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J. Immunol. 2003;171:4853–4859. doi: 10.4049/jimmunol.171.9.4853. [DOI] [PubMed] [Google Scholar]
- Berry S, Cunningham D, Michael M, et al. Preliminary safety of bevacizumab with first-line Folfox, Capox, Folfiri and capecitabine for mCRC–First B.E.A.Trial. J. Clin. Oncol. 2006;24:3534. ASCO Annual Meeting Proceedings Part I. [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Brown RA, Weiss JB, Tomlinson IW, Phillips P, Kumar S. Angiogenic factor from synovial fluid resembling that from tumours. Lancet. 1980;1:682–685. [PubMed] [Google Scholar]
- Bruce TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr. Psychiatry Rep. 2008;10:258–264. doi: 10.1007/s11920-008-0042-1. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Ceponis A, Konttinen YT, Imai S, et al. Synovial lining, endothelial and inflammatory mononuclear cell proliferation in synovial membranes in psoriatic and reactive arthritis: a comparative quantitative morphometric study. Br. J. Rheumatol. 1998;37:170–178. doi: 10.1093/rheumatology/37.2.170. [DOI] [PubMed] [Google Scholar]
- Clauss M, Gerlach M, Gerlach H, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J. Exp. Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M, Weich H, Breier G, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997;420:1–6. doi: 10.1016/s0014-5793(97)01475-0. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- DeBusk LM, Chen Y, Nishishita T, Chen J, Thomas JW, Lin PC. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2003;48:2461–2471. doi: 10.1002/art.11213. [DOI] [PubMed] [Google Scholar]
- DeBusk LM, Hallahan DE, Lin PC. Akt is a major angiogenic mediator downstream of the Ang1/Tie2 signaling pathway. Exp. Cell Res. 2004;298:167–177. doi: 10.1016/j.yexcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Ertel AN, Millender LH, Nalebuff E, McKay D, Leslie B. Flexor tendon ruptures in patients with rheumatoid arthritis. J. Hand Surg. [Am.] 1988;13:860–866. doi: 10.1016/0363-5023(88)90260-2. [DOI] [PubMed] [Google Scholar]
- Etherington PJ, Winlove P, Taylor P, Paleolog E, Miotla JM. VEGF release is associated with reduced oxygen tensions in experimental inflammatory arthritis. Clin. Exp. Rheumatol. 2002;20:799–805. [PubMed] [Google Scholar]
- Fava RA, Olsen NJ, Spencer-Green G, et al. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J. Exp. Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon U, Griosios K, Fraser A, et al. Angiopoietins, growth factors, and vascular morphology in early arthritis. J. Rheumatol. 2003;30:260–268. [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Discovery of TNF-alpha as a therapeutic target in rheumatoid arthritis: preclinical and clinical studies. Joint Bone Spine. 2002;69:12–18. doi: 10.1016/s1297-319x(01)00335-9. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Feuerherm AJ, Borset M, Seidel C, et al. Elevated levels of osteoprotegerin (OPG) and hepatocyte growth factor (HGF) in rheumatoid arthritis. Scand. J. Rheumatol. 2001;30:229–234. doi: 10.1080/030097401316909585. [DOI] [PubMed] [Google Scholar]
- FitzGerald O, Soden M, Yanni G, Robinson R, Bresnihan B. Morphometric analysis of blood vessels in synovial membranes obtained from clinically affected and unaffected knee joints of patients with rheumatoid arthritis. Ann. Rheum. Dis. 1991;50:792–796. doi: 10.1136/ard.50.11.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann. Rheum. Dis. 2005;64:971–980. doi: 10.1136/ard.2004.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, Crowson CS, Kremers HM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–58. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Sivridis E, Maltezos E, et al. Upregulated hypoxia inducible factor-1alpha and -2alpha pathway in rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2003;5:R193–R201. doi: 10.1186/ar756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierer P, Ibrahim S, Mittlmeier T, et al. Gene expression profile and synovial microcirculation at early stages of collagen-induced arthritis. Arthritis Res. Ther. 2005;7:R868–R876. doi: 10.1186/ar1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese EM, Pettit AR, Lee R, et al. Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tumour necrosis factor alpha. Ann. Rheum. Dis. 2003;62:100–107. doi: 10.1136/ard.62.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greijer AE, van der Groep P, Kemming D, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J. Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- Grosios K, Wood J, Esser R, Raychaudhuri A, Dawson J. Angiogenesis inhibition by the novel VEGF receptor tyrosine kinase inhibitor, PTK787/ZK222584, causes significant anti-arthritic effects in models of rheumatoid arthritis. Inflamm. Res. 2004;53:133–142. doi: 10.1007/s00011-003-1230-4. [DOI] [PubMed] [Google Scholar]
- Harada M, Mitsuyama K, Yoshida H, et al. Vascular endothelial growth factor in patients with rheumatoid arthritis. Scand. J. Rheumatol. 1998;27:377–380. doi: 10.1080/03009749850154429. [DOI] [PubMed] [Google Scholar]
- Harris ED., Jr Excitement in synovium: the rapid evolution of understanding of rheumatoid arthritis and expectations for therapy. J. Rheumatol. Suppl. 1992;32:3–5. [PubMed] [Google Scholar]
- Hitchon C, Wong K, Ma G, Reed J, Lyttle D, El-Gabalawy H. Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum. 2002;46:2587–2597. doi: 10.1002/art.10520. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Corke KP, Freemont AJ, Lewis CE. Expression of hypoxia-inducible factor 1alpha by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 2001;44:1540–1544. doi: 10.1002/1529-0131(200107)44:7<1540::AID-ART277>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Jain A, Kiriakidis S, Brennan F, Sandison A, Paleolog E, Nanchahal J. Targeting rheumatoid tenosynovial angiogenesis with cytokine inhibitors. Clin. Orthop. Relat. Res. 2006;446:268–277. doi: 10.1097/01.blo.0000205909.89845.f6. [DOI] [PubMed] [Google Scholar]
- Jawed S, Gaffney K, Blake DR. Intra-articular pressure profile of the knee joint in a spectrum of inflammatory arthropathies. Ann. Rheum. Dis. 1997;56:686–689. doi: 10.1136/ard.56.11.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Zhang J, Sumariwalla PS, et al. Novel splice variants derived from the receptor tyrosine kinase superfamily are potential therapeutics for rheumatoid arthritis. Arthritis Res. Ther. 2008;10:R73. doi: 10.1186/ar2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J. Clin. Oncol. 2005;23:3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- Kaplan MJ. Cardiovascular disease in rheumatoid arthritis. Curr. Opin. Rheumatol. 2006;18:289–297. doi: 10.1097/01.bor.0000218951.65601.bf. [DOI] [PubMed] [Google Scholar]
- Kay J, Matteson EL, Dasgupta B, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:964–975. doi: 10.1002/art.23383. [DOI] [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Kubo M, Kadono T, Yazawa N, Ihn H, Tamaki K. Serum concentrations of vascular endothelial growth factor in collagen diseases. Br. J. Dermatol. 1998;139:1049–1051. doi: 10.1046/j.1365-2133.1998.02563.x. [DOI] [PubMed] [Google Scholar]
- Kim JM, Ho SH, Park EJ, et al. Angiostatin gene transfer as an effective treatment strategy in murine collagen-induced arthritis. Arthritis Rheum. 2002;46:793–801. doi: 10.1002/art.10113. [DOI] [PubMed] [Google Scholar]
- Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- Koch AE, Harlow LA, Haines GK, et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J. Immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- Koch AE, Halloran MM, Hosaka S, et al. Hepatocyte growth factor. A cytokine mediating endothelial migration in inflammatory arthritis. Arthritis Rheum. 1996;39:1566–1575. doi: 10.1002/art.1780390917. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos CD, Cha EH, York JD, Peters KG. The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis. Mol. Cell. Biol. 2002;22:1704–1713. doi: 10.1128/MCB.22.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000;60:6737–6743. [PubMed] [Google Scholar]
- Kumar P, Erroi A, Sattar A, Kumar S. Weibel-Palade bodies as a marker for neovascularization induced by tumor and rheumatoid angiogenesis factors. Cancer Res. 1985;45:4339–4348. [PubMed] [Google Scholar]
- Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297–3306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]
- Lee SS, Joo YS, Kim WU, et al. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2001;19:321–324. [PubMed] [Google Scholar]
- Lu J, Kasama T, Kobayashi K, et al. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J. Immunol. 2000;164:5922–5927. doi: 10.4049/jimmunol.164.11.5922. [DOI] [PubMed] [Google Scholar]
- Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis Rheum. 1970;13:769–776. doi: 10.1002/art.1780130606. [DOI] [PubMed] [Google Scholar]
- Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- Macias I, Garcia-Perez S, Ruiz-Tudela M, Medina F, Chozas N, Giron-Gonzalez JA. Modification of pro- and antiinflammatory cytokines and vascular-related molecules by tumor necrosis factor-a blockade in patients with rheumatoid arthritis. J. Rheumatol. 2005;32:2102–2108. [PubMed] [Google Scholar]
- Maini RN, Taylor PC. Anti-cytokine therapy for rheumatoid arthritis. Annu. Rev. Med. 2000;51:207–229. doi: 10.1146/annurev.med.51.1.207. [DOI] [PubMed] [Google Scholar]
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–255. doi: 10.1016/s1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- Marron MB, Singh H, Tahir TA, et al. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor-tyrosine kinase Tie2. J. Biol. Chem. 2007;282:30509–30517. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Mechanisms and significance of bifunctional NK4 in cancer treatment. Biochem. Biophys. Res. Commun. 2005;333:316–327. doi: 10.1016/j.bbrc.2005.05.131. [DOI] [PubMed] [Google Scholar]
- Miotla J, Maciewicz R, Kendrew J, Feldmann M, Paleolog E. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab. Invest. 2000;80:1195–1205. doi: 10.1038/labinvest.3780127. [DOI] [PubMed] [Google Scholar]
- Mould AW, Tonks ID, Cahill MM, et al. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 2003;48:2660–2669. doi: 10.1002/art.11232. [DOI] [PubMed] [Google Scholar]
- Nadareishvili Z, Michaud K, Hallenbeck JM, Wolfe F. Cardiovascular, rheumatologic, and pharmacologic predictors of stroke in patients with rheumatoid arthritis: a nested, case-control study. Arthritis Rheum. 2008;59:1090–1096. doi: 10.1002/art.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M, Wauke K, Hirano D, et al. Effects of combinations of anti-rheumatic drugs on the production of vascular endothelial growth factor and basic fibroblast growth factor in cultured synoviocytes and patients with rheumatoid arthritis. Rheumatology (Oxford) 2000;39:1255–1262. doi: 10.1093/rheumatology/39.11.1255. [DOI] [PubMed] [Google Scholar]
- Nakabayashi M, Morishita R, Nakagami H, et al. HGF/NK4 inhibited VEGF-induced angiogenesis in in vitro cultured endothelial cells and in vivo rabbit model. Diabetologia. 2003;46:115–123. doi: 10.1007/s00125-002-0954-y. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Song J, Sugimoto M, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521–1529. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Eguchi K, Aoyagi T, et al. Expression of basic fibroblast growth factor in synovial tissues from patients with rheumatoid arthritis: detection by immunohistological staining and in situ hybridisation. Ann. Rheum. Dis. 1994;53:45–50. doi: 10.1136/ard.53.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- Okunishi K, Dohi M, Fujio K, et al. Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J. Immunol. 2007;179:5504–5513. doi: 10.4049/jimmunol.179.8.5504. [DOI] [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- Paleolog EM, Young S, Stark AC, McCloskey RV, Feldmann M, Maini RN. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998;41:1258–1265. doi: 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab. Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- Peters CL, Morris CJ, Mapp PI, Blake DR, Lewis CE, Winrow VR. The transcription factors hypoxia-inducible factor 1alpha and Ets-1 colocalize in the hypoxic synovium of inflamed joints in adjuvant-induced arthritis. Arthritis Rheum. 2004;50:291–296. doi: 10.1002/art.11473. [DOI] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers EF, Sano H, Lafyatis R, et al. Production of platelet derived growth factor B chain (PDGF-B/c-sis) mRNA and immunoreactive PDGF B-like polypeptide by rheumatoid synovium: coexpression with heparin binding acidic fibroblast growth factor-1. J. Rheumatol. 1991;18:7–13. [PubMed] [Google Scholar]
- Rooney M, Condell D, Quinlan W, et al. Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988;31:956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- Rothschild BM, Masi AT. Pathogenesis of rheumatoid arthritis: a vascular hypothesis. Semin. Arthritis Rheum. 1982;12:11–31. doi: 10.1016/0049-0172(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Kerkela K, Ekman N, et al. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J. Cell Biol. 2005;169:239–243. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Forough R, Maier JA, et al. Detection of high levels of heparin binding growth factor-1 (acidic fibroblast growth factor) in inflammatory arthritic joints. J. Cell Biol. 1990;110:1417–1426. doi: 10.1083/jcb.110.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Engleka K, Mathern P, et al. Coexpression of phosphotyrosine-containing proteins, platelet-derived growth factor-B, and fibroblast growth factor-1 in situ in synovial tissues of patients with rheumatoid arthritis and Lewis rats with adjuvant or streptococcal cell wall arthritis. J. Clin. Invest. 1993;91:553–565. doi: 10.1172/JCI116235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J. Natl. Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- Scott BB, Zaratin PF, Colombo A, Hansbury MJ, Winkler JD, Jackson JR. Constitutive expression of angiopoietin-1 and -2 and modulation of their expression by inflammatory cytokines in rheumatoid arthritis synovial fibroblasts. J. Rheumatol. 2002;29:230–239. [PubMed] [Google Scholar]
- Semble EL, Turner RA, McCrickard EL. Rheumatoid arthritis and osteoarthritis synovial fluid effects on primary human endothelial cell cultures. J. Rheumatol. 1985;12:237–241. [PubMed] [Google Scholar]
- Semenza GL. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Shahrara S, Volin MV, Connors MA, Haines GK, Koch AE. Differential expression of the angiogenic Tie receptor family in arthritic and normal synovial tissue. Arthritis Res. 2002;4:201–208. doi: 10.1186/ar407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen S, Korpela M, Laippala P, Mustonen J, Pasternack A. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand. J. Rheumatol. 2004;33:221–227. doi: 10.1080/03009740410005845. [DOI] [PubMed] [Google Scholar]
- Sivakumar B, Akhavani MA, Winlove CP, Taylor PC, Paleolog EM, Kang N. Synovial hypoxia as a cause of tendon rupture in rheumatoid arthritis. J. Hand Surg. [Am.] 2008;33:49–58. doi: 10.1016/j.jhsa.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Smolen J, van der Heijde D, Devogelaer J-P, et al. The Effect of Infliximab Therapy on the Prevention of New Erosions in Patients with Early Rheumatoid Arthritis. Arthritis Rheum. 2004;50(Suppl. 9):Abstract 357. [Google Scholar]
- Sone H, Kawakami Y, Sakauchi M, et al. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochem. Biophys. Res. Commun. 2001a;281:562–568. doi: 10.1006/bbrc.2001.4395. [DOI] [PubMed] [Google Scholar]
- Sone H, Sakauchi M, Takahashi A, et al. Elevated levels of vascular endothelial growth factor in the sera of patients with rheumatoid arthritis correlation with disease activity. Life Sci. 2001b;69:1861–1869. doi: 10.1016/s0024-3205(01)01264-4. [DOI] [PubMed] [Google Scholar]
- Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- Stevens CR, Blake DR, Merry P, Revell PA, Levick JR. A comparative study by morphometry of the microvasculature in normal and rheumatoid synovium. Arthritis Rheum. 1991a;34:1508–1513. doi: 10.1002/art.1780341206. [DOI] [PubMed] [Google Scholar]
- Stevens CR, Williams RB, Farrell AJ, Blake DR. Hypoxia and inflammatory synovitis: observations and speculation. Ann. Rheum. Dis. 1991b;50:124–132. doi: 10.1136/ard.50.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumariwalla P, Cao Y, Wu H, Feldmann M, Paleolog E. The angiogenesis inhibitor protease-activated kringles 1-5 reduces the severity of murine collagen-induced arthritis. Arthritis Res. Ther. 2003;5:R32–R39. doi: 10.1186/ar608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Steuer A, Gruber J, et al. Ultrasonographic and radiographic results from a two-year controlled trial of immediate or one-year-delayed addition of infliximab to ongoing methotrexate therapy in patients with erosive early rheumatoid arthritis. Arthritis Rheum. 2006;54:47–53. doi: 10.1002/art.21544. [DOI] [PubMed] [Google Scholar]
- Treuhaft PS, McCarty D. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 1971;14:475–484. doi: 10.1002/art.1780140407. [DOI] [PubMed] [Google Scholar]
- Uchida T, Nakashima M, Hirota Y, Miyazaki Y, Tsukazaki T, Shindo H. Immunohistochemical localisation of protein tyrosine kinase receptors Tie-1 and Tie-2 in synovial tissue of rheumatoid arthritis: correlation with angiogenesis and synovial proliferation. Ann. Rheum. Dis. 2000;59:607–614. doi: 10.1136/ard.59.8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doornum S, Brand C, King B, Sundararajan V. Increased case fatality rates following a first acute cardiovascular event in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2061–2068. doi: 10.1002/art.21932. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Wade M, Mapp PI, Blake DR. Focally regulated endothelial proliferation and cell death in human synovium. Am. J. Pathol. 1998;152:691–702. [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RO, Mason LJ, Feldmann M, Maini RN. Synergy between anti-CD4 and anti-tumor necrosis factor in the amelioration of established collagen-induced arthritis. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2762–2766. doi: 10.1073/pnas.91.7.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RO, Ghrayeb J, Feldmann M, Maini RN. Successful therapy of collagen-induced arthritis with TNF receptor-IgG fusion protein and combination with anti-CD4. Immunology. 1995;84:433–439. [PMC free article] [PubMed] [Google Scholar]
- Williams RO, Marinova-Mutafchieva L, Feldmann M, Maini RN. Evaluation of TNF-alpha and IL-1 blockade in collagen-induced arthritis and comparison with combined anti-TNF-alpha/anti-CD4 therapy. J. Immunol. 2000;165:7240–7245. doi: 10.4049/jimmunol.165.12.7240. [DOI] [PubMed] [Google Scholar]
- Williams RO, Paleolog E, Feldmann M. Cytokine inhibitors in rheumatoid arthritis and other autoimmune diseases. Curr. Opin. Pharmacol. 2007;7:1–6. doi: 10.1016/j.coph.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Williamson SC, Feldon P. Extensor tendon ruptures in rheumatoid arthritis. Hand Clin. 1995;11:449–459. [PubMed] [Google Scholar]
- Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J. Biol. Chem. 1998;273:18514–18521. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- Yuan HT, Venkatesha S, Chan B, et al. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 2007;21:3171–3183. doi: 10.1096/fj.07-8487com. [DOI] [PubMed] [Google Scholar]
- Yukioka K, Inaba M, Furumitsu Y, et al. Levels of hepatocyte growth factor in synovial fluid and serum of patients with rheumatoid arthritis and release of hepatocyte growth factor by rheumatoid synovial fluid cells. J. Rheumatol. 1994;21:2184–2189. [PubMed] [Google Scholar]