Abstract
The role of homocysteine, or its precursor methionine, in the formation of fibrous caps and its association with endoplasmic reticulum (ER) stress is unclear. Homocysteine can stimulate collagen accumulation and upregulate the ER stress chaperone glucose regulated protein 78 (GRP78). The aim of this study was to determine if high dietary methionine would increase fibrous caps, and that removal of an atherogenic diet would decrease the amount of ER stressed cells. New Zealand white rabbits were fed for 2, 4, or 12 weeks an atherogenic diet [1% methionine + 0.5% cholesterol (2MC, 4MC or 12MC)]; for 4 or 12 weeks a 0.5% cholesterol diet (4Ch, 12Ch); and to study plaque regression, an MC diet for 2 or 4 weeks accompanied by 10 weeks of a normal diet (2MCr, 4MCr). Endothelial function, atherosclerosis and GRP78 positive cells were studied. Endothelial function was abolished in 4MC and atherosclerosis increased 17-fold (P < 0.05) compared with 4Ch. Fibrous caps composed 48% of total plaque area in 12MC vs. 10% in 12Ch (P < 0.01), and 12MC expressed less GRP78 plaque cells vs. 12Ch (P < 0.01). Four MCr had less plaque GRP78 cells than 12MC (P < 0.05) and less endothelial GRP78 cells (P < 0.01). In addition, GRP78 positive cells were the highest in 4MC, but decreased in all other groups (P < 0.01). GRP78 positive cells within the fibrous cap inversely correlated with cap size (r2 = 0.9). These studies suggest that high dietary methionine could be beneficial for plaque stabilisation, and a normal diet also stabilises plaque and decreases the number of stressed plaque cells.
Keywords: fibrous cap, stable plaque, grp78, rabbit, methionine, cholesterol
Cardiovascular disease remains the largest cause of death in Western society. Atherosclerotic plaque stability is now recognised as a major player in patient morbidity and mortality; however, there currently is no accurate model to study atherosclerotic plaque stabilisation. An unstable plaque (thin fibrous cap, high macrophage low smooth muscle cell content) can rupture and cause thrombosis and also platelet thrombosis, and this event usually occurs on the shoulders of the plaques. The ApoE mouse is currently widely used to study atherogenesis because of its low husbandry cost, ease of which atherosclerosis develops and ease of genetic manipulation. However, there are certain pitfalls of this model that are commonly ignored. These are (a) the excessive hypercholesterolaemia (in the order of 50 mmol/l–major elevated circulating lipoprotein fraction is VLDL (very low density lipoprotein), with only minor increases in LDL Getz & Reardon 2006) (b) poor response to angiotensin II (c) the fact that mice do not share the same reverse cholesterol transport as primates and humans Ha & Barter 1982 (d) that atherosclerosis regression or stabilisation does not occur in these mice because of the lack of ApoE Getz & Reardon 2006 (e) prolonged excessive hypercholesterolaemia affecting liver function (f) contradictory effects of AT2R on blood pressure regulation Duke et al. 2005; Iwai et al. 2005 and (g) the ‘switching on’ of compensatory genes in utero (genetic compensation) when genes are manipulated (h) lack of cholesterol ester transfer protein Kee et al. 2006 and (i) dissimilar regulation of haeme-oxygenase-1Kitamuro et al. 2003. To this end, we sought to develop a new model to study plaque stabilisation using only dietary manipulation.
The role of dietary methionine in the development of atherosclerosis is unclear. Troen et al. have shown that excess dietary methionine can hasten atherosclerosis in ApoE knockout mice Troen et al. 2003 and we have previously shown similar effects in the rabbit atherosclerosis model after a 12-week diet containing excess cholesterol and methionine Zulli et al. 2003, 2004. In the clinical setting, however, the effects of high plasma homocysteine on CVD remain invalidated Kaul et al. 2006. The HOPE2 clinical trial showed that minor homocysteine lowering had no beneficial effect on clinical outcomes whereas the FIELD study showed that increased homocysteine was associated with a 23% decrease in coronary events, but no change in mortality. This raises the question as to the effect of homocysteine on plaque morphology, as homocysteine can induce oxidative stress, stimulates collagen synthesis and smooth muscle cell proliferation Lentz 2005, factors that are involved in plaque remodelling.
The normal homocysteine range is 5–15 μmol/l in the population Brattstrom & Wilcken 2000. It is accepted that there is a graded association between plasma homocysteine levels and the risk of cardiovascular disease Boushey et al. 1995; Refsum et al. 1998. In this regard, a possible mechanism of homocysteine induced disease could be via induction of endoplasmic reticulum stress (ERS) Werstuck et al. 2001. ERS occurs when the endoplasmic reticulum cannot cope with the accumulation of misfolded proteins caused by various insults, which include cholesterol, homocysteine and diabetes Aridor & Balch 1999. This triggers the unfolded protein response, and in an attempt to restore normal homeostasis, an increase in the chaperone glucose regulated protein 78 (GRP78) occurs. There are three ER chaperone categories: (a) chaperones of heat shock protein family including GRP78, GRP94 and the co-chaperones; (b), chaperone lectins such as calnexin, calreticulin and (c) substrate-specific chaperones such as Hsp47Ni & Lee 2007 GRP78 (BiP) have a conserved adenosine triphosphatase (ATPase) domain and a peptide-binding domain Hendershot 2004. The function of this chaperone is to recognise and bind to the hydrophobic residues of proteins within the unfolded regions Flynn et al. 1991. GRP78 maintains all three ER stress sensors, PERK, ATF6 and IRE1 in inactive forms in non-stressed cells Schroder & Kaufman 2005 and during unfolded protein stress, GRP78 is removed which allows the activation and transduction of the unfolded protein signals across the ER membrane to the cytosol and the nucleus. Thus changes in GRP78 positive cells can be used to determine the level of ERS. It is important to uncover the role of ERS in the initiation, progression and stabilisation of atherosclerotic plaque to provide evidence as to whether small-molecule modulators of ERS such as 4-phenylbuturic acid, salubrinal and also taurine-ursodeoxycholic acid (TUDCA) could affect plaque remodelling.
This study was designed (a) to compare the plaque cellular structure between high dietary cholesterol alone and high dietary cholesterol plus methionine at 4 and 12 weeks (b) to establish an accurate model to study atherosclerotic plaque stabilisation within a short period of time without surgical intervention (c) to quantify GRP78 positive cells during these time periods.
Methods
Male New Zealand White rabbits at 3 months of age were randomised and fed the following diet: initiation group: Group (a) received a normal rabbit chow diet supplemented with 0.5% cholesterol + 5% peanut oil (n = 4, 4Ch), group (b) received a normal rabbit chow diet supplemented 0.5% cholesterol + 1% methionine + 5% peanut oil [n = 4, (1% methionine + 0.5% cholesterol) 2MC] for 2 weeks and group (c) for 4 weeks (4MC). progression group: Group (d) received a normal rabbit chow diet supplemented with 0.5% cholesterol + 5% peanut oil (n = 5, 12Ch), for 12 weeks and group (e) received a normal rabbit chow diet supplemented 0.5% cholesterol + 1% methionine + 5% peanut oil (n = 5, 12MC) for 12 weeks Zulli et al. 2003, 2004. regression group: group (f) received a normal rabbit chow diet supplemented 0.5% cholesterol + 1% methionine + 5% peanut oil for 2 weeks and then fed a normal chow diet for 10 weeks (n = 4, 2MCr) and group (g) received a normal rabbit chow diet supplemented 0.5% cholesterol + 1% methionine + 5% peanut oil for 4 weeks and then fed a normal chow diet for 10 weeks (n = 4, 4MCr). The animals were housed in individual cages and maintained at a constant temperature of approximately 21 °C. Food and water were supplied ad libidum. The experiments were carried out according to the ‘Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996)’. The animals were then killed by an overdose intra venous injection of ketamine and xylazine via the main ear vein as previously described in our laboratory. The aorta and heart were then excised, cleaned of connective tissue and fat and used for isometric tension studies.
Isometric tension studies
Abdominal aortae were dissected into 6 × 3 mm rings and sequentially mounted between two metal hooks in organ baths attached to force displacement transducers (Grass FT03) coupled to a data acquisition system (MacLab, ADInstruments, Bellavista, NSW, Australia). The baths will be filled with Krebs solution and kept at a constant temperature of 37 °C and continuously bubbled with 95% O2/5% CO2. After 1 h, vessels were gently stretched to a resting tension of 2 g. After another hour, maximum constriction was determined by a high potassium krebs solution (124 mmol/l K+). After plateau (6 min), vessels were rinsed with krebs and 1 h later the vessel rings were precontracted with phenylephrine to approximately 30–40% of maximal contraction. After the contraction became stable, an acetylcholine concentration response curve (10−8–10−6 mol/l Ach, half log units) was performed Zulli et al. 2003.
Wall pathology
Excess rings and the rings used in the organ baths were removed from the organ bath and placed into freshly prepared 4% paraformaldehyde in PBS pH 7.3 overnight, after which the vessels were placed in PBS. Once all experiments were finished, all blood vessels were processed for paraffin embedding in one batch. This is to keep the shrinking of vessels constant in all vessels. All blood vessels were then mounted vertically in two paraffin blocks. A minimum of eight 3 mm vessels were used per animal. After this, 5 μ sections were cut, mounted on microscope slides and immunostained for endothelial nitric oxide synthase as previously described Zulli et al. 2003, 2006; or with polyclonal goat anti GRP-78 (dilution 1:250, Santa Cruz, CA, USA), antibody to RAM-11 Iwai et al. 2005 or with monoclonal anti-C/EBP homologous protein (CHOP) (dilution 1:200, ABR), or with HHF35 (1:200, ABR, Affinity Bioreagents, Thermo Fisher Scientific, Rockford, USA) to localise intimal thickening and atherosclerotic plaques. In addition, the trichrome histopathological stain was performed to identify collagen and cells Lefkowitch 2006. Wall pathology was quantified by image analysis software (mcid Elite 6.0, InterFocus Imaging, Cambridge, England). Briefly, digital images of abdominal aorta (one image per 3 mm aortic ring per animal) were collected using a Leica DC480. The length of internal elastic lamina was recorded (circumference) and the lumen area calculated using the formula: area = circumference2/12.568. The area of the plaques and intimal thickening were recorded by the MCID elite software ‘ribbon’ tool. The percentage of plaque was calculated by dividing the area of plaque by the area of lumen, which was then multiplied by 100. Macrophage content and GRP78 positive cells within plaques were determined by calculating the area of stained positive cells divided by the total area examined (proportional area × 100%). To obtain this data, the hue, saturation and intensity of the brown DAB (diaminobenzidine) reaction was selected by the mcid program as previously described in our laboratory Zulli et al. 2006, 2008; Wookey et al. 2008. Then, the whole area to be quantified (fibrous cap, whole plaque, endothelial layer) was traced using the ‘ribbon’ tool from the mcid software, and the proportional area detected by the mcid was recorded.
Data analysis
All data points analysed by anova followed by a Newman–Keuls multiple comparison post hoc test or by Students’t-test were appropriate. A P < 0.05 was accepted in all cases as significant. All data are expressed as mean ± SEM.
Results
Lipid profile
Initiation group
In the initiation group, total cholesterol levels of blood significantly increased to 22.1 ± 4.8 mmol/l in 4Ch, 24.6 ± 5.6 mmol/l in 4MC compared with the control 1.5 ± 0.1 mmol/l (P < 0.05). This increase was mainly due to a marked increase in LDL (low density lipoprotein) cholesterol (19.2 ± 4.4 mmol/l in 4Ch, 21.5 ± 5.2 in 4MC, vs. 0.56 ± 0.16 in Con, P < 0.05) and a small, significant increase in HDL (high density lipoprotein) cholesterol (2.63 ± 0.4 mmol/l in 4Ch, 2.66 ± 0.3 mmol/l in 4MC vs. 0.82 ± 0.07 mmol/l in Con, P < 0.01). Blood triglycerides and homocysteine were not significantly different between groups.
Progression group
The lipid profile for the progression group has been previously reported Zulli et al. 2003. Briefly, total plasma cholesterol in 12Ch was 39.8 ± 4.4 mmol/l and 37.8 ± 6.3 mmol/l in 12MC. Plasma homocysteine was slightly elevated in 12MC compared with the control Zulli et al. 2003.
Regression groups
In the regression groups, total cholesterol levels of blood did return to normal at the end of the 10 week normal diet regimen in both 2Wr (1.7 ± 0.3 mmol/l) and 4Wr (2.1 ± 1.1 mmol/l, Figure 1m). However, total cholesterol levels remained elevated for at least 5 weeks after the initiation of the diet. Extrapolative curves have been added to Figure 1m. Blood triglycerides were not significantly different between groups.
Figure 1.
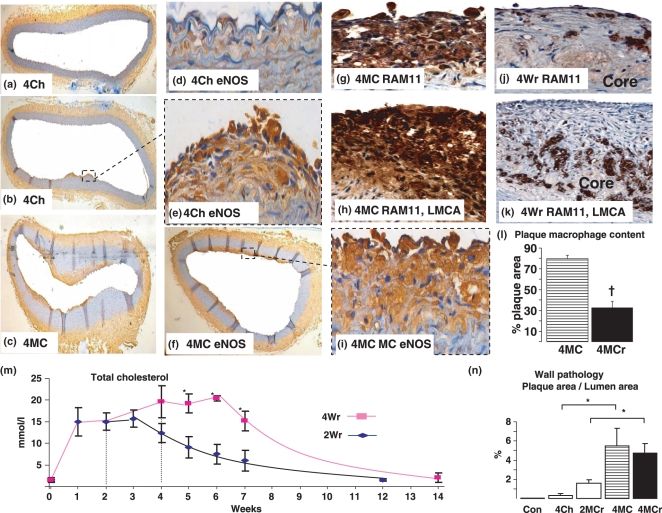
After 4 weeks of 0.5% cholesterol diet plus 5% peanut oil, atherosclerosis was not readily present in the aorta, with atherosclerosis only present in focal areas (a, b, e). Figure d appears to be hyperhomocysteinemic lesions, characterised by intimal thickening without macrophages McCully 1969. This is in stark contrast to the diffuse atherosclerosis present in the group fed 0.5% cholesterol plus 1% methionine plus 5% peanut oil for 4 weeks (c, f, g, i) and also in the left main coronary artery (h). Comparison photomicrographs for RAM11 (macrophage) in 4MC (g) and 4Wr (j) in abdominal aorta and 4MC (h) and 4Wr (k) in left main coronary artery showing lack of macrophages in regression, as quantified in (l). Plaque content was quantified and expressed as a table (n). The sustained increase in plasma cholesterol after the initiation of the normal diet (m) after the 2 or 4 week initial 0.5% cholesterol plus 1% methionine plus 5% peanut oil diet caused an increase in atherosclerosis in the vessel wall (n). This regression period also allowed the plaque to become more stable, as shown by the significant decrease in aortic plaque macrophages (stained brown, j, l) and increase in fibrous cap (Figure 2i), as well as in the left main coronary artery (k). Results are expressed as mean ± SEM, *P < 0.05.
Quantitation of wall pathology
Initiation group
Although plasma cholesterol was not significantly different between 4Ch and 4MC, foam cell accumulation in 4MC was clearly evident throughout the aortic wall (Figures 1c,f,g,i and 2a) and left main coronary artery (Figure 1h) compared with 4Ch whereby a greatly thickened sub-endothelial layer was evident (Figure 1a,d), resembling hyperhomocysteinemic lesions McCully 1969. Atherosclerosis was rarely observed in this group (Figure 1a,b). Quantitational analysis showed that atherosclerosis in the abdominal aorta significantly increased 17-fold in the 4MC group compared with 4Ch (5.5 ± 1.8%vs. 0.32 ± 0.18%, P < 0.05, Figure 1n). The plaques were composed of lipid laden foam cells [RAM11 (+) macrophages] which is typical at this time point (Figure 1g,h) and no fibrous caps were evident.
Figure 2.
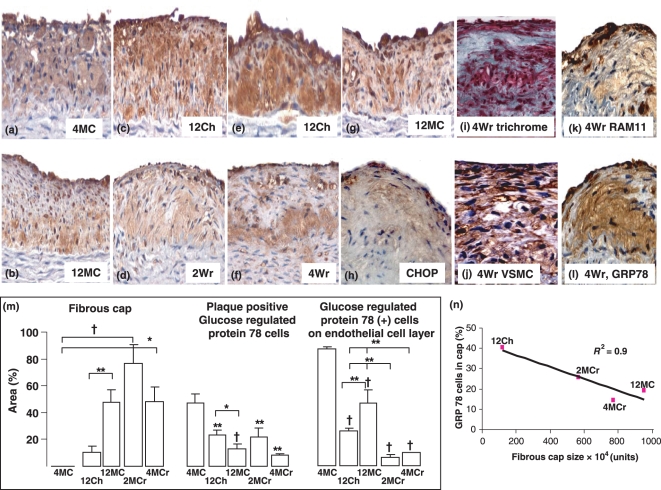
Photomicrographs of glucose regulated protein 78 (GRP78) positive cells in the initiation, progression and regression of atherosclerosis. GRP78 positive cells, most probably macrophages, are present in high amounts in aortic plaques in 4MC (a). GRP78 positive cells remained throughout the duration of 12 week dietary manipulation (12Ch – c and e; 12MC, g and b) and during the regression period (2Wr – d and 4Wr – f). CHOP immunoreactivity was also present in cells overlying endothelium and not in core (h). The trichrome stain (i) reveals collagenous cap forming (blue) with other cells (red), which are most probably VSMC (j) and some macrophages (k). Serial sections (K, RAM11 and L, GRP78) show that GRP78 positive cells are likely to be macrophages. GRP78 positive cells within plaques significantly decreased from 4 weeks onwards (m), and GRP78 positive cells overlying endothelia was scarce in the regression groups (m). Quantification of percentage area of GRP78 positive cells in the fibrous cap showed a strong correlation between fibrous cap size and cells in the cap (N, r2 = 0.9). Quantification of fibrous cap showed a significant increase in fibrous cap content in 12MC compared with 12Ch, and the regression groups also showed marked fibrous cap development (l). Results are expressed as mean ± SEM, *P < 0.05, †P < 0.01, ‡P < 0.001.
Progression group
Atherosclerotic quantification has already been published in this group Zulli et al. 2003. Briefly, there was no difference in the amount of atherosclerosis between 12Ch and 12MC. Here, we report that the major difference between the two groups is in the structure of the plaques. The fibrous cap formed by continuous cholesterol plus methionine feeding over 12 weeks was 4.8-fold higher (P < 0.01, Figure 2b,g,m) than that formed by cholesterol alone (Figure 2c,e,m). This was also associated with a 43% decrease (P < 0.05) in plaque positive GRP78 cells but a 84% increase (P < 0.01) in GRP78 positive cells binding to the plaque endothelial layer (Figure 2c,e,g,b,m).
Regression group
After the 10 week normal diet regimen, thick fibrous caps are observed in the abdominal aortic plaques in both the 2Wr (Figure 2d) and 4Wr (Figures 1j and 2f,h,i,k,l) and left main coronary artery (Figure 1k). Indeed, fibrous caps comprised 46% (P < 0.001) of total abdominal aortic plaque area in the 4Wr group compared with 4MC (Figure 2m) and plaque macrophage content decreased by 60% (P < 0.001, Figure 1l). This was associated with marked drop in GRP78 positive cells overlying atherosclerotic plaques compared with all other groups (Figures 2c, e, g, b compared with d, f, and quantified in m). It is important to note that the amount of plaques in the 4MC group and 4MCr group was not significantly different (Figure 1n); however, there were significant changes in plaque biological structure indicative of a more stable plaque phenotype (Figures 1l and 2m).
To show the collagenous and cellular nature of the cap, a trichrome stain as well as a VSMC (vascular smooth muscle cell) immunoreaction was performed. Collagen (blue) can be clearly observed overlying a cellular core (red) and is shown in Figure 1i, and VSMC were prominent throughout the plaque (Figure 2j) compared with macrophages (Figure1j, 2k). Serial sections (Figure 2j,l) clearly show RAM11 positive cells which react with the GRP78 antibody. In addition, to confirm the decrease in ER stress, the second marker CHOP was also minimally present in 4Wr.
GRP78 cells in the fibrous cap
Next, we sought to determine the amount of positive GRP78 cells in the fibrous cap. We found that, in the four groups with apparent fibrous cap formation in the atherosclerotic plaques, there was an inverse relationship between fibrous cap size and GRP78 positive cells (r2 = 0.9, Figure 2n).
Endothelial function
Endothelium dependent relaxation in response to acetylcholine was abolished in the 4W group (2.9 ± 3.8%vs. control 30.3 ± 5.7%, P<0.05). Although endothelial function in the abdominal aorta began to improve after 10 weeks of normal diet in both 2Wr (18.7 ± 4.1%) and 4Wr (6.3 ± 4%), it did not return to normal. To determine whether the abrogated endothelial function in 4W was due to a lack of eNOS (endothelial nitric oxide synthase), we immunolocalised eNOS and showed that the enzyme was clearly present in all endothelium of arteries studied in all groups, especially in the atherosclerotic plaques in both 4Ch and 4MC (Figures 1a–f,i).
Discussion
The major findings of this investigation are (a) rabbit atherosclerotic plaques in the abdominal aorta and left main coronary artery can stabilise (thick fibrous caps, low macrophage content) in 10 weeks after a 4 week atherogenic diet (b) the combination of high dietary methionine plus cholesterol exacerbates atherosclerosis formation within 4 weeks compared with the high dietary cholesterol alone but the addition of high dietary methionine to a high cholesterol diet over 12 weeks causes thicker fibrous caps compared with cholesterol alone (c) abrogated endothelial function is present in the abdominal aorta of rabbits fed high dietary cholesterol plus methionine at 4 weeks that does not return to normal after 10 weeks of normal diet (d) GRP78 positive cells within plaques are reduced during regression.
Although murine models of atherosclerosis are now established Getz & Reardon 2006; the extrapolation of results obtained from these models must be viewed with caution, as the mechanisms of atherogenesis in genetically modified murine models might well be different to that observed in humans. Thus, we need to recognise that all current studies using genetically modified animals might not be predictive of effects in humansGetz & Reardon 2006. In this regard, we have set out to develop a new model for the study of severe endothelial dysfunction and plaque stabilisation in rabbits using only dietary regimens without surgical or genetic manipulation over a short period of time. Although the rabbit as an animal model for human disease has been criticised in the past for the lack of regression or plaque stabilisation in early stages of the disease, such effects can be observed after a 2-year period Constantinides et al. 1960. In this report, we clearly show that fibrous caps overlying atherosclerotic plaques can be formed and plaque macrophages are decreased in the rabbit after 10 weeks of a normal diet, after an initial 4 week atherogenic diet. This is possible because of the large amount of atherosclerosis present in the abdominal aorta and left main coronary artery after only 4 weeks of dietary manipulation which was not previously attainable. For example, to develop such plaques, the previous studies used very high levels of cholesterol (1–2%) for extended periods of time (1–5 months) Prior et al. 1961; Clarkson 1971 to induce atherosclerosis, a regimen that ‘floods’ the animal with cholesterol leading to a ‘cholesterol storage disease’ and liver failure. Attempts to initiate regression or stabilisation of plaques at these time points over a short period of time were unsuccessful, possibly because of the lipid overload. Thus, here we report that an initial 4 week diet containing 0.5% cholesterol plus 1% methionine plus 5% peanut oil causes marked atherosclerosis in only 4 weeks and a normal diet for 10 weeks after this diet causes a more stable plaque phenotype.
The rabbit model has been extensively used to study atherosclerotic plaque stabilisation. For example, current studies include the use of photopoint photodynamic therapy, which has been shown to promote stabilisation of rabbit atherosclerotic plaques and inhibits plaque progression Waksman et al. 2008; fenofibrate intervention to improve plaque stability Jeanpierre et al. 2008 as well as the genetic hyperlipidaemic model (WHHL (Watanabe heritable hyperlipidemic) rabbit) to study the effect of statins on the plaque vulnerability index (stable plaque phenotype) Shiomi et al. 2001. In these models and the model presented here, we must stress that these plaques do not resemble type IV human coronary artery atherosclerosis Stary 2000; Stary et al. 1995, 1994. We suggest, however, that this rabbit model could be used to study factors that can influence plaque cellular biology which resembles a more stable plaque phenotype (plaque vulnerability index) without the need for surgical intervention.
We have previously reported that the combination of high dietary cholesterol (0.5%) plus methionine (1%) fed to rabbits for 12 weeks abolishes endothelial function and exacerbates atherosclerosis formation compared with high dietary cholesterol or methionine alone Zulli et al. 2003, 2004. In this study, we have also shown that severe endothelial dysfunction is present at 4 weeks as well as severe atherosclerotic involvement by the combination of the two diets. The observed atherosclerosis was markedly greater than the effects observed by cholesterol feeding alone. Indeed, in the latter group, lesions were similar to those observed by hyperhomocysteinaemia McCully 1969 rather than hypercholesterolaemia. This data clearly indicates that high dietary methionine can exacerbate early atherosclerotic development. Whether these effects are due to the increased plasma homocysteine levels observed or due to another methionine metabolite is presently unknown. However, although the combination of high dietary methionine exacerbates early atherogenesis, we report that extended diet causes a more stable atherosclerotic plaque phenotype than that observed by cholesterol alone. This could be due to the stimulatory effects of homocysteine on collagen production Majors et al. 1997; Sen et al. 2006; smooth muscle cell proliferation Taha et al. 1999 and cellular differentiation Sen et al. 2006 which are involved in establishing a more stable plaque phenotype. Such results could explain the 24% reduction in non-fatal myocardial infarction observed in the FIELD study Verges 2006; Keech et al. 2005. A more stable plaque phenotype could reduce coronary artery plaque rupture, but the increase in homocysteine might also exacerbate thrombosis Di Minno et al. 1999 leading to the 0.7% increase in total mortality increased observed in the study. Thus it could be possible that the increase in homocysteine is stimulating a more stable plaque phenotype but on the other hand, exacerbating thrombosis leading to infarcts. This theory requires further elucidation.
In this study, we observed that plasma cholesterol remained elevated for over 4 weeks after the initial 2 week or 4 week diet regimen. This clearly shows the impaired cholesterol clearance in the rabbit model and is supported by other studies showing plasma cholesterol do not return to normal in a short period of time Balkan et al. 2004; Fennessy et al. 1994. These results are in stark contrast to others that showed a 4 week normal diet after a 4 week high cholesterol diet completely restored plasma cholesterol levels Van Winkle & Levy 1970; Ohara et al. 1995. Although this could be explained by differences in fat content of the diets, a high fat, high cholesterol, high methionine diet might be a more physiologically relevant diet that feeding high cholesterol alone.
ER stress is regarded as a protective cellular response Seimon & Tabas 2008; and thus inhibiting the onset of ER stress is currently regarded as a possible novel approach for the treatment of diabetes and CVD; however, basic evidence to support this role is minimal Ozcan et al. 2006. Small chemicals, such as 4-phenyl butyric acid (PBA), and bile acid derivatives, such as TUDCA can inhibit ER stress without toxicity. PBA is a low molecular weight fatty acid that acts as a chemical chaperone reducing the load of mutant or unfolded proteins retained in the ER during cellular stress and also exerting anti-inflammatory activity. It has been used successfully for treatment of urea cycle disorders and sickle cell disease. In animal models, the addition of 4PBA or TUDCA to obese and diabetic mice resulted in the normalisation of hyperglycaemia, insulin sensitivity, fatty liver and suppression of ER stress markers Ozcan et al. 2006. Although salubrinal has been currently identified as a possible treatment for ER stress Boyce et al. 2005; recent evidence suggests that this compound can cause beta cell apoptosis Cnop et al. 2007. Information on the beneficial effects of TUDCA and 4PBA on atherosclerosis is lacking; however, both TUDCA and 4PBA can impair apoptosis, TUDCA in neuronal cells Ramalho et al. 2006 and PBA in kidney cells Choi et al. 2005. In this study, we show that cells expressing GRP78, a molecular chaperone involved in ER stress and elevated by homocysteine Outinen et al. 1999 and diabetes Parfett et al. 1990; are elevated in the early stage of atherogenesis (4 weeks) and significantly decrease over time, indicating an initial increase in ER stress cells and a decrease in ER stress cells over time. To confirm the decrease in ER stressed cells, we also performed immunohistochemistry for the CHOP Devries-Seimon et al. 2005; which is critical for cholesterol induced macrophage apoptosis Feng et al. 2003. We found that CHOP positive cells are also rare in the regressed group, indicative of less ER stressed cells. Thus, we propose that GRP78/CHOP positive cells are reduced during normal diet, as the decrease in plasma lipid would reduce excess cellular cholesterol from cells and thus reduce the UPR response Feng et al. 2003 restoring normal protein folding.
In conclusion, we show that in only 4 weeks of dietary manipulation, rabbits exhibit abrogated endothelial function and severe atherosclerosis, and that 10 weeks of normal diet after an initial 4 week atherogenic diet, restores a normal lipid profile, initiates the restoration of endothelial function and leads to a more stable atherosclerotic plaque phenotype (thick fibrous cap/low macrophage content) and a decrease in ER stressed cells. We also show that the combination of high dietary methionine plus cholesterol over 12 weeks causes a more stable plaque phenotype suggesting that methionine might be an important plaque stabiliser. Our results shed light upon the effects of methionine on atherogenesis and the possibility of using ER stress inhibitors in the rabbit model of atherosclerosis.
References
- Aridor M, Balch WE. Integration of endoplasmic reticulum signaling in health and disease. Nat. Med. 1999;5:745–751. doi: 10.1038/10466. [DOI] [PubMed] [Google Scholar]
- Balkan J, Oztezcan S, Hatipoglu A, Cevikbas U, Aykac-Toker G, Uysal M. Effect of a taurine treatment on the regression of existing atherosclerotic lesions in rabbits fed on a high-cholesterol diet. Biosci. Biotechnol. Biochem. 2004;68:1035–1039. doi: 10.1271/bbb.68.1035. [DOI] [PubMed] [Google Scholar]
- Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Brattstrom L, Wilcken DE. Homocysteine and cardiovascular disease: cause or effect? Am. J. Clin. Nutr. 2000;72:315–323. doi: 10.1093/ajcn/72.2.315. [DOI] [PubMed] [Google Scholar]
- Choi SW, Ryu OH, Choi SJ, Song IS, Bleyer AJ, Hart TC. Mutant tamm-horsfall glycoprotein accumulation in endoplasmic reticulum induces apoptosis reversed by colchicine and sodium 4-phenylbutyrate. J. Am. Soc. Nephrol. 2005;16:3006–3014. doi: 10.1681/ASN.2005050461. [DOI] [PubMed] [Google Scholar]
- Clarkson TB. Animal models for atherosclerosis. A review. N. C. Med. J. 1971;32:88–98. [PubMed] [Google Scholar]
- Cnop M, Ladriere L, Hekerman P, et al. Selective inhibition of eukaryotic translation initiation factor 2{alpha} dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J. Biol. Chem. 2007;282:3989–3997. doi: 10.1074/jbc.M607627200. [DOI] [PubMed] [Google Scholar]
- Constantinides P, Booth J, Carlson G. Production of advanced cholesterol atherosclerosis in the rabbit. Arch. Pathol. 1960;70:712–724. [PubMed] [Google Scholar]
- Devries-Seimon T, Li Y, Yao PM, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Minno G, Coppola A, Mancini FP, Margaglione M. Homocysteine, platelet function and thrombosis. Haematologica. 1999;84(Suppl EHA-4):61–63. [PubMed] [Google Scholar]
- Duke LM, Evans RG, Widdop RE. AT2 receptors contribute to acute blood pressure-lowering and vasodilator effects of AT1 receptor antagonism in conscious normotensive but not hypertensive rats. Am. J. Physiol. 2005;288:H2289–H2297. doi: 10.1152/ajpheart.01096.2004. [DOI] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Fennessy PA, Campbell JH, Campbell GR. An angiotensin converting enzyme inhibitor, perindopril, prevents progression of preformed atherosclerotic lesions in the cholesterol-fed rabbit. Clin. Sci. (Lond.) 1994;87:685–691. doi: 10.1042/cs0870685. [DOI] [PubMed] [Google Scholar]
- Flynn GC, Pohl J, Flocco MT, Rothman JE. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- Ha YC, Barter PJ. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. B. 1982;71:265–269. doi: 10.1016/0305-0491(82)90252-8. [DOI] [PubMed] [Google Scholar]
- Hendershot LM. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. 2004;71:289–297. [PubMed] [Google Scholar]
- Iwai M, Chen R, Li Z, et al. Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation. 2005;112:1636–1643. doi: 10.1161/CIRCULATIONAHA.104.525550. [DOI] [PubMed] [Google Scholar]
- Jeanpierre E, Le Tourneau T, Zawadzki C, et al. Beneficial effects of fenofibrate on plaque thrombogenicity and plaque stability in atherosclerotic rabbits. Cardiovasc. Pathol. 2008 doi: 10.1016/j.carpath.2008.03.001. Apr 22. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kaul S, Zadeh AA, Shah PK. Homocysteine hypothesis for atherothrombotic cardiovascular disease: not validated. J. Am. Coll. Cardiol. 2006;48:914–923. doi: 10.1016/j.jacc.2006.04.086. [DOI] [PubMed] [Google Scholar]
- Kee P, Caiazza D, Rye KA, Barrett PH, Morehouse LA, Barter PJ. Effect of inhibiting cholesteryl ester transfer protein on the kinetics of high-density lipoprotein cholesteryl ester transport in plasma: in vivo studies in rabbits. Arterioscler. Thromb. Vasc. Biol. 2006;26:884–890. doi: 10.1161/01.ATV.0000201064.89581.35. [DOI] [PubMed] [Google Scholar]
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- Kitamuro T, Takahashi K, Ogawa K, et al. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J. Biol. Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- Lefkowitch JH. Special stains in diagnostic liver pathology. Semin. Diagn. Pathol. 2006;23:190–198. doi: 10.1053/j.semdp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J. Thromb. Haemost. 2005;3:1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- Majors A, Ehrhart LA, Pezacka EH. Homocysteine as a risk factor for vascular disease. Enhanced collagen production and accumulation by smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1997;17:2074–2081. doi: 10.1161/01.atv.17.10.2074. [DOI] [PubMed] [Google Scholar]
- McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. am. J. Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y, Peterson TE, Sayegh HS, Subramanian RR, Wilcox JN, Harrison DG. Dietary correction of hypercholesterolemia in the rabbit normalizes endothelial superoxide anion production. Circulation. 1995;92:898–903. doi: 10.1161/01.cir.92.4.898. [DOI] [PubMed] [Google Scholar]
- Outinen PA, Sood SK, Pfeifer SI, et al. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999;94:959–967. [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfett CL, Brudzynski K, Stiller C. Enhanced accumulation of mRNA for 78-kilodalton glucose-regulated protein (GRP78) in tissues of nonobese diabetic mice. Biochem. Cell Biol. 1990;68:1428–1432. doi: 10.1139/o90-206. [DOI] [PubMed] [Google Scholar]
- Prior JT, Kurtz DM, Ziegler DD. The hypercholesteremic rabbit. An aid to understanding arteriosclerosis in man? Arch. Pathol. 1961;71:672–684. [PubMed] [Google Scholar]
- Ramalho RM, Borralho PM, Castro RE, Sola S, Steer CJ, Rodrigues CM. Tauroursodeoxycholic acid modulates p53-mediated apoptosis in Alzheimer’s disease mutant neuroblastoma cells. J. Neurochem. 2006;98:1610–1618. doi: 10.1111/j.1471-4159.2006.04007.x. [DOI] [PubMed] [Google Scholar]
- Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 2008 doi: 10.1194/jlr.R800032-JLR200. Oct 25. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen U, Moshal KS, Tyagi N, Kartha GK, Tyagi SC. Homocysteine-induced myofibroblast differentiation in mouse aortic endothelial cells. J. Cell. Physiol. 2006;209:767–774. doi: 10.1002/jcp.20752. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Hirouchi Y, Enomoto M. Stability of atheromatous plaque affected by lesional composition: study of WHHL rabbits treated with statins. Ann. NY Acad. Sci. 2001;947:419–423. doi: 10.1111/j.1749-6632.2001.tb03977.x. [DOI] [PubMed] [Google Scholar]
- Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler. Thromb. Vasc. Biol. 2000;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. 1994;14:840–856. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- Taha S, Azzi A, Ozer NK. Homocysteine induces DNA synthesis and proliferation of vascular smooth muscle cells by a hydrogen peroxide-independent mechanism. Antioxid. Redox Signal. 1999;1:365–369. doi: 10.1089/ars.1999.1.3-365. [DOI] [PubMed] [Google Scholar]
- Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc. Natl Acad. Sci. USA. 2003;100:15089–15094. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle M, Levy L. Further studies on the reversibility of serum sickness cholesterol-induced atherosclerosis. J. Exp. Med. 1970;132:858–867. doi: 10.1084/jem.132.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verges B. Fenofibrate therapy and cardiovascular protection in diabetes: recommendations after FIELD. Curr. Opin. Lipidol. 2006;17:653–658. doi: 10.1097/01.mol.0000252612.21602.e3. [DOI] [PubMed] [Google Scholar]
- Waksman R, McEwan PE, Moore TI, et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008;52:1024–1032. doi: 10.1016/j.jacc.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Werstuck GH, Lentz SR, Dayal S, et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Invest. 2001;107:1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wookey PJ, Zulli A, Buxton BF, Hare DL. Calcitonin receptor immunoreactivity associated with specific cell types in diseased radial and internal mammary arteries. Histopathology. 2008;52:605–612. doi: 10.1111/j.1365-2559.2008.02979.x. [DOI] [PubMed] [Google Scholar]
- Zulli A, Widdop RE, Hare DL, Buxton BF, Black MJ. High methionine and cholesterol diet abolishes endothelial relaxation. Arterioscler. Thromb. Vasc. Biol. 2003;23:1358–1363. doi: 10.1161/01.ATV.0000080686.39871.54. [DOI] [PubMed] [Google Scholar]
- Zulli A, Hare DL, Buxton BF, Black MJ. High dietary methionine plus cholesterol exacerbates atherosclerosis formation in the left main coronary artery of rabbits. Atherosclerosis. 2004;176:83–89. doi: 10.1016/j.atherosclerosis.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Zulli A, Buxton BF, Black MJ, Ming Z, Cameron A, Hare DL. The immunoquantification of caveolin-1 and eNOS in human and rabbit diseased blood vessels. J. Histochem. Cytochem. 2006;54:151–159. doi: 10.1369/jhc.5A6677.2005. [DOI] [PubMed] [Google Scholar]
- Zulli A, Buxton BF, Black MJ, Hare DL. Embryonic stem cells markers are present within rabbit atherosclerotic plaques. Histol. Histopathol. 2008;23:741–746. doi: 10.14670/HH-23.741. [DOI] [PubMed] [Google Scholar]