Abstract
A disintegrin and metalloprotease-15 (ADAM-15) is a potential novel regulator of inflammatory response and tissue remodelling, which is thought to have the ability to attenuate the cardiac function resulting from myocardial infarction (MI). Therefore, the aim of our study was to investigate the expression of ADAM-15 in rat MI. Wistar rats were subjected to MI by ligation of the left anterior descending coronary artery. Euthanasia was performed at 1, 3, 7 and 14 days following MI. The mRNA and protein expression levels of ADAM-15 were detected respectively by reverse transcription-polymerase chain reaction (RT-PCR) and Western blot. The localization of ADAM-15 protein was observed by immunohistochemistry. Compared with sham-MI, the expression of ADAM-15 in MI increased at day 1, reached to maximum at day 3, decreased at day 7 and day 14 gradually. In addition, we also found that the localization of ADAM-15 was mainly at cardiac myocytes in the border area of MI and some macrophages in the border and infarcted areas. This study revealed a significant difference of ADAM-15 expression in rat MI and indicated that ADAM-15 maybe one of the important factors involved in inflammatory response and cardiac remodelling of rat MI.
Keywords: A disintegrin and metalloprotease-15, cardiac remodelling, inflammatory response, myocardial infarction
Acute myocardial infarction (MI) is one of the most common emergencies in clinical practice and is also a potential cause of death events (Herlitz et al. 2001); therefore, continuous efforts have been made to identify novel events, which may help to elucidate its mechanisms. Myocardial infarction is a complicated process, which can be affected by age, gender, diabetes, inflammatory response, cardiac remodelling and so on (Zornoff et al. 2000; Maekawa et al. 2004; Kruk et al. 2008; Van den Borne et al. 2009). An excessive inflammatory response following MI could affect the healing process and cardiac remodelling (Maekawa et al. 2004; Kruk et al. 2008; Van den Borne et al. 2008). Moreover, both inflammatory response and cardiac remodelling can cause deterioration of heart function (Fedak et al. 2005; Van den Borne et al. 2009). However, the molecular basis of the inflammatory response and cardiac remodelling in MI is still a matter of debate. Its understanding could have an important therapeutic impact in future.
A disintegrin and metalloproteases (ADAMs) are a large family of membrane-bound glycoproteins which consist of prodomain, metalloprotease domain, disintegrin domain, cysteine-rich domain, epidermal growth factor (EGF)-like domain, transmembrane domain, and cytoplasmic domain (Kratzschmar et al. 1996). ADAMs have been known to play important role in physiological and pathological processes such as proteolysis, adhesion, fertilization, fusion, migration, proliferation and cleavage-secretion of inflammatory factors (Black & White 1998; Schlondorff & Blobel 1999; Ding Rocks et al. 2008). Furthermore, some studies have confirmed that ADAMs could underlie inflammatory response and structural remodelling by regulating cell–cell and cell–matrix interactions (McCulloch et al. 2000; Arndt et al. 2002; Fedak et al. 2003, 2004, 2006; Worley et al. 2007; Charrier-Hisamuddin et al. 2008). Although previous studies have demonstrated that ADAMs such as ADAM-9, 10, 12, 15, 17 and 19 are expressed in myocardium (Arndt et al. 2002; Fedak et al. 2006), there is little data in evaluating these molecules in MI.
Human ADAM-15 is the only ADAM that has the RGD motif, an Arg–Gly–Asp tripeptide sequence, which can bind to integrin and participate in structural remodelling (Howard et al. 1999). Some studies have demonstrated that human ADAM-15 is mainly involved in the pathological process of inflammatory response and structural remodelling in some diseases such as atherosclerosis (Herren et al. 1997; Al-Fakhri et al. 2003), retinopathy of prematurity (Horiuchi et al. 2003), rheumatoid arthritis (Böhm et al. 2005) and inflammatory bowel disease (Mosnier et al. 2006). Furthermore, Arndt et al. and Fedak et al., respectively, have confirmed that the increased ADAM-15 expression is one of the molecular mechanisms, which may contribute to the pathological process of atrial fibrillation (AF) and dilated cardiomyopathy (DCM) (Arndt et al. 2002; Fedak et al. 2006). However, the role of ADAM-15 in MI is not well understood.
In this study, we investigated the expression of ADAM-15 in rat MI and elucidated its possible mechanisms in the pathological process of MI. It will provide a novel insight into the mechanisms, which influence the inflammatory response and cardiac remodelling following MI.
Materials and methods
Animals
We used female Wistar rats, weighing 200–250 g, from Laboratory Animal Facility of Harbin Medical University. The animals were kept in cages, and fed on standard commercial feed and water ad libitum, with controlled light in 12 h cycles, temperature (approximately 24 °C) and humidity. The experimental protocols were approved by the Animal Experiment Ethics Committee of Harbin Medical University.
Myocardial infarction
Myocardial infarction was produced as per the method previously described (Ben-Dor et al. 2007; Wang et al. 2007). The rats were anaesthetized with a single intraperitoneal injection of 10% chloral hydrate in a dose of 3 ml/kg. Intubation was performed and the rats were ventilated with room air using a rodent mechanical ventilator (Harvard Apparatus, 55–7058). The chest was opened by left thoracotomy through the fourth intercostal space to expose the heart. The pericardium was opened and the proximal left anterior descending coronary artery was ligated with a silk 6-0 suture near its origin between the pulmonary outflow tract and the edge of the left atrium, which ensured permanent coronary occlusion. Regional MI was confirmed by observing a rapid change from reddish to a whitish-dark color on the anterior surface of the left ventricle (LV). The sham-operated animals underwent the same procedure, but without the ligation of the coronary artery.
Reverse transcription-polymerase chain reaction (RT-PCR)
Rats were anaesthetized with 10% chloral hydrate at 1, 3, 7, and 14 days following surgically induced MI. The heart was stopped at diastole by injection of 10% KCl and removed. The LV free wall was dissected (Maekawa et al. 2004; Tang et al. 2006), washed with cold phosphate-buffered saline (PBS), quickly frozen in liquid nitrogen, and stored at −80 °C until use. Total RNA was isolated according to the direction of Trizol (Invitrogen Corp., Carlsbad, CA, USA), and dissolved in Rnase-free water (1‰ diethylpyrocarbonate). The reverse transcription proceeded as per the direction (Promega Corp., Madison, WI, USA). The primer sequences of ADAM-15 for PCR were: 5′-ATGGACCACTCGACAAGCA-3′ and 5′-GCAGAACTCAGGCAAATCG-3′. The PCR reaction was inactivated for 5 min at 94 °C, 30 s of denaturation at 94 °C, 30 s of annealing at 50 °C, and 1 min of extension at 72 °C for 30 cycles, followed by an incubation at 72 °C for 5 min. PCR products were analysed by running on a 2.0% agarose gel and using the tanon GIS2010 Analysis (tanon GIS 2010 Analysis, Shanghai, China) software.
Western blot
Total proteins were extracted as described previously (Fedak et al. 2003). Total tissue homogenates were harvested by lysing in RIPA: 1 × PBS, 1% NP40, 0.1% SDS, 5 mM EDTA, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate and 1/100 phenylmethanesulfonyl fluoride (PMSF). 50 μg of the total protein, which was assayed by Bradford Protein Assay kit (Beyotime Corp., Haimen, JS, China), was fractionated by 8% SDS-PAGE and electrotransferred onto a polyvinylidene difluoride membrane (PVDF, Millipore Corp., Billerica, MA, USA). The membranes were blocked with 5% non-fat dry milk in 20 mM Tris-HCl (pH 8.8), 500 mM NaCl, and 0.05% Tween 20 (TBST, TBS containing 0.05% Tween 20) for 1 h at room temperature, then incubated with the rabbit polyclonal antibody for ADAM-15 (1:400, Santa Cruz Biotech, Santa Cruz, CA, USA) overnight at 4 °C. After being washed with TBST, the membranes were incubated with a goat anti-rabbit IgG (1:1000, Solarbio, Beijing, China) for 1 h at room temperature. Detection was done by reaction with freshly prepared diaminobenzidine (DAB) (Santa Cruz Biotech) substrate solution. Blots were scanned and analysed using the tanon GIS2010 Analysis software.
Histology
Rats were anaesthetized with 10% chloral hydrate at 1, 3, 7, and 14 days following MI. The heart functioning was stopped at diastole by injection of 10% KC1. Hearts from all animals were excised and soaked with 10% formalin for at least 24 h. Measurements were performed on midventricular sections about 5–6 mm from the apex, on the assumption that the left midventricular slice showed a close linear relation with the sum of the area measurements from all heart sections (Zornoff et al. 2000). The sections were embedded in paraffin, sectioned into 5 μm and stained with haematoxylin and eosin (H&E), or for immunohistochemical study.
Immunohistochemistry
Sections were deparaffinized and rehydrated in a graded alcohol series, and antigen retrieval was performed in 10 mm citrate buffer pH 6.0 at 100 °C for 15 min. Incubation with rabbit polyclonal antibody for ADAM-15 (1:100, Santa Cruz Biotech) was performed in a moist chamber at 37 °C for 1 h. Polyvalent anti-rabbit IgG (Santa Cruz Biotech) was incubated at room temperature for 30 min. Followed by another 30 min of incubation with horseradish peroxidase (Santa Cruz Biotech) at room temperature. Immunostaining was visualized by DAB solution. Washings between steps were performed using PBS (Arndt et al. 2002; Campos et al. 2008). The sham-MI group was euthanized 14 days after surgery. We have confirmed that sham operations have no time effect on any parameter measured.
Histological evaluation
Stained sections were photographed with a JVC digital camera (TK-C 1381, Nikon, Sendai, Japan) mounted on an Olympus microscope (BH-2, Olympus, Tokyo, Japan). Multiple digital images were taken and staining was analysed using motic image software (motic images 2000, Xiamen, FJ, China). The number of ADAM-15-postive cells was counted by the total positive stained area of the myocardium to the total area in five standard randomly selected different fields.
Statistical analysis
All statistical analyses were performed with spss 15.0 software (SPSS Inc., Chicago, IL, USA). Statistical differences between means were determined by analysis of variance (anova) followed by least significant difference (LSD) tests. Values are expressed as mean ± SEM, with P<0.05 considered to be significant.
Results
ADAM-15 mRNA expression
ADAM-15 was observed at mRNA levels. In this study, we showed that ADAM-15 mRNA expression was not at the same level in rat MI at 1, 3, 7 and 14 days. As shown in Figure 1, compared with sham-MI (0.322 ± 0.061), the mRNA expression of ADAM-15 increased at day 1 (0.377 ± 0.139; P>0.05 vs. sham-MI), reached statistical significance at day 3 (1.048 ± 0.235; P<0.01 vs. sham-MI, P<0.01 vs. day 1), decreased at day 7 (0.863 ± 0.126; P ≤0.01 vs. sham-MI, P ≤0.01 vs. day 1, P>0.05 vs. day 3) and reduced gradually at day 14 (0.6 ± 0.108; P ≤0.05 vs. sham-MI, P ≤0.05 vs. day 1, P<0.01 vs. day 3, P<0.05 vs. day 7). The time-course of ADAM-15 mRNA expression was expressed as the ratio of ADAM-15 to β-actin.
Figure 1.
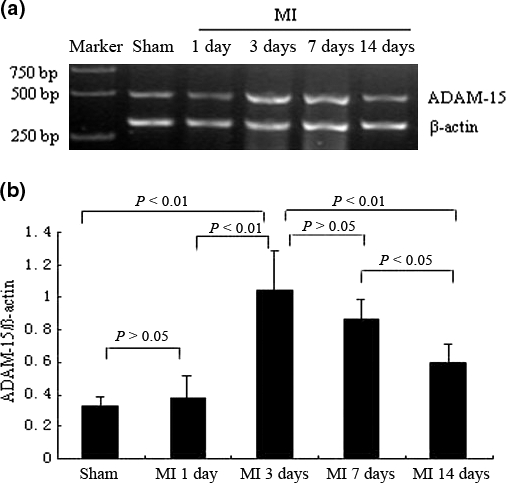
The mRNA expression of A disintegrin and metalloprotease-15 (ADAM-15) in rat myocardial infarction (MI). (a) reverse transcription-polymerase chain reaction (RT-PCR) analysis of ADAM-15 mRNA expression; and (b) The relative abundance of ADAM-15 by RT-PCR analysis is shown in the graph as percentage (mean ± SEM) from different time-course groups (n = 7).
ADAM-15 protein expression
The protein expression of ADAM-15 was detected by Western blot. As shown in Figure 2, ADAM-15 protein was expressed in a pattern, which was approximately correlated with the mRNA pattern. Compared with sham-MI (0.471 ± 0.029), the protein expression of ADAM-15 increased immediately at day 1 (0.71 ± 0.108; P<0.01 vs. sham-MI), reached to maximum at day 3 (1.071 ± 0.102; P<0.01 vs. sham-MI, P<0.01 vs. day 1), fell thereafter at day 7 (0.852 ± 0.127; P ≤0.01 vs. sham-MI, P ≥0.05 vs. day 1, P<0.05 vs. day 3), and reduced by degrees at day 14 (0.46 ± 0.041; P ≥0.05 vs. sham-MI, P ≤0.01 vs. day 1, P<0.01 vs. day 3, P<0.01 vs. day 7). The time-course of ADAM-15 protein expression was expressed as the ratio of ADAM-15 to β-actin.
Figure 2.
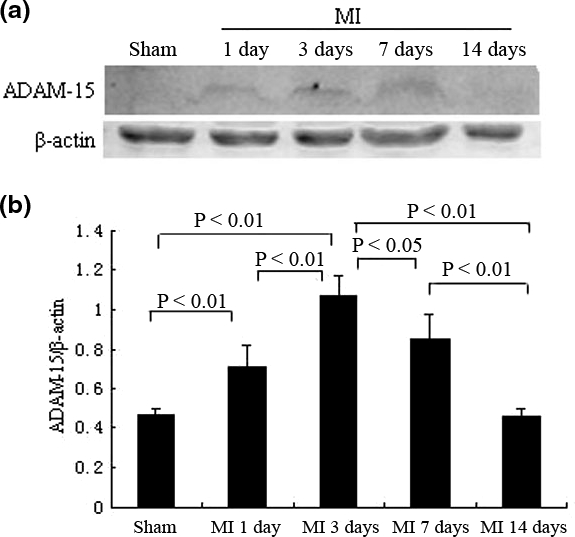
The protein expression of A disintegrin and metalloprotease-15 (ADAM-15) in rat myocardial infarction (MI). (a) Representative examples of ADAM-15 immunoblots are depicted; and (b) Bar graph showing relative abundance of ADAM-15 was by Western blot analysis in a time–course manner (n = 7).
Histology
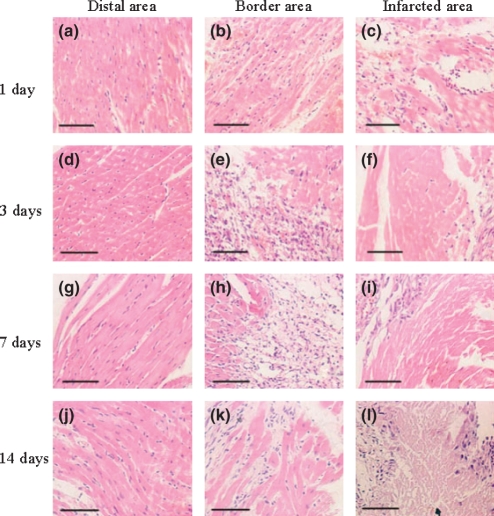
The H&E picture of sham-MI was not shown because its character was similar to that of the distal area following MI at day 1 (Figure 3a). Furthermore, there were no obvious changes in the distal area at day 1, 3, 7 and 14 following MI (Figure 3a, d, g, j). There were few macrophages in the border area at day 1 (Figure 3b), and the infarcted area was largely occupied by coagulative necrosis and haemorrhage (Figure 3c). There were intramural haemorrhage with widespread macrophages, neutrophils and fibroblast infiltration in the border area (Figure 3e), and the coagulative necrosis was aggravated in the infarcted area at day 3 (Figure 3f). Compared with day 3, less inflammatory cells infiltration and more fibroblast could be seen in the border area at day 7 (Figure 3h), while the myocardium was completely necrotic with fibroblasts in the infarcted area (Figure 3i). At day 14, it was characterized by fibrosis with interstitial collagen fibres, and a little infiltration of inflammatory cells in the border area (Figure 3k). There were multiple foci of myolysis and fibrosis in the infarcted area (Figure 3l).
Figure 3.
Haematoxylin and eosin (H&E) staining of rat myocardial infarction (MI). Histological features in distal, border and infarcted areas at 1, 3, 7 and 14 days of rat MI. (Bar = 50 μm).
Immunohistochemistry
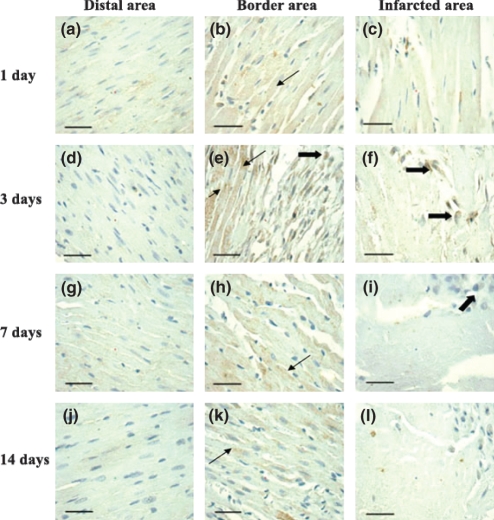
As shown in Figure 4, ADAM-15 was mainly expressed at cardiac myocytes (arrow) in the border area (Figure 4b, e, h, k) and some macrophages (thick-arrow) both in the border area (Figure 4e) and infarcted area (Figure 4f, i). In distal (Figure 4a, d, g, j) and infarcted areas (Figure 4c, f, i, l), the stained sections were weakly to moderately labelled. In addition, the time-course of ADAM-15 expression by the immunohistochemical staining in the border area was noted to correspond with those of RT-PCR and Western blot. Compared with sham-MI (0.039 ± 0.016), ADAM-15 expression increased at day 1 (0.116 ± 0.026; P<0.01 vs. sham-MI), reached maximum at day 3 (0.26 ± 0.052; P<0.01 vs. sham-MI, P<0.01 vs. day 1), and declined at day 7 (0.15 ± 0.035; P ≤0.01 vs. sham-MI, P ≥0.05 vs. day 1, P<0.01 vs. day 3), then fell again at day 14 (0.136 ± 0.023; P ≤0.01 vs. sham-MI, P ≥0.05 vs. day 1, P<0.01 vs. day 3, P>0.05 vs. day 7) (Figure 5).
Figure 4.
The localization of A disintegrin and metalloprotease-15 (ADAM-15) in rat myocardial infarction (MI) detected by immunohistochemistry. The representative localization of ADAM-15 (brown stained) were observed under light microscopy. ADAM-15 was mainly expressed at cardiac myocytes (arrow) in the border area and some macrophages (thick-arrow) both in the border area and infarcted area. (Bar = 40 μm).
Figure 5.
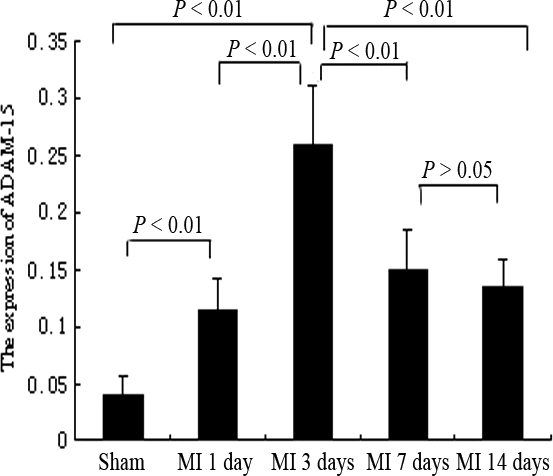
Semi-quantitative analysis of A disintegrin and metalloprotease-15 (ADAM-15) positive staining in border areas of rat myocardial infarction (MI) was in the graph as relative percentage (mean ± SEM). Similar results were obtained from three independent experiments.
Discussion
Inflammatory response and cardiac remodelling influence the pathological process of MI. They are major factors, which affect the progression of MI (Maekawa et al. 2004; Kruk et al. 2008). For defining new therapeutic targets that control the progression, novel biomolecules and mechanisms must be discovered (Fedak et al. 2006). This study indicated, for the first time, that ADAM-15 was not conserved in rat MI. Our results showed that: (i) ADAM-15 increased at day 1, reached to maximum level at day 3, then gradually decreased at day 7 and day 14 following MI; (ii) The localization of ADAM-15 was mainly at cardiac myocytes in the border area and some macrophages in the border and infarcted areas. Therefore, we consider that the time-course of ADAM-15 expression was similar to those of some inflammatory cells and remodelling gene or protein expressions in MI, indicating that it may be essential for inflammatory response and cardiac remodelling in MI.
Following MI, the heart undergoes a dynamic healing process, characterized by infiltration of inflammatory cells, activation of matrix metalloproteases (MMPs), degradation of extracellular matrix (ECM), and deposition of collagen, which are associated with LV dysfunction (Yang et al. 2002; Jugdutt 2003; Wang et al. 2007). Factors which impair the process of MI may deteriorate heart function. Inflammatory response is the necessary first step in wound healing, nevertheless exaggerated inflammatory cell infiltration may be detrimental (Gao et al. 2005). ADAMs are a unique class of MMPs (Spinale 2002). They are involved in inflammatory response and structural remodelling given their documented ability to activate cytokines and inflammatory factors (Fedak et al. 2003, 2004). Previous studies have indicated that ADAM-15 participated in some inflammatory disease or inflammatory response (Herren et al. 1997; Böhm et al. 1999, 2005; Al-Fakhri et al. 2003; Horiuchi et al. 2003; Mosnier et al. 2006). In this study, we observed that ADAM-15 increased at day 1, reached to maximum level at day 3, gradually decreased at day 7 and day 14 following MI (Figures 1, 2, 4, 5). The tendency was identical with that of the inflammatory response in MI, which has been confirmed in previous study (Yang et al.2002). It has been shown that inflammatory cells such as macrophages are major source of early inflammatory response (Gao et al. 2005). In this study, we also found from the H&E staining that some macrophages infiltrated in early inflammatory response following MI. Moreover, the result of immunohistochemistry showed that ADAM-15 was expressed in some macrophages in the border and infarcted areas. Taken together, these findings implicated that ADAM-15 may be one of the genes involved in the inflammatory response of MI.
ADAMs can profoundly affect tissue architectures just like MMPs (Spinale 2002; Fedak et al. 2006), which may provide a novel mechanism of cardiac remodelling. ADAM-15 can cleave type IV collagen and gelatin, just like MMP-2 and MMP-9 (Arndt et al. 2002; Martin et al. 2002; Fedak et al. 2006). Previous studies showed that MMP-2 and MMP-9 were mainly expressed at cardiac myocytes in MI border area (Wang et al. 2007; Van den Borne et al. 2009). Our results showed that ADAM-15 was mainly expressed at cardiac myocytes in MI border area. Furthermore, we confirmed that the time-course of ADAM-15 expression following MI was similar to those of MMP-2 and MMP-9 expression, which has been studied before (Wang et al. 2007). Besides that, ADAM-15 can bind to integrin αvβ3 and α5β1 in an RGD-dependent manner (Zhang et al. 1998; Nath et al. 1999; Elsherif et al. 2008), and to α9β1 in an RGD-independent manner (Eto et al. 2000, 2002). Integrin β1 is an important integrin in the heart, which is involved in cardiac fibrosis, heart failure (Shai et al. 2002; Elsherif et al. 2008) and ischaemic cardiomyopathy (ICM) (Pfister et al. 2007). In heart, ADAM-15 maybe the shedding enzyme of integrin β1 (Arndt et al. 2002; Fedak et al. 2006). Therefore, we presumed that the function of ADAM-15 involving in cardiac remodelling may be like MMPs, or through integrin β1 and other integrins in RGD-dependent or independent manner. However, the mechanisms need to be further studied.
Every domain of ADAM-15 has its own function: the catalytic activation is the metalloprotease domain; the disintegrin and cysteine-rich domain may mediate cell–cell or cell–matrix interactions, and the cytoplasmic domain may have a role in signalling or intracellular transportation (Schlondorff & Blobel 1999; Martin et al. 2002; Horiuchi et al. 2003; Elsherif et al. 2008). ADAM-15 has many functions such as: proliferation, adhesion and angiogenesis (McCulloch et al. 2000; Poghosyan et al. 2002) except inflammatory response and cardiac remodelling. Therefore, those functions of ADAM-15 may also have some important roles in the MI pathological process, which need to be further studied.
Conclusion
This study revealed a significant difference of ADAM-15 expression in rat MI and indicated that ADAM-15 may be one of the important factors involved in inflammatory response and cardiac remodelling. Therefore, it is necessary to further elucidate the underlying mechanisms of ADAM-15 in rat MI; eventually, it would be more helpful in clinical practice of human MI in the future.
Acknowledgments
This work was supported in part by a grant from the Innovation Fund of Heilung-kiang Province (YJSCX2008-111HLJ). We thank all of the members of our laboratory for technical assistance and helpful discussions.
References
- Al-Fakhri N, Wilhelm J, Hahn M, et al. Increased expression of disintegrin-metalloproteinases ADAM-15 and ADAM-9 following upregulation of integrins alpha5beta1 and alphavbeta3 in atherosclerosis. J. Cell. Biochem. 2003;89:808–823. doi: 10.1002/jcb.10550. [DOI] [PubMed] [Google Scholar]
- Arndt M, Lendeckel U, Röcken C, et al. Altered expression of ADAMs (A Disintegrin And Metalloproteinase) in fibrillating human atria. Circulation. 2002;105:720–725. doi: 10.1161/hc0602.103639. [DOI] [PubMed] [Google Scholar]
- Ben-Dor I, Hardy B, Fuchs S, et al. Repeated low-dose of erythropoietin is associated with improved left ventricular function in rat acute myocardial infarction model. Cardiovasc. Drugs Ther. 2007;21:339–346. doi: 10.1007/s10557-007-6049-8. [DOI] [PubMed] [Google Scholar]
- Black R, White J. ADAMs: focus on the protease domain. Curr. Opin. Cell Biol. 1998;10:654–659. doi: 10.1016/s0955-0674(98)80042-2. [DOI] [PubMed] [Google Scholar]
- Böhm BB, Aigner T, Gehrsitz A, Blobel CP, Kalden JR, Burkhardt H. Up-regulation of MDC15 (metargidin) messenger RNA in human osteoarthritic cartilage. Arthritis Rheum. 1999;42:1946–1950. doi: 10.1002/1529-0131(199909)42:9<1946::AID-ANR21>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Böhm BB, Aigner T, Roy B, Brodie TA, Blobel CP, Burkhardt H. Homeostatic effects of the metalloproteinase disintegrin ADAM15 in degenerative cartilage remodeling. Arthritis Rheum. 2005;52:1100–1109. doi: 10.1002/art.20974. [DOI] [PubMed] [Google Scholar]
- Campos SG, Zanetoni C, Scarano WR, Vilamaior PS, Taboga SR. Age-related histopathological lesions in the Mongolian gerbil ventral prostate as a good model for studies of spontaneous hormone-related disorders. Int. J. Exp. Pathol. 2008;89:13–24. doi: 10.1111/j.1365-2613.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier-Hisamuddin L, Laboisse CL, Merlin D. ADAM-15: a metalloprotease that mediates inflammation. FASEB J. 2008;22:641–653. doi: 10.1096/fj.07-8876rev. [DOI] [PubMed] [Google Scholar]
- Ding Rocks N, Paulissen G, El Hour M, et al. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie. 2008;90:369–379. doi: 10.1016/j.biochi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Elsherif L, Huang MS, Shai SY, et al. Combined deficiency of dystrophin and beta1 integrin in the cardiac myocyte causes myocardial dysfunction, fibrosis and calcification. Circ. Res. 2008;102:1109–1117. doi: 10.1161/CIRCRESAHA.108.173153. [DOI] [PubMed] [Google Scholar]
- Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin α9β1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J. Biol. Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- Eto K, Huet C, Tarui T, et al. Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1: implications for sperm-egg binding and other cell interactions. J. Biol. Chem. 2002;277:17804–17810. doi: 10.1074/jbc.M200086200. [DOI] [PubMed] [Google Scholar]
- Fedak PW, Altamentova SM, Weisel RD, et al. Matrix remodeling in experimental and human heart failure: a possible regulatory role for TIMP-3. Am. J. Physiol. Heart. Circ. Physiol. 2003;284:H626–H634. doi: 10.1152/ajpheart.00684.2002. [DOI] [PubMed] [Google Scholar]
- Fedak PW, Smookler DS, Kassiri Z, et al. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation. 2004;110:2401–2409. doi: 10.1161/01.CIR.0000134959.83967.2D. [DOI] [PubMed] [Google Scholar]
- Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure: from molecules to man (part I) Cardiovasc. Pathol. 2005;14:1–11. doi: 10.1016/j.carpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Fedak PW, Moravec CS, McCarthy PM, et al. Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation. 2006;113:238–245. doi: 10.1161/CIRCULATIONAHA.105.571414. [DOI] [PubMed] [Google Scholar]
- Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc. Res. 2005;65:469–477. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Herlitz J, Karlson BW, Sjölin M, Lindqvist J. Ten year mortality in subsets of patients with an acute coronary syndrome. Heart. 2001;86:391–396. doi: 10.1136/heart.86.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herren B, Raines EW, Ross R. Expression of a disintegrin-like protein in cultured human vascular cells and in vivo. FASEB J. 1997;11:173–180. doi: 10.1096/fasebj.11.2.9039960. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Weskamp G, Lum L, et al. Potential role for ADAM15 in pathological neovascularization in mice. Mol. Cell. Biol. 2003;23:5614–5624. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L, Nelson KK, Maciewicz RA, Blobel CP. Interaction of the metalloprotease disintegrins MDC9 and MDC15 with two SH3 domain-containing proteins, endophilin I and SH3PX1. J. Biol. Chem. 1999;274:31693–31699. doi: 10.1074/jbc.274.44.31693. [DOI] [PubMed] [Google Scholar]
- Jugdutt BL. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- Kratzschmar J, Lum L, Blobel CP. Metargidin, a membraneanchored metalloprotease-disintegrin protein with an RGD integrin binding sequence. J. Biol. Chem. 1996;271:4593–4596. doi: 10.1074/jbc.271.9.4593. [DOI] [PubMed] [Google Scholar]
- Kruk M, Przyłuski J, Kalińczuk Ł, et al. Myocardial Infarction Registry Group. Association of non-specific inflammatory activation with early mortality in patients with ST-elevation acute coronary syndrome treated with primary angioplasty. Circ. J. 2008;72:205–211. doi: 10.1253/circj.72.205. [DOI] [PubMed] [Google Scholar]
- Maekawa Y, Anzai T, Yoshikawa T, et al. Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2004;44:1510–1520. doi: 10.1016/j.jacc.2004.05.083. [DOI] [PubMed] [Google Scholar]
- Martin J, Eynstone LV, Davies M, Williams JD, Steadman R. The role of ADAM15 in glomerular mesangial cell migration. J. Biol. Chem. 2002;277:33683–33689. doi: 10.1074/jbc.M200988200. [DOI] [PubMed] [Google Scholar]
- McCulloch DR, Harvey M, Herington AC. The expression of the ADAMs proteases in prostate cancer cell lines and their regulation by dihydrotestosterone. Mol. Cell. Endocrinol. 2000;167:11–21. doi: 10.1016/s0303-7207(00)00305-1. [DOI] [PubMed] [Google Scholar]
- Mosnier JF, Jarry A, Bou-Hanna C, Denis MG, Merlin D, Laboisse CL. ADAM15 upregulation and interaction with multiple binding partners in inflammatory bowel disease. Lab. Invest. 2006;86:1064–1073. doi: 10.1038/labinvest.3700465. [DOI] [PubMed] [Google Scholar]
- Nath D, Slocombe PM, Stephens PE, et al. Interaction of metargidin (ADAM-15) with αvβ3 and α5β1 integrins on different haemopoietic cells. J. Cell Sci. 1999;112:579–587. doi: 10.1242/jcs.112.4.579. [DOI] [PubMed] [Google Scholar]
- Pfister R, Acksteiner C, Baumgarth J, et al. Loss of beta1D-integrin function in human ischemic cardiomyopathy. Basic Res. Cardiol. 2007;102:257–264. doi: 10.1007/s00395-006-0640-1. [DOI] [PubMed] [Google Scholar]
- Poghosyan Z, Robbins SM, Houslay MD, Webster A, Murphy G, Edwards DR. Phosphorylation-dependent interactions between ADAM15 cytoplasmic domain and Src family protein-tyrosine kinases. J. Biol. Chem. 2002;277:4999–5007. doi: 10.1074/jbc.M107430200. [DOI] [PubMed] [Google Scholar]
- Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci. 1999;112:3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- Shai SY, Harpf AE, Babbitt CJ, et al. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ. Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ. Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur. J. Cardiothorac. Surg. 2006;30:353–361. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Van den Borne SW, Cleutjens JP, Hanemaaijer R. Increased matrix metalloproteinase-8 and -9 activity in patients with infarct rupture after myocardial infarction. Cardiovasc. Pathol. 2009;18:37–43. doi: 10.1016/j.carpath.2007.12.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wang F, Keimig T, He Q, et al. Augmented healing process in female mice with acute myocardial infarction. Gend. Med. 2007;4:230–247. doi: 10.1016/s1550-8579(07)80043-x. [DOI] [PubMed] [Google Scholar]
- Worley JR, Hughes DA, Dozio N, Gavrilovic J, Sampson MJ. Low density lipoprotein from patients with Type 2 diabetes increases expression of monocyte matrix metalloproteinase and ADAM metalloproteinase genes. Cardiovasc. Diabetol. 2007;6:21–25. doi: 10.1186/1475-2840-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Liu YH, Yang XP, Xu J, Kapke A, Carretero OA. Myocardial infarction and cardiac remodelling in mice. Exp. Physiol. 2002;87:547–555. doi: 10.1113/eph8702385. [DOI] [PubMed] [Google Scholar]
- Zhang XP, Kamata T, Yokoyama K, Puzon-McLaughlin W, Takada Y. Specific interaction of the recombinant disintegrin-like domain of MDC-15 (metargidin, ADAM-15) with integrin αvβ3. J. Biol. Chem. 1998;273:7345–7350. doi: 10.1074/jbc.273.13.7345. [DOI] [PubMed] [Google Scholar]
- Zornoff LA, Matsubara BB, Matsubara LS, Paiva SA, Spadaro J. Early rather than delayed administration of lisinopril protects the heart after myocardial infarction in rats. Basic Res. Cardiol. 2000;95:208–214. doi: 10.1007/s003950050183. [DOI] [PubMed] [Google Scholar]