Abstract
Ischaemia/reperfusion (I/R) is a major cause of heart failure. Recently cardiac stem cells (CSCs) were proposed as the most appropriate cell type for heart disease therapy. However, it is still unclear whether I/R can stimulate the CSCs homing to the injured myocardium. Male Sprague–Dawley rats were subjected to a 30-min ischaemia followed by reperfusion of different intervals. RT-PCR, western blotting and immunohistochemistry were performed to detect stem cell factor (SCF) expression at mRNA and protein levels respectively. Activation of nuclear factor-κB (NF-κB) was determined by electrophoretic mobility shift assay. To assess the homing of CSCs in vivo, BrdU-labelled CSCs were injected into AV-groove before induction of ischaemia and examined by immunofluorescent staining in the injured myocardium after I/R. From day 3 to day 6 after reperfusion, the accumulation of CSCs was significantly elevated in the injured area, which was matched with the increased SCF expression during I/R. Pretreatment of rats with NF-κB inhibitor, N-acetyl-l-cysteine (NAC) not only suppressed NF-κB activation induced by I/R but also attenuated SCF expression. Further analysis revealed that I/R induced phosphorylation of IκBα after 15 min of reperfusion, and the raised phosphor-IκBα returned to the basal level at 2 h of reperfusion. In simulated I/R(SI/R) in vitro, it enhanced NF-κB activation and SCF expression in cultured neonatal rat cardiomyocytes, which was markedly inhibited by NF-κB decoy oligodeoxynucleotide or NAC. Taken together, our results demonstrated that I/R induced CSCs homing to the injured myocardium by stimulating myocardial SCF expression via activation of NF-κB.
Keywords: cardiac stem cell, homing, ischaemia/reperfusion, nuclear factor-κB, stem cell factor
Ischaemia, which is caused by coronary artery occlusion, followed by reperfusion can evoke myocardial ischaemia/reperfusion (I/R) injury with the potential to induce cardiac dysfunction (Onai et al. 2004; Hu et al. 2007). Many treatments for ischaemic diseases, including coronary artery bypass graft surgery, angioplasty and thrombolytic therapy of the heart etc., can possibly lead to I/R injury (Neely et al. 1996; Levitsky 2006). In recent years, the possibilities of stem cell-mediated cardiac repair seem to be promising and inspiring. Comparing the advantages and disadvantages of different cell populations involved in myocardial repair, cardiac stem cells (CSCs) have been considered as the most appropriate cell type and interventional approach to reconstitute the myocardium and improve cardiac function after myocardial injury (Oh et al. 2003; Linke et al. 2005; Torella et al. 2005). Beltrami et al. firstly reported that CSCs are a pure population of cloned c-kit-positive cells that can give rise to cardiomyocytes, smooth muscle cells and vascular endothelium (Beltrami et al. 2003). Despite these landmark studies, it is still unclear whether I/R can stimulate the CSCs homing to injured regions, which may contribute to the improvement of cardiac function.
The activation and migration of stem cells, as we know, were usually regulated by some chemoattractant factors, such as stem cell factor (SCF), the ligand of the c-kit receptor. In early foetal life, c-kit+ cells colonized the yolk sack, liver and probably other organs, and the worthy of special attention was that the colonized organs all expressed SCF (Teyssier-Le Discorde et al. 1999). SCF/c-kit signalling pathway was involved in the proliferation, differentiation, survival as well as migration of stem and progenitor cells (Heissig et al. 2002; Vandervelde et al. 2005; Wang et al. 2007). The previous work in our laboratory reported that SCF mediated the CSCs migration after myocardial infarction (Kuang et al. 2008). However, it remains entirely unknown about the signal transduction pathways responsible for regulating the SCF expression during I/R.
The nuclear factor-κB (NF-κB) is a ubiquitous inducible transcription factor that plays key roles in regulating inflammatory and immune responses as well as stress responses (Li et al. 1999; Baldwin 2001). As far as the heart is concerned, NF-κB signalling pathway has been linked to several pathological processes in the myocardium including cardiomyocyte proinflammatory cytokine release, I/R injury, hypertrophy and apoptosis (Valen et al. 2001; Valen 2004; Hall et al. 2006) and NF-κB was also involved in the regulation of downstream genes expression during I/R (Li et al. 1999; Xiao 2004; Moss et al. 2007). Da Silva’s study firstly identified a NF-κB responsive element located in the first intron of the SCF gene (Da Silva et al. 2003), which provided the possibility of NF-κB involved in the regulation of SCF transcription, accordingly we wondered whether NF-κB activation can really play a role in regulating SCF expression during I/R.
Therefore, the aim of this study was to investigate whether I/R can induce CSCs homing to the injured myocardium by stimulating myocardial SCF expression via activation of NF-κB.
Methods
Animal models and experimental protocols
The procedure for rat I/R model was performed as previously described with a minor modification (Gao et al. 2002b). In brief, Male Sprague–Dawley rats (250–300 g, purchased from the Animal Experimental Centre of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China) were anaesthetized with intraperitoneal sodium pentobarbital. After the rats were fully anaesthetized, they underwent a left lateral thoracotomy and myocardial ischaemia was produced by exteriorizing the heart and then placing a suture (6.0 nylon) to make a slipknot around the left anterior descending coronary artery (LAD). After a 30-min ischaemia, the slipknot was released and the myocardium was reperfused for different intervals. At the end of reperfusion, the coronary artery was again occluded and 1 ml of 2% Evans blue dye was injected into the left ventricular cavity. The injured myocardium by I/R was Evan’s blue stained negative area (area-at-risk, AAR) and on the basis of visual inspection it was excised for the further experiments. Additionally, 2,3,5-triphenyltetrazolium chloride (TTC) staining was performed on sections from AAR to assess the infarct size. All procedures were performed in accordance with the Guidelines of the Hubei Council of Animal Care and approved by the Animal Use Subcommittee at the Huazhong University of Science and Technology, China.
Cardiac haemodynamics
Haemodynamic measurements were made in a separate group of animals exposed to a 30-min ischaemia and then released the slipknot for the reperfusion. After 3 weeks of reperfusion, rats were anaesthetized again and the right carotid artery was instrumented with 1.4F microtip pressure transducer catheter. After determination of heart rate and arterial blood pressure, the catheter was advanced into the left ventricle (LV) for the measurement of LV systolic pressures (LVSP) and end-diastolic pressures (LVEDP) as well as the maximal rate of pressure development (+dP/dtmax) and rate of relaxation (−dP/dtmin) of LV.
Isolation and culture of CSCs from the adult rat heart
The CSCs were isolated by magnet-activated cell sorting (MACS) system (Dynal Biotech, Oslo, Norway) from the hearts of male Sprague–Dawley rats as described previously (Beltrami et al. 2003; Kuang et al. 2008). Briefly, The heart was excised and the aorta was cannulated rapidly, followed by perfused with Ca2+-free Tyrode solution for 10 min, and then digested by 0.5 mg/ml collagenase (Sigma, St. Louis, MO, USA) and 0.05 mg/ml trypsin (Difco, Kansas, MO, USA) at 37 °C for 30 min. Next, the heart tissue was chopped and cell suspension was filtered with a strainer (Becton Dickson, Franklin Lakes, NJ, USA). Afterwards, cells were incubated with a rabbit anti-c-kit antibody (1:150, Santa Cruz, CA, USA) and separated by using immunomagnetic microbeads (Dynal Biotech). These CSCs were cultured for 3–5 days with Dulbecco’s MEM (Invitrogen, Carlsbad, CA, USA) containing foetal calf serum, bFGF and LIF at 37 °C. After recovery, they were used for subsequent experiments.
Assessment of CSC migration in vivo during I/R
To assess the migration of CSCs in vivo after I/R, the cultured CSCs were labelled with BrdU (Zymed, San Francisco, CA, USA) as described previously (Barbash et al. 2003), and then the labelled cells (2 × 106) were injected into AV-groove before induction of ischaemia. After reperfusion, at least five frozen sections from the injured myocardium of per heart were randomly selected and detected by immunofluorescent staining with mouse anti-BrdU (Zymed) antibody. For each section 10 fields in peri-infarcted areas were randomly chosen by using a defined rectangular field area (×40 objective) to count the BrdU+ cells and then calculate the number of the BrdU+ cells per mm2. Imaging the cells by transmitted light and fluorescence inverted microscope (IX71; Olympus, Tokyo, Japan) linked to a personal computer.
Isolation of neonatal rat cardiomyocytes and the simulated I/R treatment
Ventricular myocytes were isolated from 1- to 2-day-old Sprague–Dawley rats and submitted to primary culture as reported previously (Yue et al. 1998). Simulated I/R (SI/R) was performed as described with a minor modification (Yue et al. 2000; Gao et al. 2002a). Twenty-four hours after plating, ischaemia of the cardiomyocytes was induced by replacing medium with a serum-/glucose-free DMEM and exposing cells to an air-tight chamber continually gassed with 95%N2–5%CO2 at 37 °C for 2 h; the medium was then replaced with the maintenance medium and exposed to normoxic environment for 4 h to cause reperfusion injury. Control cells were left in normoxic incubator at 37 °C with a normal glucose for 6 h.
ELISA
A sensitive ELISA procedure was used to quantify immunoreactive SCF released into the supernatant of cultured cardiac myocytes. The ELISA was performed according to the instructions provided by the manufacturer (R&D systems, Minneapolis, MN, USA). A polystyrene microplate (96-wells) was coated with a rabbit polyclonal anti-SCF antibody and recombinant rat SCF was used as the standard.
Immunohistochemical staining
After I/R, heart slices from injured myocardium were prepared for immunohistochemical staining by using rabbit polyclonal antibodies (1:100; Pepro Tech EC Ltd, London, UK) against rat SCF overnight at 4 °C (for negative control studies, the antibodies were substituted by phosphate-buffered saline). Endogenous peroxidase was blocked by 0.3% H2O2 for 20 min at room temperature and the biotin-conjugated anti-rabbit immunoglobulin (1:200, Dako, Glostrup, Denmark) was used as the secondary antibody. After incubation with streptavidin peroxidase, visualization of peroxidase localization was performed using diaminobenzidine-hydrogen peroxide (DAB-H2O2) substrate to give a brown colour.
RNA extraction and RT-PCR
Total RNA was extracted with Trizol reagent (Invitrogen) following the manufacturer’s protocols. RT-PCR was carried out by using the pairs of primers (Invitrogen) as following for semiquantitative assessment, SCF: sense, 5′-TGTTTTGCCTAGTCATTGTTG-3′; anti-sense, 5′-TGTCATTCCTAAGGGAACTG-3′, yielding a 404 bp product (Konrad et al. 2005); β-actin: sense, 5′-CGTTGACATCCGTAAAGA-3′; anti-sense, 5′-AGCCACCAATCCACACAG-3′, yielding a 173 bp product. The products of PCR were separated by 1.5% agarose gel electrophoresis and visualized under UV using gel documentation system (Bio-Rad Gel Doc1000, Bio-Rad, Hercules, CA, USA). The β-actin was used as an internal standard to verify equal PCR product loading for each experiment.
Western blotting analysis
Tissue samples were homogenized and separated by SDS–polyacrylamide gel (12.5%) electrophoresis followed by electrophoretic transfer of proteins from the gel to a nitrocellulose membrane (Bio-Rad). The membranes were probed for the following primary antibodies: rabbit anti-SCF (1:500) or mouse anti-phospho-IκBα (1:400) or mouse anti-IκBα antibody (1:500) (all from Santa Cruz Biotechnology) overnight at 4 °C. Bands were visualized by using corresponding horseradish peroxidase (HRP)-conjugated anti-biotin antibody and enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA).
Electrophoretic mobility shift assay
Nuclear proteins were isolated by a method described previously (Nho & O’Dwyer 2004). Electrophoretic mobility shift assay (EMSA) was performed to determine the NF-κB-DNA binding activity. In brief, nuclear proteins (10 μg) were incubated with the reaction buffer for 20 min at room temperature, followed by incubation with oligonucleotide containing the consensus sequence for the NF-κB-DNA binding site (5′-AGAGTGGGAATTTCCACTCA-3′) (Ueda et al. 1997) (Invitrogen). The reaction mixture was separated in a non-denaturing polyacrylamide gel (6%) that was later stained by SYBR Green EMSA staining solution from Molecular Probes (Invitrogen) with continuous, gentle agitation for about 20 min, protected from light. Then washed the gel in 150 ml of dH2O and visualized the stained nucleic acids and documented the image under UV using gel documentation system (Bio-Rad Gel Doc1000).
Statistical analysis
All data are expressed as mean ± SEM. For analysis of differences between two groups, Student’s t test was performed. For multiple groups, anova was carried out followed by Student–Newman–Keuls test. The level of statistical significance was set at P<0.05.
Results
SCF expression in the injured myocardium during I/R
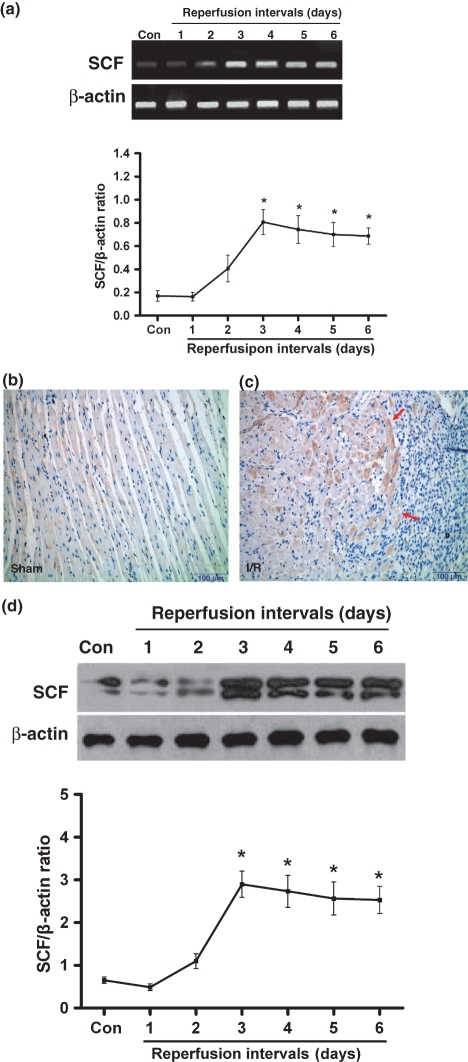
As shown in Figure 1a, semiquantitative analysis of SCF mRNA expression in the ischaemic/reperfused myocardium by rats was illustrated. Low level of constitutive SCF mRNA expression was noted in the sham-operated group and significant up-regulation of SCF mRNA was occurred in the I/R group after 3 days of reperfusion. No significant induction of SCF mRNA was noted with shorter reperfusion intervals (1–2 days of reperfusion). To further confirm this result, SCF expression was detected at protein level by immunohistochemical staining (Figure 1b,c) and western blotting analysis (Figure 1d). The results manifested that SCF protein expression was markedly increased after 3 days of reperfusion compared with the sham-operated.
Figure 1.
SCF expression in rat ischaemic samples undergoing different reperfusion intervals. (a) SCF mRNA expression by RT-PCR. (b) SCF protein expression by immunohistochemical staining in sham or (c) at 3 days after I/R (↑indicates SCF expression in the peri-infarcted myocardium). (d) SCF protein levels by western blotting analysis. n=5–7 per group. Results are depicted as mean ± SEM. *Means P<0.05 vs. sham-operated (control).
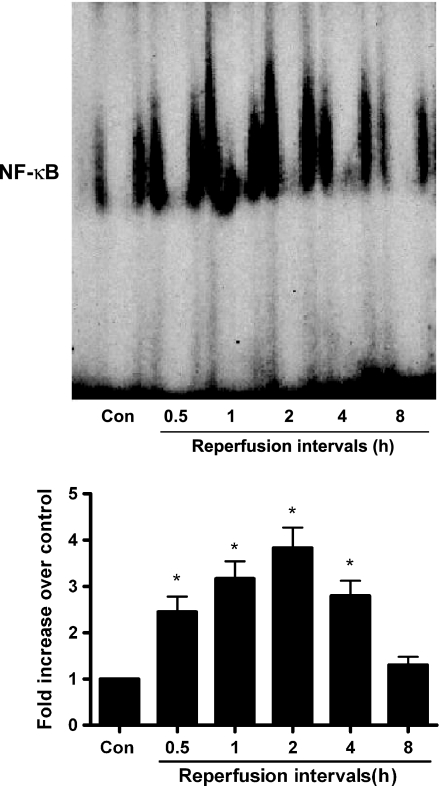
NF-κB-DNA binding activity in the injured myocardium during I/R
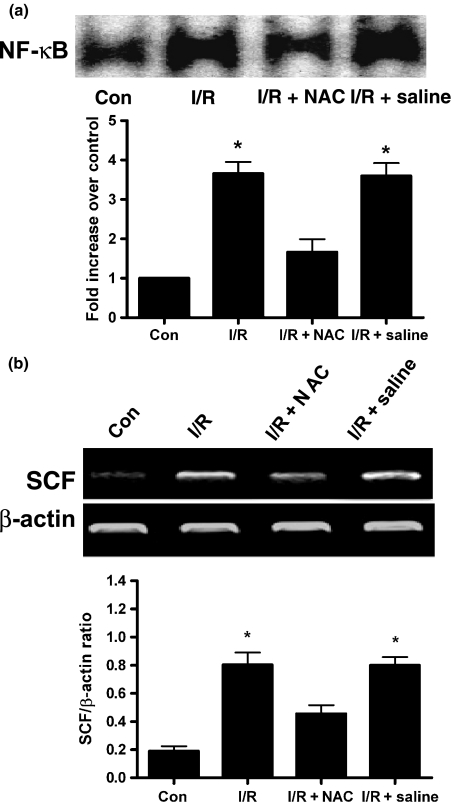
To investigate whether NF-κB was activated in the injured heart tissues during I/R, EMSA was performed. As shown in Figure 2, NF-κB activation was evident at 30 min of reperfusion and reached its maximum at 2 h of reperfusion. To further determine whether NF-κB activation was necessary for I/R-mediated SCF expression, an inhibitor of NF-κB activation, N-acetyl-l-cysteine (NAC) (Sigma) was administered to rats intrapenetorially 30 min before ischaemia and at the beginning of reperfusion (Kin et al. 2006). As shown in Figure 3, NAC treatment significantly blocked the I/R-stimulated NF-κB activation as well as abolished the enhanced SCF mRNA expression.
Figure 2.
NF-κB-DNA binding activity in rat injured tissues during I/R. Upon I/R, nuclei proteins were isolated and EMSA was performed to determine NF-κB-DNA binding activity. n=5 to 7 per group. Results are depicted as mean ± SEM. *Means P<0.05 vs. sham-operated (control).
Figure 3.
Effect of inhibitor NAC on NF-κB-DNA binding activity and SCF mRNA expression in rat injured tissues after I/R injury. Thirty minutes prior to the induction of ischaemia and at the beginning of reperfusion, rats were twice treated with the inhibitor of NF-κB, NAC (200 mg/kg) or saline (0.9% NaCl) by intraperitoneal injection. (a) Nuclear proteins were isolated for EMSA after 2 h of reperfusion to investigate the effect of NAC on NF-κB activation. (b) Total RNA was prepared for RT-PCR after 3 days of reperfusion to examine the effect of NAC on SCF expression. n=5–7 per group. Results are depicted as mean ± SEM. *Means P<0.05 vs. sham-operated (control) or I/R + NAC.
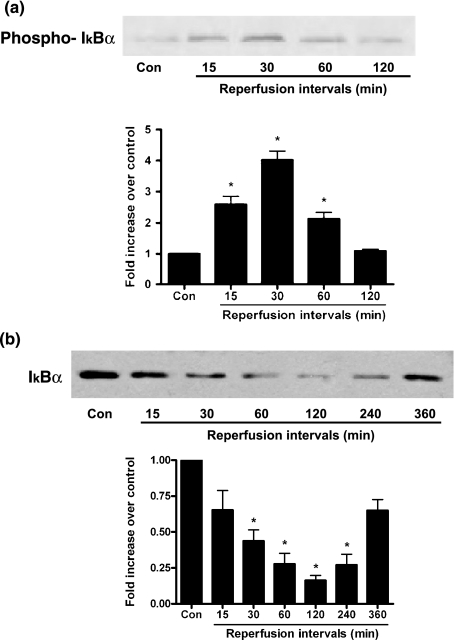
Phosphorylation of IκBα in the injured myocardium during I/R
The activation of NF-κB might be caused by enhanced phosphorylation and degradation of the inhibitor protein IκBα. The level of phospho-IκBα was significantly elevated in the injured myocardium after 15 min of reperfusion and reached a peak at 30 min of reperfusion (Figure 4a). Moreover, we also investigated the total protein levels of IκBα, as shown in Figure 4b, the 30 min of reperfusion treatment caused an obvious reduction of IκBα protein content and reached its minimum at 2 h of reperfusion.
Figure 4.
Representative figures of Phospho-IκBα and IκBα protein expression during I/R. Total proteins were prepared from rat injured tissues during I/R and western blotting analysis was performed to determine the protein levels of (a) phospho-IκBα, (b) total IκBα levels. n=5–7 per group. Results are depicted as mean ± SEM. *Means P<0.05 vs. sham-operated (control).
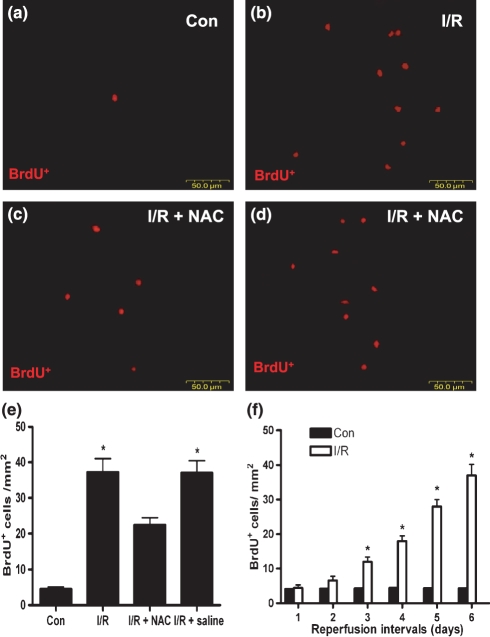
CSCs homing to the injured myocardium during I/R
To understand whether the increased SCF led to the more accumulation of CSCs in the injured area after I/R, the assessment of CSCs homing in vivo was performed by using BrdU-labelled CSCs. Following the extending of reperfusion interval, statistically significant increase of CSCs in the peri-infarcted area was noted after 3 days of reperfusion compared with the sham-operated and for shorter reperfusion intervals, there was no obvious difference (Figure 5f). Moreover, the present results also showed pretreatment of rats with NAC to weaken I/R-induced SCF expression had an obvious decrease on CSCs homing to the injured myocardium compared with non-NAC treatment (Figure 5a–e).
Figure 5.
Representative figures of BrdU-labelled CSCs accumulation in peri-infacted myocardium. The migrated CSCs were detected by using TRITC-conjugated goat anti-mouse IgG after 6 days of reperfusion. (a) sham-operated group (control). (b) I/R group. (c) I/R + NAC group. (d) I/R + saline group. (f) The trend of enhanced CSCs accumulation during the reperfusion intervals. n=5–7 per group. Results are depicted as mean ± SEM. *Means P<0.05 vs. sham-operated (control) or I/R + NAC.
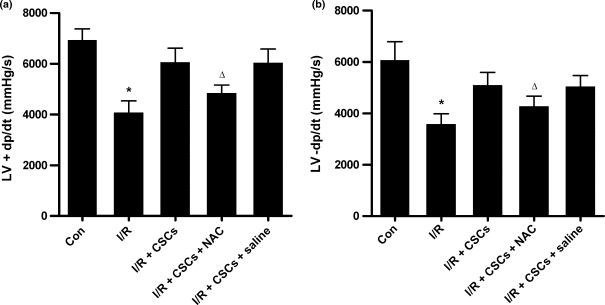
Cardiac function
To ascertain that CSCs homing to the injured myocardium during I/R was related with the change of cardiac function, the cardiac function was measured at 3 weeks after I/R. As shown in Figure 6, injection of CSCs into the AV-groove resulted in the recovery of cardiac function after I/R, and the reduced CSCs homing caused by pretreatment with NAC led to the decreased LV +dP/dtmax and −dP/dtmin compared with non-NAC treatment. However, there were no significant differences in heart rate, mean arterial pressure, or LVSP and LVEDP between NAC treatment and non-NAC treatment after I/R.
Figure 6.
Cardiac function was measured at 3 weeks of reperfusion after a 30-min ischaemia. The changes of LV +dP/dtmax and −dP/dtmin were shown in (a) and (b) respectively. n=5–7 per group. Results are depicted as mean ± SEM. *Means P<0.05 vs. sham-operated (control) or I/R + CSCs; Δ means P<0.05 vs. I/R + CSCs or I/R + CSCs + saline.
NF-κB activation involved in SI/R-induced SCF expression in cultured cardiomyocytes
To confirm the causative role of NF-κB activation in the myocardial SCF expression induced by SI/R, the cardiomyocytes were transiently transfected with the NF-κB decoy oligodeoxynucleotide (ODN) (Invitrogen) (Vos et al. 2000) or treated with NAC before SI/R, and then NF-κB activity as well as SCF expression were examined in the cells. As shown in Figure 7, treatment with NF-κB decoy ODN or NAC markedly contributed to the blockage of the NF-κB activation, and subsequently attenuated the SI/R-induced myocardial SCF expression examined by RT-PCR and ELISA respectively.
Figure 7.
NF-κB activation and SCF expression in cultured cardiomyocytes exposed to SI/R. Twenty-four hours after plating, the myocytes were randomized to receive one of the following treatments before exposed to SI/R: (i) Control; (ii) Simulated I/R (SI/R); (iii) SI/R + NF-κB decoy ODN (cardiomyocytes were transiently transfected with lipofectin containing 1 mM decoy ODN); (iv) SI/R + NAC (10 mM). (a) Activation of NF-κB by EMSA. (b) SCF mRNA expression by RT-PCR. (c) SCF protein expression by ELISA on the supernatant of the cultured cardiomyocytes. Results are depicted as mean ± SEM from five independent experiments. *Means P<0.05 vs. SI/R.
Discussion
The study of Fazel et al. suggested that stem cell-mediated myocardial repair was dependent on the activation of the c-kit receptor (Fazel et al. 2006), which was expressed on bone marrow stem cells (BMSCs) and CSCs, and can be activated by its ligand SCF. Administration of SCF can promote lin-c-kit+ BMSCs homing to the injured myocardium (Lutz et al. 2008). However, concerning the ability of exogenous stem cells to acquire cardiac cell lineages and reconstitute the myocardium lost during the process of heart injury, there always exists a controversy (Balsam et al. 2004; Murry et al. 2004; Nygren et al. 2004). In recent years, the recognition that the adult heart possesses a cardiac stem cell pool that can regenerate myocytes and coronary vessels has raised the unique possibility to rebuild the injured heart (Leri et al. 2005; Linke et al. 2005). However, it remains unclear whether CSCs can home to the injured myocardium during I/R to contribute the effective repair.
In this study, our results showed that notable up-regulation of SCF at mRNA and protein levels was occurred in the injured myocardium of rats after suffering a 30-min coronary occlusion and 3 days of reperfusion, which was just coincident with the increase of BrdU-labelled CSCs that exactly started statistically significant accumulating after 3 days of reperfusion. Meanwhile, pretreatment of rats with NF-κB inhibitor attenuated the I/R-induced SCF expression, leading to the reduced CSCs accumulation in the injured myocardium. What’s more, the cardiac function was measured to illustrate the ability of CSCs to promote the myocardial repair after I/R, and our data documented that more accumulation of CSCs in the injured area really contributed to the recovery of cardiac function. Accordingly, on the basis of the present results, we have the ambition to believe that I/R-induced SCF expression can effectively attract CSCs homing to the injured area and potentially mediate a therapeutic effect.
Although SCF played a pivotal role in chemotaxis and homing of CSCs during I/R, at present, it remains entirely unknown about the signal transduction mechanism underlying the SCF expression in this process. A previous study had revealed that there existed the NF-κB binding site in the SCF gene (Da Silva et al. 2003; Fazel et al. 2008); however, the role of the transcription factor in I/R-induced SCF expression is still unknown. In this study, several lines of evidence clearly indicated that NF-κB was responsible for I/R-stimulated SCF expression. Firstly, the results from EMSA clearly demonstrated that NF-κB was activated during I/R. Secondly, the finding that inhibiting the activation of NF-κB significantly reversed I/R-induced SCF expression, suggested that the activated NF-κB was necessary for SCF expression during I/R.
Under normal conditions, NF-κB in the cytoplasm is maintained in an inactive form because of binding to the inhibitor proteins known as IκBs, which retain NF-κB dimers in the cytoplasm (Scheidereit 2006). Among the seven IκB family members – IκBα, IκBβ, BCL-3, IκBε, IκBγ and the precursor proteins p100 and p105, IκBα is the best-characterized form of IκBs (Hayden & Ghosh 2004). The pathways of NF-κB activation have been intensely illustrated by many investigators. Most studies (Karin & Lin 2002; Xiao 2004; Luo et al. 2005) have demonstrated that upon stimulation, IκBα is rapidly phosphorylated, leading to the ubiquitination and subsequent degradation of IκBα. The liberated NF-κB dimers then translocate to the nucleus, where they regulate the transcription of target genes. To elucidate the activation mechanisms of NF-κB during I/R injury, we explored the level of phospho-IκBα at first, and the results demonstrated that phospho-IκBα was significantly elevated after 15 min of reperfusion, and returned to the basal level at 2 h of reperfusion. Further investigation revealed that the total IκBα protein was significantly decreased after 30 min of reperfusion in heart tissues, therefore, the rapid phosphorylation of IκBα protein might serve as a signal for degradation of this inhibitory protein during the early stage of I/R. Based on the present results, it was deduced that the initial increase in the level of phospho-IκBα and a subsequent decrease in IκBα protein expression may be responsible for the activation of NF-κB, eventually leading to the enhanced SCF expression.
Additionally, the cultured cardiomyocytes were exposed to SI/R to ascertain the causative role of NF-κB activation in the myocardial SCF expression in vitro. Our data documented that SI/R markedly induced the SCF expression of cardiomyocytes, in which NF-κB activation was greatly involved.
Taken together, in this study we reported that CSCs can be recruited to repair the injured myocardium by the increased endogenous myocardial SCF via NF-κB activation during I/R. However, the study of Stephanie et al. demonstrated that ageing can impair the beneficial effect of SCF on postmyocardial infarction remodelling (Lehrke et al. 2006). Therefore, exploration of favourable or unfavourable factors that influence the effect of SCF will be indispensable to optimize the future strategy of CSCs therapy on I/R-induced heart disease.
Acknowledgments
This study was supported in part by research grants 30470710 from the National Natural Science Foundation of China, NCET-04-0711 from the Program for New Century Excellent Talents in University, and 2005ABB009 from the Natural Science Foundation of Hubei.
References
- Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J. Clin. Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Da Silva CA, Heilbock C, Kassel O, Frossard N. Transcription of stem cell factor (SCF) is potentiated by glucocorticoids and interleukin-1beta through concerted regulation of a GRE-like and an NF-kappaB response element. FASEB J. 2003;17:2334–2336. doi: 10.1096/fj.03-0136fje. [DOI] [PubMed] [Google Scholar]
- Fazel S, Cimini M, Chen L, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J. Clin. Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel SS, Chen L, Angoulvant D, et al. Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. FASEB J. 2008;22:930–940. doi: 10.1096/fj.07-8636com. [DOI] [PubMed] [Google Scholar]
- Gao F, Gao E, Yue TL, et al. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002a;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- Gao F, Yue TL, Shi DW, et al. p38 MAPK inhibition reduces myocardial reperfusion injury via inhibition of endothelial adhesion molecule expression and blockade of PMN accumulation. Cardiovasc. Res. 2002b;53:414–422. doi: 10.1016/s0008-6363(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J. Mol. Cell. Cardiol. 2006;41:580–591. doi: 10.1016/j.yjmcc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Dai S, Wu WJ, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kin H, Wang NP, Halkos ME, Kerendi F, Guyton RA, Zhao ZQ. Neutrophil depletion reduces myocardial apoptosis and attenuates NFkappaB activation/TNFalpha release after ischemia and reperfusion. J. Surg. Res. 2006;135:170–178. doi: 10.1016/j.jss.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Konrad L, Munir Keilani M, Cordes A, et al. Rat Sertoli cells express epithelial but also mesenchymal genes after immortalization with SV40. Biochim. Biophys. Acta. 2005;1722:6–14. doi: 10.1016/j.bbagen.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kuang D, Zhao X, Xiao G, et al. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res. Cardiol. 2008;103:265–273. doi: 10.1007/s00395-007-0690-z. [DOI] [PubMed] [Google Scholar]
- Lehrke S, Mazhari R, Durand DJ, et al. Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ. Res. 2006;99:553–560. doi: 10.1161/01.RES.0000238375.88582.d8. [DOI] [PubMed] [Google Scholar]
- Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol. Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- Levitsky S. Protecting the myocardial cell during coronary revascularization. The William W. L. Glenn Lecture. Circulation. 2006;114:I339–I343. doi: 10.1161/CIRCULATIONAHA.105.001685. [DOI] [PubMed] [Google Scholar]
- Li C, Browder W, Kao RL. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am. J. Physiol. 1999;276:H543–H552. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- Linke A, Muller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl. Acad. Sci. U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death–a new approach to cancer therapy. J. Clin. Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M, Rosenberg M, Kiessling F, et al. Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit + bone marrow-derived stem cells. Cardiovasc. Res. 2008;77:143–150. doi: 10.1093/cvr/cvm027. [DOI] [PubMed] [Google Scholar]
- Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH. IKKbeta inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2248–H2253. doi: 10.1152/ajpheart.00776.2007. [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Neely CF, DiPierro FV, Kong M, Greelish JP, Gardner TJ. A1 adenosine receptor antagonists block ischemia-reperfusion injury of the heart. Circulation. 1996;94:II376–II380. [PubMed] [Google Scholar]
- Nho CW, O’Dwyer PJ. NF-kappaB activation by the chemopreventive dithiolethione oltipraz is exerted through stimulation of MEKK3 signaling. J. Biol. Chem. 2004;279:26019–26027. doi: 10.1074/jbc.M309022200. [DOI] [PubMed] [Google Scholar]
- Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai Y, Suzuki J, Kakuta T, et al. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004;63:51–59. doi: 10.1016/j.cardiores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25:6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- Teyssier-Le Discorde M, Prost S, Nandrot E, Kirszenbaum M. Spatial and temporal mapping of c-kit and its ligand, stem cell factor expression during human embryonic haemopoiesis. Br. J. Haematol. 1999;107:247–253. doi: 10.1046/j.1365-2141.1999.01725.x. [DOI] [PubMed] [Google Scholar]
- Torella D, Ellison GM, Nadal-Ginard B, Indolfi C. Cardiac stem and progenitor cell biology for regenerative medicine. Trends Cardiovasc. Med. 2005;15:229–236. doi: 10.1016/j.tcm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J. Biol. Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- Valen G. Signal transduction through nuclear factor kappa B in ischemia-reperfusion and heart failure. Basic Res. Cardiol. 2004;99:1–7. doi: 10.1007/s00395-003-0442-7. [DOI] [PubMed] [Google Scholar]
- Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J. Am. Coll. Cardiol. 2001;38:307–314. doi: 10.1016/s0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J. Mol. Cell. Cardiol. 2005;39:363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Vos IH, Govers R, Grone HJ, et al. NFkappaB decoy oligodeoxynucleotides reduce monocyte infiltration in renal allografts. FASEB J. 2000;14:815–822. doi: 10.1096/fasebj.14.5.815. [DOI] [PubMed] [Google Scholar]
- Wang CH, Verma S, Hsieh IC, et al. Stem cell factor attenuates vascular smooth muscle apoptosis and increases intimal hyperplasia after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2007;27:540–547. doi: 10.1161/01.ATV.0000257148.01384.7d. [DOI] [PubMed] [Google Scholar]
- Xiao W. Advances in NF-kappaB signaling transduction and transcription. Cell. Mol. Immunol. 2004;1:425–435. [PubMed] [Google Scholar]
- Yue TL, Wang C, Romanic AM, et al. Staurosporine-induced apoptosis in cardiomyocytes: a potential role of caspase-3. J. Mol. Cell. Cardiol. 1998;30:495–507. doi: 10.1006/jmcc.1997.0614. [DOI] [PubMed] [Google Scholar]
- Yue TL, Wang C, Gu JL, et al. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ. Res. 2000;86:692–699. doi: 10.1161/01.res.86.6.692. [DOI] [PubMed] [Google Scholar]