Abstract
The present study examined if nicotine enhances contextual fear conditioning when the training context is either a background stimulus or a foreground stimulus. In the background conditioning experiment, mice were trained using two auditory conditioned stimulus (CS; 30 s, 85 dB white noise)–footshock unconditioned stimulus (US; 2 s, 0.57 mA) pairings and tested 24 h later. In the foreground conditioning experiment, mice were trained with two presentations of a footshock US (2 s, 0.57 mA) and tested 24 h later. Mice received 0.09 mg/kg nicotine before training and testing. For both the foreground and background conditioning experiments, nicotine enhanced contextual conditioning. No enhancement of the auditory CS–US association was seen. These results demonstrate that nicotine enhances contextual fear conditioning regardless of whether the context is a background stimulus or a foreground stimulus during conditioning.
Keywords: Learning, Hippocampus, Nicotine, Mice, Acetylcholine, Context
Nicotine can enhance many cognitive processes including contextual fear conditioning. In previous studies that examined the effects of acute nicotine administration on fear conditioning [4,5,7,9–11,22], mice were trained using auditory CS–footshock US pairings. Such training results in the formation of two associations: an association between the CS and the US (cued fear conditioning), and an association between the context and the US (contextual fear conditioning). These studies consistently report that nicotine enhances contextual fear conditioning, and nicotine does not enhance cued fear conditioning.
The data from these studies have been interpreted as evidence for nicotine-associated enhancement of contextual fear conditioning. However, another interpretation is possible. As discussed by Rescorla and Wagner [19], Pavlovian conditioning occurs “against a background of uncontrolled stimuli”, namely the context. Thus, during fear conditioning when a CS, such as an auditory stimulus, is paired with a US, the context becomes a background stimulus. If conditioning occurs in the absence of a CS, however, the context can become a foreground stimulus [14,15]. Research suggests that some of the biochemical processes involved in consolidation of foreground contextual fear conditioning could differ from those that support the consolidation of background contextual fear conditioning [21]. Because previous studies that examined the effects of nicotine on contextual fear conditioning have all used a training protocol in which the context is a background stimulus [4,5,7,9–11,22], it is unclear if nicotine enhances the formation of contextual associations or if nicotine enhances background stimulus conditioning. Thus, in order to examine if nicotine enhances contextual fear conditioning or if nicotine enhances background conditioning, the present study compared the effects of nicotine on fear conditioning when the context was a foreground stimulus versus when the context was a background stimulus during training. If nicotine enhances contextual fear conditioning, then nicotine should enhance contextual conditioning when the context is a foreground stimulus and when the context is a background stimulus. If nicotine only enhances background conditioning, then no enhancement of contextual conditioning by nicotine should be seen when the context is a foreground stimulus.
Male, C57BL/6 mice (n = 8 per group; 8–12 weeks of age; Jackson Laboratories) were housed in groups of four. Mice were maintained on a 12-h light/12-h dark cycle (lights on 7:00 a.m.) and allowed ad libitum access to food and water. Training and testing procedures occurred between 8:00 a.m. and 5:00 p.m. All behavioral procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Nicotine hydrogen tartrate salt (Sigma Co., St. Louis, MO, USA) was dissolved in saline and administered via intraperitoneal injection. Mice received injections (0.01 ml/g of body weight) of saline or 0.09 mg/kg of nicotine (reported as freebase), a dose shown previously to enhance contextual fear conditioning in C57BL/6 mice [4,7–11], 5 min before training and testing.
Training and testing occurred in two identical conditioning chambers (16.5 cm wide × 21.0 cm long × 15.9 cm high) housed in Igloo ice chests (54cm long × 30 cm high × 27 cm deep). All walls of the chambers, which were interfaced with an IBM computer running MED-PC software to control stimulus administration, were constructed from clear Plexiglas. During training and testing for freezing to the context, the floors of the conditioning chambers were constructed of metal rods connected to a shock generator and scrambler (MED Associates, St. Albans, VT, USA). Ventilation fans that provided air exchange and background noise (65 dB) were mounted on the back wall of each ice chest, and a light was mounted at the top of each ice chest. In addition, speakers for delivering the CS (85 dB white noise) were mounted on the right wall of each ice chest.
Testing for fear to the CS also occurred in the training chambers. However, changes were made in order to alter the context; the metal rod chamber floors were removed and replaced with a flat piece of Plexiglas, a striped piece of Plexiglas was placed along the back wall of each chamber, a vanilla olfactory cue was present. In addition, mice were not tested for fear to the CS in the chamber in which they were trained in and tested for contextual fear.
Mice were trained and tested according to procedures used by Gould and Wehner [11]. Freezing, the absence of movement except for respiration [1], was assessed during training and testing using a time sampling procedure. Each mouse’s behavior was assessed every 10 s for 1 s and scored as freezing or active.
In Experiment 1, mice were placed in the conditioning chambers. After the first 120 s, during which baseline freezing was assessed, the mice received two paired presentations of a CS (30 s, 85 dB white noise) with a co-terminating US (2 s, 0.57 mA footshock). Immediate freezing was observed during the 120 s intertrial interval (ITI). The training session ended with a 30 s period that occurred after the final CS–US pairing. In Experiment 2, mice were trained using two US alone presentations separated by 120 s. As in Experiment 1, baseline freezing was assessed during the first 120 s of the training session, and immediate freezing was assessed during the 120 s ITI. The session ended with a 30 s period that occurred after the final US presentation.
Testing occurred 24 h later. Mice were placed in the conditioning chambers, and contextual fear conditioning was assessed over 5 min. One hour later, mice were placed in altered training chambers. Cued fear conditioning was assessed over 6 min. During the first 180 s freezing in the absence of the CS (preCS) was assessed. Freezing in the altered context in the continuous presence of the CS was assessed during the final 180 s. For Experiment 2, freezing to the CS was not expected but was measured to verify that freezing to the CS in mice trained with a CS (i.e., Experiment 1) was not masked by a startle response to the CS but instead was related to the CS–US training.
Data were analyzed using SPSS version 11.0. Independent samples t-tests were performed to assess differences between treatment groups.
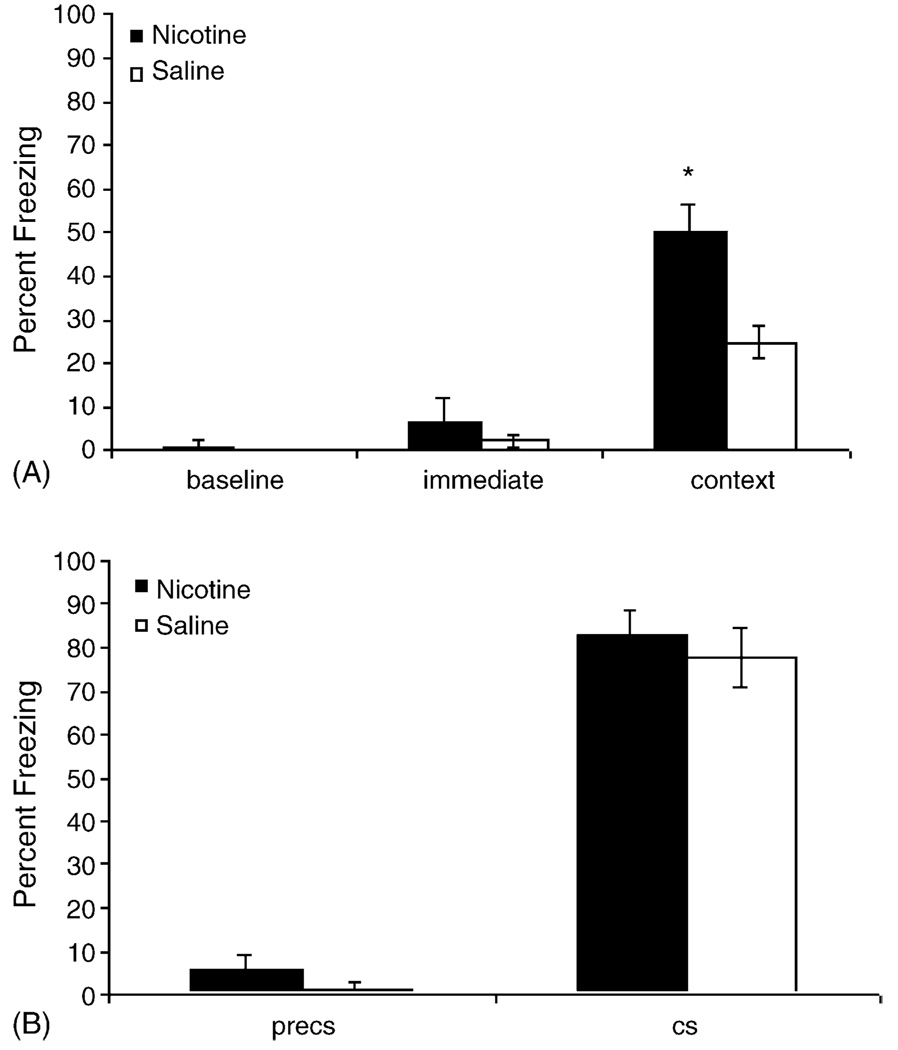
The effect of acute nicotine (0.09 mg/kg) administration on background contextual fear conditioning was examined. Mice were trained using two CS–US pairings, and nicotine or saline was administered before training and testing.
Fig. 1 depicts the results of the background contextual fear conditioning experiment. Consistent with previous research [4,5,7,9–11,22], mice treated with nicotine before training and testing demonstrated higher levels of contextual fear conditioning than mice treated with saline before training and testing (t(14) = 3.893, p = 0.002). There was no effect of nicotine administration on cued fear conditioning (p > 0.05). In addition, there were no differences between the treatment groups in baseline freezing, immediate freezing (both assessed during training), and preCS freezing (assessed during testing; p > 0.05 for all comparisons). These data suggest that differences between the treatment groups in contextual fear conditioning were not due to baseline differences in locomotor activity, sensitivity to shock, or generalized freezing.
Fig. 1.
Acute nicotine administration (0.09 mg/kg) enhances freezing to the context when the context is a background stimulus during training. There were no effects of nicotine administration on baseline freezing, immediate freezing, preCS freezing, or freezing to the CS (*p < 0.05).
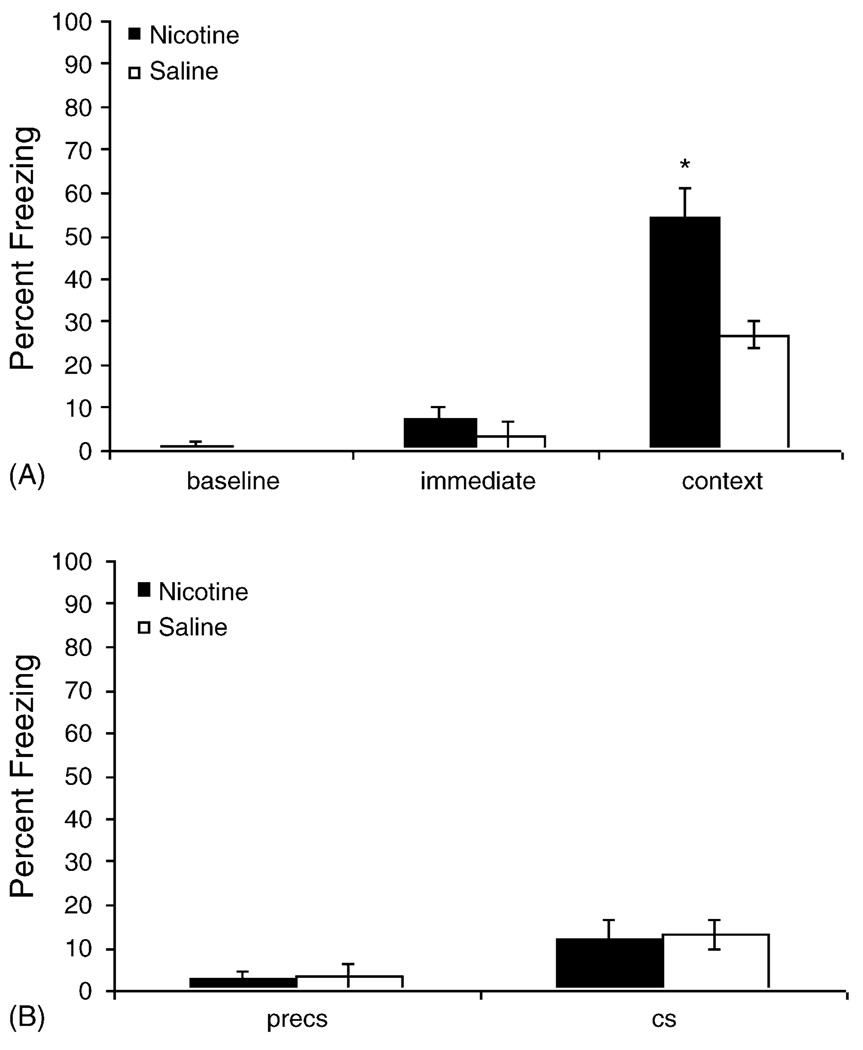
In order to examine if nicotine enhances contextual fear conditioning when the context is the foreground training stimulus, mice were trained using two US alone presentations. No CS was presented during training. As in Experiment 1, nicotine (0.09 mg/kg) or saline was administered before training and testing.
Fig. 2 depicts the results of foreground contextual fear conditioning experiment. An independent samples t-test revealed that mice that received nicotine before training and testing demonstrated higher levels of freezing to the context than mice treated with saline before training and testing (t(14) = 4.137, p = 0.001). There were no differences between groups in freezing to the CS (p > 0.05). In fact, levels of freezing to the auditory stimulus were very low as expected because the auditory CS was not presented during training. This low level of freezing to an unconditioned auditory stimulus suggests that the freezing to the CS observed in Experiment 1 reflects the formation of a CS–US association and not a startle response to the CS. No differences existed between groups in baseline, immediate, and preCS freezing (p > 0.05 for all comparisons).
Fig. 2.
Nicotine (0.09 mg/kg) administration before training and testing enhances contextual fear conditioning when the context is a foreground training stimulus. There were no effects of nicotine on baseline or immediate freezing. Freezing to an altered context (preCS) and to a white noise (CS) were also examined in order to verify that freezing in mice trained with a CS results from CS–US training and is not due to a startle response. Freezing to the altered context (preCS) and freezing to the white noise (CS) were minimal. There were no differences in preCS and CS freezing between saline-treated and nicotine-treated mice (*p < 0.05).
The present study demonstrates that nicotine enhances contextual fear conditioning when the context is a background stimulus and when the context is a foreground stimulus. These results argue against the possibility that nicotine is only enhancing background conditioning and strengthen the contention made by previous studies [4,5,7,11–13,22] that nicotine is enhancing contextual conditioning. Studies indicating that alterations in the neural circuitry and the second messenger signaling cascades that support contextual fear conditioning produce similar effects on foreground contextual fear conditioning and background contextual fear conditioning provide additional evidence for these conclusions. For example, mice with altered hippocampal mossy fiber projections [3,20] and reduced levels of hippocampal PKC activity [6,23] have deficits in contextual fear conditioning regardless of whether the context is a foreground or a background stimulus [16].
The present study also replicates research indicating that nicotine does not enhance cued fear conditioning [4,5,7,9–11,22]. It is unlikely that this null effect of nicotine on cued fear conditioning is due to a ceiling effect, since a previous study from our lab demonstrated that freezing to the CS was not enhanced by nicotine following training (i.e., one shock presentation) that produced lower levels of conditioning [8]. The difference in the effects of nicotine on cued versus contextual fear conditioning suggests that nicotine may differentially alter the neural circuitry underlying contextual fear conditioning compared to the neural circuitry underlying cued fear conditioning. Studies comparing the neural circuitry underlying cued and contextual fear conditioning have indicated that many similar brain areas are involved in the two types of conditioning; however, the hippocampus is only necessary for contextual fear conditioning. In support, hippocampal lesions have been shown to disrupt foreground and background contextual fear conditioning [2,12,13,17].
Two studies directly compared foreground and background contextual fear conditioning in subjects with altered hippocampal function [16,18]. Paylor et al. [16] found that altered hippocampal function disrupted both foreground and background contextual fear conditioning. Philips and Ledoux [18], however, found that background contextual fear conditioning was disrupted, and foreground contextual conditioning was unaffected by altered hippocampal function. It is possible that the findings from these studies diverge because of methodological differences; subjects that were pre-exposed to the training chambers for 20 min the day before training demonstrated deficits in background contextual conditioning but not foreground contextual conditioning [18], while subjects that were not pre-exposed to the training chambers [16] demonstrated disrupted foreground and background contextual fear conditioning [16]. Thus, under certain circumstances contextual fear conditioning may not be critically dependent on the hippocampus. Overall, these data and data indicating that nicotine enhances contextual but not cued fear conditioning suggest that the hippocampus is a potential site where nicotine may act to enhance contextual fear conditioning. Current studies in our lab are investigating this hypothesis.
Acknowledgements
The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 T.G.), the Pennsylvania Department of Health (T.G.), and Temple University (T.G.). Jennifer Davis was supported by a NIH/NIDA training grant (T32DA07237).Part of this study was Miss Jessica Porter’s undergraduate research.
References
- 1.Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J. Comp. Physiol. Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard RJ, Fial RA. Effects of limbic lesions on passive avoidance and reactivity to shock. J. Comp. Physiol. Psychol. 1968;66:606–612. doi: 10.1037/h0026512. [DOI] [PubMed] [Google Scholar]
- 3.Crusio WE, Schwegler H, van Abeelen JH. Behavioral responses to novelty and structural variation of the hippocampus in mice. II. Multivariate genetic analysis. Behav., Brain Res. 1989;32:81–88. doi: 10.1016/s0166-4328(89)80075-0. [DOI] [PubMed] [Google Scholar]
- 4.Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. doi: 10.1007/s00213-005-0047-y. in press. [DOI] [PubMed] [Google Scholar]
- 5.Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J. Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fordyce DE, Wehner JM. Determination of hippocampal protein kinase C using a frozen tissue method: comparison of synaptosomal and total activity in C57BL/6 and DBA/2 mice. Biochem. Biophys. Res. Commun. 1992;188:690–694. doi: 10.1016/0006-291x(92)91111-3. [DOI] [PubMed] [Google Scholar]
- 7.Gould TJ. Nicotine produces a within subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr. Physiol. Behav. Sci. 2003;38:124–132. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- 8.Gould TJ, Feiro O, Moore D. Nicotine enhancement of trace cued fear conditioning but not delay cued fear conditioning in C57BL/6J mice. Behav. Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol. Learn. Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 10.Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav. Neurosci. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- 11.Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav. Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short-and long-term contextual fear. Behav. Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 13.Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav. Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- 14.Odling-Smee FJ. Background stimuli and the inter-stimulus interval during Pavlovian conditioning. Q. J. Exp. Psychol. 1975;27:387–392. doi: 10.1080/14640747508400498. [DOI] [PubMed] [Google Scholar]
- 15.Odling-Smee FJ. The role of background stimuli during Pavlovian conditioning. Q. J. Exp. Psychol. 1975;27:201–209. doi: 10.1080/14640747508400480. [DOI] [PubMed] [Google Scholar]
- 16.Paylor R, Tracy R, Wehner JM, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav. Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- 17.Phillips RG, Ledoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 18.Phillips RG, Ledoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn. Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- 19.Rescorla A, Wagner AR. A theory of Pavlovian Conditioning: variation in the effectiveness of reinforcement and non reinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 20.Schopke R, Wolfer DP, Lipp HP, Leisinger-Trigona MC. Swimming navigation and structural variations of the infrapyramidal mossy fibers in the hippocampus of the mouse. Hippocampus. 1991;1:315–328. doi: 10.1002/hipo.450010322. [DOI] [PubMed] [Google Scholar]
- 21.Stiedl O, Palve M, Radulovic J, Birkenfeld K, Spiess J. Differential impairment of auditory and contextual fear conditioning by protein synthesis inhibition in C57BL/6N mice. Behav. Neurosci. 1999;113:496–506. doi: 10.1037//0735-7044.113.3.496. [DOI] [PubMed] [Google Scholar]
- 22.Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Wehner JM, Sleight S, Upchurch M. Hippocampal protein kinase C activity is reduced in poor spatial learners. Brain Res. 1990;523:181–187. doi: 10.1016/0006-8993(90)91485-y. [DOI] [PubMed] [Google Scholar]