Abstract
In the renal cortex, the connecting tubule (CNT) returns to the glomerular hilum and contacts the afferent arteriole (Af-Art). Increasing Na delivery to the CNT dilates the Af-Art by activating epithelial Na channels, a process that we call connecting tubule glomerular feedback (CTGF). However, the mediator(s) of CTGF are unknown. We tested the hypothesis that Na reabsorption by the CNT induces release of arachidonic acid metabolites that diffuse to and dilate the Af-Art. Microdissected rabbit Af-Arts and adherent CNTs were simultaneously microperfused. CTGF was measured as the increase in diameter of norepinephrine-preconstricted Af-Arts in response to switching NaCl concentration in the lumen of the CNT from 10 to 80 mmol/L. Under control conditions, CTGF was repeatable and completely reversed norepinephrine-induced vasoconstriction. In the presence of 5,8,11,14-eicosatetraynoic acid, an inhibitor of arachidonic acid metabolism, CTGF was completely blocked (−0.7 ± 0.3 versus 7.3 ± 0.5 µm), suggesting that arachidonic acid metabolites mediate CTGF. Because both cyclooxygenase-derived prostaglandins and epoxygenase-derived epoxyeicosatrienoic acids are known vasodilatory arachidonic acid metabolites, we tested whether indomethacin or MS-PPOH (a cyclooxygenase and an epoxygenase inhibitor) could block CTGF. Both indomethacin and MS-PPOH partially blocked CTGF (2.3 ± 0.8 versus 6.5 ± 0.5 µm, and 2.9 ± 0.8 versus 6.6 ± 1.1 µm, respectively). When combined, they completely blocked CTGF (−0.4 ± 0.3 versus 6.6 ± 1.1 µm). We confirmed these findings by using the epoxyeicosatrienoic acid antagonist 14,15-EEZE. The combination of indomethacin plus 14,15-EEZE completely abolished CTGF (−0.3 ± 0.2 versus 8.0 ± 1.0 µm). We conclude that increasing Na concentrations in the CNT stimulate release of prostaglandins and epoxyeicosatrienoic acids, which mediate CTGF.
Keywords: afferent arteriole, connecting tubule, tubuloglomerular feedback, EETs, cyclooxygenase
In humans and all other mammals studied to date, there is a transitional region of varying length between the distal convoluted tubule and the cortical collecting duct, called the connecting tubule (CNT). Faarup1 in 1965 examined serial sections of the rat kidney and commented that the “intercalated part” of the distal tubule returned to the vascular pole of the glomerulus and accompanied the afferent arteriole (Af-Art) for varying distances. Based on our immunohistochemical studies of kallikrein (located in the CNT) and renin (located mainly in the Af-Art), we concluded that, in rats, the CNT is consistently in close proximity to the Af-Art.2 Vio et al3 reported that this association also occurs in humans. In the renal outer cortex, it reportedly occurs in 66% to 100% of nephrons, whereas in the middle cortex and inner cortex it occurs less frequently.2,4
Based on this morphology, we found that a cross-talk exists between the CNT and the Af-Art.2,5 We obtained direct evidence of this by simultaneously perfusing a microdissected rabbit Af-Art and its adherent CNT.5 Increasing NaCl in the CNT from 10 to 80 mmol/L markedly dilated the preconstricted Af-Art. We called this effect “connecting tubule glomerular feedback” (CTGF). We also found that Na entry into the CNT via the epithelial Na channel (ENaC) is required to induce CTGF and that inhibiting NO production in the CNT potentiates CTGF.
Resetting and desensitization of tubuloglomerular feedback (TGF) are known to occur under hyperperfusion and favor Na excretion when Na intake is high.6 Several studies have recently shown that, in addition to myogenic response and TGF, at least one other mechanism of Af-Art tone regulation is present under physiological conditions in vivo.7–12 Therefore, part of TGF resetting could be explained by the existence of a separate mechanism that responds to the same stimulus (distal delivery of Na) but counteracts TGF. This matches our finding that, in contrast to TGF, which causes Af-Art constriction when distal delivery of Na is increased, CTGF causes its dilation. Furthermore, there is in vivo evidence that the distal tubule regulates Af-Art tone. Morsing et al13 performed in vivo micropuncture studies of TGF with and without interrupting distal tubule flow and found that interruption of flow significantly enhanced the maximum decrease in stop flow pressure. This suggests that, in addition to TGF, there is also cross-talk between the Af-Art and either the distal convoluted tubule, CNT, or cortical collecting duct in vivo. Cross-talk between CNT and Af-Art is a novel mechanism that may contribute to the regulation of renal blood flow and GFR. However, the mediator(s) of CTGF are unknown.
Arachidonic acid (AA) is released from tissue phospholipids by a variety of phospholipases. Once free, AA is processed by cyclooxygenases (COXs), cytochrome P450 oxygenases, or lipooxygenases to form compounds that are capable of regulating renal and vascular function. Thus, AA plays a central role in eicosanoid production in mammalian cells for review, see Reference 14). We hypothesized that Na reabsorption by the CNT induces release of AA metabolites, which diffuse to and dilate the Af-Art. To test this hypothesis, we simultaneously perfused a microdissected rabbit Af-Art and its adherent CNT, thereby avoiding the confounding influence of the multiple systemic factors that regulate renal microcirculation.
Methods
Rabbits were fed standard chow (Ralston Purina) and tap water ad libitum and were anesthetized with ketamine (100 mg IM), xylazine (20 mg IM), and pentobarbital (30 mg IV). We used rabbits because the CNT in this species is well demarcated, and microdissection of the CNT and attached Af-Art is easier than in rats or mice. To isolate and microperfuse the Af-Art and CNT, we used methods similar to those described previously.5,15,16 The kidneys were sliced along the corticomedullary axis, and slices were placed in ice-cold minimum essential medium (Gibco Laboratories) containing 5% BSA (Sigma). Using fine forceps, a single superficial Af-Art with its glomerulus intact was dissected together with its adherent CNT. The microdissected complex was transferred with a micropipette to a temperature-regulated perfusion chamber mounted on an inverted microscope with Hoffmann modulation. Both the Af-Art and CNT were cannulated with an array of glass pipettes, as described previously.6 The methods of cannulating and perfusing the tubular segment are similar to those originally described by Burg.17 The Af-Art was perfused with minimum essential medium oxygenated with room air and containing 5% BSA. Intraluminal pressure was measured by Landis technique and maintained at 60 mm Hg. The CNT was perfused with either 10 or 80 mmol/L of NaCl. The basic perfusion solution contained (in mmol/L): 4 KHCO3, 10 HEPES, 0.5 Na acetate, 0.5 Na lactate, 0.5 K2HPO4, 1.2 MgSO4, 1.0 CaCO3, and 5.5 glucose, adding 1 mol/L of NaCl to achieve the desired final NaCl concentration. The driving force to maintain the tubular perfusion rate was provided by hydrostatic pressure causing a tubular flow of ≈20 nL/min. The bath was superfused with minimum essential medium containing 0.15% BSA at a rate of 1 mL/min.
Microdissection and cannulation of the Af-Art and CNT were completed within 90 minutes at 8°C, after which the temperature was gradually raised to 37°C. Once it was stable, a 30-minute equilibration period was allowed before taking any measurements. Images of the Af-Art were displayed at resolutions up to 10 pixels per micrometer. Because isolated arteries have little or no tone, and because our preliminary studies showed that increasing NaCl in the CNT perfusate causes only modest dilatation of the Af-Art unless it was preconstricted, we decided to perform all of the CTGF experiments in Af-Arts preconstricted with norepinephrine (NE) at 2 to 5 × 10−7 mol/L. Af-Art diameter was measured in the region of maximal response to NE at 3 sites separated by 3 to 5 µm and expressed as the average of these 3 measurements. Diameter was recorded at 5-second intervals with a video camera and measured with a computer equipped with a Metavue image analysis system (MDS Analytic Technologies).
Values are expressed as means ± SEMs. Paired t tests were used to compare CTGF (Δ Af-Art diameter) between the control and experimental periods. Hochberg’s step-up procedure was used to adjust the P values for multiple comparisons so that the family-wise type I error rate, predefined as 0.05, was controlled.
Results
To show that the CTGF response was stable over time, we performed control experiments by repeatedly switching NaCl from 10 to 80 mmol/L. In the first CTGF response, Af-Art diameter increased from 10.1 ± 1.3 µm to 17.3 ± 1.6 µm (n = 6; P < 0.05). When the solution was switched back to 10 mmol/L of NaCl, diameter returned to preconstricted levels. In the second CTGF response, Af-Art diameter increased from 10.8 ± 1.4 to 17.0 ± 1.0 µm. These data indicate that the CTGF response is stable over time.
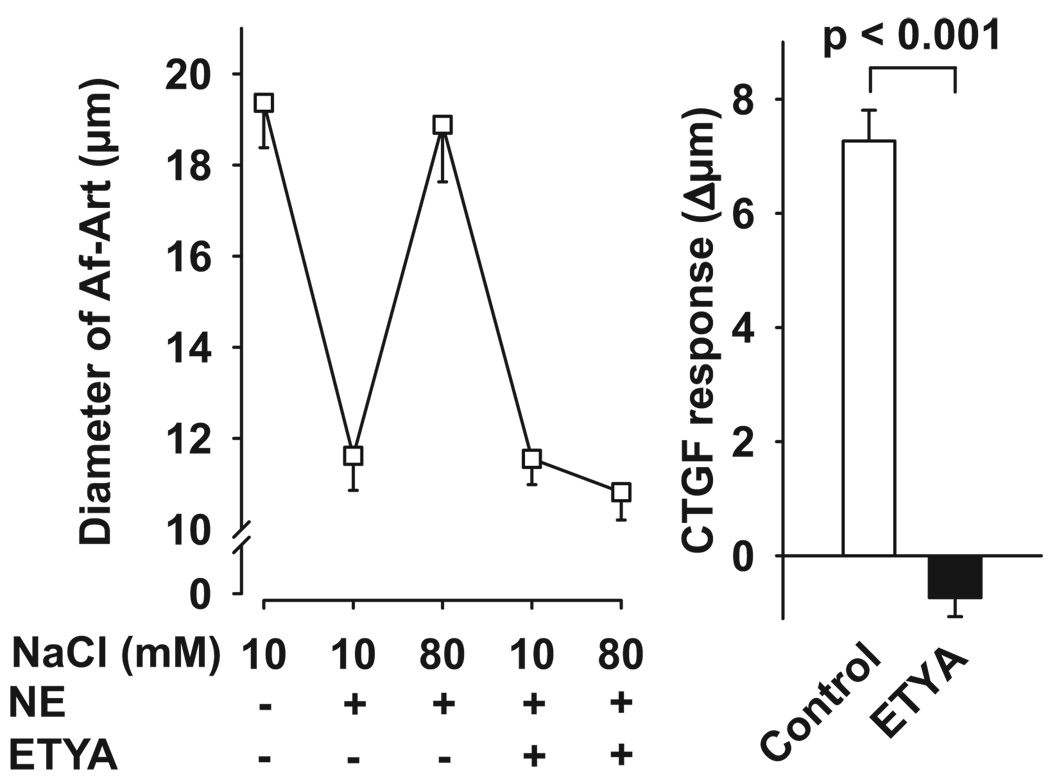
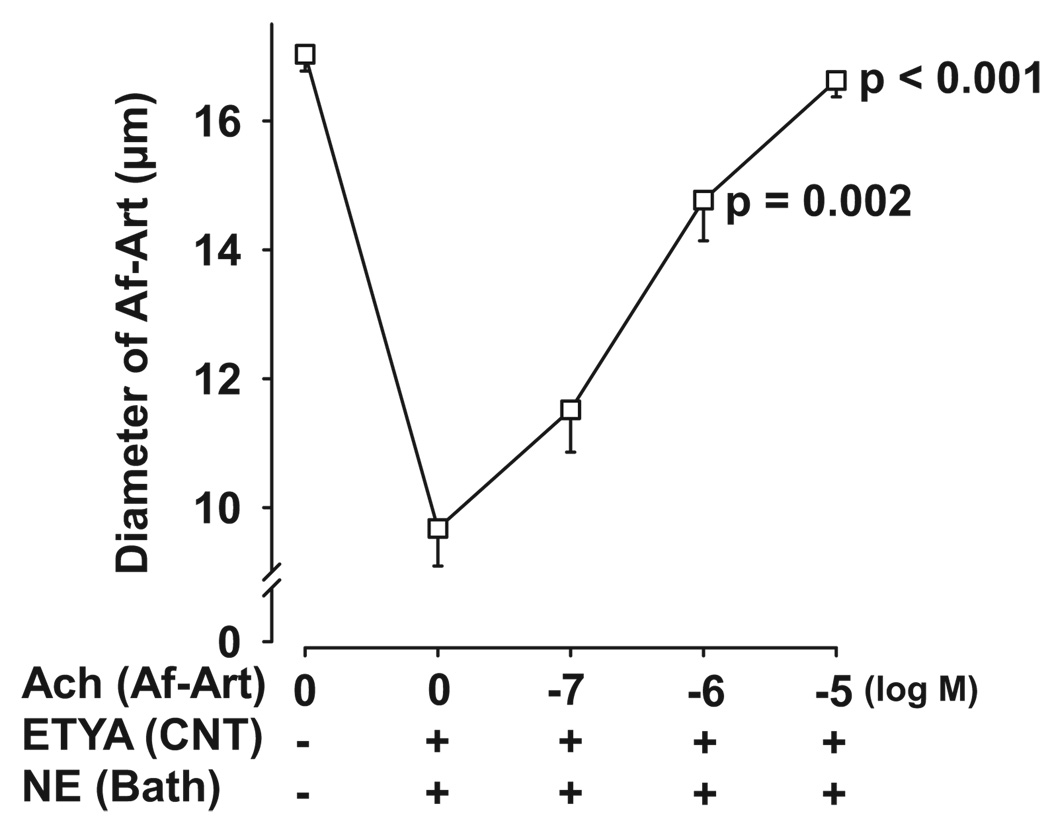
To study the possible mediators of Af-Art dilation induced by CTGF, we first tested 5,8,11,14-eicosatetraynoic acid (ETYA), a nonmetabolizable analogue of AA. ETYA was added to the CNT lumen during CTGF. As shown in Figure 1, during the control CTGF, increasing NaCl concentration in the CNT dilated preconstricted Af-Arts from 11.6 ± 0.8 to 18.9 ± 1.3 µm. We defined CTGF response as the Δ change in Af-Art diameter (in micrometers) in response to high Na in the CNT. Thus, control CTGF was 7.3 ± 0.5 µm. When ETYA (12.5 µmol/L) was added to the CNT, it completely blocked the CTGF response (control: 7.3 ± 0.5 µm; ETYA: −0.7 ± 0.3 µm), suggesting that CTGF is mediated by AA metabolites. To test whether ETYA perfusion in the CNT blocks CNT-initiated vasodilation (CTGF) without directly impairing the vascular dilator mechanisms, we performed a dose-response curve to the CTGF-independent vasodilator acetylcholine (Ach), given in the lumen of the Af-Art, while perfusing the attached CNT with ETYA (12.5 µmol/L). As shown in Figure 2, Ach induced complete dilation of the Af-Art, showing that ETYA perfusion in the CNT does not affect Af-Art reactivity.
Figure 1.
Left, Effect of adding ETYA to the CNT lumen (thereby inhibiting AA metabolism) on Af-Art dilatation induced by high NaCl in the CNT. Right, CTGF response measured as changes in Af-Art diameter in the absence (control) and presence of ETYA (12.5 µmol/L). ETYA completely blocked the preconstricted Af-Art dilation induced by high NaCl (n = 6).
Figure 2.
Dose-response curve to Ach in the Af-Art during perfusion of ETYA (12.5 µmol/L) in the CNT. ETYA perfusion in the CNT did not prevent Af-Art dilatation induced by the CTGF-independent vasodilator Ach. P values are vs 0 Ach (n = 5).
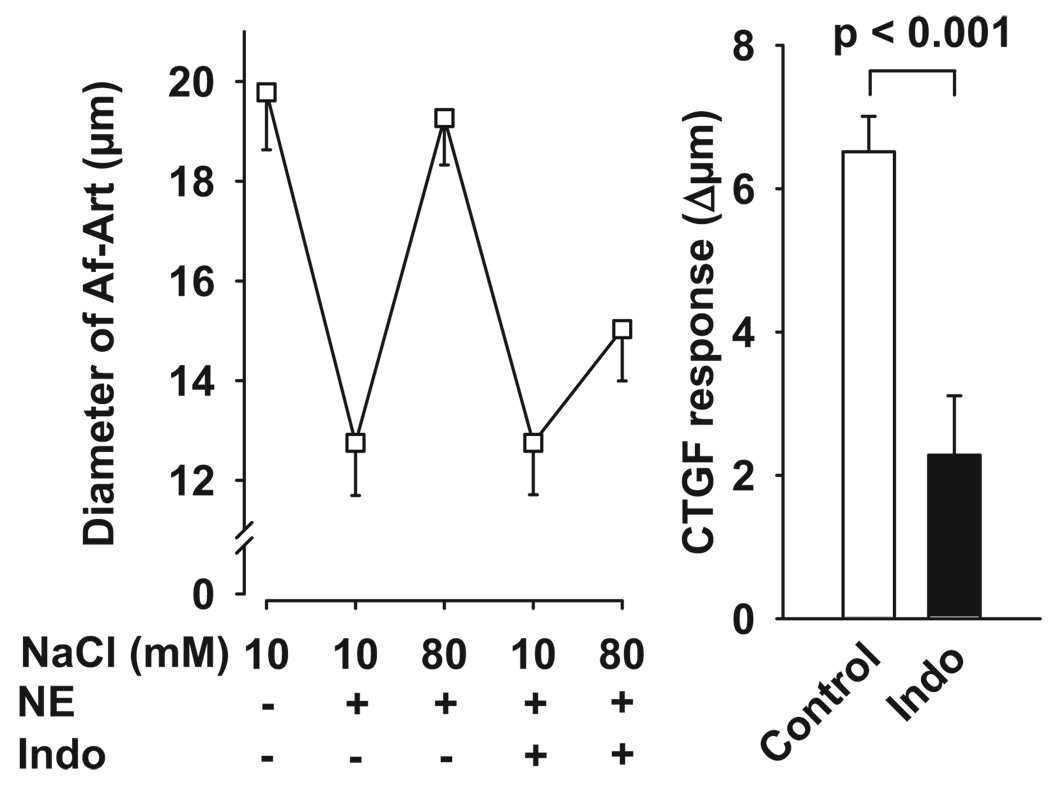
To study whether prostaglandins (PGs) mediate CTGF, we next tested the ability of indomethacin (which blocks the COX pathway) to block CTGF-induced Af-Art dilation. As shown in Figure 3, during the control CTGF, increasing NaCl concentration in the CNT dilated preconstricted Af-Arts from 12.8 ± 1.1 to 19.3 ± 0.9 µm. The addition of indomethacin (5 × 10−5 mol/L) to the CNT perfusate did not affect the preconstricted Af-Art diameter when CNT was perfused with 10 mmol/L NaCl but partially blocked the dilatation induced by 80 mmol/L of NaCl (control: 6.5 ± 0.5 µm; indomethacin: 2.3 ± 0.8 µm), suggesting that PGs participate in CTGF but also that other vasodilatory metabolites of AA could play a role.
Figure 3.
Left, Effect of adding the COX inhibitor indomethacin to the CNT lumen on Af-Art dilatation induced by high NaCl in the CNT. Right, CTGF response measured as changes in Af-Art diameter in the absence (control) and presence of indomethacin (50 µmol/L). Indomethacin partially blocked the preconstricted Af-Art dilation induced by high NaCl (n = 6).
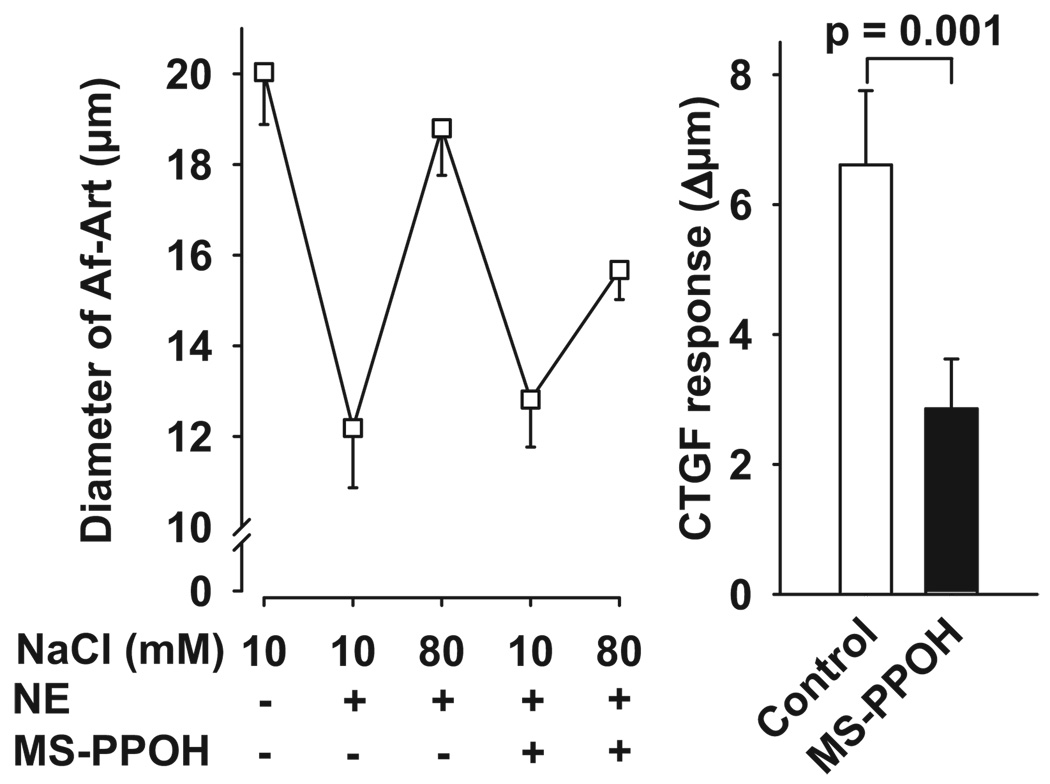
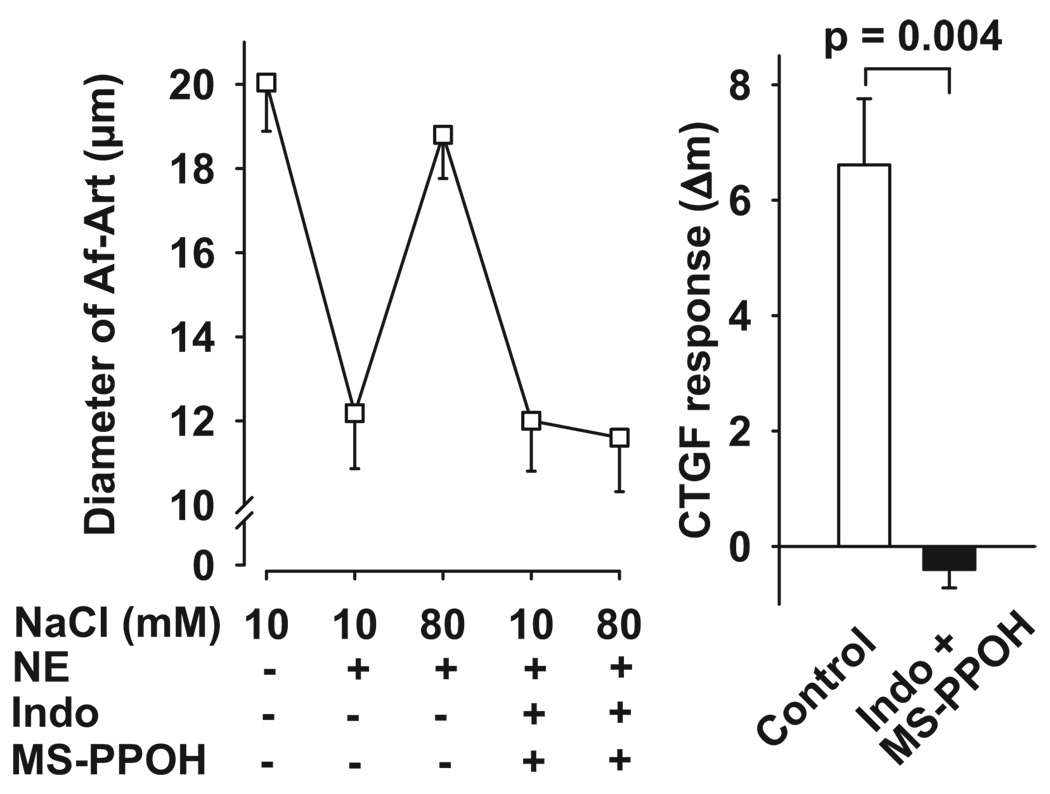
We then tested the effect of adding an epoxygenase inhibitor (MS-PPOH) to the CNT lumen during CTGF, thus blocking the generation of epoxyeicosatrienoic acids (EETs). As shown in Figure 4, during the control CTGF, increasing NaCl concentration in the CNT dilated preconstricted Af-Arts from 12.2 ± 1.3 to 18.8 ± 1.0 µm. Blockade of EET generation by the addition of MS-PPOH (10−6 mol/L) to the CNT perfusate did not affect preconstricted Af-Art diameter when the CNT was perfused with 10 mmol/L of NaCl but partially blocked the dilatation induced by 80 mmol/L of NaCl (control: 6.6 ± 1.1 µm; MS-PPPOH: 2.9 ± 0.8 µm), suggesting that EETs also partially mediate CTGF.
Figure 4.
Left, Effect of adding the epoxygenase inhibitor MS-PPOH to the CNT lumen on Af-Art dilatation induced by high NaCl in the CNT. Right, CTGF response measured as changes in Af-Art diameter in the absence (control) and presence of MS-PPOH (1 µmol/L). MS-PPOH partially blocked the dilatation of preconstricted Af-Art induced by high NaCl (n = 6).
Next we added both indomethacin and MS-PPOH to the CNT perfusate. During the control period, high NaCl dilated preconstricted Af-Arts from 12.2 ± 1.3 to 18.8 ± 1.0 µm. When both indomethacin and MS-PPOH were present in the CNT lumen, the dilatation induced by 80 mmol/L of NaCl was completely blocked (control: 6.6 ± 1.1 µm; indomethacin+MS-PPOH: −0.4 ± 0.3 µm; Figure 5).
Figure 5.
Left, Effect of adding both indomethacin and MS-PPOH to the CNT lumen on Af-Art dilatation induced by high NaCl in the CNT. Right, CTGF response measured as changes in Af-Art diameter in the absence (control) and presence of indomethacin and MS-PPOH. The combined treatment completely blocked the dilatation of preconstricted Af-Art induced by high NaCl (n = 6).
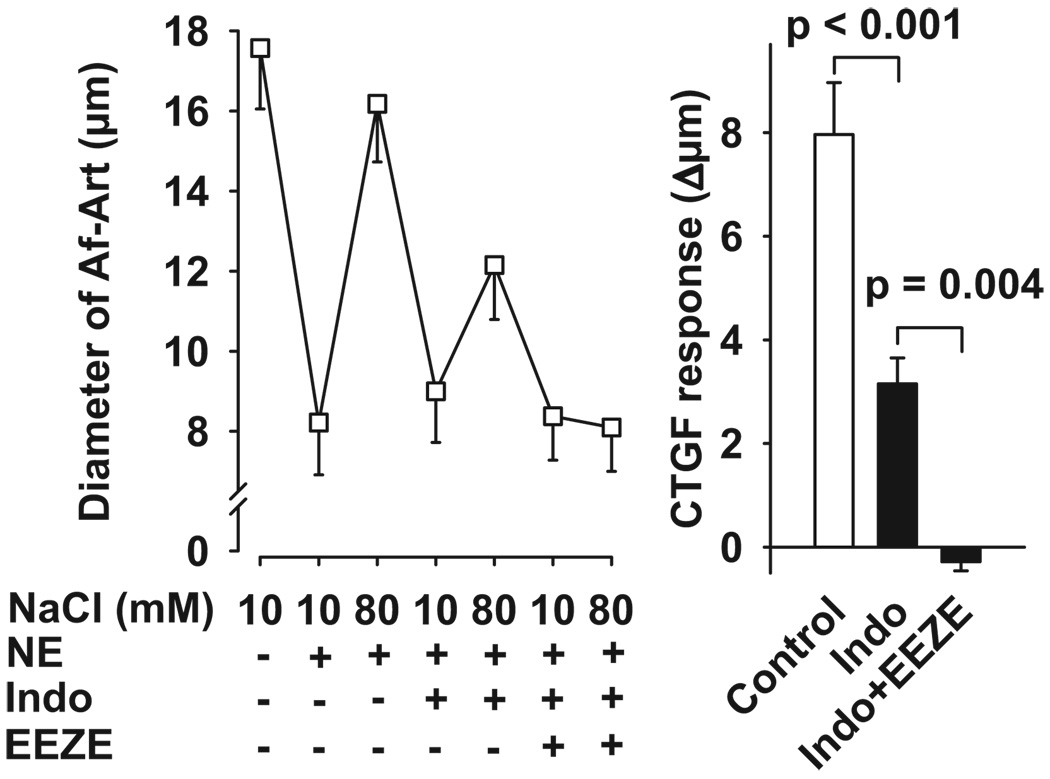
Finally, to confirm the involvement of epoxygenase-derived EETs in CTGF-induced Af-Art dilation, we used the EET selective antagonist 14,15-EEZE18,19 in the presence of indomethacin. As shown in Figure 6, during the control period, high NaCl dilated preconstricted Af-Arts from 8.2 ± 1.3 to 16.2 ± 1.5 µm. Indomethacin alone partially blocked CTGF (control: 8.0 ± 1.0 µm; indomethacin: 3.2 ± 0.5 µm). The addition of 14,15-EEZE (10−5 mol/L) to the bath, while continuing indomethacin treatment, completely blocked CTGF (control: 8.0 ± 1.0 µm; indomethacin+14,15-EEZE: −0.3 ± 0.2 µm), conforming that EETs mediate CTGF.
Figure 6.
Left, Effect of adding the selective EET antagonist 14,15-EEZE to the bath in the presence of indomethacin in the CNT on Af-Art dilatation induced by high NaCl in the CNT. Right, CTGF response measured as changes in Af-Art diameter in the absence (control) and presence of indomethacin alone or with 14,15-EEZE. The combined treatment completely blocked the dilatation of preconstricted Af-Art induced by high NaCl (n = 6).
Discussion
We investigated the possible mediators of CTGF and found that 2 classes of AA metabolites, EETs and PGs, are involved. Inhibiting either COX or epoxygenase in the CNT partially blocked CTGF, but inhibiting both or combining a COX inhibitor with a selective EET antagonist (14,15-EEZE) completely blocked CTGF.
We previously provided direct evidence of cross-talk between CNT and Af-Art.5 It is initiated by increasing NaCl concentration in the lumen of the CNT, which stimulates Na transport via ENaC in the CNT and dilates the Af-Art. Although the signal sent from the CNT to the Af-Art is unknown, there are several likely candidates. Although NO is a known vasodilator in the Af-Art, it was ruled out as the signal that the CNT sends to the Af-Art, because NOS inhibition in the CNT did not block but rather potentiated CTGF-induced Af-Art dilation.5 These data, together with evidence that NO decreases Na reabsorption by ENaC in the cortical collecting duct,20,21 suggest that NO inhibits Na reabsorption in the CNT, ie, the trigger for CTGF.
The contribution of AA metabolites to the control of blood flow and renal hemodynamics has been recognized for over 2 decades. AA, an unsaturated fatty acid, is a normal constituent of membrane phospholipids and is released from them by phospholipase A2. AA can be further processed by COX, lipoxygenase, and cytochrome P450 enzymes to yield metabolites that act as potential autocrine regulators.22,23 To test whether AA metabolites mediate CTGF, we first used ETYA, a nonmetabolizable analogue of AA. When ETYA was added to the perfusate and bath, it completely blocked CTGF. These data show that at least one AA metabolite mediates CTGF. However, because ETYA is not specific, it cannot distinguish between different AA pathways.
COX catalyzes oxygenation and peroxidation of AA to generate endoperoxides, the immediate precursors of a series of bioactive PGs. When we added the COX inhibitor indomethacin to the CNT perfusate, it blocked > 50% of CTGF response. Thus, vasodilatory PGs generated by COXs are likely mediators of CTGF. However, as opposed to ETYA, which completely blocked CTGF, indomethacin only partially blocked CTGF response. These data suggest that, besides PGs, other AA metabolites also mediate CTGF. Indomethacin is a nonselective COX inhibitor; therefore, our data do not indicate which COX isoform, COX-1 or COX-2, mediates CTGF. However, COX-1 is expressed in significant amounts in the CNT, whereas COX-2 is absent,24 suggesting that CTGF is mediated by COX-1–derived PGs. The primary vasodilators that originate from metabolism of AA catalyzed by COXs are PGI2 and PGE2. Future studies are needed to examine which PGs and which receptors are involved in CTGF.
AA metabolites produced by cytochrome P450 enzymes have generated widespread interest because of their potent second messenger functions and cellular effects. In the kidney, they play major roles in the regulation of vascular tone, Na transport, and TGF.25,26 Enzymes of the CYP1A and CYP2C families (epoxygenases) catalyze the formation of EETs from AA25,26 and are all expressed in the kidney, including the distal tubular epithelial cells.27 11,12-EET and 14,15-EET are vasodilators. In our studies, we found that blocking epoxygenase activity by adding MS-PPOH to the CNT lumen inhibited CTGF. These data demonstrate that cytochrome P450 epoxygenase-dependent metabolites, such as EETs, play an important role in CTGF.
It has been reported that EETs inhibit ENaC in the cortical collecting duct28; therefore, if EETs in the CNT acted in an autocrine manner, they could inhibit ENaC and block CTGF. However, we found that EETs do not block CTGF but actually mediate it, suggesting that they act in a paracrine manner to cause Af-Art dilation. To confirm that EETs mediate CTGF in a paracrine fashion, we added a selective EET antagonist, 14,15-EEZE, only to the bath (not to the CNT perfusate) in the presence of indomethacin and found that, although it did not alter Af-Art diameter when the CNT was perfused with low NaCl, it blocked high-NaCl–induced dilatation. These data suggest that EETs partially mediate CTGF by diffusing from the CNT to the Af-Art. Consistent with our studies, Imig et al29 reported that adventitial administration of 11,12-EET and 14,15-EET dilates the Af-Art.
In summary, using isolated perfused CNT and Af-Art, we found that, when AA metabolism was inhibited by ETYA, CTGF was completely blocked. Both COX and epoxygenase inhibitors partially blocked CTGF. When an EET antagonist was added to the bath in the presence of indomethacin, CTGF was eliminated. We conclude that, during CTGF, PGs and EETs mediate Af-Art dilation.
Perspectives
CTGF is a novel regulatory mechanism of the renal microcirculation that, in physiological situations, may account for part of the Af-Art dilatation and increased glomerular filtration rate observed during high-salt intake, perhaps by antagonizing or resetting TGF, thereby favoring Na excretion. In pathological situations, the potential effects of CTGF are complex and may depend on renal hemodynamics. On one hand, if intraglomerular pressure is elevated, such as in diabetes mellitus, CTGF response could worsen glomerular hypertension by dilating the Af-Art, contributing to glomerulosclerosis, proteinuria, and progressive renal failure. On the other hand, CTGF could oppose vasoconstrictor stimuli, such as angiotensin II, and may ameliorate glomerular damage in renal ischemia. Thus, given the physiological and pathophysiological implications of Af-Art resistance in the control of renal function and injury, understanding the mechanisms that control Af-Art tone is of great physiological and pathological significance.
Acknowledgments
Sources of Funding
This work was supported by a grant from the National Institutes of Health (HL28982). Y.R. was supported in part by the American Heart Association.
Footnotes
Disclosures
None.
References
- 1.Faarup P. On the morphology of the juxtaglomerular apparatus. Acta Anat. 1965;60:20–38. doi: 10.1159/000142634. [DOI] [PubMed] [Google Scholar]
- 2.Barajas L, Powers K, Carretero OA, Scicli AG, Inagami T. Immunocytochemical localization of renin and kallikrein in the rat renal cortex. Kidney Int. 1986;29:965–970. doi: 10.1038/ki.1986.94. [DOI] [PubMed] [Google Scholar]
- 3.Vio CP, Figueroa CD, Caorsi I. Anatomical relationship between kallikrein-containing tubules and the juxtaglomerular apparatus in the human kidney. Am J Hypertens. 1988;1:269–271. doi: 10.1093/ajh/1.3.269. [DOI] [PubMed] [Google Scholar]
- 4.Dørup J, Morsing P, Rasch R. Tubule-tubule and tubule-arteriole contacts in rat kidney distal nephrons. A morphologic study based on computer-assisted three-dimensional reconstructions. Lab Invest. 1992;67:761–769. [PubMed] [Google Scholar]
- 5.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int. 2007;71:1116–1121. doi: 10.1038/sj.ki.5002190. [DOI] [PubMed] [Google Scholar]
- 6.Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274:R263–R279. doi: 10.1152/ajpregu.1998.274.2.R263. [DOI] [PubMed] [Google Scholar]
- 7.Just A, Arendshorst WJ. Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback. J Physiol. 2005;569:959–974. doi: 10.1113/jphysiol.2005.094888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Just A, Ehmke H, Toktomambetova L, Kirchheim HR. Dynamic characteristics and underlying mechanisms of renal blood flow autoregulation in the conscious dog. Am J Physiol Renal Physiol. 2001;280:F1062–F1071. doi: 10.1152/ajprenal.2001.280.6.F1062. [DOI] [PubMed] [Google Scholar]
- 9.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R619–R631. doi: 10.1152/ajpregu.00766.2002. [DOI] [PubMed] [Google Scholar]
- 10.Just A, Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol. 2007;293:F1489–F1500. doi: 10.1152/ajprenal.00256.2007. [DOI] [PubMed] [Google Scholar]
- 11.Wronski T, Seeliger E, Persson PB, Forner C, Fichtner C, Scheller J, Flemming B. The step response: a method to characterize mechanisms of renal blood flow autoregulation. Am J Physiol Renal Physiol. 2003;285:F758–F764. doi: 10.1152/ajprenal.00420.2002. [DOI] [PubMed] [Google Scholar]
- 12.Just A. Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1–R17. doi: 10.1152/ajpregu.00332.2006. [DOI] [PubMed] [Google Scholar]
- 13.Morsing P, Velazquez H, Ellison D, Wright FS. Resetting of tubuloglomerular feedback by interrupting early distal flow. Acta Physiol Scand. 1993;148:63–68. doi: 10.1111/j.1748-1716.1993.tb09532.x. [DOI] [PubMed] [Google Scholar]
- 14.Irvine RF. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982;204:3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito S, Carretero OA. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int. 1990;38:1206–1210. doi: 10.1038/ki.1990.335. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Ren Y. Evidence for the role of nitric oxide in macula densa control of glomerular hemodynamics. J Clin Invest. 1993;92:1093–1098. doi: 10.1172/JCI116615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burg MB. Perfusion of isolated renal tubules. Yale J Biol Med. 1972;45:321–326. [PMC free article] [PubMed] [Google Scholar]
- 18.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-epoxyeicosa-5(Z)-enoic acid. A selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic-mSI. A 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003;42:555–561. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- 20.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol. 1995;6:89–94. doi: 10.1681/ASN.V6189. [DOI] [PubMed] [Google Scholar]
- 21.Stoos BA, Carretero OA, Garvin JL. Endothelial-derived nitric oxide inhibits sodium transport by affecting apical membrane channels in cultured collecting duct cells. J Am Soc Nephrol. 1994;4:1855–1860. doi: 10.1681/ASN.V4111855. [DOI] [PubMed] [Google Scholar]
- 22.Currie MG, Needleman P. Renal arachidonic acid metabolism. Annu Rev Physiol. 1984;46:327–341. doi: 10.1146/annurev.ph.46.030184.001551. [DOI] [PubMed] [Google Scholar]
- 23.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Ann Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 24.Campean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse) Am J Physiol Renal Physiol. 2003;285:F19–F32. doi: 10.1152/ajprenal.00443.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hoagland KM, Maier KG, Moreno C, Yu M, Roman RJ. Cytochrome P450 metabolites of arachidonic acid: novel regulators of renal function. Nephrol Dial Transplant. 2001;16:2283–2285. doi: 10.1093/ndt/16.12.2283. [DOI] [PubMed] [Google Scholar]
- 26.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 27.Tsao C-C, Coulter SJ, Chien A, Luo G, Clayton NP, Maronpot R, Goldstein JA, Zeldin DC. Identification and localization of five CYP2Cs in murine extrahepatic tissues and their metabolism of arachidonic acid to regio- and stereoselective products. J Pharmacol Exp Ther. 2001;299:39–47. [PubMed] [Google Scholar]
- 28.Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol. 2004;124:719–727. doi: 10.1085/jgp.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]