Abstract
The results of recent studies indicate that, in healthy men and women beyond ~65 years of age, the energy-producing pathways in skeletal muscle may combine with changes in motor unit behavior and muscle contractile properties to provide a unique environment for resisting muscle fatigue.
Keywords: bioenergetics, activation, acidosis, physical activity, muscle fiber type, contraction, economy
INTRODUCTION
Skeletal Muscle Fatigue
Muscle fatigue, generally defined as the loss of force- or power-producing capacity in response to contractile activity, is a fundamental characteristic of skeletal muscle. Muscular endurance, defined as the duration that a given target force can be maintained, is a related characteristic. In the intact organism, a host of factors can influence the development of muscle fatigue. Because the ability to maintain force output is a critically-important aspect of neuromuscular function, an understanding of fatigue is essential to understanding the biology of senescence.
In humans, fatigue is typically expressed as the fall of maximal force or power relative to an individual’s baseline. This approach is particularly important when comparing fatigue in two populations who may have marked differences in muscle mass and strength, which is generally the case with young and older adults. By expressing fatigue as a relative drop in force or power, the confounding influence of differences in muscle size and strength can be eliminated, and fatigue and its mechanisms can be examined in relative isolation. The goal of this review is to provide an integrative perspective that suggests how changes in the senescent neuromuscular system may act together to increase its ability to resist fatigue under some conditions.
Does Older Muscle Fatigue Less Than Young?
Given the aging of our population, and the advances in medicine that have extended the average lifespan of humans tremendously over the past century, the study of whether and how older skeletal muscle resists fatigue has become an important and active area of research. Studies conducted in the past decade generally indicate that older muscle fatigues relatively less than young muscle. Healthy older men and women have shown a greater ability to maintain maximal force production under a variety of conditions, and in several muscle groups (4, 7, 19, 22, 27, 29, 30). Likewise, endurance time is often shown to be longer in older compared with younger adults (13).
Despite substantial literature that suggests an advantage in terms of fatigue resistance on the part of older muscle, this is not always observed. Several studies suggest that there is no difference in fatigue between young and old (25, 26), and some recent reports have observed more fatigue in the elderly (1, 26). The discrepant results across studies could be attributable to differences in contraction mode, protocol, muscle group or subject characteristics. Older adults may be more susceptible to fatigue during high-velocity dynamic contractions. Likewise, there is emerging evidence that the age of the older subjects may be important in the fatigue response (26). The health and habitual physical activity of the study groups are important design considerations that also can affect the results. Full clarification of these discrepancies awaits further study. This review will focus on a discussion of the circumstances that might allow the aged neuromuscular system to be well-suited for maintaining force production during muscular activity. Studies of muscle fatigue in the ankle dorsiflexor muscles, which are important for locomotion and can be problematic in falls in the elderly, are highlighted.
MECHANISMS OF FATIGUE RESISTANCE IN OLD AGE
Determining the Mechanisms of Muscle Fatigue In Vivo
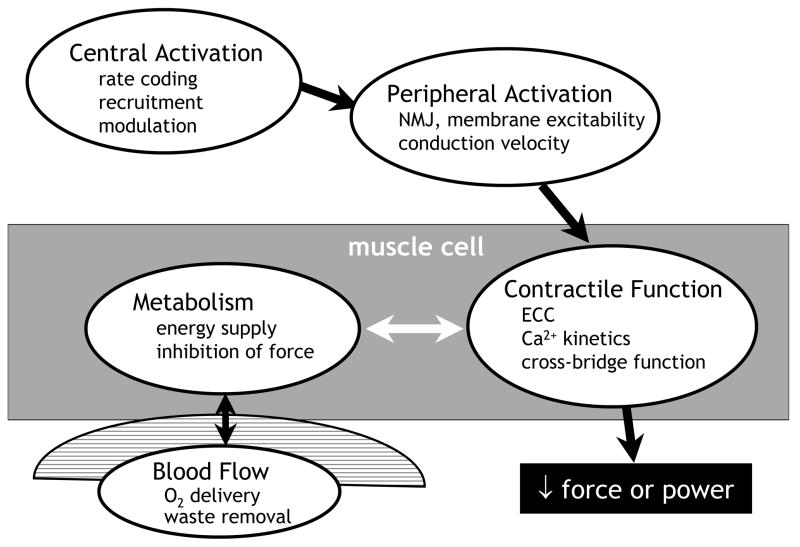
The study of fatigue and its mechanisms can be approached in vivo using a combination of techniques that, together, provide a powerful means for quantifying changes in multiple systems during muscular activity. A working model of the potential sites of failure that can result in fatigue is presented in Figure 1. The strength of this approach is that it integrates measures of central and peripheral activation, intracellular energy metabolism and contractile function simultaneously, in vivo and, to a large extent, non-invasively.
Figure 1.
Potential sites of failure during muscular activity. A model of the pathway of force production, useful for studies of the mechanisms of fatigue in vivo. Impairment at any site could result in a decrease in force or power output, indicating muscle fatigue. See text for details.
Several methods have been developed to assess the completeness of voluntary activation of muscle during maximal contractions. Some of these methods are more suitable to the study of fatigue than others, as the ability to obtain the measure during and immediately following a fatigue protocol is critical. Central activation failure, which constitutes a failure to activate all motor units at optimal discharge rates, can be evaluated by comparing the fall of force during a maximal voluntary contraction (MVC), which requires full functioning of the entire pathway of force production, to the fall of electrically-stimulated force, which evaluates changes distal to the point of stimulation. Due to their greater reliability than twitch forces, which are more variable and can disappear during fatigue, tetanic trains are the measure of choice for assessing force-producing capability in the periphery. Obtaining a tetanus has the additional advantage of providing information about the rates of force production and relaxation.
Another approach to quantifying the completeness of central activation is the central activation ratio: [MVC/(MVC + superimposed stimulated force)]. While we and others have successfully used the central activation ratio to detect central fatigue during isometric protocols, its application to dynamic contractions can be problematic. At this time, the best measure of central activation failure during dynamic contractions may be a comparison of the fall of dynamic MVC and that of a separate measure of dynamic tetanic force. Other techniques, such as twitch interpolation and transcranial magnetic stimulation, can also be very useful under the right conditions. In general, studies of fatigue that employ high-intensity contractions are most likely to result in central activation failure in vivo.
Peripheral activation failure is generally observed as a fall in the amplitude or area of the compound muscle action potential, or m-wave, which is elicited in response to a single, supramaximal stimulation of a peripheral motor nerve. A fall in m-wave amplitude suggests a loss of excitability at the neuromuscular junction or along the muscle membrane, while an increase in the duration of the negative peak of the m-wave in response to fatiguing contractions suggests a slowing of neuromuscular propagation. Comparisons of changes in the H-reflex to changes in the m-wave provides additional insight as to changes in spinal vs. peripheral excitability during fatigue. Peripheral activation failure typically does not play an important role in fatigue during voluntary contractions in humans.
Skeletal muscle energetics can be quantified non-invasively and continuously during various fatigue protocols using phosphorus magnetic resonance spectroscopy (MRS; (19, 21). In studies of fatigue, it is especially useful to monitor those metabolites, such as inorganic phosphate (Pi), proton (H+) and diprotonated inorganic phosphate (H2PO4−) that have been shown at the molecular, cellular and whole-muscle (6, 8, 19, 24) levels to inhibit force production. The synthesis rates of intracellular adenosine triphosphate (ATP) by the creatine kinase reaction, glycolysis and oxidative phosphorylation also can be determined using MRS (16). In all but the most exhaustive contraction protocols, [ATP] is conserved through buffering by phosphocreatine (PCr), via the creatine kinase reaction. Thus, ATP production matches its use, which allows the cost of contractions to be determined from changes in PCr and H+ (21).
An adequate blood supply is vital to the maintenance of force production, both for the delivery of oxygen and the removal of the by-products of metabolism. Elimination of blood flow by cuff ischemia results in greater fatigue than during free-flow conditions. Transient occlusion of blood flow due to intramuscular pressure on the vasculature during contractions has been suggested to be one mechanism by which stronger muscles might fatigue more than weaker muscles.
In vivo, whole-muscle contractile properties are assessed indirectly from the rates of force development and relaxation, as well as from the total force produced during an electrically-elicited contraction. In unfatigued muscle, slower force development and relaxation are observed in muscles with greater type I myosin heavy chain content. The key observation is whether these rates, and the total force produced during the stimulated contraction, decrease during fatigue. Changes in the rate of force development can reflect slower calcium release or slowing of cross-bridge cycling due to elevated levels of Pi or H+ (8). Slowed force relaxation can also occur as a result of the effects of metabolite accumulation on cross-bridge kinetics and the resequestration of calcium by the sarcoplasmic reticulum. It is common to observe slowing of force relaxation in response to fatiguing contractions in humans.
Baseline Alterations in the Aged Neuromuscular System That May Affect Fatigue
There are many long-term changes in the unfatigued neuromuscular system of older adults that may affect their ability to resist muscle fatigue. In the central nervous system, lower maximal motor unit discharge rates have been observed during MVCs in the unfatigued muscles of older compared with young adults (5, 15). In contrast to the decrease in maximal motor unit discharge rates (MUDR), the ability to recruit all motor units during an MVC appears to be unperturbed in healthy older adults. This preservation of full voluntary activation is important, as the assumption of recruitment of all motor units is critical to the accurate interpretation of fatigue and its mechanisms during contractions.
Comparable deficits in baseline MVC and electrically-stimulated force in old compared to young, along with similar specific strength (MVC · cross-sectional area−1) across age groups (17), are consistent with the concept that most of the isometric strength differences in young and old arise from differences in the periphery, mainly muscle size. Indeed, fat-free muscle cross-sectional area (CSA, cm2) was shown to explain ~66% of the variation in ankle dorsiflexor strength in healthy older subjects (17), highlighting the dominant role of sarcopenia in the muscle weakness of old age. It is notable, however, that recent longitudinal studies (9, 10) reported decreased specific strength during dynamic contractions as the participants aged. The observations that single-fiber specific tension was not decreased at the older time point, along with the greater loss of specific strength at higher contraction velocities (9), suggests that, while muscle mass is clearly important, neural factors also may be important in any age-related changes in specific strength during dynamic contractions.
In the periphery, there is a small (~8–10%), but potentially significant shift in fiber type number toward more type I fibers in older compared with younger adults (14). The magnitude of this shift, which is thought to arise from a loss of type II motor units with some reinnervation of muscle fibers by type I motor neurons, is roughly similar across several muscle groups, and appears to be accompanied by a relatively greater atrophy of type II fibers. The net result is a slowing of whole-muscle contractile properties in older adults (19, 28), which manifests as a left-ward shift in the force-frequency curve (27, 28). While type I fibers are generally more fatigue-resistant compared to type II fibers, differences in functional characteristics between type I and type IIA, the dominant type II isoform in human muscle, may be related more to contractile speeds than to fatigue resistance, per se.
Despite the shift to a slower, theoretically more oxidative muscle, lower activities of oxidative enzymes have been reported in older muscle (12), likely reflecting lower mitochondrial content in the elderly. However, in studies in which subjects’ habitual physical activity levels are addressed (3, 18), similar oxidative capacities have been found in young and old, suggesting that healthy older muscle can maintain its capacity to generate energy by oxidative phosphorylation. Likewise, a preserved vascular bed is generally observed in older muscle (11), which helps ensure an adequate supply of oxygen and clearance of metabolites during fatiguing contractions.
During dynamic contractions, older adults exhibit decreased power production that becomes more pronounced as contraction velocity increases, an effect that may be more apparent in some muscle groups than in others (23). This velocity-dependent loss of power may result both from central and peripheral changes in older adults. The impact of slowed voluntary contractions on muscle fatigue in older adults has recently become an area of intense interest (1, 26), and will no doubt provide important new information about the malleability of this characteristic in the aging neuromuscular system.
Mechanisms of Age-Related Fatigue Resistance
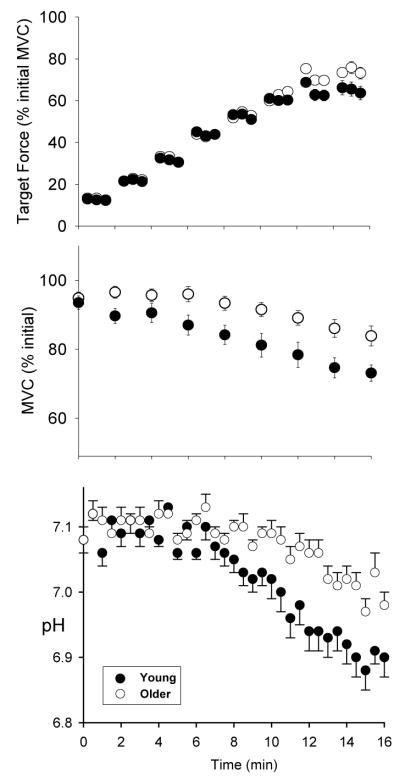
The integrative approach described above and illustrated in Figure 1 was first applied to the study of muscle fatigue in aging using an incremental protocol designed to progress from steady-state conditions to the onset of fatigue, quantified as the fall of MVC (19). Healthy, relatively sedentary men and women aged 21–40 or 65–80 years were recruited, and the groups were matched for similar physical activity levels using accelerometers. Following 16 minutes of dorsiflexor contractions during which force, activation, contractile properties and energetics were measured, the older group had fatigued less than the young group (Figure 2). Central and peripheral activation failure were not sources of fatigue in either age group during this protocol. Likewise, while the older adults had slower contractile properties at baseline compared to the young adults, slowing in the rates of force development and relaxation did not differ across age groups during fatigue. Rather, the difference in fatigue across age groups had a metabolic basis. The older men and women had a smaller decrease in intracellular pH (Figure 2), and accumulated less Pi and H2PO4− compared with the young. In both groups, the fall of MVC was strongly associated with [H2PO4−]. The smaller degree of acidosis in the older group suggested that they relied relatively more on oxidative metabolism to provide energy for contractions, which allowed them to avoid the accumulation of fatigue-inducing metabolites and thereby maintain force production more effectively than the young.
Figure 2.
Target force, fatigue and intracellular pH in young (solid symbols) and older (open symbols) groups during incremental contractions. MVC; maximal voluntary contraction, obtained every 2 min. While both groups attained target level through 12 min of contractions (top panel), the older group had less fatigue (fall of MVC, middle panel) and acidosis (bottom panel) compared to young. Modified from J Appl Physiol 93:1813–1823, 2002. Used with permission.
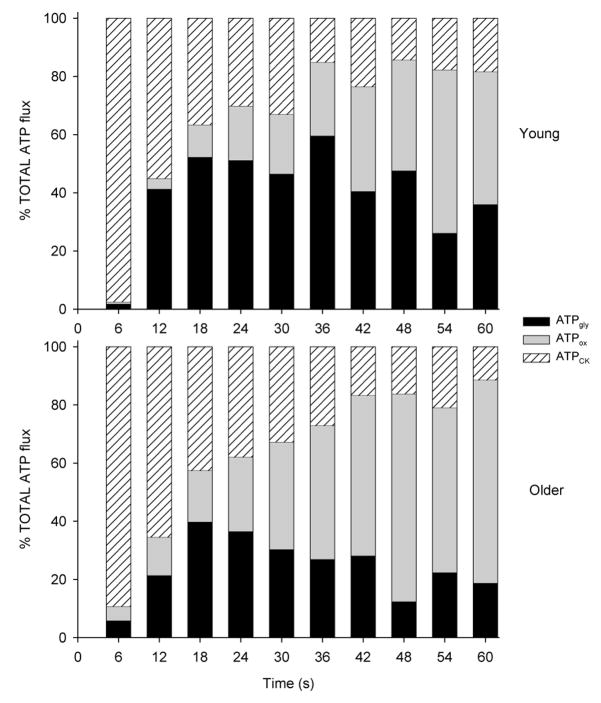
To pursue the possibility of a greater reliance on oxidative energy production by older muscle, we next compared ATP production rates by the creatine kinase (CK) reaction, glycolysis and oxidative phosphorylation in healthy young and older men (20). During an MVC sustained for 60 s, the older group generated relatively more ATP (% total) by oxidative phosphorylation and relatively less by glycolysis compared to the young group (Figure 3). In addition, the total energy cost of the contraction was lower in the older group. Thus, these results provided novel evidence of a relatively greater contribution to ATP production from oxidative metabolism in the muscle of healthy older men.
Figure 3.
Relative contribution from the 3 pathways of adenosine triphosphate (ATP) production during a sustained maximal voluntary contraction (MVC) in young (top) and older (bottom) groups. Percentages of total ATP from the creatine kinase reaction (hatched bar), oxidative phosphorylation (gray bar) and glycolysis (black bar) are shown. Older group generated relatively more ATP from oxidative phosphorylation than young group. J Appl Physiol 99:1736–1744, 2005. Used with permission.
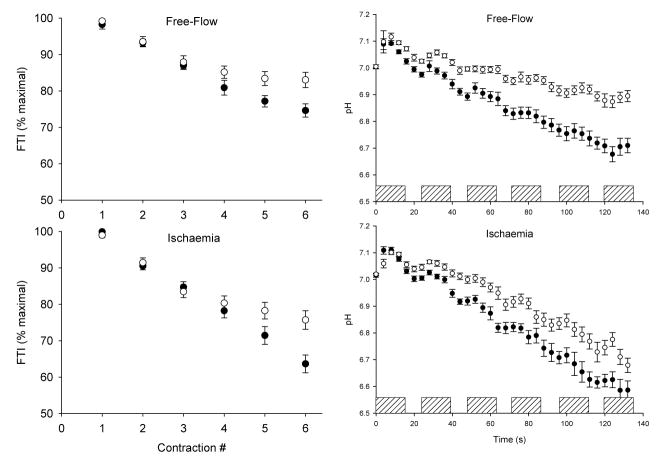
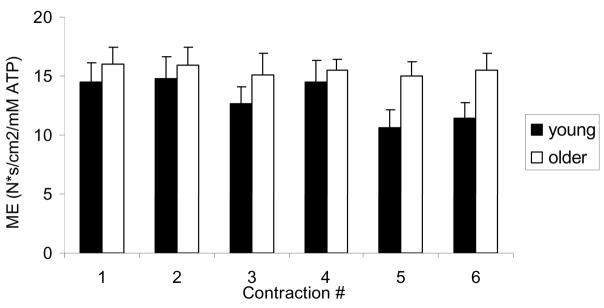
The next question to address was whether this reliance on oxidative energy production in older muscle represented a “preference” for oxidative phosphorylation or a limitation in glycolytic function. To distinguish these possibilities, healthy young and older men and women performed an intermittent MVC protocol (12 s on/12 s off) twice, once with blood flow intact and once with flow occluded by a pressure cuff placed around the thigh and maintained at 220 mmHg (21). The use of ischemic contractions enabled us to remove the muscle’s ability to generate ATP oxidatively and determine whether older muscle was capable of generating sufficient ATP via anaerobic glycolysis. While the expected difference across age groups in fatigue and acidosis was observed during free-flow contractions, we were somewhat surprised to observe that, despite removing the oxidative energy supply, the older group also showed less fatigue and intracellular acidosis compared to the young during ischemic contractions (Figure 4). Once again, there was a difference across age groups in the pathways of ATP generation during free-flow conditions, such that the young group generated more ATP by glycolysis. However, there was no difference between groups in glycolytic flux during ischemia, indicating that glycolytic function was unimpaired in the older group, and suggesting that the greater reliance on oxidative metabolism during free-flow conditions represents a “preference” rather than a limitation in healthy older muscle. As in the earlier study that used submaximal contractions (19), the accumulation of H2PO4− was strongly associated with the fall of force during these MVCs. Analysis of a subset of 16 young and 16 older subjects in whom maximal muscle CSA was obtained by magnetic resonance imaging revealed that, overall, the older group had higher metabolic economy (Ns · cm−2 · mM ATP) during the free-flow protocol than the young group (Figure 5). These data provide the first evidence of an age-related difference in metabolic economy in human skeletal muscle in vivo under conditions in which a difference in fatigue was also observed.
Figure 4.
Fatigue (left) and intracellular pH (right) during intermittent dorsiflexion maximal voluntary contractions (MVC) under free-flow (top) and ischemic (bottom) conditions. Open symbols indicate older group; solid symbols indicate young group. Hatched bars indicate contraction. FTI; force-time integral. Data are mean ± SE. Modified from J Physiol 583.3:1093–1105, 2007. Used with permission.
Figure 5.
Metabolic economy in young (solid bars) and older (open bars) groups during intermittent maximal voluntary contractions (MVC). Older group had a higher metabolic economy (ME) than young group. Data are mean ± SE. See text for details.
Because intramuscular pressure can cause transient occlusion of the vasculature and thereby interrupt oxygen delivery during strong contractions, it has been suggested that a portion of the relative fatigue resistance of older adults may be related to the lower muscle forces they produce compared to the young. To address the question of whether muscle strength or the interruption of blood flow contributes to differences in fatigue during intermittent maximal contractions, young and older men performed MVCs (50% duty cycle, 5 s on/5 s off) with blood flow intact and during ischemia (4). Again, the older group had less fatigue during both free-flow and ischemic conditions. When subgroups of young and old were matched for similar baseline strength, these differences in fatigue remained. Notably, the greater fatigue exhibited in the young during ischemia was accompanied by central and peripheral activation failure, which were not observed in the older group. While it is generally accepted that metabolic changes in the periphery during muscular work can influence central motor drive via afferent feedback (2), little is known about this process in the aged neuromuscular system. Overall, these results suggest that the smaller metabolic perturbation observed in older muscle (19–21) may also confer an advantage by minimizing changes in central motor drive during voluntary muscle contractions.
It is important to note that, as in all studies of muscle fatigue, the task performed will influence the sites of failure. As a result, there may be a point at which the advantage older subjects may have in maintaining voluntary activation is abolished. In a separate study using intermittent MVCs with a 70% duty cycle, greater fatigue was again observed in young than old, but in this case central fatigue was not different across groups (30). As in our study using a 50% duty cycle (4), baseline strength was not predictive of fatigue. Collectively, these studies suggest that the source of the fatigue differences in healthy young and older adults resides mainly in the muscle, and is independent of blood flow.
In summary, an age-related fatigue resistance has been observed during submaximal and maximal voluntary contractions in the ankle dorsiflexor muscles of healthy older adults. This has been the case for both isometric and dynamic contraction protocols (22), with and without blood flow (4, 21). Although cross-sectional study designs are not without their limitations, a key design element in these studies has been the matching of habitual physical activity levels in young and old study groups, all of whom consisted of relatively sedentary, un-medicated volunteers. We have determined that fatigue during both sub-maximal (19) and maximal (21) contraction protocols is associated with a greater reliance on oxidative phosphorylation, less acidosis and lower accumulation of Pi and H2PO4− in older compared to young muscle.
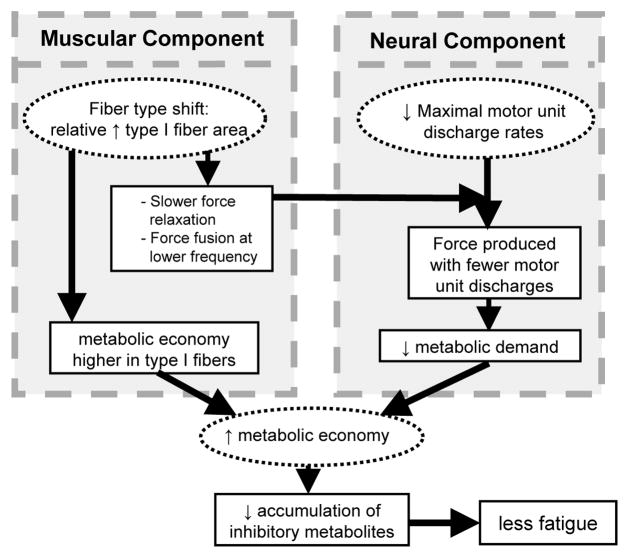
The results of these studies suggest a new hypothesis to explain this age-related fatigue resistance, which is illustrated in Figure 6. We hypothesize that higher metabolic economy is a critical mechanism of the enhanced fatigue resistance of older muscle, as it would allow older muscle to meet its energetic needs with a smaller perturbation of intracellular metabolism. Both muscular and neural factors could contribute to a difference in metabolic economy across age groups. The small increase in the proportion of type I fibers, which have been shown to be more economical than type II fibers (31), results in slower contractile properties and a leftward shift in the force-frequency relationship, thereby allowing older muscle to produce similar relative force with lower MUDR. Fewer depolarizations could translate to lower energy costs for ion pumping, thereby decreasing the total ATP cost of contractions. These neural and muscular factors could combine to reduce the energetic cost of contractions, allow relatively greater use of oxidative metabolism, and delay because fewer byproducts of glycolysis would accumulate in the muscle. Studies to test this novel hypothesis are underway.
Figure 6.
Hypothetical sources and impact of increased metabolic economy in older skeletal muscle. Proposed scenario that may provide older muscle an advantage in terms of fatigue resistance, with the mechanisms of this advantage residing in both the neural and muscular systems. See text for details.
The Paradox: Fatigue Resistance Despite Declining Physical Function in the Elderly?
There are at least two important reasons to study muscle fatigue in the elderly. The first is to address the fundamental questions: Independent of sarcopenia, how does an aged neuromuscular system respond to the demand for continued force production? Are the mechanisms of fatigue different in young and old? A second reason for the study of fatigue in the elderly is more pragmatic. While the inevitable loss of muscle mass is well-known, if not yet fully understood, clarification of potential changes in the quality of old muscle, including fatigue resistance, is equally compelling. Large-scale longitudinal data suggest a decrease in whole-muscle, but not single-cell, quality (i.e., specific strength) in advanced age (9, 10). Additional in vivo markers of muscle quality, such as motor unit behavior, metabolic economy and fatigue resistance may be acting to preserve physical function in the face of declining strength.
The design of interventions that mitigate the loss of neuromuscular function in old age first requires an understanding of how and why muscle fatigue is altered in healthy older adults. Then the questions can be asked: To what extent do changes in lifestyle (e.g., decreased physical activity), health and environment affect the various factors involved in muscle fatigue in older adults? Further, how do changes in muscle fatigue in the elderly impact physical function and the ability to live an independent lifestyle? Under what conditions might an age-related fatigue advantage be lost? As the mechanisms of fatigue in healthy elders become more clear, work has begun to systematically examine how poor health, various medications, deconditioning, very old age and severe sarcopenia affect the ability of the aged neuromuscular system to resist muscle fatigue in wide range of situations.
CONCLUSION
An integrated approach to the study of muscle fatigue has provided an exciting opportunity to examine the mechanisms of human skeletal muscle fatigue in vivo, and the effects of old age on these mechanisms. By incorporating information from multiple systems, we can determine how these systems interact to produce force, and under what conditions the various components fail. The combination of lower maximal MUDRs, slower contractile properties, and relatively greater reliance on oxidative metabolism suggests a scenario in which older muscle may be better equipped to resist fatigue than young muscle. Thus, we suggest a neuro-energetic basis for fatigue resistance in the elderly that is centered on higher metabolic economy in the older neuromuscular system. Elucidation of the conditions under which this advantage is asserted, as well as the factors that contribute to its loss due to disease, senescence or changes in lifestyle, awaits further investigation.
Acknowledgments
Disclosure of funding: Funding was provided by NIH/NIA R01 AG21094 and NIA K02 AG023582.
The author gratefully acknowledges the enthusiasm and assistance of past and present members of the Muscle Physiology Lab, as well as all study participants who gave their time and effort to support this work.
References
- 1.Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 2.Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol (Lond) 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chilibeck PD, Paterson DH, McCreary CR, Marsh GD, Cunningham DA, Thompson RT. The effects of age on kinetics of oxygen uptake and phosphocreatine in humans during exercise. Exp Physiol. 1998;83:107–117. doi: 10.1113/expphysiol.1998.sp004087. [DOI] [PubMed] [Google Scholar]
- 4.Chung LH, Callahan DM, Kent-Braun JA. Age-related resistance to skeletal muscle fatigue is preserved during ischemia. J Appl Physiol. 2007;103:1628–1635. doi: 10.1152/japplphysiol.00320.2007. [DOI] [PubMed] [Google Scholar]
- 5.Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–852. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- 6.Debold EP, Beck SE, Warshaw DM. The effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol Cell Physiol. 2008;295:C173–179. doi: 10.1152/ajpcell.00172.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol. 2000;78:781–790. [PubMed] [Google Scholar]
- 8.Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- 9.Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol. 2008;105:637–642. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 11.Hepple RT, Vogell JE. Anatomic capillarization is maintained in relative excess of fiber oxidative capacity in some skeletal muscles of late middle-aged rats. J Appl Physiol. 2004;96:2257–2264. doi: 10.1152/japplphysiol.01309.2003. [DOI] [PubMed] [Google Scholar]
- 12.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol. 1998;85:1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- 13.Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol. 2005;99:890–897. doi: 10.1152/japplphysiol.00243.2005. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsson A, Borg K, Edstrom L. Fiber-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropath. 1990;80:459–468. doi: 10.1007/BF00294604. [DOI] [PubMed] [Google Scholar]
- 15.Kamen G, Sison SV, Duke Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79(6):1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- 16.Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q. 1994;10:43–63. [PubMed] [Google Scholar]
- 17.Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1999;87:22–29. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- 18.Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol. 2000;89:1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- 19.Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- 20.Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99:1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol. 2007;583:1093–1105. doi: 10.1113/jphysiol.2007.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–975. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- 23.Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol. 2003;95:2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- 24.Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischemic muscle contractions in humans. J Physiol. 2006;577.1:353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindstrom B, Lexell J, Gerdle B, Downham D. Skeletal muscle fatigue and endurance in young and old men and women. J Gerontol A Biol Sci Med Sci. 1997;52:B59–66. doi: 10.1093/gerona/52a.1.b59. [DOI] [PubMed] [Google Scholar]
- 26.McNeil CJ, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci. 2007;62:624–629. doi: 10.1093/gerona/62.6.624. [DOI] [PubMed] [Google Scholar]
- 27.Narici MV, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol. 1991;71:1277–1281. doi: 10.1152/jappl.1991.71.4.1277. [DOI] [PubMed] [Google Scholar]
- 28.Ng AV, Kent-Braun JA. Slowed muscle contractile properties are not associated with a decreased EMG/force relationship in older humans. J Gerontol A Biol Sci Med Sci. 1999;54:B452–B458. doi: 10.1093/gerona/54.10.b452. [DOI] [PubMed] [Google Scholar]
- 29.Rubinstein S, Kamen G. Decreases in motor unit firing rate during sustained maximal-effort contractions in young and older adults. J Electromyogr Kinesiol. 2005;15:536–543. doi: 10.1016/j.jelekin.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Russ DW, Towse TF, Wigmore DM, Lanza IR, Kent-Braun JA. Contrasting influences of age and sex on muscle fatigue. Med Sci Sports Exerc. 2008;40:234–241. doi: 10.1249/mss.0b013e31815bbb93. [DOI] [PubMed] [Google Scholar]
- 31.Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J Physiol. 1996;493(Pt 2):299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]