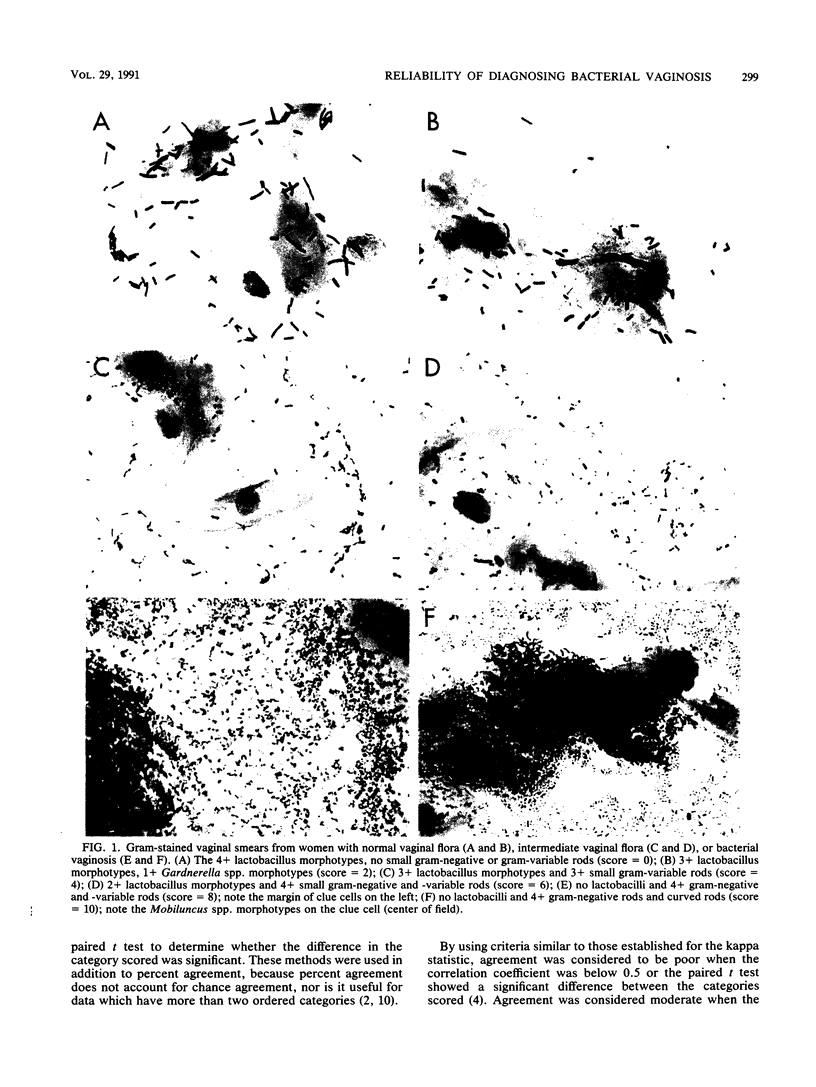
Abstract
The purpose of the study was to examine intercenter variability in the interpretation of Gram-stained vaginal smears from pregnant women. The intercenter reliability of individual morphotypes identified on the vaginal smear was evaluated by comparing them with those obtained at a standard center. A new scoring system that uses the most reliable morphotypes from the vaginal smear was proposed for diagnosing bacterial vaginosis. This scoring system was compared with the Spiegel criteria for diagnosing bacterial vaginosis. The scoring system (0 to 10) was described as a weighted combination of the following morphotypes: lactobacilli, Gardnerella vaginalis or bacteroides (small gram-variable rods or gram-negative rods), and curved gram-variable rods. By using the Spearman rank correlation to determine intercenter variability, gram-positive cocci had poor agreement (0.23); lactobacilli (0.65), G. vaginalis (0.69), and bacteroides (0.57) had moderate agreement; and small (0.74) and curved (0.85) gram-variable rods had good agreement. The reliability of the 0 to 10 scoring system was maximized by not using gram-positive cocci, combining G. vaginalis and bacteroides morphotypes, and weighting more heavily curved gram-variable rods. For comparison with the Spiegel criteria, a score of 7 or higher was considered indicative of bacterial vaginosis. The standardized score had improved intercenter reliability (r = 0.82) compared with the Spiegel criteria (r = 0.61). The standardized score also facilitates future research concerning bacterial vaginosis because it provides gradations of the disturbance of vaginal flora which may be associated with different levels of risk for pregnancy complications.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsel R., Totten P. A., Spiegel C. A., Chen K. C., Eschenbach D., Holmes K. K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983 Jan;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- DUNKELBERG W. E., Jr DIAGNOSIS OF HEMOPHILUS VAGINALIS VAGINITIS BY GRAM-STAINED SMEARS. Am J Obstet Gynecol. 1965 Apr 1;91:998–1000. doi: 10.1016/0002-9378(65)90569-7. [DOI] [PubMed] [Google Scholar]
- GARDNER H. L., DUKES C. D. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol. 1955 May;69(5):962–976. [PubMed] [Google Scholar]
- Gravett M. G., Hummel D., Eschenbach D. A., Holmes K. K. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986 Feb;67(2):229–237. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- Gravett M. G., Nelson H. P., DeRouen T., Critchlow C., Eschenbach D. A., Holmes K. K. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA. 1986 Oct 10;256(14):1899–1903. [PubMed] [Google Scholar]
- Hillier S. L., Martius J., Krohn M., Kiviat N., Holmes K. K., Eschenbach D. A. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988 Oct 13;319(15):972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- Krohn M. A., Hillier S. L., Eschenbach D. A. Comparison of methods for diagnosing bacterial vaginosis among pregnant women. J Clin Microbiol. 1989 Jun;27(6):1266–1271. doi: 10.1128/jcm.27.6.1266-1271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclure M., Willett W. C. Misinterpretation and misuse of the kappa statistic. Am J Epidemiol. 1987 Aug;126(2):161–169. doi: 10.1093/aje/126.2.161. [DOI] [PubMed] [Google Scholar]
- Martius J., Krohn M. A., Hillier S. L., Stamm W. E., Holmes K. K., Eschenbach D. A. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstet Gynecol. 1988 Jan;71(1):89–95. [PubMed] [Google Scholar]
- Mazzulli T., Simor A. E., Low D. E. Reproducibility of interpretation of Gram-stained vaginal smears for the diagnosis of bacterial vaginosis. J Clin Microbiol. 1990 Jul;28(7):1506–1508. doi: 10.1128/jcm.28.7.1506-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam S., Fitzgerald B. L. Semiquantitative culture of Gardnerella vaginalis in laboratory determination of nonspecific vaginitis. J Clin Microbiol. 1983 Aug;18(2):344–347. doi: 10.1128/jcm.18.2.344-347.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel C. A., Amsel R., Eschenbach D., Schoenknecht F., Holmes K. K. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med. 1980 Sep 11;303(11):601–607. doi: 10.1056/NEJM198009113031102. [DOI] [PubMed] [Google Scholar]
- Spiegel C. A., Amsel R., Holmes K. K. Diagnosis of bacterial vaginosis by direct gram stain of vaginal fluid. J Clin Microbiol. 1983 Jul;18(1):170–177. doi: 10.1128/jcm.18.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason J. L., Gelbart S. M., Wilcoski L. M., Peterson A. K., Jilly B. J., Hamilton P. R. Proline aminopeptidase activity as a rapid diagnostic test to confirm bacterial vaginosis. Obstet Gynecol. 1988 Apr;71(4):607–611. [PubMed] [Google Scholar]
- Totten P. A., Amsel R., Hale J., Piot P., Holmes K. K. Selective differential human blood bilayer media for isolation of Gardnerella (Haemophilus) vaginalis. J Clin Microbiol. 1982 Jan;15(1):141–147. doi: 10.1128/jcm.15.1.141-147.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. H., Krohn M. A., Hillier S. L., Eschenbach D. A. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 1990 Jan;75(1):52–58. [PubMed] [Google Scholar]