Abstract
Objective
Folic acid supplementation has been shown to be an effective agent for improving endothelial function, a prognostic factor for cardiovascular disease; but its effects on systolic and diastolic blood pressure in hypertensive individuals has been met with mixed results. Therefore, the purpose of this study was to provide a comprehensive meta-analysis of randomized controlled trials to investigate the effect of high-dose folic acid supplementation on blood pressure and endothelial function in hypertensive patients.
Methods
Twelve randomized controlled trials published between 1970 and December 2007 were identified using Medline and a manual search. All 12 studies used hypertensive subjects who were supplemented with at least 5000 μg/d of folic acid for between 2 and 16 weeks. Three separate meta-analyses were carried out using a random-effects model, and the overall effect sizes were calculated for changes in systolic and diastolic blood pressures and for changes in endothelial function as measured through the percentage of change in flow-mediated dilation.
Results
The pooled estimate of effect of folic acid supplementation on systolic and diastolic blood pressure was −2.03 mm Hg (95% confidence interval [CI], −3.63 to −0.43; P = .04) and 0.01 mm Hg (95% CI, −1.12 to 1.13; not significant), respectively. The pooled estimate of effect of folic acid supplementation on change in flow-mediated dilation was 1.61% (95% CI, 1.27 to 1.96; P = .000).
Conclusion
Based upon the studies used in this meta-analysis, supplementation with at least 5000 μg/d of folic acid, for a minimum of 6 weeks, can lower systolic blood pressure slightly; but the real clinical benefit is achieved through improved endothelial function.
Key indexing terms: Folic acid, Blood pressure, Hypertension, Endothelium, Meta-analysis, Chiropractic
Introduction
Hypertension affects at least one quarter of the adult population in the United States and is an important determinant of the incidence of coronary heart disease.1 It has been demonstrated that low serum folate levels are related to increased cardiovascular risk.2-4 Epidemiologic evidence suggests that a deficiency of folic acid may lead to hypertension, and a negative association between plasma folic acid and blood pressure (BP) has been reported.5 However, this association only suggests that the intake of extra folic acid lowers BP; and clinical trials performed to assess the effect of folic acid supplementation on BP in hypertensive individuals have been met with mixed results.6-12
Folic acid has also been shown to significantly improve endothelial dysfunction.13-16 These findings are clinically significant because endothelial dysfunction is observed to play an active role in the pathophysiologic mechanisms leading to coronary heart disease.17,18 An important noninvasive surrogate of endothelial dysfunction is flow-mediated dilation (FMD), which has been demonstrated to be a good predictor of subsequent cardiovascular events.19,20 This widely applied surrogate measure of coronary heart disease risk involves occlusion of the brachial artery with a pneumatic tourniquet for 5 minutes. Afterward, the pressure is released; and the reactive hyperemia that results, which is due to the increased brachial artery blood flow and subsequent shear stress on the endothelial walls, is imaged and measured using high-resolution ultrasound. A number of recent studies have shown that folic acid supplementation can result in a significant improvement in FMD6,11,21,22; however, a couple of studies have shown no such findings,23-25 especially those studies that used low-dose folic acid supplementation (400-800 μg).26-28
To address the inconsistency in the literature, a meta-analysis was conducted recently that showed that folic acid improved endothelial function via improvements in FMD; but this article failed to conduct an analysis on the more traditional clinical measures of systolic and diastolic BP.29 Furthermore, the authors of this meta-analysis confounded their overall estimate of effect by including in their pooled analysis the studies that used low-dose folic acid supplementation. Based on these shortcomings, it was deemed necessary to conduct a comprehensive meta-analysis of randomized placebo-controlled trials that used high-dose folic acid supplementation (5000-10 000 μg/d) to determine if reductions in systolic and diastolic BP as well as an increase in percentage of change in FMD (%FMD) in patients with hypertension were at all possible.
Methods
Selection of studies
A comprehensive PubMed literature search was performed to locate relevant randomized controlled trials published between 1966 through December 2007. The following headings were combined using the following Boolean operation: (Folic Acid OR Folate) AND (Blood Pressure OR Hypertension OR Hypertensive OR Endothelial OR Flow Mediated). The search was restricted to key terms located in the title/abstract and was also restricted to studies published in English-language journals. Only full-length original journal articles were considered. No attempt was made to include abstracts or unpublished studies. A manual search was also conducted by using reference lists from original research papers and review articles.
To be included in the meta-analysis, a study had to meet the following criteria: (1) the study was conducted using hypertensive human subjects (systolic BP >130 mm Hg); (2) there was at least single-blinded, random allocation of study participants to either folic acid treatment or placebo-controlled groups; (3) folic acid was given orally with a minimum dose of 5000 μg/d; (4) the intervention was equal to or greater than 2 weeks and less than or equal to 16 weeks; and (5) the study reported the mean BP changes for systolic and diastolic BPs and/or the %FMD in both the treatment and control groups. Studies that reported either BP or %FMD changes alone, assuming all other criteria were met, were included in the study.
Twelve studies met the eligibility criteria and were included in the meta-analysis.6-12,21-25 Although 45 potentially relevant studies were identified and screened, 33 trials did not meet the eligibility criteria for the meta-analysis. Major reasons for exclusion of studies were (1) cointervention with other therapies (10 trials); (2) nonhypertensive subject populations (6 trials); (3) not randomized (3 trials); (4) a treatment duration of less than 2 weeks or greater than 12 weeks (3 trials); (5) folic acid dose less than 5000 μg (2 trials); (6) use of children as subjects (2 trials); (7) study populations overlapped with other published studies (1 trials); and (8) an absence of data to calculate the net mean change in BP or FMD from baseline to end of follow-up (6 trials). Fig 1 shows the number of studies that were identified and excluded at different stages of the selection process.
Fig 1.
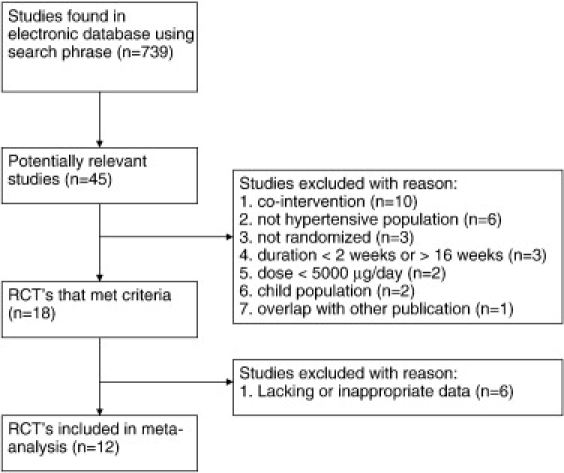
Study selection process for inclusion in the meta-analysis investigating the effects of folic acid supplementation on BP and %FMD changes in hypertensive patients.
Two studies7,8 included in the BP analysis that did report measures on FMD were excluded from the FMD analysis because they did not report %FMD, but had reported their results as the baseline diameter in millimeters and the diameter increase postarterial occlusion in micrometers. The %FMD could have been calculated from the available data; but the variance would have to be estimated, and such an estimate would have introduced an unknown amount of uncertainty into the meta-analysis calculation. Therefore, the decision was made to exclude these 2 articles from the %FMD analysis.
Data abstraction and statistical analysis
Information on sample size, participant characteristics, study design, folic acid dosage, duration, and treatment results were abstracted from the 12 clinical trials and entered into a Microsoft Excel (Microsoft Inc, Redmond, WA) spreadsheet. To calculate the overall effect size, each study was weighted by the reciprocal of the variance for either BP or %FMD changes. Variances for BP net changes between treatment and control groups were provided for 2 studies, whereas the variances for the remaining 10 studies were calculated using the variances at baseline and at the end of follow-up based on the methodology of Follmann et al.30 In this method, a correlation coefficient of 0.5 between initial and final measures was assumed. Within each trial, equal variance was assumed between the control and intervention groups, as well as between the beginning and end of each trial. For parallel and crossover trials, net changes in BP or FMD were calculated as (BP or FMD at end of follow-up in the treatment group − BP or FMD at baseline in the treatment group) − (BP or FMD at end of follow-up in the control group − BP or FMD at baseline in the control group).
Estimates of the mean effect of folic acid supplementation on BP and FMD as well as the corresponding 95% confidence intervals (CIs) were calculated using random-effects models. The assumption of heterogeneity implied by the use of the random-effects model was plausible because of differences between trials in such aspects as duration of the trial, dosages used, and sample populations that differed by age and sex. To examine potential publication bias, sample size was plotted against effect size. Data analysis was performed using Comprehensive Meta-Analysis software version 2.0 (Biostat, Englewood, NJ).
Results
Participant characteristics and study designs
Participant and study design characteristics for the 8 randomized controlled trials included in the BP meta-analysis are presented in Table 1. Collectively, the 8 trials that were conducted between 1991 and 2007 included a total of 293 subjects (222 in both the folic acid supplementation and control groups). All of the trials were conducted in adults, with an age range of 32 to 58.9 years. Men were the majority in 7 of the 8 trials, with the pooled population being made up of 83.1% men. Four trials had a parallel double-blind design, and 4 used a crossover double-blind design. The study duration varied from 2 to 16 weeks, with a median duration of 6 weeks. Folic acid supplementation for 6 of the 8 trials was 5000 μg/d, whereas 2 trials used 10 000 μg/d. The baseline systolic and diastolic BPs ranged from 131 to 139 mm Hg and 73 to 83 mm Hg, respectively. The weighted average baseline systolic and diastolic BPs were 133.2 and 76.8 mm Hg, respectively.
Table 1.
Population and baseline characteristics of the 12 trials included in the BP and FMD meta-analyses
| Source and Year (ref) | Sample Size | Mean Age, y | Male, % | Study Design | Folic Acid/d, mg | Duration, wk | Baseline Measures |
||
|---|---|---|---|---|---|---|---|---|---|
| Systolic BP, mm Hg | Diastolic BP, mm Hg | % FMD | |||||||
| Woo et al 199921 | 17 | 54 | 88 | XD | 10 | 8 | 5.7 | ||
| Thambyrajah et al 200023 | 100 | 61.5 | 73 | PD | 5 | 12 | 3.7 | ||
| Title et al 20006 | 50 | 58.9 | 80 | PD | 5 | 16 | 132 | 80 | 3.0 |
| Doshi et al 20017 | 50 | 57 | 88 | XD | 5 | 6 | 133 | 73 | |
| Thambyrajah et al 200124 | 86 | 63.2 | 87 | PD | 5 | 12 | 3.6 | ||
| Doshi et al 20028 | 33 | 55.5 | 90 | PD | 5 | 6 | 132 | 73 | |
| Lekakis et al 200422 | 34 | 56.5 | 85 | PD | 5 | 4 | 139 | 78 | 5.2 |
| Woodman et al 200425 | 26 | 49 | 69 | XD | 5 | 8 | 7.2 | ||
| Mangoni et al 20059 | 26 | 56.5 | 54 | PD | 5 | 4 | 137 | 77 | |
| Williams et al 200510 | 41 | 32 | 100 | XD | 5 | 3 | 131 | 74 | |
| Title et al 200611 | 19 | 54.5 | 47 | XD | 10 | 2 | 133 | 83 | 3.1 |
| Moens et al 200712 | 40 | 56.5 | 92.5 | XD | 10 | 6 | 131 | 76 | 4.0 |
XD, Crossover double blind; PD, parallel double blind.
Participant and study design characteristics for the 8 randomized controlled trials included in the FMD meta-analysis are presented in Table 1. Collectively, the 8 trials that were conducted between 1991 and 2007 included a total of 372 subjects (237 in both the folic acid supplementation and control groups). All of the trials were conducted in adults, with an age range of 49 to 63.2 years. Men were the majority in 7 of the 8 trials, with the pooled population being made up of 79.4% men. Four trials had a parallel double-blind design, and 4 used a crossover double-blind design. The study duration varied from 2 to 16 weeks, with a median duration of 6 weeks. Folic acid supplementation for 5 of the 8 trials was 5000 μg/d, whereas 3 trials used 10 000 μg/d. The baseline %FMD change ranged between 3.0% and 5.7%, with a weighted average of 4.1%.
Net change in BP and %FMD
The individual trial results along with the 95% CIs and the overall pooled estimates of effect in both systolic and diastolic BPs are shown in Figs 2 and 3, respectively. Folic acid's effects on systolic BP illustrate that 5 of the 8 trials presented with an intervention-related trend toward a reduction in systolic BP, but with only 1 of the 5 showing a statistically significant reduction (P < .05) in BP when compared with the control group (Fig 2). For diastolic BP, a trend toward intervention-related reduction was observed in only 3 of the 8 trials, but with none of these showing a statistically significant reduction in BP when compared with the control group (Fig 3). The overall pooled estimate of effect of folic acid supplementation on systolic BP resulted in a pressure reduction of 2.03 mm Hg (95% CI, −3.63 to −0.43; P = .04). This reduction in BP was just barely statistically significant. The overall pooled estimate of effect of folic acid supplementation on diastolic BP resulted in an increase in pressure of 0.01 mm Hg (95% CI, −1.12 to 1.13; not significant).
Fig 2.
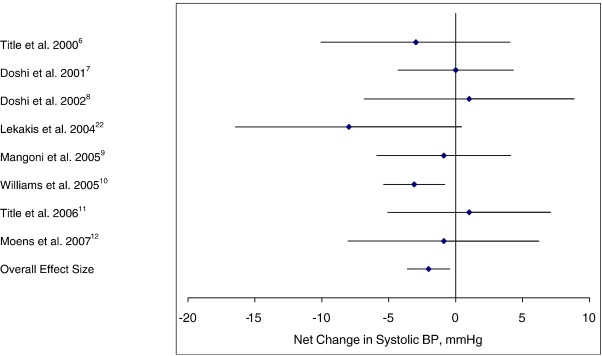
Net change (and 95% CI) in systolic BP associated with folic acid supplementation. The overall effect size is weighted by the inverse of the total variance of each trial.
Fig 3.
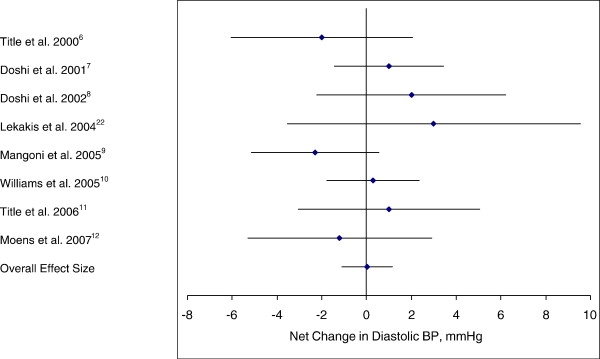
Net change (and 95% CI) in diastolic BP associated with folic acid supplementation. The overall effect size is weighted by the inverse of the total variance of each trial.
The individual trial results for the change in %FMD and the pooled estimate of effect are presented in Fig 4. Folic acid's effects on %FMD showed that all 8 of the trials presented with an intervention-related trend toward an increase in %FMD, with 5 of the 8 showing statistically significant increases (P < .05) when compared with their control groups (Fig 4). The overall pooled estimate of effect of folic acid supplementation on %FMD change resulted in an increase of 1.61% (95% CI, 1.27 to 1.96; P = .000).
Fig 4.
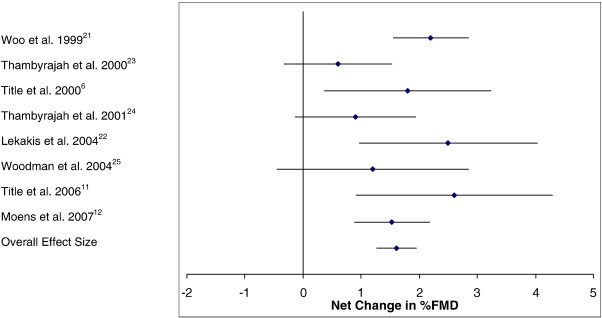
Net change (and 95% CI) in %FMD associated with folic acid supplementation. The overall effect size is weighted by the inverse of the total variance of each trial.
Both the BP and %FMD plots of sample size vs effect size showed a typical “funnel” shape with little variation in effect size for large sample studies and increasing spread of effect size with smaller sample sizes. In both cases, the distribution of effects sizes seen in the individual studies was symmetrically distributed around the pooled mean effect size.
Discussion
This is the first meta-analysis to provide a comprehensive examination of the effects of folic acid supplementation on both BP and %FMD together. The overall effect sizes observed in this meta-analysis included a 2.03 mm Hg reduction in systolic BP, a 0.01 mm Hg increase in diastolic BP, and a 1.61 % increase in %FMD change. Although the reduction in systolic BP was just barely statistically significant, this small change could potentially translate to a 4% reduction in coronary heart disease risk.31-33 Furthermore, the significant increase in %FMD could similarly translate to a 5% reduction in coronary heart disease risk.34 In 1 epidemiologic study comparing endothelial dysfunction with cardiovascular risk prediction, it was shown that patients who had a past cardiovascular event possessed %FMD measures that were 1.80% less than patients who had no past cardiovascular events.35 The effects of folic acid supplementation on systolic BP and %FMD are consistent with epidemiologic observations that there exists an inverse relationship between BP and plasma folate status.36 It has not escaped our notice that this meta-analysis supports that folic acid supplementation has the potential to positively impact cardiovascular disease rates.
One possible explanation for folic acid's nonsignificant observable effect on diastolic BP may be because the baseline diastolic BP values were starting below 90 mm Hg, and so there was no considerable room for improvement when subjects were supplemented with folic acid. Another possible reason is that the folic acid dose used was too small to achieve an observable effect, as it was illustrated that 30 000 μg/d was able to drop diastolic BP from 75 to 70 mm Hg in a patient population with coronary artery disease.37
The %FMD change with high-dose folic acid supplementation (5000-10 000 μg/d) determined in this meta-analysis was comparable with the finding in a previous meta-analysis (1.61% vs 1.42%, respectively).29 The authors of this previous meta-analysis also showed that low-dose folic acid supplementation (400-800 μg/d) had no appreciable effect on changes in %FMD, as the pooled effect size for 4 of these studies was only −0.07%. This suggests that folic acid's effects on %FMD change are achievable when supplemental doses are in the range of 5000 μg/d and above.
In regard to mechanism of action, the primary mechanism proposed for the effect of folic acid on endothelial function is a reduction in plasma homocysteine concentrations by remethylation of homocysteine back to methionine.38 Homocysteine increases oxidative stress by increasing superoxide anion production that results in a down-regulation of nitric oxide (NO) production.39,40 Several studies have observed homocysteine to be positively associated with BP,41,42 and a couple of studies have shown that homocysteine-lowering vitamin treatments have shown a decrease in BP.16,43 Hypertension has been shown to be associated with impaired NO production.44 It has been shown that endothelial dysfunction can be induced in healthy subjects, as measured by using FMD, with oral methionine load–induced hyperhomocysteinemia.38,45 Hyperhomocysteinemia has emerged as an independent risk factor for cardiovascular disease; and it was shown that, for each 3 μmol/L increase in plasma homocysteine, the risk of coronary heart disease increased approximately 11%.46 In this meta-analysis, the average weighted decrease in homocysteine was calculated to be approximately 2.5 μmol/L. This reduction in homocysteine should therefore translate to a reduction in coronary heart disease risk by approximately 9%, which is somewhat higher than the surmised average calculations made previously in the first paragraph based on the observed decrease in systolic BP and increase in %FMD change. It therefore remains controversial as to whether the endothelial dysfunction is mediated directly by homocysteine, as numerous studies have shown that the acute administration of folic acid improved endothelial function without any effects on homocysteine concentrations.12,16,47 If the effects of folic acid on %FMD change are largely independent of a homocysteine-lowering effect, then some other mechanisms must contribute to the benefit of high-dose folic acid supplementation on endothelial dysfunction; and 3 such possibilities will be further addressed.
First, folic acid was shown to have antioxidant properties that can directly scavenge superoxide anions that are capable of destroying the enzyme endothelial nitric oxide synthase (eNOS), its cofactor tetrahydrobiopterin (BH4), and its NO product.7,47 Hypertension is associated with an imbalance in antioxidant status, suggesting that oxidative stress is an important driving factor in the pathogenesis of hypertension. However, folic acid's scavenging potency is 20-fold lower than the scavenging ability of vitamin C,48 a well-known antioxidant and known chemical stabilizer of BH4.49-51 Furthermore, folic acid's antioxidant ability has been questioned because it was shown to be unable to reduce the cellular concentrations of malondialdehyde, an end product of lipid peroxidation.6,7
Second, the eNOS cofactor BH4 plays a crucial role in regulating NO and superoxide production.52-55 Normally, BH4 concentrations are adequate and eNOS will transform l-arginine into NO; but reactive oxygen species such as superoxide can oxidize BH4 to its inactive metabolite BH2. This of course leads to a reduction in BH4 availability and promotes eNOS uncoupling.56 The uncoupling of eNOS results in the generation of superoxide rather than NO, which contributes to vascular oxidative stress, and further reduces NO availability.57 Hence, folic acid may rescue or stabilize BH4 by stimulating the regeneration of BH2 to BH4, thereby leading to an increased BH4 concentration that can recouple the eNOS enzyme and therefore increase NO production.58 However, a recent study found that folic acid was able to reverse the endothelial dysfunction induced by BH4 depletion independently of either the regeneration or stabilization of BH4.59 This last finding therefore leads us to our third possible mechanism of action.
Third, folic acid may directly improve NO production by enhancing the enzymatic activity of eNOS.48,60 This is a result of folic acid containing a pteridine ring, which is molecularly similar to the ring structure found in BH4. This ring structure allows folic acid to bind to the pteridine-binding site of eNOS, therefore mimicking BH4 and resulting in an increased synthesis of NO. Fig 5 illustrates all of the mechanisms described in the preceding paragraphs.
Fig 5.
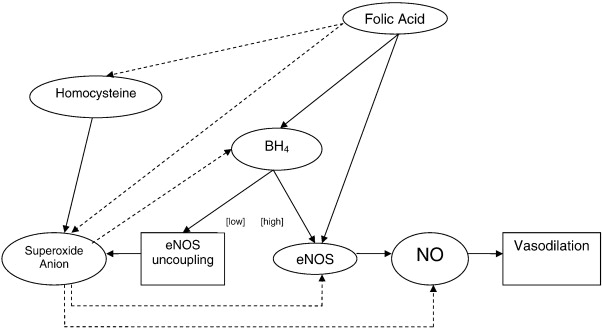
Proposed mechanism showing the relationship between folic acid and endothelial function. Solid lines represent increase in synthesis, action, or positive influence; dashed lines represent decrease or inhibition of synthesis, action, or negative influence. [high] indicates high concentrations of BH4; [low], low concentrations of BH4.
Another possible effect of folic acid on endothelial dysfunction is that the effects are mediated through non-NO pathways. However, studies that have investigated folic acids action on other markers of endothelial dysfunction have shown no possible role.61 This study failed to show any significant alteration in blood-borne markers of endothelial dysfunction such as E-selectin, von Willebrand factor, or thrombomodulin. Another more recent study also found no changes in plasma markers such as C-reactive protein, intercellular adhesion molecule, vascular cell adhesion molecule, interleukin-18, or tumor necrosis factor–α.11
Although a reduction in systolic BP and an increase in %FMD by folic acid supplementation may possibly translate to a drop in coronary heart disease risk by approximately 5%, a recent meta-analysis of 12 randomized controlled trials using 16 958 participants had found that folic acid supplementation had no effect on reducing the risk of coronary heart disease.62 It should be noted that half of the studies included in this meta-analysis used folic acid dosages that were much less than 5000 μg/d. This begs the question then that the changes in BP and %FMD, along with the concomitant changes in coronary heart disease risk, may only be observed when folic acid doses are in the order of 5000 μg/d or greater. Several ongoing trials with large sample sizes might provide a definitive answer to this important clinical and public health question.63,64
Because high-dose folic acid supplementation at doses of 5000 to 10 000 μg/d may be necessary to achieve the clinical benefits in reducing coronary heart disease rates, there is concern that, in populations with vitamin B-12 deficiencies (such as the elderly and vegans), high-dose folic acid supplementation may mask the hematologic manifestations of the B-12 deficiency and propagate the progression of central and peripheral neurologic damage. Of course, this can be ameliorated with the inclusion of vitamin B-12 with folic acid supplements. There is also a concern that high-dose folic acid therapy might lead to an increase in the incidence of colon or prostate carcinogenesis.65 Two studies have reported an increased risk of prostate cancer in association with high folate intake, but these results were not statistically significant and were limited to early-stage cancers.66,67 It has also been suggested that high intakes of folic acid may not lead to de novo carcinogenesis, but may only increase the rate of proliferation of already established tumor cells.68,69
A major limitation of this study is the pooling of clinical trials that include a considerable amount of heterogeneity in design and population characteristics. Furthermore, not having evenly matched baseline BPs could confound the results, as populations with higher baseline BPs could possibly exhibit more of a hypotensive effect with folic acid supplementation. Confounders also included differences between studies with folic acid supplementation dose (ranged between 5000 and 10 000 μg/d) and study duration (ranged between 2 and 16 weeks) and patient population clinical morbidities, as 5 of the 12 clinical trials used patients with coronary heart disease, 2 with type 2 diabetes, 2 with essential hypertension, and 1 each with chronic kidney disease, hypercholesterolemia, and hyperhomocysteinemia.
Conclusion
In summary, this meta-analysis has shown that supplementation with 5000 to 10 000 μg/d of folic acid for 6 weeks can lower systolic BP and increase %FMD changes. These findings are important because such changes may subsequently result in a reduction in the incidence of coronary heart disease. Although the changes in systolic BP were modest, any small sustained change can have beneficial effects, especially in light of the low cost and absence of toxicity even when folic acid supplementation is in the range of 5000 μg/d.70 However, in light of the current epidemiologic studies showing no clinical benefit of folic acid supplementation on coronary heart disease risk, further clinical trials investigating the effects of high-dose folic acid on hard clinical cardiovascular end points, beyond BP and %FMD changes, are required.
References
- 1.Burt V.L., Whelton P., Roccella E.J. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25(3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 2.Robinson K., Arheart K., Refsum H. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation. 1998;97(5):437–443. doi: 10.1161/01.cir.97.5.437. [DOI] [PubMed] [Google Scholar]
- 3.Bunout D., Petermann M., Hirsch S. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition. 2000;16(6):434–438. doi: 10.1016/s0899-9007(00)00289-6. [DOI] [PubMed] [Google Scholar]
- 4.Voutilainen S., Rissanen T.H., Virtanen J., Lakka T.A., Salonen J.T. Kuopio Ischemic Heart Disease Risk Factor Study. Low dietary folate intake is associated with an excess incidence of acute coronary events: the Kuopio Ischemic Heart Disease Risk Factor Study. Circulation. 2001;103(22):2674–2680. doi: 10.1161/01.cir.103.22.2674. [DOI] [PubMed] [Google Scholar]
- 5.Forman J.P., Rimm E.B., Stampfer M.J., Curhan G.C. Folate intake and the risk of incident hypertension among US women. JAMA. 2005;293(3):320–329. doi: 10.1001/jama.293.3.320. [DOI] [PubMed] [Google Scholar]
- 6.Title L.M., Cummings P.M., Giddens K., Genest J.J., Nassar B.A. Effect of folic acid and antioxidant vitamins on endothelial dysfunction in patients with coronary artery disease. J Am Coll Cardiol. 2000;36(3):758–765. doi: 10.1016/s0735-1097(00)00809-3. [DOI] [PubMed] [Google Scholar]
- 7.Doshi S.N., McDowell I.F., Moat S.J. Folate improves endothelial function in coronary artery disease: an effect mediated by reduction of intracellular superoxide? Arterioscler Thromb Vasc Biol. 2001;21(7):1196–1202. doi: 10.1161/hq0701.092000. [DOI] [PubMed] [Google Scholar]
- 8.Doshi S.N., McDowell I.F., Moat S.J. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002;105(1):22–26. doi: 10.1161/hc0102.101388. [DOI] [PubMed] [Google Scholar]
- 9.Mangoni A.A., Sherwood R.A., Asonganyi B., Swift C.G., Thomas S., Jackson S.H. Short-term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens. 2005;18(2 Pt 1):220–226. doi: 10.1016/j.amjhyper.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Williams C., Kingwell B.A., Burke K., McPherson J., Dart A.M. Folic acid supplementation for 3 wk reduces pulse pressure and large artery stiffness independent of MTHFR genotype. Am J Clin Nutr. 2005;82(1):26–31. doi: 10.1093/ajcn.82.1.26. [DOI] [PubMed] [Google Scholar]
- 11.Title L.M., Ur E., Giddens K., McQueen M.J., Nassar B.A. Folic acid improves endothelial dysfunction in type 2 diabetes—an effect independent of homocysteine-lowering. Vasc Med. 2006;11(2):101–109. doi: 10.1191/1358863x06vm664oa. [DOI] [PubMed] [Google Scholar]
- 12.Moens A.L., Claeys M.J., Wuyts F.L. Effect of folic acid on endothelial function following acute myocardial infarction. Am J Cardiol. 2007;99(4):476–481. doi: 10.1016/j.amjcard.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy M.F., McDowell I.F., Ramsey M.W., Brownlee M., Newcombe R.G., Lewis M.J. Oral folate enhances endothelial function in hyperhomocysteinaemic subjects. Eur J Clin Invest. 1999;29(8):659–662. doi: 10.1046/j.1365-2362.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- 14.Verhaar M.C., Wever R.M., Kastelein J.J. Effects of oral folic acid supplementation on endothelial function in familial hypercholesterolemia. A randomized placebo-controlled trial. Circulation. 1999;100(4):335–338. doi: 10.1161/01.cir.100.4.335. [DOI] [PubMed] [Google Scholar]
- 15.Wilmink H.W., Stroes E.S., Erkelens W.D. Influence of folic acid on postprandial endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2000;20(1):185–188. doi: 10.1161/01.atv.20.1.185. [DOI] [PubMed] [Google Scholar]
- 16.Mangoni A.A., Sherwood R.A., Swift C.G., Jackson S.H. Folic acid enhances endothelial function and reduces blood pressure in smokers: a randomized controlled trial. J Intern Med. 2002;252(6):497–503. doi: 10.1046/j.1365-2796.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 17.Suwaidi J.A., Hamasaki S., Higano S.T., Nishimura R.A., Holmes D.R., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 18.Schächinger V., Britten M.B., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 19.Moens A.L., Goovaerts I., Claeys M.J., Vrints C.J. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127(6):2254–2263. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T., Kawano H., Miyamoto S. Prognostic value of flow-mediated dilation of the brachial artery in patients with cardiovascular disease. Intern Med. 2006;45(9):575–579. doi: 10.2169/internalmedicine.45.1534. [DOI] [PubMed] [Google Scholar]
- 21.Woo K.S., Chook P., Lolin Y.I., Sanderson J.E., Metreweli C., Celermajer D.S. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol. 1999;34(7):2002–2006. doi: 10.1016/s0735-1097(99)00469-6. [DOI] [PubMed] [Google Scholar]
- 22.Lekakis J.P., Papamichael C.M., Papaioannou T.G. Oral folic acid enhances endothelial function in patients with hypercholesterolaemia receiving statins. Eur J Cardiovasc Prev Rehabil. 2004;11(5):416–420. doi: 10.1097/00149831-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Thambyrajah J., Landray M.J., McGlynn F.J., Jones H.J., Wheeler D.C., Townend J.N. Does folic acid decrease plasma homocysteine and improve endothelial function in patients with predialysis renal failure? Circulation. 2000;102(8):871–875. doi: 10.1161/01.cir.102.8.871. [DOI] [PubMed] [Google Scholar]
- 24.Thambyrajah J., Landray M.J., Jones H.J., McGlynn F.J., Wheeler D.C., Townend J.N. A randomized double-blind placebo-controlled trial of the effect of homocysteine-lowering therapy with folic acid on endothelial function in patients with coronary artery disease. J Am Coll Cardiol. 2001;37(7):1858–1863. doi: 10.1016/s0735-1097(01)01235-9. [DOI] [PubMed] [Google Scholar]
- 25.Woodman R.J., Celermajer D.E., Thompson P.L., Hung J. Folic acid does not improve endothelial function in healthy hyperhomocysteinaemic subjects. Clin Sci (Lond) 2004;106(4):353–358. doi: 10.1042/CS20030296. [DOI] [PubMed] [Google Scholar]
- 26.Pullin C.H., Ashfield-Watt P.A., Burr M.L. Optimization of dietary folate or low-dose folic acid supplements lower homocysteine but do not enhance endothelial function in healthy adults, irrespective of the methylenetetrahydrofolate reductase (C677T) genotype. J Am Coll Cardiol. 2001;38(7):1799–1805. doi: 10.1016/s0735-1097(01)01668-0. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch S., Pia D., Yanez P. Hyperhomocysteinemia and endothelial function in young subjects: effects of vitamin supplementation. Clin Cardiol. 2002;25:495–501. doi: 10.1002/clc.4960251105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olthof M.R., Bots M.L., Katan M.B., Verhoef P. Effect of folic acid and betaine supplementation on flow-mediated dilation: a randomized, controlled study in healthy volunteers. PLoS Clin Trials. 2006;1(2):e10. doi: 10.1371/journal.pctr.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Bree A., van Mierlo L.A., Draijer R. Folic acid improves vascular reactivity in humans: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2007;86(3):610–617. doi: 10.1093/ajcn/86.3.610. [DOI] [PubMed] [Google Scholar]
- 30.Follmann D., Elliott P., Suh I., Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 31.Franklin S.S., Larson M.G., Khan S.A. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103(9):1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 32.Bowman T.S., Gaziano J.M., Kase C.S., Sesso H.D., Kurth T. Blood pressure measures and risk of total, ischemic, and hemorrhagic stroke in men. Neurology. 2006;67(5):820–823. doi: 10.1212/01.wnl.0000233981.26176.e1. [DOI] [PubMed] [Google Scholar]
- 33.Menotti A., Lanti M., Nedeljkovic S. The relationship of age, blood pressure, serum cholesterol and smoking habits with the risk of typical and atypical coronary heart disease death in the European cohorts of the Seven Countries Study. Int J Cardiol. 2006;106(2):157–163. doi: 10.1016/j.ijcard.2004.12.092. [DOI] [PubMed] [Google Scholar]
- 34.Witte D.R., Westerink J., de Koning E.J., van der Graaf Y., Grobbee D.E., Bots M.L. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol. 2005;45(12):1987–1993. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 35.Brevetti G., Silvestro A., Schiano V., Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108(17):2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 36.Stehouwer C.D., van Guldener C. Does homocysteine cause hypertension? Clin Chem Lab Med. 2003;41(11):1408–1411. doi: 10.1515/CCLM.2003.216. [DOI] [PubMed] [Google Scholar]
- 37.Tawakol A., Migrino R.Q., Aziz K.S. High-dose folic acid acutely improves coronary vasodilator function in patients with coronary artery disease. J Am Coll Cardiol. 2005;45(10):1580–1584. doi: 10.1016/j.jacc.2005.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Usui M., Matsuoka H., Miyazaki H., Ueda S., Okuda S., Imaizumi T. Endothelial dysfunction by acute hyperhomocyst(e)inaemia: restoration by folic acid. Clin Sci (Lond) 1999;96(3):235–239. [PubMed] [Google Scholar]
- 39.McDowell I.F., Lang D. Homocysteine and endothelial dysfunction: a link with cardiovascular disease. J Nutr. 2000;130(2S Suppl):369S–372S. doi: 10.1093/jn/130.2.369S. [DOI] [PubMed] [Google Scholar]
- 40.Pruefer D., Scalia R., Lefer A.M. Homocysteine provokes leukocyte-endothelium interaction by downregulation of nitric oxide. Gen Pharmacol. 1999;33(6):487–498. doi: 10.1016/s0306-3623(99)00045-2. [DOI] [PubMed] [Google Scholar]
- 41.Kahleová R., Palyzová D., Zvára K. Essential hypertension in adolescents: association with insulin resistance and with metabolism of homocysteine and vitamins. Am J Hypertens. 2002;15(10 Pt 1):857–864. doi: 10.1016/s0895-7061(02)02984-9. [DOI] [PubMed] [Google Scholar]
- 42.Lim U., Cassano P.A. Homocysteine and blood pressure in the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2002;156(12):1105–1113. doi: 10.1093/aje/kwf157. [DOI] [PubMed] [Google Scholar]
- 43.van Dijk R.A., Rauwerda J.A., Steyn M., Twisk J.W., Stehouwer C.D. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: a 2-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2001;21(12):2072–2079. doi: 10.1161/hq1201.100223. [DOI] [PubMed] [Google Scholar]
- 44.Kedziora-Kornatowska K., Czuczejko J., Pawluk H. The markers of oxidative stress and activity of the antioxidant system in the blood of elderly patients with essential arterial hypertension. Cell Mol Biol Lett. 2004;9(4A):635–641. [PubMed] [Google Scholar]
- 45.Bellamy M.F., McDowell I.F., Ramsey M.W. Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation. 1998;98(18):1848–1852. doi: 10.1161/01.cir.98.18.1848. [DOI] [PubMed] [Google Scholar]
- 46.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 47.Verhaar M.C., Wever R.M., Kastelein J.J., van Dam T., Koomans H.A., Rabelink T.J. 5-Methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97(3):237–241. doi: 10.1161/01.cir.97.3.237. [DOI] [PubMed] [Google Scholar]
- 48.Stroes E.S., van Faassen E.E., Yo M. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res. 2000;86(11):1129–1134. doi: 10.1161/01.res.86.11.1129. [DOI] [PubMed] [Google Scholar]
- 49.Huang A., Vita J.A., Venema R.C., Keaney J.F. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275(23):17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 50.Baker T.A., Milstien S., Katusic Z.S. Effect of vitamin C on the availability of tetrahydrobiopterin in human endothelial cells. J Cardiovasc Pharmacol. 2001;37(3):333–338. doi: 10.1097/00005344-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Heller R., Unbehaun A., Schellenberg B., Mayer B., Werner-Felmayer G., Werner E.R. l-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276(1):40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 52.Xia Y., Tsai A.L., Berka V., Zweier J.L. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273(40):25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 53.Vásquez-Vivar J., Kalyanaraman B., Martásek P. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95(16):9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vásquez-Vivar J., Kalyanaraman B., Martásek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. 2003;37(2):121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 55.Alp N.J., Channon K.M. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(3):413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 56.Stroes E., Hijmering M., van Zandvoort M., Wever R., Rabelink T.J., van Faassen E.E. Origin of superoxide production by endothelial nitric oxide synthase. FEBS Lett. 1998;438(3):161–164. doi: 10.1016/s0014-5793(98)01292-7. [DOI] [PubMed] [Google Scholar]
- 57.Dixon L.J., Morgan D.R., Hughes S.M. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation. 2003;107(13):1725–1728. doi: 10.1161/01.CIR.0000066283.13253.78. [DOI] [PubMed] [Google Scholar]
- 58.Verhaar M.C., Stroes E., Rabelink T.J. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22(1):6–13. doi: 10.1161/hq0102.102190. [DOI] [PubMed] [Google Scholar]
- 59.Moat S.J., Clarke Z.L., Madhavan A.K., Lewis M.J., Lang D. Folic acid reverses endothelial dysfunction induced by inhibition of tetrahydrobiopterin biosynthesis. Eur J Pharmacol. 2006;530(3):250–258. doi: 10.1016/j.ejphar.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 60.Hyndman M.E., Verma S., Rosenfeld R.J., Anderson T.J., Parsons H.G. Interaction of 5-methyltetrahydrofolate and tetrahydrobiopterin on endothelial function. Am J Physiol Heart Circ Physiol. 2002;282(6):H2167–H2172. doi: 10.1152/ajpheart.00935.2001. [DOI] [PubMed] [Google Scholar]
- 61.Doshi S.N., Moat S.J., Lewis M.J., McDowell I.F., Giddings J.C., Goodfellow J. Short-term high-dose folic acid does not alter markers of endothelial cell damage in patients with coronary heart disease. Int J Cardiol. 2004;94(2-3):203–207. doi: 10.1016/j.ijcard.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Bazzano L.A., Reynolds K., Holder K.N., He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296(22):2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 63.Bostom A.G., Carpenter M.A., Kusek J.W. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006;152(3):448.e1–448.e7. doi: 10.1016/j.ahj.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 64.B-Vitamin Treatment Trialists' Collaboration Homocysteine-lowering trials for prevention of cardiovascular events: a review of the design and power of the large randomized trials. Am Heart J. 2006;151(2):282–287. doi: 10.1016/j.ahj.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 65.Cole B.F., Baron J.A., Sandler R.S. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 66.Hultdin J., Van Guelpen B., Bergh A., Hallmans G., Stattin P. Plasma folate, vitamin B12, and homocysteine and prostate cancer risk: a prospective study. Int J Cancer. 2005;113(5):819–824. doi: 10.1002/ijc.20646. [DOI] [PubMed] [Google Scholar]
- 67.Stevens V.L., Rodriguez C., Pavluck A.L., McCullough M.L., Thun M.J., Calle E.E. Folate nutrition and prostate cancer incidence in a large cohort of US men. Am J Epidemiol. 2006;163(11):989–996. doi: 10.1093/aje/kwj126. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y.I. Role of folate in colon cancer development and progression. J Nutr. 2003;133(11 Suppl 1):3731S–3739S. doi: 10.1093/jn/133.11.3731S. [DOI] [PubMed] [Google Scholar]
- 69.Kim Y.I. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80:1123–1128. doi: 10.1093/ajcn/80.5.1123. [DOI] [PubMed] [Google Scholar]
- 70.Campbell N.R. How safe are folic acid supplements? Arch Intern Med. 1996;156(15):1638–1644. [PubMed] [Google Scholar]


