Abstract
The chicken is the most extensively studied species in birds and thus constitutes an ideal reference for comparative genomics in birds. Comparative cytogenetic studies indicate that the chicken has retained many chromosome characters of the ancestral avian karyotype. The homology between chicken macrochromosomes (1–9 and Z) and their counterparts in more than 40 avian species of 10 different orders has been established by chromosome painting. However, the avian homologues of chicken micro-chromosomes remain to be defined. Moreover, no reciprocal chromosome painting in birds has been performed due to the lack of chromosome-specific probes from other avian species. Here we have generated a set of chromosome-specific paints using flow cytometry that cover the whole genome of the stone curlew (Burhinus oedicnemus, Charadriiformes), a species with one of the lowest diploid number so far reported in birds, as well as paints from more microchromosomes of the chicken. A genome-wide comparative map between the chicken and the stone curlew has been constructed for the first time based on reciprocal chromosome painting. The results indicate that extensive chromosome fusions underlie the sharp decrease in the diploid number in the stone curlew. To a lesser extent, chromosome fissions and inversions occurred also during the evolution of the stone curlew. It is anticipated that this complete set of chromosome painting probes from the first Neoaves species will become an invaluable tool for avian comparative cytogenetics.
Keywords: chicken, stone curlew, Burhinus oedicnemus, reciprocal chromosome painting, chromosome rearrangements
Introduction
Current avian taxonomy recognizes about 9000 extant species. In spite of extensive comparative studies, evolutionary relationships among major avian groups remain a contentious issue due to the rapid radiation of most modern birds (Livezey and Zusi 2007; Hackett et al. 2008). Only two basal divergences in the tree of living birds are supported consistently by both morphological and molecular phylogenetic studies. The first divergence took place approximately 100–120 million years ago, leading to the separation of Paleognathae (ratites and tinamous) from all other remaining birds (Neognathae). The second divergence split the neognaths and gave birth to Galloanserae (chickens, ducks, and allies) and Neoaves (other neognaths) about 100 million years ago (van Tuinen et al. 2000; Livezey and Zusi 2001; van Tuinen and Hedges 2001; Mayr and Clarke 2003; Fain and Houde 2004). The timing of species radiation within Neoaves is under debate. Molecular dating suggests a Cretaceous radiation, while fossil evidence seems to support a Tertiary radiation (Hedges et al. 1996; Cooper and Penny 1997; Cracraft 2001; van Tuinen and Hedges 2001; Feduccia 2003; Ericson et al. 2006; Brown et al. 2008). However, new fossil evidence also supports a Cretaceous origin of neoavian lineages (Clarke et al. 2005). The rapid diversification of most modern avian orders in the late Cretaceous has hampered the attempt to rebuild the tree of living birds (Edwards et al. 2005). More evidence from different studies is required to understand better the evolution of modern bird orders.
Comparative cytogenetic analyses have suggested a high degree of conservatism in the avian karyotypes; most birds studied so far have high diploid numbers ranging from 2n=76 to 2n=84, comprising several pairs of macrochromosomes and a uniquely large number of microchromosomes. Only a small number of species, such as some Falconiformes, have exceptional karyotypes with relatively few microchromosomes and extensive rearrangements of macrochromosomes (Christidis 1990; Bed’Home et al. 2003; Nanda et al. 2006; also see reviews in Burt 2002, Griffin et al. 2007). Despite the apparent conservatism of avian chromosomes, rearrangements such as chromosomal fusions and fissions have been uncovered using fluorescence in-situ hybridization (FISH) with the chicken (Gallus gallus domesticus, 2n=78) chromosomes 1–9 and Z paint probes. So far, more than 40 avian species, which belong to 10 orders, have been investigated by this approach (Shetty et al. 1999; Schmid et al. 2000; Raudsepp et al. 2002; Shibusawa et al. 2002, 2004a, b, Guttenbach et al. 2003; Kasai et al. 2003; Derjusheva et al. 2004; Itoh and Arnold 2005; de Oliveira et al. 2005; Nanda et al. 2006, 2007; Nishida-Umehara et al. 2007; Nishida et al. 2008), providing insights into avian karyotype evolution and phylogenetic relationships (reviewed in Griffin et al. 2007).
Nevertheless, the origin and evolution of avian microchromosomes remains to be better addressed, as so far only two studies have used any GGA microchromosome probes in cross-species painting experiments (Derjusheva et al. 2004; de Oliveira et al. 2005). Avian microchromosomes were regarded as accessory elements that lacked genes and centromeres (Newcomer 1955). However, microchromosomes represent approximately 18–23% of the female chicken genome, and more recent studies indicate that microchromosomes have functional centromeres and telomeres, and are early-replicating, gene rich, and enriched for CpG islands (Auer et al. 1987; Solovei et al. 1994; Smith and Burt 1998; McQueen et al. 1996; Smith et al. 2000). Comparative gene mapping between the genomes of chicken, human, mouse and zebrafish has demonstrated that four ancient syntenies (homologous to chicken chromosomes 6, 14, 19 and 28) have been conserved in fish, birds and mammals for over 400 million years, and a ‘fusion–fission model’ of microchromosome evolution was proposed (Burt et al. 1999; Burt 2002). While comparative molecular cytogenetics has the potential to unravel the evolutionary origin of avian microchromosomes, this has been hampered by the lack of painting probes that cover the entire avian genome of a given avian species and, in particular, lack of probes from avian microchromosomes. Although a complete set of probes from mostly microdissected chicken microchromosomes has been established (Masabanda et al. 2004), such probes are often not suitable for cross-species painting due to their poor genomic coverage.
Here we have generated, by bivariate flow sorting and DOP-PCR amplification, a complete set of avian chromosome-specific painting probes that cover all chromosomes of the stone curlew (Burhinus oedicnemus, BOE), a Neoaves species with one of the lowest diploid numbers so far reported in birds. Moreover, we have prepared painting probes from more GGA microchromosomes in addition to GGA1–9 and ZW. Reciprocal chromosome painting between GGA and BOE, two species belonging to different orders (Galliformes and Charadriiformes) with highly contrasting karyotypes, has been conducted. A genome-wide comparative map between GGA and BOE has been established for the first time.
Materials and methods
Cell culture, metaphase preparation and chromosome nomenclature
Fibroblast cell lines derived from a female GGA embryo and a female BOE were grown in DMEM medium enriched with 15% fetal bovine serum. Metaphase preparations for flow sorting and in-situ hybridization were generated by the method described previously (Yang et al. 1995). GGA chromosomes were numbered according to the widely accepted nomenclature (Ladjali-Mohammedi et al. 1999). The chromosomes of BOE were arranged according to their relative size, from the largest to the smallest.
Flow sorting and generation of chromosome-specific paint probes
Chromosome preparations for bivariate flow sorting were stained with Hoechst 33258 (Sigma, St Louis MO, USA) and Chromomycin A3 (Sigma). BOE chromosomes were sorted on a FACStar Plus (Becton Dickinson, Franklin Lakes, NJ, USA) equipped with two lasers as described previously (Carter et al. 1990; Yang et al. 1995), and GGA chromosomes were sorted on a flow cytometer (MoFlo, DAKO, Glostrup, Denmark DAKO) as previously described by Ng and Carter (2006). Chromosome-specific paints for GGA and BOE were generated from flow-sorted chromosomes using DOP-PCR (Telenius et al. 1992). Probes were obtained by labeling primary PCR products with biotin-16-dUTP (Roche, Basel, Switzerland), FITC-12-dUTP (Roche), digoxigenin-11-dUTP (Roche) or Cy3-dUTP (Amersham, Little Chalfont, Bucks, UK) in a second round of PCR amplification.
Fluorescence in-situ hybridization
Reciprocal cross-species chromosome painting between GGA and BOE, and post-hybridization detection followed the procedure for mammals (Yang et al. 1997, 2004). In brief, metaphase chromosome preparations were treated for 5 min in 1% pepsin and then aged for 1 h at 65°C. Chromosomal DNA was denatured for 2 min in 70% formamide, 2× SSC (65–67°C); the probes were denatured at 75°C for 10 min, and annealed at 37°C for 20–60 min. After 2–3 days’ hybridization at 37°C, biotin-labeled probes were visualized using a layer of Cy3-a or Cy5-avidin (1:1000 dilution; Amersham); FITC-labeled probes were detected with a layer of rabbit anti-FITC (1:200; DAKO) followed by a layer of goat anti-rabbit-FITC antibodies (1:100; Vector Laboratories, Burlingame, CA, USA); digoxigenin-labeled probes were visualized using monoclonal anti-digoxin antibody produced in mouse (1:500; Sigma) and FITC-conjugated goat anti-mouse IgG (1:200; Sigma). After detection, slides were mounted in Vectashield mounting medium with DAPI (4′,6-diamidino-2-phenylindole, Vector Laboratories). Digital images were acquired using either the CytoVision system (Applied Imaging, Newcastle upon Tyne, UK) or SmartCapture 2 (Digital Scientific, Cambridge, UK) with a CCD camera mounted on a microscope (Zeiss, Axioplan 2 Imaging). Hybridization signals were assigned to specific chromosomes or chromosome regions defined by DAPI-banding patterns.
Results
The conventional analysis of BOE karyotype
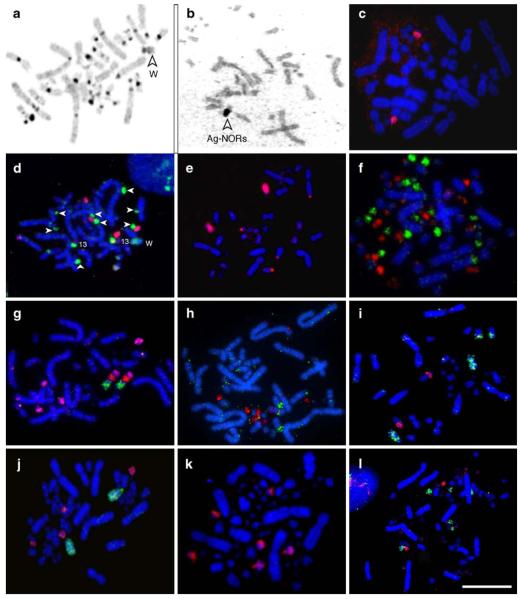
Bulatova et al. (1971) first reported the karyotype of BOE with 2n=40 from a specimen with unknown sex, which showed three pairs of dot-like microchromosomes. However, the BOE cell line investigated here appears to have a 2n=42 karyotype with four pairs of dot-like microchromosomes in the majority of metaphases analyzed (Fig. 1a). The avian microchromosomes contain GC-rich DNA (Auer et al. 1987) and thus are difficult to visualize using AT-rich sequence preferring 4′,6-diamidino-2-phenylindole (DAPI) as the counterstain. To better visualize all chromosomes of BOE, in addition to DAPI we simultaneously stained BOE chromosomes with propidium iodide (PI), which has no sequence preference and thus allows a better differentiation of the GC-rich microchromosomes than DAPI (Fig. 1b,c). Of the 60 metaphases karyotyped, 41 showed a diploid number of 2n=42. The presence of four pairs of dot-like microchromosomes was also confirmed by chromosome painting with BOE probes (Fig. 1d,1e). Our results thus suggest that the diploid number of BOE is 2n=42 rather than 2n=40 as reported previously. Such a discrepancy could be due to the ambiguity in identifying the dot-like microchromosomes or could reflect a true polymorphism in the 2n of different BOE populations or, less probably, a culture-induced artifact in the cell line.
Fig. 1.
(a) The DAPI-banded karyotype of the stone curlew (Burhinus oedicnemus, BOE, 2n=42). (b) The BOE metaphase stained with PI (propidium iodide). (c) The same metaphase as (b) stained with DAPI; arrows point to the dot-like microchromosomes. (d) The probe from BOE 17+18+19+20 hybridized to BOE 17, 18, 19 and 20; note the cross-hybridization signals on BOE 13 and W chromosomes. (e) The same metaphase as (d) stained with DAPI; arrows indicates the dot-like microchromosomes. Scale bar represents 10 μm
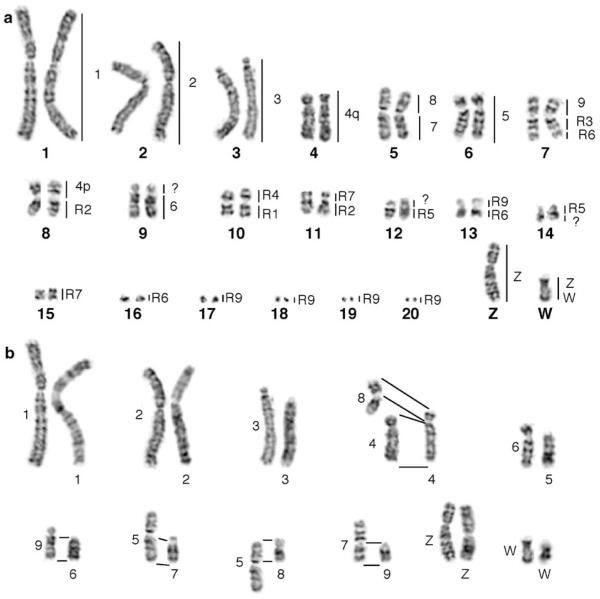
The G-banded karyotype of BOE is shown in Fig. 2a, consisting of 14 pairs of meta-, submeta-, and acrocentric macrochromosomes, 6 pairs of microchromosomes, and one medium-sized submetacentric Z, and one smaller submetacentric W. C-bands are mainly localized at the centromeric regions, and the entire short arm of W (Fig. 3a). An interstitial C-band is also found in the long arm of W. The Ag-NOR staining demonstrates the presence of one pair of nucleolar organizer regions (NORs) on chromosome 13 (Fig. 3b), which is further confirmed by FISH with a Chinese pangolin (Manis pentadactyla) BAC clone containing ribosomal DNA (Fig. 3c).
Fig. 2.
(a) The G-banded karyotype of BOE with hybridization patterns of the chicken (Gallus gallus, GGA, 2n=78) painting probes to the right of each BOE chromosome. Chromosome numbers of BOE are indicated below the chromosomes. (b) Comparison of homologous G-banded macrochromosomes between GGA and BOE. GGA macrochromosomes (1–9, Z and W chromosomes) are on the right, and their chromosome numbers are given below. The homologous BOE chromosomes are on the left, and their chromosomal numbers are given to the left
Fig. 3.
C-banding, Ag-NOR staining, and FISH examples. (a) C-banded BOE metaphase (the arrow shows the W chromosome). (b) Ag-NOR staining of BOE chromosomes (the arrow indicates the association of the two chromosomes with NORs). (c) The probe from a Chinese pangolin (Manis pentadactyla) BAC clone containing ribosomal DNA hybridized to BOE chromosome 13. (d) BOE 15+16 (red) and 17+18+19+20 (green) probes hybridized to BOE metaphase; note that the latter also gave cross-hybridization signals to BOE 13p proximal and W (see Fig. 1d and e for details); arrows show BOE 17–20. (e) BOE 7 (red) probe hybridized to BOE metaphase. (f) GGA probes R7 (green) and R9 (red) hybridized to GGA metaphase. (g) GGA 9 (green), R3 (red) and R6 (pink) probes hybridized to BOE chromosomes 7, 13q and 16q. (h) GGA probes R7 (green) and R9 (red) hybridized to BOE chromosomes 11, 13, 15, 17–20; (i) GGA probes 4 (green) and 9 (red) hybridized to BOE chromosomes 4, 7 and 8. (j) BOE probes 6 (green) and 10 (red) hybridized to GGA chromosomes 5 and two pairs of microchromosomes. (k) BOE probe 7 hybridized to GGA chromosomes 9 and two pairs of microchromosomes. (l) BOE probes 15+16 (green) and 17+18+19+20 (red) hybridized to 4 pairs of GGA microchromosomes. Scale bar represents 10 μm
The BOE flow karyotype and characterization
The 42 chromosomes of BOE were resolved into 19 flow-peaks (Fig. 4a). Identification of the chromosomal content of each peak was achieved by hybridizing paints derived from flow-sorted BOE chromosomes to its own metaphases (Fig. 3d,e, Fig. 4b,c). All except two peaks each contained one type of BOE chromosome. Chromosomes 15 and 16 were sorted into one peak, as were chromosomes 17, 18, 19 and 20. In addition to signals on four dot-like microchromosomes (BOE 17, 18, 19, 20), the paint derived from the peak containing 17+18+19+20 also gave cross-hybridization signals on BOE 13 (i.e., the NOR-bearing chromosome) and the W chromosome (Fig. 1d, Fig. 3d). Moreover, the two homologues of chromosome 14 were each sorted in a different flow peak. The painting results suggest that a set of probes covering all BOE chromosomes has been generated.
Fig. 4.
(a) Bivariate flow karyotype of the stone curlew (B. oedicnemus) with chromosome assignments. (b) Seven-color chromosome painting of the stone curlew metaphase. (c) The same metaphase with DAPI counterstain. Scale bar represents 10 μm
The new flow karyotype of GGA and characterization
The karyotype of GGA comprises 78 chromosomes. Only GGA 1–9, the Z and W chromosomes are distinguishable (Fig. 2b). Chromosome paint probes from GGA 1–9, the Z chromosome, and one microchromosome have been generated by bivariate flow sorting (Griffin et al. 1999). In addition, six paints, each containing 2–3 or multiple GGA microchromosomes, were also generated in this study. However, these paints from GGA microchromosomes were not used widely in subsequent chromosome painting studies. To obtain more painting probes from GGA microchromosomes, the GGA chromosomes were sorted again using modified protocols (Ng and Carter 2006). Figure 5a and b show the new GGA flow karyotype. Besides the painting probes from GGA1–9, Z and W chromosomes, two chromosomal arm paints (GGA 1p and 2p) and eight paints from GGA microchromosomes were also obtained. Among the eight GGA microchromosome paints, three paints (GGA R1, R3 and R4) each contained one microchromosome, two paints (GGA R2 and R5) each comprised two microchromosomes, one paint (GGA R6) included three microchromosomes, the other two paints (GGA R7, R9) each contained multiple microchromosomes (Fig. 3f). The paint from GGA R8 did not give specific-signals on GGA chromosomes.
Fig. 5.
Bivariate flow karyotypes of the chicken (G. gallus) with chromosome assignments: (a) macrochromosomes; (b) microchromosomes
Reciprocal chromosome painting between GGA and BOE
To define the evolutionarily conserved chromosomal segments between GGA and BOE, chromosome painting probes GGA 1–9, the sex chromosomes (Z and W) and the microchromosomes were hybridized onto BOE. The whole set of BOE chromosome-specific probes were also hybridized onto GGA metaphases. The FISH examples are shown in Fig. 3g–l, and the results of reciprocal chromosome painting between GGA and BOE are summarized in Fig. 2 and Table 1.
Table 1.
Chromosomal correspondence between the chicken and the stone curlew revealed by FISH with stone curlew chromosome-specific paints
| Chromosome | |
|---|---|
|
| |
| Stone curlew (B. oedicnemus, 2n=42) |
Chicken (Gallus gallus, 2n=78) |
| 1 | 1 |
| 2 | 2 |
| 3 | 3 |
| 4 | 4q |
| 5 | 7, 8 |
| 6 | 5 |
| 7 | 9, 2 MICs |
| 8 | 4p, 1 MIC |
| 9 | 6, 1 MIC |
| 10 | 2 MICs |
| 11 | 2 MICs |
| 12 | 2 MICs |
| 13 | 2 MICs |
| 14 | 2 MICs |
| 15+16 | 3 MICs |
| 17+18+19+20 | 1 MIC |
| Z | Z |
| W | W, Zqter |
MIC: microchromosome
Probes from GGA 1–3 and 5 each painted one pair of BOE autosomes (BOE1–3 and 6 respectively). Paints from GGA 6–9 and some microchromosomes (GGA R1-R6) each hybridized to one chromosomal segment of BOE (Fig. 3g). The paints derived from multiple GGA microchromosomes (GGA R7, R9) each gave signals on two or more BOE chromosomes or chromosomal segments (Fig. 3h). GGA 4 probe gave signals on two pairs of BOE chromosomes (Fig. 3i), similar to results found in other birds. The GGA Z probe hybridized to the Z and W chromosomes of BOE (Fig. 6a), and the W chromosome probe of GGA gave signals on the W chromosome and also some microchromosomes of BOE (Fig. 6b). In total, probes from GGA autosomes revealed 27 homologous segments in the BOE genome (Fig. 2a).
Fig. 6.
(a) The Z probe of GGA painted the Z and W chromosomes of BOE. (b) The W probe of GGA gave signals on the W chromosome of BOE; note the cross-hybridization signals on some microchromosomes of BOE. (c) The Z probe of BOE painted onto the Z chromosomes of GGA. (d) The W probe of GGA gave signals on the W chromosome; note the cross-hybridization signals on Zqter and some microchromosomes of GGA. Scale bar represents 10 μm
Probes from five BOE chromosomes (BOE 1–4 and 6) each painted one GGA chromosome or chromosomal segment (GGA 1–3, 5 and 4q) respectively. Probes from BOE 5 and 8–14 chromosomes each hybridized two GGA chromosomes or chromosome segments (Fig. 3j). BOE 7 probe painted three pairs of GGA chromosomes (Fig. 3k). The probe from BOE 15+16 gave signals on three pairs of GGA chromosomes, whereas the probe from BOE 17+18+19+20 hybridized only to one pair of GGA chromosomes (Fig. 3l). The Z chromosome probe of BOE painted most Z chromosome of GGA but not the Zqter (Fig. 6c), and the W chromosome paint of BOE hybridized to the whole W chromosome and the GGA Zqter and some microchromosomes (Fig. 6d). As a whole, painting probes representing the twenty BOE autosomes revealed 28 homologous chromosomal segments in the GGA genome (Fig. 2b, Table 1).
Discussion
Recent cross-species chromosome painting studies in birds suggested that the GGA karyotype may have retained the avian ancestral state, because in most species studied so far, even in palaeognathous birds (Shetty et al. 1999; Guttenbach et al. 2003; Nishida-Umehara et al. 2007), the macrochromosomes show complete homology to GGA chromosomes 1–3 and 5–9. Only GGA chromosome 4 represents the derived form as it corresponds to one pair of macrochromosomes and an additional pair of microchromosomes in most bird species so far studied. It was proposed that segments homologous to GGA chromosomes 1, 2, 3, 4q, 5, 6, 7, 8, 9, 4p and Z represent the ancestral chromosomes 1–10 + Z for all birds (reviewed in Griffin et al. 2007). Moreover, avian chromosomal evolution and karyotypic phylogenetic relationships were also analyzed in detail based on previously available chromosome painting results with the paints from GGA chromosomes 1–9 and the Z chromosome (reviewed in Griffin et al. 2007).
To date, only GGA 1–9 and Z chromosome paints have been used widely in all avian chromosome painting studies. The availability of a second set of avian probes that cover all BOE chromosomes, and the additional types of GGA microchromosome paints presented in this study, allow us to compare directly the karyotypic differences between a typical (GGA) and an atypical (BOE) avian genome, and to understand better the course of avian chromosomal evolution, especially the origin and evolution of avian microchromosomes.
Chromosomal rearrangements in BOE
Although BOE has an atypical avian karyotype (with only a few microchromosomes), and is one of the species with the smallest diploid number in birds, our chromosome painting results demonstrate that the homologues of GGA chromosomes 1, 2, 3, 4q and 5 remain as whole chromosomes in the BOE genome (BOE 1, 2, 3, 4 and 6). The probes from GGA chromosomes 6–9 and some microchromosomes (GGA R1-R6) each painted one chromosomal segment of BOE, and reverse chromosome painting with BOE probes 5, 8–14 each hybridized to two GGA chromosomes, indicating that the chromosomes of BOE 5, 8–14 each resulted from the fusion (centromere–centromere or centromere–telomere fusion) of two GGA chromosomes (Fig. 2a). BOE 7 was painted by three GGA probes (Fig. 3g), and the probe of BOE 7 gave signals on three GGA chromosomes (Fig. 3k). These results demonstrated that BOE 7 originated from the fusion of three chicken chromosomes (GGA 9 and two microchromosomes).
Comparing chromosome painting results between GGA and BOE with those between GGA and other birds, we found that the main mechanism for chromosomal rearrangements in BOE was chromosomal fusion. As with other avian species studied so far, reciprocal translocations were not detected in the BOE genome. Differences between the karyotypes of GGA and BOE resulted from at least 12 chromosomal fusions between homologues of GGA 4p, 6–9, and some microchromosomes. The most notable fusions were between ancestral chromosomes 7 and 8 (BOE 5), and between ancestral chromosome 10 and two microchromosomes (BOE7). These fusion events apparently belong to tandem (centromere–telomere) fusions accompanied by centromere inactivation and have not been reported in other birds. As found in all Galliformes and other birds (reviewed in Griffin et al. 2007), the ancestral chromosome 4 (GGA 4q) is also conserved intact in BOE. The ancestral chromosome 10 (GGA 4p) was usually found as an unassigned microchromosome in some birds (Shibusawa et al. 2004a), but a fusion between the ancestral chromosome 10 (GGA 4p) and a microchromosome had occurred in BOE. Thus extensive centrome–centromere and centromere–telomere fusions appear to be the main mechanism resulting in the dramatic karyotype reorganization in BOE and the substantial reduction in the diploid number. Besides chromosomal fusions, intrachromosomal rearrangements were also found in BOE by the comparison of G-banded chromosomes between GGA and BOE. Apparent inversions were observed between BOE 6 and GGA 5, between BOE 9 and GGA 6, and between Z chromosomes of BOE and GGA (Fig. 2b).
Homology between BOE and GGA microchromosomes
The origin and evolution of microchromosomes in birds remain a mystery. Besides the Aves, microchromosomes are also found in some primitive amphibians, most reptiles, and even some fish, suggesting that some microchromosomes were already present in the common ancestor that gave rise to birds and other terrestrial vertebrates (reviewed in Burt 2002). Comparative gene mapping between the genomes of GGA, human, mouse and zebrafish provides evidence that more than half the GGA microchromosomes may represent ancestral syntenies and at least 10 avian microchromosomes are the product of chromosome fission (Burt 2002). However, the correspondence between microchromosomes of different avian species remains poorly studied, except for two chromosome painting studies with the probe pool from 19 GGA microchromosomes and the nine largest microchromosomes probes. The probe pool representing 19 out of the 29 GGA microchromosomes delineated 16 regions on 15 different chromosomes of the Harpy eagle (2n=58). Nine of 15 Harpy eagle chromosomes showed homology exclusively to GGA microchromosomes (de Oliveira et al. 2005). Paints from two or three GGA microchromosomes hybridized to the same number of corresponding microchromosomes in pigeon and two passerine birds (Derjusheva et al. 2004)
To define the origin and evolution of avian microchromosomes, we performed reciprocal chromosome painting between GGA and BOE microchromosomes. The probe from BOE 15+16 gave signals on three pairs of GGA microchromosomes, indicating that one of these chromosomes corresponds to one homologous GGA and the other to two homologous GGA microchromosomes. Also the probe from the four smallest dot-like BOE microchromosomes (BOE17–20) hybridized only to one pair of GGA microchromosomes (Fig. 3l). This appears to suggest that the microchromosome fission could have occurred during BOE chromosomal evolution, i.e., the four BOE microchromosomes might be the product of three fissions of one ancestral microchromosome. In addition, reverse painting using the probes from GGA multiple microchromosomes (GGA R7 and R9) gave fewer hybridization signals in BOE genome than in GGA genome (Fig. 3f, h), suggesting further fusion of some GGA microchromosomes in BOE, or, less likely, the loss of some ancestral microchromosomes in BOE. However, the apparent discrepancy in the numbers of hybridization signals observed in the BOE and GGA genomes with GGA R7 and R9 and BOE (17+18+19+20) probes in comparative painting experiments is most likely due to that the hybridization signals from some microchromosomes were either too weak or too small to detect, considering the 100 million years or so divergence between the chicken and stone curlew lineages. Further investigation is needed to fully resolve the correspondence between the microchromosomes of BOE and GGA.
Z–W homology in birds
Previous chromosome painting studies have shown that the Z chromosome paint of GGA hybridized to the Z chromosome of other birds across different avian orders, indicating that the Z chromosome is a highly conserved ancient chromosome (reviewed in Griffin et al. 2007). Moreover, the hybridization of GGA Z to W chromosomes was found both in female ratites (Shetty et al. 1999; Nishida-Umehara et al. 2007; Nanda et al. 2008) and in some female Neoaves such as the California condor (Raudsepp et al. 2002), the chaffinch and the red wing (Derjusheva et al. 2004), the Harpy eagle (de Oliveira et al. 2005), and the golden pheasant, the mallard duck, the graylag goose, the common coot, the common moorhen and the ringneck dove (Nanda et al. 2008). Here, we also demonstrate the successful hybridization of the GGA Z chromosome probe to both the Z and W chromosomes of BOE (Fig. 6a). Like the human Y chromosome, which has limited use in cross-species painting in placental mammals due to its rapid divergence, the W chromosome paint of GGA has not been widely used in cross-species painting in birds, possibly due to an assumed limitation when applied to distantly related species (Raudsepp et al. 2002). Our results demonstrate that the W chromosome paint of GGA can hybridize successfully to the W chromosome of BOE and the W chromosome paint of BOE gave signals on the W chromosome and Zqter of GGA (Fig. 6b,d).
Data from gene mapping in the chicken and other birds support the evolution of avian sex chromosomes from a pair of autosomes, possibly 60–100 million years ago (Fridolfsson et al. 1998). In ratites, the W chromosome is indistinguishable from the Z chromosome. The lack of genetic divergence between the ratite Z and W chromosomes thus could have contributed to the successful hybridization of the GGA Z probe to both the Z and W chromosomes of ratites (Shetty et al. 1999; Nishida-Umehara et al. 2007). However, the non-ratite species have evolved dimorphic Z and W chromosomes. This has raised the question whether the hybridization of GGA Z chromosome probe to both the Z and W chromosomes of non-ratite species indeed indicates the existence of homologous regions between their Z and W chromosomes. In our study, the W probe from BOE and GGA hybridized to the W chromosomes of each other respectively, and did not give signals on the whole Z chromosome. Further investigations are required to understand better the homology between the sex chromosomes of GGA and non-ratite species. In spite of the synteny conservation in avian Z chromosomes as demonstrated by chromosome painting, at least three heteromorphic Z chromosomes have been reported so far among different avian lineages: a metacentric Z in the GGA, the common black bird, the ringneck dove and the common barn owl (Nanda et al. 2008), an acrocentric Z in some Falco species and the wood duck (Nanda et al. 2008; Nishida et al. 2008), and a submetacentric Z in some other birds (Griffin et al. 2007; Nanda et al. 2008; and this study). As also shown by Nanda et al. (2008), inversions seem to have played an important role in the evolution of avian Z chromosomes.
The potential utility of BOE and GGA paints
The basal placement of flow peak R9 in the GGA flow karyotype indicates that R9 must contain the so called D-group GGA chromosomes (Masabanda et al. 2004)—the smallest chromosomes in the chicken karyotype. Despite continuous mapping efforts, the D-group chromosomes still have no DNA sequences assigned to them. The availability of probe R9, together with the comparative map between R9 and its BOE homologues will facilitate the assignment of chicken DNA sequences of D-group chromosomal origin (including DNA contigs and linkage groups that have no chromosomal assignment) by FISH. The probes derived from R9 and its corresponding BOE chromosomes can also be used to screen chicken DNA libraries for additional DNA clones of potential D-group chromosomal origin. Moreover, our BOE painting probes represent the first set of probes that cover the whole genome of a given species in birds. Considering that most living birds, like BOE, belong to Neoaves, it is anticipated that the BOE probes will find an even wider application in avian comparative cytogenetics and genome evolution studies than the GGA probes.
In summary, we have successfully generated a complete set of painting probes from BOE and also additional paints for GGA microchromosomes, which allowed us to establish the first genome-wide comparative chromosome maps between BOE and GGA by reciprocal chromosome painting. The results reveal that the dramatic decrease of BOE diploid number is mainly due to extensive chromosome fusions. In addition, fissions of ancestral microchromosomes could have also occurred during BOE chromosome evolution. The establishment of chromosome homologies between GGA and BOE will facilitate the integration of previous chromosome painting data and the transfer of gene mapping data from GGA to other birds.
Acknowledgements
We thank Dr. Norin Chai (Paris Museum of Natural History) for providing the tissue biopsy of a female Burhinus oedicnemus and Dr. Indrajit Nanda for a critical reading of the manuscript. The study was supported partly by a Royal Society International Short Visit award to MAFS and WN, and a grant from the National Natural Science Foundation of China. The Cambridge Resource Centre for Comparative Genomics was funded by the Wellcome Trust. N.P.C., B.L.N. and F.Y. are supported by the Wellcome Trust.
Abbreviations
- BOE
Burhinus oedicnemus
- DAPI
4′,6-diamidino-2-phenylindole
- DOP-PCR
degenerate oligonucleotide-primed PCR
- GGA
Gallus gallus
- PI
propidium iodide
Contributor Information
Wenhui Nie, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan, PRC.
Patricia C. M. O’Brien, Cambridge Resource Centre for Comparative Genomics, Department of Veterinary Medicine, University of Cambridge, Cambridge CB3 0ES, UK
Vitaly Volobouev, Muséum National d’Histoire Naturelle, Origine, Structure et Evolution de la Biodiversité, Paris, France.
Nigel P. Carter, Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton Cambridge CB10 1SA, UK
Malcolm A. Ferguson-Smith, Cambridge Resource Centre for Comparative Genomics, Department of Veterinary Medicine, University of Cambridge, Cambridge CB3 0ES, UK
Fengtang Yang, Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton Cambridge CB10 1SA, UK.
References
- Auer H, Mayr B, Lambrou M, Schleger W. An extended chicken karyotype, including the NOR chromosome. Cytogenet Cell Genet. 1987;45:218–221. doi: 10.1159/000132457. [DOI] [PubMed] [Google Scholar]
- Bed’Home B, Coullin P, Guillier-Gencik S, Moulin S, Bernheim A, Volobouev V. Characterization of the atypical karyotype of the black-winged kite Elanus caeruleus (Falconiformes: Accipitridae) by means of classical and molecular cytogenetic techniques. Chromosome Res. 2003;11:335–343. doi: 10.1023/a:1024091923939. [DOI] [PubMed] [Google Scholar]
- Brown JW, Rest JS, García-Moreno J, Sorenson MD, Mindell DP. Strong mitochondrial DNA support for a Cretaceous origin of modern avian lineages. BMC Biology. 2008;6:6. doi: 10.1186/1741-7007-6-6. doi:10.1186/1741-7007-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulatova N, Panov E, Radjabli S. Description of karyotypes of some birds from the USSR fauna. Proc USSR Acad Sci. 1971;199:1420–1423. [Google Scholar]
- Burt DW. Origin and evolution of avian microchromosomes. Cytogenet Genome Res. 2002;96:72–112. doi: 10.1159/000063018. [DOI] [PubMed] [Google Scholar]
- Burt DW, Bruley C, Dunn IC, et al. The dynamics of chromosome evolution in birds and mammals. Nature. 1999;402:411–413. doi: 10.1038/46555. [DOI] [PubMed] [Google Scholar]
- Carter NP, Ferguson-Smith ME, Affara NA, Briggs H, Ferguson-Smith MA. Study of X chromosome abnormality in XX males using bivariate flow karyotype analysis and flow sorted dot blots. Cytometry. 1990;11:202–207. doi: 10.1002/cyto.990110123. [DOI] [PubMed] [Google Scholar]
- Christidis L. Aves. In: John B, Kayano H, Levan A, editors. Animal cytogenetics. vol. 4. Gebrüder Borntraeger; Berlin: 1990. Chordata 3. [Google Scholar]
- Clarke JA, Tambussi CP, Noriega JI, Ericson GM, Ketcham RA. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 2005;433:305–308. doi: 10.1038/nature03150. [DOI] [PubMed] [Google Scholar]
- Cooper A, Penny D. Mass survival of birds across the Cretaceous–Tertiary boundary: molecular evidence. Science. 1997;275:1109–1113. doi: 10.1126/science.275.5303.1109. [DOI] [PubMed] [Google Scholar]
- Cracraft J. Avian evolution, Gondwana biogeography and the Cretaceous–Tertiary mass extinction event. Proc R Soc B. 2001;268:459–469. doi: 10.1098/rspb.2000.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira EHC, Habermann FA, Lacerda O, Sbalqueiro IJ, Wienberg J, Müller S. Chromosome reshuffling in birds of prey: the karyotype of the world’s largest eagle (Harpy eagle, Harpia harpyja) compared to that of the chicken (Gallus gallus) Chromosoma. 2005;114:338–343. doi: 10.1007/s00412-005-0009-5. [DOI] [PubMed] [Google Scholar]
- Derjusheva S, Kurganova A, Habermann F, Gaginskaya E. High chromosome conservation detected by comparative chromosome painting in chicken, pigeon and passerine birds. Chromosome Res. 2004;12:715–723. doi: 10.1023/B:CHRO.0000045779.50641.00. [DOI] [PubMed] [Google Scholar]
- Edwards SV, Jennings W Bryan, Shedlock AM. Phylogenetics of modern birds in the era of genomics. Proc R Soc B. 2005;272:979–992. doi: 10.1098/rspb.2004.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson PGP, Anderson CL, Britton T, et al. Diversification of Neoaves: Integration of molecular sequence data and fossils. Biol Letters. 2006;2:543–547. doi: 10.1098/rsbl.2006.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain MG, Houde P. Parallel radiations in the primary clades of birds. Evolution. 2004;58:2558–2573. doi: 10.1111/j.0014-3820.2004.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Feduccia A. “Big bang” for tertiary birds? Trends Ecol Evol. 2003;18:172–176. [Google Scholar]
- Fridolfsson A, Cheng H, Copeland NG, et al. Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc Natl Acad Sci USA. 1998;95:8147–8152. doi: 10.1073/pnas.95.14.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DK, Haberman F, Masabanda J, et al. Micro-and macrochromosome paints generated by flow cytometry and microdissection: tools for mapping the chicken genome. Cytogenet Cell Genet. 1999;87:278–281. doi: 10.1159/000015449. [DOI] [PubMed] [Google Scholar]
- Griffin DK, Robertson LBW, Tempest HG, Skinner BM. The evolution of the avian genome as revealed by comparative molecular cytogenetics. Cytogenet Genome Res. 2007;117:64–77. doi: 10.1159/000103166. [DOI] [PubMed] [Google Scholar]
- Guttenbach M, Nanda I, Feichtinger W, Masabanda JS, Griffin DK, Schmid M. Comparative chromosome painting of chicken autosomal paints 1–9 in nine different bird species. Cytogenet Genome Res. 2003;103:173–184. doi: 10.1159/000076309. [DOI] [PubMed] [Google Scholar]
- Hackett SJ, Kimball RT, Reddy S. A phylogenomic study of birds reveals their evolutionary history. Science. 2008;320:1763–1768. doi: 10.1126/science.1157704. [DOI] [PubMed] [Google Scholar]
- Hedges SB, Parker PH, Sibley CG, Kumar S. Continental breakup and the ordinal diversification of birds and mammals. Nature. 1996;381:226–229. doi: 10.1038/381226a0. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Arnold AP. Chromosomal polymorphism and comparative painting analysis in the zebra finch. chromosome Res. 2005;13:47–56. doi: 10.1007/s10577-005-6602-x. [DOI] [PubMed] [Google Scholar]
- Kasai F, Garcia C, Arruga MV, Ferguson-Smith MA. Chromosome homologybetween chicken (Gallus gallus domesticus) and the red-legged partridge (Alectoris rufa); evidence of the occurrence of a neocentromere during evolution. Cytogenet Genome Res. 2003;102:326–330. doi: 10.1159/000075770. [DOI] [PubMed] [Google Scholar]
- Ladjali-Mohammedi K, Bitgood JJ, Tixier-Boichard M, De Leon FA Ponce. International system for standardized avian karyotypes (ISSAK): standardized banded karyotypes of the domestic fowl (Gallus gallus domesticus) Cytogenet Cell Genet. 1999;86:271–276. doi: 10.1159/000015318. [DOI] [PubMed] [Google Scholar]
- Livezey BC, Zusi RL. Higher-order phylogenetics of modern Aves based on comparative anatomy. Netherlands J Zool. 2001;51:179–205. [Google Scholar]
- Livezey BC, Zusi RL. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zool J Linn Soc. 2007;149:1–95. doi: 10.1111/j.1096-3642.2006.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masabanda JS, Burt DW, O’Brien PCM, et al. Molecular cytogenetic definition of the chicken genome: the first complete avian karyotype. Genetics. 2004;166:1367–1373. doi: 10.1534/genetics.166.3.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr G, Clarke J. The deep divergences of neornithine birds: a phylogenetic analysis of morphological characters. Cladistics. 2003;19:527–553. doi: 10.1111/j.1096-0031.2003.tb00387.x. [DOI] [PubMed] [Google Scholar]
- McQueen HA, Fantes J, Cross SH, Clark VH, Archibald AL, Bird AP. CpG islands of chicken are concentrated on microchromosomes. Nature Genet. 1996;12:321–324. doi: 10.1038/ng0396-321. [DOI] [PubMed] [Google Scholar]
- Nanda I, Karl E, Volobouev V, Griffin DK, Schartl M, Schmid M. Extensive gross genomic rearrangements between chicken and Old World vultures (Falconiformes: Accipitridae) Cytogenet Genome Res. 2006;112:286–295. doi: 10.1159/000089883. [DOI] [PubMed] [Google Scholar]
- Nanda I, Karl E, Griffin DK, Schartl M, Schmid M. Chromosome repatterning in three representative parrots (Psittaciformes) inferred from comparative chromosome painting. Cytogenet Genome Res. 2007;117:43–53. doi: 10.1159/000103164. [DOI] [PubMed] [Google Scholar]
- Nanda I, K Schlegelmilch, Haaf T, Schartl M, Schmid M. Synteny conservation of the Z chromosome in 14 avian species (11 families) supports a role for Z dosage in avian sex determination. Cytogenet Genome Res. 2008;122:150–156. doi: 10.1159/000163092. [DOI] [PubMed] [Google Scholar]
- Newcomer EH. Accessory chromosomes in the domestic fowl. Genetics. 1955;40:587–588. [Google Scholar]
- Ng BL, Carter NP. Factors affecting flow karyotype resolution. Cytometry Part A. 2006;69A:1028–1036. doi: 10.1002/cyto.a.20330. [DOI] [PubMed] [Google Scholar]
- Nishida-Umehara C, Tsuda Y, Ishijima J, Ando J, Fujiwara A, Matsuda Y, Griffin DK. The molecular basis of chromosome orthologies and sex chromosomal differentiation in palaeognathous birds. Chromosome Res. 2007;15:721–734. doi: 10.1007/s10577-007-1157-7. [DOI] [PubMed] [Google Scholar]
- Nishida C, Ishijima J, Kosaka A, et al. Characterization of chromosome structures of Falconinae (Falconidae, Falconiformes, Aves) by chromosome painting and delineation of chromosome rearrangements during their differentiation. Chromosome Res. 2008;16:171–181. doi: 10.1007/s10577-007-1210-6. [DOI] [PubMed] [Google Scholar]
- Raudsepp T, Houck ML, O’Brien PC, Ferguson-Smith MA, Ryder OA, Chowdhary BP. Cytogenetic analysis of California condor (Gymnogyps californianus) chromosomes: comparison with chicken (Gallus gallus)macrochromosomes. Cytogenet Genome Res. 2002;98:54–60. doi: 10.1159/000068532. [DOI] [PubMed] [Google Scholar]
- Schmid M, Nanda I, Guttenbach M, et al. First report on chicken genes and chromosomes 2000. Cytogenet Cell Genet. 2000;90:169–218. doi: 10.1159/000056772. [DOI] [PubMed] [Google Scholar]
- Shetty S, Griffin DK, Graves JAM. Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Res. 1999;7:289–295. doi: 10.1023/a:1009278914829. [DOI] [PubMed] [Google Scholar]
- Shibusawa M, Nishida-Umehara C, Masabanda J, Griffin DK, Isobe T, Matsuda Y. Chromosome rearrangements between chicken and guinea fowl defined by comparative chromosome painting and FISH mapping of DNA clones. Cytogenet Genome Res. 2002;98:225–230. doi: 10.1159/000069813. [DOI] [PubMed] [Google Scholar]
- Shibusawa M, Nishida-Umehara C, Tsudzuki M, Masabanda J, Griffin DK, Matsuda Y. A comparative karyological study of the blue-breasted quail (Coturnix chinensis, Phasianidae) and California quail (Callipepla californica, Odontophoridae) Cytogenet Genome Res. 2004a;106:82–90. doi: 10.1159/000078569. [DOI] [PubMed] [Google Scholar]
- Shibusawa M, Nishibori M, Nishida-Umehara C, et al. Karyotypic evolution in the Galliformes: An examination of the process of karyotypic evolution by comparison of the molecular cytogenetic findings with the molecular phylogeny. Cytogenet Genome Res. 2004b;106:111–119. doi: 10.1159/000078570. [DOI] [PubMed] [Google Scholar]
- Smith J, Burt DW. Parameters of the chicken genome. Anim Genet. 1998;29:290–294. doi: 10.1046/j.1365-2052.1998.00334.x. [DOI] [PubMed] [Google Scholar]
- Smith J, Bruley CK, Paton IR, Dunn I, Jones CT, Windsor D, Morrice DR, Law AS, Masabanda J, Sazanov A, Waddington D, Fries R, Burt DW. Differences in gene density on chicken macrochromosomes and microchromosomes. Anim Genet. 2000;31:96–103. doi: 10.1046/j.1365-2052.2000.00565.x. [DOI] [PubMed] [Google Scholar]
- Solovei I, Gaginskaya ER, Macgregor HC. The arrangement and transcription of telomere DNA sequences at the ends of lampbrush chromosomes of birds. chromosome Res. 1994;2:460–470. doi: 10.1007/BF01552869. [DOI] [PubMed] [Google Scholar]
- Telenius H, Pelmear AH, Tunnacliffe A, Carter NP, Behmel A, Ferguson-Smith MA, Nordenskjold M, Pfragner R, Ponder BA. Cytogenetic analysis by chromosome painting using DOP-PCR amplified flow-sorted chromosomes. Genes Chromosomes Cancer. 1992;4:2576–2263. doi: 10.1002/gcc.2870040311. [DOI] [PubMed] [Google Scholar]
- van Tuinen M, Hedges SB. Calibration of avian molecular clocks. Mol Biol Evol. 2001;18:206–213. doi: 10.1093/oxfordjournals.molbev.a003794. [DOI] [PubMed] [Google Scholar]
- van Tuinen M, Sibley CG, Hedges SB. The early history of modern birds inferred from DNA sequences of nuclear and mitochondrial ribosomal genes. Mol Biol Evol. 2000;17:451–457. doi: 10.1093/oxfordjournals.molbev.a026324. [DOI] [PubMed] [Google Scholar]
- Yang F, Carter NP, Shi L, Ferguson-Smith MA. A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma. 1995;103:642–652. doi: 10.1007/BF00357691. [DOI] [PubMed] [Google Scholar]
- Yang F, Müller S, Just R, Ferguson-Smith MA, Wienberg J. Comparative chromosome painting in mammals: human and the Indian muntjac (Muntiacus muntjak vaginalis) Genomics. 1997;39:396–401. doi: 10.1006/geno.1996.4497. [DOI] [PubMed] [Google Scholar]
- Yang FT, Fu BY, O’Brien PCM, Nie WH, Ryder OA, Ferguson-Smith MA. Refined genome-wide comparative map of the domestic horse, donkey and human based on cross-species chromosome painting: Insight into the occasional fertility of mules. Chromosome Res. 2004;12:65–76. doi: 10.1023/b:chro.0000009298.02689.8a. [DOI] [PubMed] [Google Scholar]