Abstract
The development and progression of systemic lupus erythematosus (SLE) is strongly associated with complement activation and deposition. The anaphylatoxin C3a is a product of complement activation with immunomodulatory properties, and the receptor for C3a (C3aR) is not only expressed by granulocytes and antigen presenting cell populations, but it is also strongly up-regulated in lupus prone mice with active nephritis. In order to characterize the role of the C3aR in inflammatory nephritis, we bred C3aR knock-out mice onto the MRL/lpr genetic background (C3aR KO MRL). Compared to control MRL/lpr mice, C3aR KO MRL mice had elevated autoantibody titers and an earlier onset of renal injury. At 8 weeks, renal expression of a wide range of chemokines and chemokine receptors was increased in C3aR KO MRL kidneys compared to controls. Only the expression of MCP-1 was significantly decreased in the C3aR KO MRL mice. The increased chemokine and chemokine receptor expression seen in the C3aR KO MRL mice was associated with a more rapid rise in serum creatinine and the acceleration of renal fibrosis. However, loss of the C3aR had little impact on long-term kidney injury and did not alter survival. These findings suggest that activation of the C3aR plays a protective, not pathologic, role in the early phase of inflammatory nephritis in the MRL/lpr model of SLE.
Keywords: Systemic Lupus Erythematosus, Complement, Transgenic/Knockout Mice
Introduction
Systemic lupus erythematosus is an autoimmune disorder where production of auto-antibodies, circulating immune complexes, systemic complement activation, and auto-reactive T-cells are associated with multi-system injury including nephritis, arthritis, serositis, dermatitis, and blood dyscrasias. Lupus nephritis is mediated in part by deposition and local production of immune complexes and complement activation products. Complement proteins participate at several levels in disease pathogenesis (Liszewski, 1989). Genetic deficiencies in the early components of the classical complement pathway, C1 inhibitor, C1q/r/s, C2, or C4, confer increased risk for developing disease (Manderson, 2004). While complement activation enhances the clearance of pathological immune complexes, the deposition of complement activation products contributes to inflammation, fibrosis, and local tissue injury (Couser, 1985).
The complement system is a cascade of enzymatic reactions with multiple immunologic effects, including cell lysis, B-cell activation, leukocyte recruitment, and clearance of apoptotic cells and immune complexes. Anaphylatoxins, C3a and C5a, are generated during complement activation. Activation of complement leads to the formation of C3b and C3a, and in turn C3b production leads to the generation of C5a and the membrane attack complex. Traditionally, C3a has been thought to be pro-inflammatory, enhancing cytokine production, mediating eosinophil chemotaxis, leukocyte degranulation, histamine release, and increased vascular permeability (Fischer, 1999; Wetsel, 1995). The effects of C3a require interaction with a specific receptor (C3aR), a 55-kDa protein of the rhodopsin family of G protein-coupled seven-transmembrane receptors. C3aR is expressed on leukocytes including, mast cells, eosinophils, dendritic cells, and some B-lymphocytes (Fischer, 1997; Kirchhoff, 2001; Werfel, 2000; Zwirner, 1999). In the kidney, C3aR expression is limited to glomerular podocytes and proximal tubular epithelial cells (Bao, 2005a; Braun, 2004; Peake, 1999). C3a/C3aR interactions on proximal tubular epithelial cells result in an increase in TGF-β and type I collagen production (Braun, 2004; Peake, 1999).
The MRL/MpJ-Tnfrsf6lpr (MRL) mouse is a widely used and extensively studied mouse strain which develops a severe spontaneous autoimmune disease similar to SLE (Hicks, 2006). The lpr mutation, a retroviral transposon insertion in the FAS gene, results in loss of FAS function and thus a defect in FAS mediated apoptosis (Kono, 2000; Nose, 2000), massive lymphoproliferation with the generation of auto-reactive T-cells, autoantibody formation, and circulating immune complexes (Hicks, 2006). The ensuing autoimmune disease is characterized by lymphadenopathy, complement activation, severe immune complex renal disease, and 50% lethality by 20 to 24 wks (Andrews, 1978). C3aR levels have been reported to be up-regulated in the kidneys of MRL mice as early as 6 wks, long before the development of nephritis (Bao, 2005a).
Based on the hypothesis that C3a acting via the C3aR may have a major functional role mediating disease progression in SLE, mice with a targeted deletion of the C3aR gene were bred onto the MRL/lpr genetic background (here after referred to as C3aR KO MRL). Comparative analyses of immunologic responses and indices of renal injury were then performed between MRL/lpr control (CTRL MRL) and C3aR KO MRL mice. Experimental data contained in this report suggest that loss of the C3aR results in accelerated onset, but not increased severity, of renal injury; thus, the activation of the C3aR is more likely to be protective than pathologic in the MRL/lpr model.
Materials and Methods
Mice
MRL mice (Jackson Laboratories, Bar Harbor, ME) and C3aR KO C57BL/6 mice (Kildsgaard, 2000a) maintained in our animal colony were employed for backcrossing. The gene encoding the C3aR maps to chromosome 6 (64.8cM) (Hollmann, 2007), a region which is not known to contain epigenetic modifiers for autoantibody formation, lymphoproliferation, or nephritis (Kono, 2006; Nguyen, 2002). F9 generation C3aR+/−MRL mice were then intercrossed to obtain homozygous C3aR−/− MRL/lpr (C3aR KO MRL) mice and C3aR+/+ MRL/lpr controls (CTRL MRL). Genotyping was confirmed by PCR for all F9 mice used in this study (data not shown). Only female mice were used for the studies. These studies were approved by the UTHSC-H Animal Welfare Committee.
Immunophenotyping
Leukocytes were obtained for FACS analysis from spleens (16 and 20 wks), peripheral blood (20 wks), and cervical lymph nodes (20 wks). Cell populations were characterized with the following markers (eBiosciences, San Diego, CA): CD3 (clone 145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD11b (M1/70), CD19 (6D5), CD25 (PC61.5), (CD45/RB220 (RA3-6B2), and CD62L (MEL-14), and GR-1(Ly-6G). A minimum of 10,000 events were collected and analyzed on a FACSCaliber using CellQuest software (BD Biosciences, San Diego, CA).
Measurement of serum C3 levels and auto-antibody titers
Serum levels of C3 and titers of antibodies specific for double stranded DNA were measured by ELISA as previously reported (Wenderfer, 2005). For the non-quantitative C3 ELISA, goat polyclonal antisera specific for mouse C3 (Cappel/MP Biomedical, Solon, OH) was used for both capture and for detection, and results were compared between sera from C3aR KO MRL mice, CTRL MRL mice and pooled serum from non-autoimmune C57BL/6 mice. For autoantibody responses, end-point titers were measured by serial dilutions. Results are shown as fold differences in A450 between C3aR KO MRL and CTRL MRL serum at a 1/100 dilution.
Renal Function
Serum and urine was obtained from mice at 20 wks immediately prior to histologic analysis. Serum and urine creatinine was determined using a modified alkaline picrate method (Exocell, Philadelphia, PA); Urinary protein concentration was determined by BCA assay (Thermo Scientific, Rockford, IL) and normalized for urinary creatinine concentration.
Histologic Analysis
Renal tissue was fixed in PBS buffered 4% formalin, dehydrated and embedded in paraffin. Four micron sections were stained with Periodic Acid Schiff (PAS) or Sirius red/picric acid stain (Grimm, 2003). Glomerular and tubular injury was scored as previously described (Wenderfer, 2005), except that tubulointerstitial disease in the renal cortex was also quantified by measuring the cortical fractional interstitial fibrosis volume of Sirius red staining in 10 high powered fields (hpf) per mouse (excluding glomeruli) (Grimm, 2003) using Image Pro software (Media Cybernetics, Inc., Bethesda, MD).
Immunostaining
OCT embedded snap-frozen 4 micron sections were stained with the following antibodies: FITC conjugated anti-mouse C3 (ICN, Aurora, OH), IgG (Jackson ImmunoResearch, West Grove, PA), CD8, and CD4 (BD Biosciences). Control staining was also performed using matched isotypes or IgG (data not shown). A minimum of 10 glomeruli and 10 hpfs were scored per animal in a blinded manner as described previously (Wenderfer, 2005).
Chemokine and receptor array
Quantitative mRNA expression analysis of chemokines and their receptors was performed with the mouse chemokine and receptor RT2 profiler PCR array (SA Bioscience Corporation, Frederick, MD). Kidney cortexes of different groups of mice (n=3/group) were collected after cardiac perfusion with HBSS. Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) and followed by Turbo DNA-free treatment (Ambion, Austin, TX) and RNeasy Mini Kit clean up (Qiagen, Valencia, CA). Equal amounts of RNA from each sample were then converted to cDNA using RT2 First Strand Kit (SABiosciences, Frederick, MD). Quantitative real-time PCR was performed according to the manufacturer’s protocol using RT2 profiler PCR array PAMM-022 (SA Biosciences) on the Applied Biosystems 7900. Data were analyzed using ΔΔCt method to determine the expression level of each gene of interest normalized to the expression level of housekeeping gene controls. Fold differences in gene expression was calculated by normalizing expression in C3aR KO MRL mice to CTRL MRL mice to as 2(−ΔΔCt).
Statistical Analyses
Data were analyzed using Sigma Stat software version 3.0 (Jandel Scientific, San Rafael, CA). Survival analysis was performed using log-rank analysis. Data in tables are presented as means ± standard error. Comparisons between groups were performed using the Student’s t test. A gene-wise, two-sample, t-test was done for each transcript to identify statistical differences in expression between C3aR KO MRL mice to CTRL MRL mice in vivo. P-values < 0.05 were assumed to be statistically significant.
Results
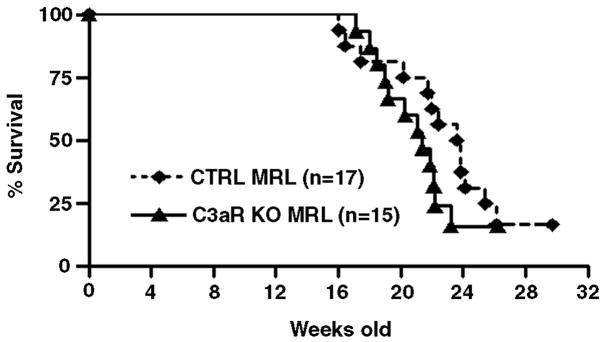
Survival analysis was performed for up to 30 wks to study the effect of progressive renal disease in these strains. Mean survival in the C3aR KO MRL mice was 21.4 wks, compared to 23.7 wks for CTRL MRL mice. Kaplan-Meier analysis revealed a trend towards decreased survival in C3aR KO MRL mice (Figure 1); however, this did not reach statistical significance.
Figure 1. No survival benefit for C3aR KO MRL mice.
C3aR KO MRL mice (solid line) and CTRL MRL mice (dashed line) were followed for 30 wks in order to assess survival using Kaplan-Meier analysis. C3aR KO MRL mice had a mean survival time of 21.4 ± 1.0 wks compared to 23.7 ± 1.2 wks for CTRL MRL mice (p-value = 0.2, Log-Rank).
Both C3aR KO MRL and CTRL MRL mice developed systemic inflammation with multi-organ involvement. Inflammatory infiltrates in the lungs, liver, and intestines were assessed at 20 wks and were equivalent in C3aR KO MRL mice and their CTRL MRL littermates (data not shown). C3aR KO MRL mice developed dermatitis at rates comparable to CTRL MRL mice. Serum C3 levels were also measured using a semi-quantitative ELISA. C3aR KO MRL mice and CTRL MRL littermates had equivalent levels of circulating C3, with no apparent depletion compared to levels seen in pooled mouse serum (data not shown).
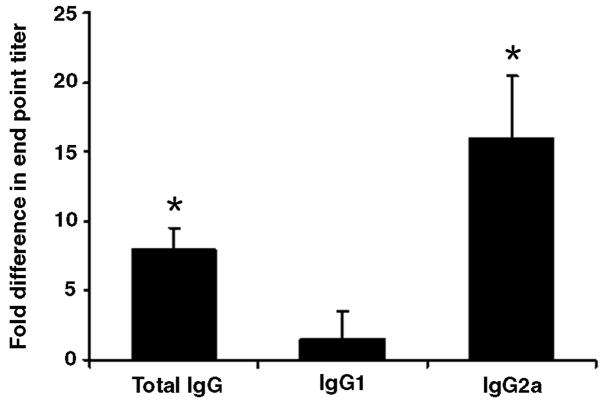
Characterization of splenic leukocytes at 16 wks and 20 wks revealed no significant differences between C3aR KO MRL and CTRL MRL mice in absolute splenocyte number or T-cell subsets (Table 1). The expression of T-cell activation markers as well as the proportion of CD4+CD25+ Treg cells was also similar between groups (data not shown). With respect to auto-antibody production, C3aR KO MRL mice developed slightly higher anti-double stranded DNA antibody titers, which was predominantly IgG2a (Figure 2).
Table 1.
T-cell subsets at 20 wks
| CTRL MRL (n = 6) | C3aR KO MRL (n = 6) | |
|---|---|---|
| Splenocyte number (× 106) | 250 ± 25 | 372 ± 35 * |
| Splenic DN T-cells | 44 ± 1% | 37 ± 1% * |
| CD4+ Splenocytes | 15 ± 1% | 13 ± 1% * |
| CD8+ Splenocytes | 14 ± 1% | 13 ± 1% * |
| CD4+/CD8+ ratio | 1.1 ± 0.1 | 1.0 ± 0.1 * |
p-value >0.05 for all parameters tested. The absolute cell number was calculated after mechanical disruption of whole spleen, while the proportion of CD4+, CD8+, or DN T-cells (CD4−CD8−) cells were determined by FACS analysis, gating on the CD3+ population,)
Figure 2. Elevated auto-antibody titers in C3aR KO MRL mice.
Serum from 20 wk C3aR KO MRL mice was compared with serum from CTRL MRL mice. End-point titers for anti-double stranded DNA antibodies (total IgG, IgG1, and IgG2a) were measured by ELISA using serial dilution. End point tiers of IgG were 8-fold higher and of IgG2a were 16-fold higher than in CTRL MRL mice. Data are means ± SEM for six samples per group (* p-value < 0.05).
The renal injury seen in MRL mice is a chronic progressive disease characterized by abnormal proteinuria and renal failure. At 20 wks, C3aR KO MRL mice had a 20% increase in proteinuria compared to CTRL MRL littermates (5.5 mg/mg vs. 4.6 mg/mg creatinine respectively)(Table 2). In addition, serum creatinine concentrations were 50% higher in C3aR KO MRL mice (1.5 mg/dl) compared to control mice (1.0 mg/dl).
Table 2.
Renal function at 20 wks
| CTRL MRL (n = 10) | C3aR KO MRL (n = 6) | p-value | |
|---|---|---|---|
| Serum Creatinine (mg/dl) | 1.0 ± 0.18 | 1.5 ± 0.2 | < 0.01 |
| Urine Protein/Creatinine (mg/mg) | 4.6 ± 0.9 | 5.5 ± 0.6 | < 0.05 |
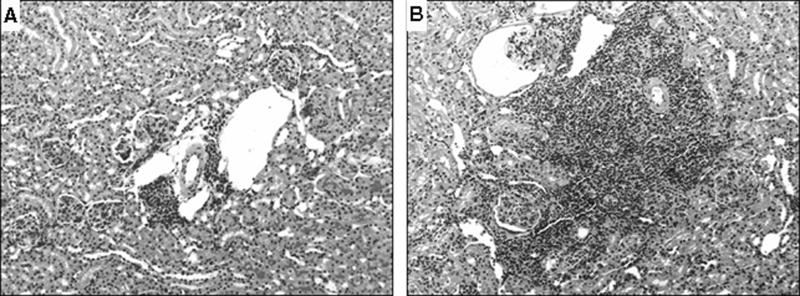
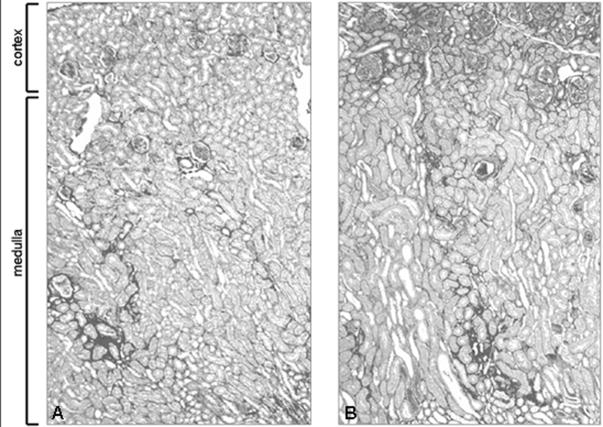
Histologically, there were clear differences in the patterns of renal injury between the two strains (Table 3). At 16 wks, C3aR KO MRL mice had more glomerular crescents and more severe intra-renal vasculitis compared to CTRL MRL mice (Figure 3). While there were no significant differences in the severity of tubulo-interstitial infiltrates, the C3aR KO MRL mice had significantly more interstitial fibrosis than control mice with the greatest increase in Sirius red staining surrounding superficial rather than deep cortical glomeruli (Figure 4) By 20 wks, the differences in renal injury between the two strains became non-significant, although there was a trend toward more injury in the C3aR KO MRL mice in regard to both glomerular crescents and glomerulosclerosis. At 20 wks, there were no differences in the pattern or the intensity of glomerular IgG or C3 staining between the mouse strains, nor were there difference in CD4+ or CD8+ T-cell infiltrates (Table 4).
Table 3.
Renal histopathology
| CTRL MRL (n = 6) | C3aR KO MRL (n = 6) | p-value | |
|---|---|---|---|
| Crescents | |||
| 16wks | 8 ± 4 | 19 ± 9 | 0.22 |
| 20wks | 4 ± 2 | 11 ± 8 | 0.48 |
| Sclerosis | |||
| 16wks | 14 ± 6 | 19 ± 4 | 0.52 |
| 20wks | 13 ± 10 | 30 ± 10 | 0.29 |
| TID – PAS a | |||
| 16wks | 1.3 ± 0.1 | 1.6 ± 0.3 | 0.21 |
| 20wks | 1.4 ± 0.6 | 1.6 ± 0.4 | 0.79 |
| TID – Sirius a | |||
| 16wks | 25.3 ± 2.0 | 31.4 ± 1.3 | 0.03 |
| 20wks | 25.2 ± 2.1 | 29.3 ± 3.0 | 0.30 |
| vasculitis | |||
| 16wks | 1.1 ± 0.2 | 2.3 ± 0.2 | 0.003 |
| 20wks | 2.1 ± 0.5 | 2.5 ± 0.3 | 0.51 |
abbreviations: TID = tubulointerstitial disease, PAS = periodic acid Schiff stain
Figure 3. Increased peri-vascular infiltrates in the C3aR KO MRL kidney.
Representative images of paraffin sections from 16 wk mouse kidneys stained with PAS are shown. Medium sized arteries (black arrow) and veins (open arrow) from CTRL MRL (A) and C3aR KO MRL kidneys (B) are shown at 100x. Leukocytic infiltrates are larger in C3aR KO MRL kidneys compared with CTRL MRL kidneys.
Figure 4. Increased tubulo-interstitial fibrosis in the C3aR KO MRL kidney.
Representative images of paraffin sections from 16 wk CTRL MRL (A) and C3aR KO MRL (B) mouse kidneys stained with Sirius red are shown at 50x. C3aR KO MRL mice have increased tubulo-interstitial staining of the cortex. Staining of areas around large vessels in the medulla was not significantly different between C3aR KO MRL and CTRL MRL mice.
Table 4.
Immunofluorescence staining at 20 wks
| CTRL MRL (n = 10) | C3aR KO MRL (n = 7) | |
|---|---|---|
| C3 | 2.42 ± 0.13 | 2.20 ± 0.12 * |
| IgG | 2.04 ± 0.28 | 1.65 ± 0.25 * |
| Periglomerular CD4+ cells | 25.6 ± 4.5 | 21.4 ± 7.6 * |
| Intraglomerular CD4+ cells | 1.2 ± 0.1 | 1.3 ± 0.3 * |
| Peritubular CD4+ cells | 35.8 ± 2.9 | 42.5 ± 4.4 * |
p-value > 0.05
In order to explore the mechanism of the early onset of renal injury seen in C3aR KO MRL mice, chemokine and chemokine receptor expression was analyzed using qRT-PCR. Expression was measured at 8 wks, prior to onset of overt renal injury, and at 14 wks, just before histological differences were greatest between strains. These data are summarized in Figure 5. At 8wks, only a single chemokine (Ccl2/MCP-1) was significantly down-regulated at the mRNA level in C3aR KO MRL kidneys compared to controls. Conversely, significant increases in expression were detected at 8 weeks in four Ccl (Ccl5/RANTES, Ccl7, Ccl8, and Ccl19), three Cxcl (Cxcl5, Cxcl13, and Cxcl15), and one Xcl (Xcl1) class chemokines. There were also significant increases in five chemokine receptors (Ccr1l1, Ccr6, Ccr7, Ccr8, and Cxcr5). The differences in expression detected at 8wks disappeared by 14wks, at which time the trend was towards decreased mRNA expression in the C3aR KO MRL kidney. This was the result of relatively static levels of chemokine and chemokine receptor expression in the C3aR KO MRL mice with concurrent increases in expression in the CTRL MRL mice.
Figure 5. Early induction of chemokine receptors and ligands mRNA in C3aR KO MRL kidneys.
Quantitative mRNA expression analysis was performed on kidney cortex of C3aR KO MRL and CTRL MRL mice at both 8 and 14 wks (n = 3 per strain). Fold differences, at each time point for each gene expressed, was calculated by comparing data collected from C3aR KO MRL mice to data from CTL MRL mice, after normalization for housekeeping gene expression (‡ p-value < 0.05, * p-value < 0.005).
Discussion
The pathogenic role of complement in the MRL model is complex. While human data strongly suggested a pathogenic role for C3 in lupus nephritis, MRL mice deficient in C3 paradoxically develop slightly more severe disease (Sekine, 2001). The absence of C3 was not associated with changes in renal pathology, the degree of lymphoproliferation, the titers of autoantibodies, or the levels of circulating immune complexes. Survival at 24 wks was similar, but there was a trend toward decreased survival in C3 deficient MRL mice. There was also increased proteinuria and glomerular IgG deposition in the C3 deficient MRL mice, which was thought to be due to the loss of C3 dependent clearance of circulating immune complexes and apoptotic bodies.
Cleavage of C3 not only generates products involved to IC clearance, but also the anaphylatoxin C3a. The anaphylatoxins are one of the primary effector mechanisms of complement activation, and the major complement anaphylatoxins C3a and C5a exert their effects via binding to the specific receptors. Previously, we described attenuated disease in C5aR KO MRL mice (Wenderfer, 2005). Here, we report that C3aR KO MRL mice develop accelerated onset of renal disease compared to CTRL MRL mice. Similar to C3 deficient MRL mice (Sekine, 2001), C3aR KO MRL mice had an earlier onset of renal injury compared to CTRL MRL mice and a trend toward decreased survival. C3 deficient MRL mice and C3aR KO MRL mice both developed exacerbated tubulo-interstitial fibrosis. While the glomerular immune complex deposition typical for the MRL model is unaffected by C3a/C3aR interactions, the loss of C3aR resulted in earlier onset of perivascular infiltrates and chronic tubulo-interstitial changes. As the complement split product C3a is the only known ligand for the C3aR, the results suggest that C3a generation is protective in the MRL mouse.
The opposing effects of the anaphylatoxin receptors C3aR and C5aR in disease progression in MRL mice is not unexpected, based on reports in other models using C3aR and C5aR KO mice. C3aR KO mice have exacerbated endotoxemia from LPS treatment (Kildsgaard, 2000b) and worse outcome after gram-negative bacteremia (Hollmann, 2007), whereas C5aR KO mice have attenuated responses to cecal ligation and puncture (Riedemann, 2004) as well as gram-negative bacteremia and endotoxic shock (Hollmann, 2007). The receptors also play opposing roles in Aspergillus-induced airway inflammation (Drouin, 2002; Kohl, 2006), Pseudomonas pneumonia (Hopken, 1996; Mueller-Ortiz, 2006), and contact dermatitis (Kawamoto, 2004; Tsuji, 2000). Opposing effects could be mediated either by differential expression of C3aR or C5aR on individual cell types, separate signaling pathways within the same cell, or via counter-regulation of anaphylatoxin receptor expression (Sayah, 2003; Schraufstatter, 2002;Melendi, 2007 Settmacher, 1999).
Previous studies have suggested that complement anaphylatoxins might regulate fibrosis (Addis-Lieser, 2005; Bao, 2003; Boor, 2007). The accelerated tubulo-interstitial fibrosis in C3aR KO MRL mice, combined with previous studies demonstrating reductions in renal fibrosis with loss of C5aR function (Bao, 2003; Welch, 2002; Wenderfer, 2005), suggest that chronic complement activation modulates the development of renal fibrosis, with the C5aR activation driving fibrosis and C3aR activation limiting its progression.
Serum autoantibody titers were elevated in C3aR KO MRL mice, and this increase was more prominent for the IgG2a isotype. In contrast, C5aR KO MRL mice were previously shown to have elevated IgG1 and depressed IgG2a anti-dsDNA antibody titers. Disease in lupus-prone mice is thought to be a Th1-mediated disease (Balomenos, 1998; Schwarting, 1999; Takahashi, 1996; Yin, 2002), where the IgG2a subtype is more prevalent. Therefore, the isotype predominance may simply reflect the overall disease activity in this model.
The results described here differ from those published previously showing that MRL mice treated with a C3a analog, SB290157, had preservation of renal function, delayed onset of proteinuria, and decreased tubulo-interstitial leukocyte infiltrates (Bao, 2005a). Bao et al treated MRL mice continuously from 13 to 19wks of age with 60mcg/gm/day of SB290157. At the time, SB290157 was thought to be a specific antagonist of C3aR (Ames, 2001). However, both agonist and antagonist activity of SB290157 has been described in vitro (Mathieu, 2005; Purwar, 2006). SB290157 appears to act as an antagonist when receptor density is low, while it has agonist activity in cell lines expressing higher levels of the C3aR, suggesting that it is a partial agonist (Mathieu, 2005). This may be particularly relevant in the MRL mouse, where renal C3aR expression is markedly increased early in the disease process (Bao, 2005a). Paradoxical effects of SB290157 have been reported in other disease models as well (Baelder, 2005; Bautsch, 2000; Drouin, 2002; Humbles, 2000). In addition to the partial agonist affects, SB290157 can lead to dose dependent neutropenia (Proctor, 2004). While the MRL mice treated with SB290157 were not tested for neutropenia, significant reductions in the numbers of renal infiltrating neutrophils were reported (Bao, 2005a). Thus it is possible that prolonged exposure to SB290157 may result in chronic neutropenia and improved survival independent of C3aR inhibition.
The data on patterns of chemokine and chemokine receptor expression were consistent with the pathologic findings in this study. At 8 wks, there were significant increases in transcript expression of several pro-inflammatory chemokines and chemokine receptors in C3aR KO MRL mice compared to CTRL MRL mice. The differences of expression level disappeared at 14 wks. The reduction in Ccl2/MCP-1 in the C3aR KO kidney’s is consistent with prior reports that MCP-1 induction is in part C3a dependent (Ahamed, 2004; Venkatesha, 2005). However, as the C3aR is thought to play a major role in granulocyte chemotaxis, it was surprising that the granulocyte chemotactic proteins, Cxcl5 (Wuyts, 1996) and Cxcl15 (Rossi, 1999) and the granulocyte receptor Cxcr1/IL8Ra (Fan, 2007; Moepps, 2006) were up-regulated early in C3aR KO MRL kidneys. This suggests that there may be an intrinsic compensatory response mediating granulocyte chemotaxis in the C3AR KO mice.
There were two sets of chemokine and cognate receptor pairs up-regulated (Ccl19/Ccr7 and Cxcl13/Cxcr5). CCR7 is expressed in many lymphoid tissues and activates B- and T-lymphocytes. Ccl19/Ccr7 interaction enhances migration of dendritic cells and T-cells to and within lymphoid tissues (Davalos-Misslitz, 2007; Debes, 2005), promotes renal fibrosis in the unilateral ureteral obstruction model (Sakai, 2006), and also drives production of bronchial-associated lymphoid tissue (Kocks, 2007). A similar mechanism may explain the large perivascular lymphoid infiltrates seen in the MRL kidney and accelerated in the C3aR KO MRL kidney. Cxcl13/Cxcr5 interaction has been implicated in human lupus and animal models of inflammatory nephritis (Adalid-Peralta, 2008; Duan, 2008; Steinmetz, 2008). Ccr6 and Ccr8 were also up-regulated, and are more restricted to cells of the adaptive immune response: CCR6 on dendritic cells, inactivated memory T-cells, and Th17 cells (Acosta-Rodriguez, 2007; Schutyser, 2003), CCR8 on monocytes and activated T-cells (Cosmi, 2001), and CXCR5 on follicular dendritic cells, T- and B-cells. Cell migration via CCR6, CCR7, CCR8, and CXCR5 is important in germinal center formation and delayed type hypersensitivity responses (Arnold, 2007; Hardtke, 2005; Jakubzick, 2006; Qu, 2004; Steinmetz, 2008; Varona, 2001) and may contribute to formation of lymphoid follicles in inflamed MRL kidneys. Further investigation into regulation of chemokine and chemokine receptor expression by anaphylatoxins will be required to determine whether these are direct or indirect effects.
Conclusions
Compared to CTRL MRL littermates, C3aR KO MRL mice have acceleration in the onset of renal injury and more severe interstitial fibrosis. This is associated with a significant broadly based increase in chemokines and chemokine receptor expression at 8 wks. However, the enhanced expression of chemokines and chemokine receptors declined markedly by 14 wks and by 20 wks the differences in glomerular injury were modest. Based on these data, it appears that activation of the C3aR in the MRL/lpr mouse functions primarily to delay the onset of chronic renal inflammation and fibrosis. The C3aR is neither sufficient nor required as a pathogenic mediator as the loss of C3aR expression had little overall impact on the severity of auto-immunity or long-term survival in the MRL/lpr model of lupus nephritis.
Acknowledgments
The authors would like to thank Todd Triplett for his technical assistance and Dr. Irma Gigli for her guidance and review of the manuscript. Supported in part by NIH grants DK61929 (SEW), AI025011, HL074333, AI068795 (RAW); DK071057, DK072322, and DK062197 (MCB).
Glossary
- C3aR
C3a receptor
- DN
double negative
- dsDNA
double stranded DNA
- fB
factor B
- fD
factor D
- hpf
high power field
- KO
knock out
- MRL
MRL/MpJ-Tnfrsf6lpr
- PAS
periodic acid Schiff
- SLE
systemic lupus erythematosus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing t helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Adalid-Peralta L, Mathian A, Tran T, Delbos L, Durand-Gasselin I, Berrebi D, Peuchmaur M, Couderc J, Emilie D, Koutouzov S. Leukocytes and the kidney contribute to interstitial inflammation in lupus nephritis. Kidney Int. 2008;73:172–180. doi: 10.1038/sj.ki.5002625. [DOI] [PubMed] [Google Scholar]
- Addis-Lieser E, Kohl J, Chiaramonte MG. Opposing regulatory roles of complement factor 5 in the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2005;175:1894–1902. doi: 10.4049/jimmunol.175.3.1894. [DOI] [PubMed] [Google Scholar]
- Ahamed J, Venkatesha RT, Thangam EB, Ali H. C3a enhances nerve growth factor-induced nfat activation and chemokine production in a human mast cell line, hmc-1. J Immunol. 2004;172:6961–6968. doi: 10.4049/jimmunol.172.11.6961. [DOI] [PubMed] [Google Scholar]
- Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, Settmacher B, Klos A, Erhard KF, Cousins RD, Sulpizio AC, Hieble JP, McCafferty G, Ward KW, Adams JL, Bondinell WE, Underwood DC, Osborn RR, Badger AM, Sarau HM. Identification of a selective nonpeptide antagonist of the anaphylatoxin c3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol. 2001;166:6341–6348. doi: 10.4049/jimmunol.166.10.6341. [DOI] [PubMed] [Google Scholar]
- Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold CN, Campbell DJ, Lipp M, Butcher EC. The germinal center response is impaired in the absence of t cell-expressed cxcr5. Eur J Immunol. 2007;37:100–109. doi: 10.1002/eji.200636486. [DOI] [PubMed] [Google Scholar]
- Baelder R, Fuchs B, Bautsch W, Zwirner J, Kohl J, Hoymann HG, Glaab T, Erpenbeck V, Krug N, Braun A. Pharmacological targeting of anaphylatoxin receptors during the effector phase of allergic asthma suppresses airway hyperresponsiveness and airway inflammation. J Immunol. 2005;174:783–789. doi: 10.4049/jimmunol.174.2.783. [DOI] [PubMed] [Google Scholar]
- Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in mrl-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Osawe I, Haas M, Quigg RJ. Signaling through up-regulated c3a receptor is key to the development of experimental lupus nephritis. J Immunol. 2005a;175:1947–1955. doi: 10.4049/jimmunol.175.3.1947. [DOI] [PubMed] [Google Scholar]
- Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol. 2005b;35:2496–2506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- Bao L, Zhou J, Holers VM, Quigg RJ. Excessive matrix accumulation in the kidneys of mrl/lpr lupus mice is dependent on complement activation. J Am Soc Nephrol. 2003;14:2516–2525. doi: 10.1097/01.asn.0000089831.96794.0b. [DOI] [PubMed] [Google Scholar]
- Bautsch W, Hoymann HG, Zhang Q, Meier-Wiedenbach I, Raschke U, Ames RS, Sohns B, Flemme N, Meyer zu Vilsendorf A, Grove M, Klos A, Kohl J. Cutting edge: Guinea pigs with a natural c3a-receptor defect exhibit decreased bronchoconstriction in allergic airway disease: Evidence for an involvement of the c3a anaphylatoxin in the pathogenesis of asthma. J Immunol. 2000;165:5401–5405. doi: 10.4049/jimmunol.165.10.5401. [DOI] [PubMed] [Google Scholar]
- Boor P, Konieczny A, Villa L, Schult AL, Bucher E, Rong S, Kunter U, van Roeyen CR, Polakowski T, Hawlisch H, Hillebrandt S, Lammert F, Eitner F, Floege J, Ostendorf T. Complement c5 mediates experimental tubulointerstitial fibrosis. J Am Soc Nephrol. 2007;18:1508–1515. doi: 10.1681/ASN.2006121343. [DOI] [PubMed] [Google Scholar]
- Braun MC, Reins RY, Li TB, Hollmann TJ, Dutta R, Rick WA, Teng BB, Ke B. Renal expression of the c3a receptor and functional responses of primary human proximal tubular epithelial cells. J Immunol. 2004;173:4190–4196. doi: 10.4049/jimmunol.173.6.4190. [DOI] [PubMed] [Google Scholar]
- Cosmi L, Annunziato F, Maggi E, Romagnani S, Manetti R. Chemoattractant receptors expressed on type 2 t cells and their role in disease. Int Arch Allergy Immunol. 2001;125:273–279. doi: 10.1159/000053827. [DOI] [PubMed] [Google Scholar]
- Couser WG, Baker PJ, Adler S. Complement and the direct mediation of immune glomerular injury: A new perspective. Kidney Int. 1985;28:879–890. doi: 10.1038/ki.1985.214. [DOI] [PubMed] [Google Scholar]
- Davalos-Misslitz AC, Rieckenberg J, Willenzon S, Worbs T, Kremmer E, Bernhardt G, Forster R. Generalized multi-organ autoimmunity in ccr7-deficient mice. Eur J Immunol. 2007;37:613–622. doi: 10.1002/eji.200636656. [DOI] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor ccr7 required for t lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin c3a receptor suppresses th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169:5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- Duan B, Niu H, Xu Z, Sharpe AH, Croker BP, Sobel ES, Morel L. Intrafollicular location of marginal zone/cd1d(hi) b cells is associated with autoimmune pathology in a mouse model of lupus. Lab Invest. 2008;88:1008–1020. doi: 10.1038/labinvest.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hedrick JA, Lundell D, Lira SA, Hipkin RW. Murine cxcr1 is a functional receptor for gcp-2/cxcl6 and interleukin-8/cxcl8. J Biol Chem. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- Fischer WH, Hugli TE. Regulation of b cell functions by c3a and c3a(desarg): Suppression of tnf-alpha, il-6, and the polyclonal immune response. J Immunol. 1997;159:4279–4286. [PubMed] [Google Scholar]
- Fischer WH, Jagels MA, Hugli TE. Regulation of il-6 synthesis in human peripheral blood mononuclear cells by c3a and c3a(desarg) J Immunol. 1999;162:453–459. [PubMed] [Google Scholar]
- Grimm PC, Nickerson P, Gough J, McKenna R, Stern E, Jeffery J, Rush DN. Computerized image analysis of sirius red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol. 2003;14:1662–1668. doi: 10.1097/01.asn.0000066143.02832.5e. [DOI] [PubMed] [Google Scholar]
- Hardtke S, Ohl L, Forster R. Balanced expression of cxcr5 and ccr7 on follicular t helper cells determines their transient positioning to lymph node follicles and is essential for efficient b-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- Hicks J, Bullard DC. Review of autoimmune (lupus-like) glomerulonephritis in murine models. Ultrastruct Pathol. 2006;30:345–359. doi: 10.1080/01913120600932677. [DOI] [PubMed] [Google Scholar]
- Hollmann TJ, Mueller-Ortiz SL, Braun MC, Wetsel RA. Disruption of the c5a receptor gene increases resistance to acute gram-negative bacteremia and endotoxic shock: Opposing roles of c3a and c5a. Mol Immunol. 2007 doi: 10.1016/j.molimm.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopken UE, Lu B, Gerard NP, Gerard C. The c5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. A role for the c3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Yalcindag A, Laouini D, Brodeur S, Bryce P, Lu B, Humbles AA, Oettgen H, Gerard C, Geha RS. The anaphylatoxin c3a downregulates the th2 response to epicutaneously introduced antigen. J Clin Invest. 2004;114:399–407. doi: 10.1172/JCI19082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: Targeted disruption of the c3a receptor gene demonstrates a novel protective anti-inflammatory role for c3a in endotoxin-shock. J Immunol. 2000a;165:5406–5409. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: Targeted disruption of the c3a receptor gene demonstrates a novel protective anti-inflammatory role for c3a in endotoxin-shock. J Immunol. 2000b;165:5406–5409. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- Kirchhoff K, Weinmann O, Zwirner J, Begemann G, Gotze O, Kapp A, Werfel T. Detection of anaphylatoxin receptors on cd83+ dendritic cells derived from human skin. Immunology. 2001;103:210–217. doi: 10.1046/j.1365-2567.2001.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory t cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007;204:723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, Whitsett JA, Gerard C, Sfyroera G, Lambris JD, Wills-Karp M. A regulatory role for the c5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono DH, Theofilopoulos AN. Genetics of systemic autoimmunity in mouse models of lupus. Int Rev Immunol. 2000;19:367–387. doi: 10.3109/08830180009055504. [DOI] [PubMed] [Google Scholar]
- Kono DH, Theofilopoulos AN. Genetics of sle in mice. Springer Semin Immunopathol. 2006;28:83–96. doi: 10.1007/s00281-006-0030-7. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Kahl LE, Atkinson JP. The functional role of complement genes in systemic lupus erythematosus and sjogren’s syndrome. Curr Opin Rheumatol. 1989;1:347–352. doi: 10.1097/00002281-198901030-00018. [DOI] [PubMed] [Google Scholar]
- Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- Mathieu MC, Sawyer N, Greig GM, Hamel M, Kargman S, Ducharme Y, Lau CK, Friesen RW, O’Neill GP, Gervais FG, Therien AG. The c3a receptor antagonist sb 290157 has agonist activity. Immunol Lett. 2005;100:139–145. doi: 10.1016/j.imlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Melendi GA, Hoffman SJ, Karron RA, Irusta PM, Laham FR, Humbles A, Schofield B, Pan CH, Rabold R, Thumar B, Thumar A, Gerard NP, Mitzner W, Barnum SR, Gerard C, Kleeberger SR, Polack FP. C5 modulates airway hyperreactivity and pulmonary eosinophilia during enhanced respiratory syncytial virus disease by decreasing c3a receptor expression. J Virol. 2007;81:991–999. doi: 10.1128/JVI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Pickering MC, Warren J, Fossati-Jimack L, Cortes-Hernandez J, Cook HT, Botto M, Walport MJ. C1q deficiency and autoimmunity: The effects of genetic background on disease expression. J Immunol. 2002;168:2538–2543. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- Moepps B, Nuesseler E, Braun M, Gierschik P. A homolog of the human chemokine receptor cxcr1 is expressed in the mouse. Mol Immunol. 2006;43:897–914. doi: 10.1016/j.molimm.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Mueller-Ortiz SL, Hollmann TJ, Haviland DL, Wetsel RA. Ablation of the complement c3a anaphylatoxin receptor causes enhanced killing of pseudomonas aeruginosa in a mouse model of pneumonia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L157–165. doi: 10.1152/ajplung.00358.2005. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Limaye N, Wakeland EK. Susceptibility genes in the pathogenesis of murine lupus. Arthritis Res. 2002;4(Suppl 3):S255–263. doi: 10.1186/ar583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose M, Nishihara M, Kamogawa J, Terada M, Nakatsuru S. Genetic basis of autoimmune disease in mrl/lpr mice: Dissection of the complex pathological manifestations and their susceptibility loci. Rev Immunogenet. 2000;2:154–164. [PubMed] [Google Scholar]
- Peake PW, O’Grady S, Pussell BA, Charlesworth JA. C3a is made by proximal tubular hk-2 cells and activates them via the c3a receptor. Kidney Int. 1999;56:1729–1736. doi: 10.1046/j.1523-1755.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- Proctor LM, Arumugam TV, Shiels I, Reid RC, Fairlie DP, Taylor SM. Comparative anti-inflammatory activities of antagonists to c3a and c5a receptors in a rat model of intestinal ischaemia/reperfusion injury. Br J Pharmacol. 2004;142:756–764. doi: 10.1038/sj.bjp.0705819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwar R, Wittmann M, Zwirner J, Oppermann M, Kracht M, Dittrich-Breiholz O, Gutzmer R, Werfel T. Induction of c3 and ccl2 by c3a in keratinocytes: A novel autocrine amplification loop of inflammatory skin reactions. J Immunol. 2006;177:4444–4450. doi: 10.4049/jimmunol.177.7.4444. [DOI] [PubMed] [Google Scholar]
- Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, Sanchez-Torres C, Bromberg J, Charo IF, Jung S, Lira SA, Randolph GJ. Role of ccr8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of c5a in lps-induced il-6 production by neutrophils during sepsis. Faseb J. 2004;18:370–372. doi: 10.1096/fj.03-0708fje. [DOI] [PubMed] [Google Scholar]
- Rossi DL, Hurst SD, Xu Y, Wang W, Menon S, Coffman RL, Zlotnik A. Lungkine, a novel cxc chemokine, specifically expressed by lung bronchoepithelial cells. J Immunol. 1999;162:5490–5497. [PubMed] [Google Scholar]
- Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (slc/ccl21)/ccr7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayah S, Jauneau AC, Patte C, Tonon MC, Vaudry H, Fontaine M. Two different transduction pathways are activated by c3a and c5a anaphylatoxins on astrocytes. Brain Res Mol Brain Res. 2003;112:53–60. doi: 10.1016/s0169-328x(03)00046-9. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Trieu K, Sikora L, Sriramarao P, DiScipio R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002;169:2102–2110. doi: 10.4049/jimmunol.169.4.2102. [DOI] [PubMed] [Google Scholar]
- Schutyser E, Struyf S, Van Damme J. The cc chemokine ccl20 and its receptor ccr6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Schwarting A, Tesch G, Kinoshita K, Maron R, Weiner HL, Kelley VR. Il-12 drives ifn-gamma-dependent autoimmune kidney disease in mrl-fas(lpr) mice. J Immunol. 1999;163:6884–6891. [PubMed] [Google Scholar]
- Sekine H, Graham KL, Zhao S, Elliott MK, Ruiz P, Utz PJ, Gilkeson GS. Role of mhc-linked genes in autoantigen selection and renal disease in a murine model of systemic lupus erythematosus. J Immunol. 2006;177:7423–7434. doi: 10.4049/jimmunol.177.10.7423. [DOI] [PubMed] [Google Scholar]
- Sekine H, Reilly CM, Molano ID, Garnier G, Circolo A, Ruiz P, Holers VM, Boackle SA, Gilkeson GS. Complement component c3 is not required for full expression of immune complex glomerulonephritis in mrl/lpr mice. J Immunol. 2001;166:6444–6451. doi: 10.4049/jimmunol.166.10.6444. [DOI] [PubMed] [Google Scholar]
- Settmacher B, Bock D, Saad H, Gartner S, Rheinheimer C, Kohl J, Bautsch W, Klos A. Modulation of c3a activity: Internalization of the human c3a receptor and its inhibition by c5a. J Immunol. 1999;162:7409–7416. [PubMed] [Google Scholar]
- Steinmetz OM, Velden J, Kneissler U, Marx M, Klein A, Helmchen U, Stahl RA, Panzer U. Analysis and classification of b-cell infiltrates in lupus and anca-associated nephritis. Kidney Int. 2008;74:448–457. doi: 10.1038/ki.2008.191. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Fossati L, Iwamoto M, Merino R, Motta R, Kobayakawa T, Izui S. Imbalance towards th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in mrl mice. J Clin Invest. 1996;97:1597–1604. doi: 10.1172/JCI118584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji RF, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli TE, Gerard C, Askenase PW. Early local generation of c5a initiates the elicitation of contact sensitivity by leading to early t cell recruitment. J Immunol. 2000;165:1588–1598. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutierrez J, Torres M, Martinez AC, Marquez G. Ccr6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001;107:R37–45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha RT, Berla Thangam E, Zaidi AK, Ali H. Distinct regulation of c3a-induced mcp-1/ccl2 and rantes/ccl5 production in human mast cells by extracellular signal regulated kinase and pi3 kinase. Mol Immunol. 2005;42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Garnier G, Circolo A, Wetsel RA, Ruiz P, Holers VM, Boackle SA, Colten HR, Gilkeson GS. Modulation of renal disease in mrl/lpr mice genetically deficient in the alternative complement pathway factor b. J Immunol. 2000;164:786–794. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
- Welch TR, Frenzke M, Witte D, Davis AE. C5a is important in the tubulointerstitial component of experimental immune complex glomerulonephritis. Clin Exp Immunol. 2002;130:43–48. doi: 10.1046/j.1365-2249.2002.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC. C5a receptor deficiency attenuates t cell function and renal disease in mrllpr mice. J Am Soc Nephrol. 2005;16:3572–3582. doi: 10.1681/ASN.2005040373. [DOI] [PubMed] [Google Scholar]
- Werfel T, Kirchhoff K, Wittmann M, Begemann G, Kapp A, Heidenreich F, Gotze O, Zwirner J. Activated human t lymphocytes express a functional c3a receptor. J Immunol. 2000;165:6599–6605. doi: 10.4049/jimmunol.165.11.6599. [DOI] [PubMed] [Google Scholar]
- Wetsel RA. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr Opin Immunol. 1995;7:48–53. doi: 10.1016/0952-7915(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Wuyts A, Haelens A, Proost P, Lenaerts JP, Conings R, Opdenakker G, Van Damme J. Identification of mouse granulocyte chemotactic protein-2 from fibroblasts and epithelial cells. Functional comparison with natural kc and macrophage inflammatory protein-2. J Immunol. 1996;157:1736–1743. [PubMed] [Google Scholar]
- Yin Z, Bahtiyar G, Zhang N, Liu L, Zhu P, Robert ME, McNiff J, Madaio MP, Craft J. Il-10 regulates murine lupus. J Immunol. 2002;169:2148–2155. doi: 10.4049/jimmunol.169.4.2148. [DOI] [PubMed] [Google Scholar]
- Zwirner J, Gotze O, Begemann G, Kapp A, Kirchhoff K, Werfel T. Evaluation of c3a receptor expression on human leucocytes by the use of novel monoclonal antibodies. Immunology. 1999;97:166–172. doi: 10.1046/j.1365-2567.1999.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]