Abstract
Objective
The objective of this study was to detail the epidemiologic characteristics and natural history of HIV-1 Natural Viral Suppressors (NVS), a cohort of HIV-1 infected individuals who are able to suppress viral replication to undetectable levels in the absence of therapy.
Design and Methods
HIV-1 patients who met the NVS criteria were enrolled into a prospective study. The incidence and prevalence of NVS were calculated by performing a chart review on all patients seen in one clinic in a 10 year period. Cumulative probability of progression-free survival was calculated by Kaplan-Meier product limit method.
Results
Forty individuals enrolled in the study. The median year of diagnosis was 1994, and individuals demonstrated a median 6.7 years of HIV-1 viral suppression and CD4 count of 795 cells/ul. NVS had an incidence of 1.1% (95%CI, 0.0–2.1) and prevalence of 1.5% (95% CI, 0.8–2.1). Only one patient (2.5%) has progressed. Within the first 10 years for follow-up having met the definition of NVS, 95.1% (95% CI 86.5%–100%) of the NVS continued to control their viral loads to undetectable levels.
Conclusions
The NVS cohort has demonstrated remarkable stability and a low rate of progression over many years. Detailed evaluations of viral-host immune regulatory factors associated with persistent HIV-1 natural viral suppression, as well as loss of such suppression, has the potential to provide important new insight in HIV pathogenesis and future immune regulatory targeted preventive and therapeutic research.
Keywords: HIV, natural viral suppressor, elite controller, elite suppressor, natural history
INTRODUCTION
For over a decade, there have been many studies on HIV-infected individuals who control their infection without antiretrovirals in the hopes of better understanding the pathogenesis and treatment of HIV. Initially, this was done in Long Term Nonprogressors (LTNP); however, at the definition of LTNP was established viral load testing was not performed routinely. Thus, individuals in LTNP cohorts demonstrated heterogeneous viral loads, and the studies on such cohorts often had conflicting results.1–4 We have recently described a cohort of HIV-1 infected individuals, Natural Viral Suppressors (NVS), who have the ability to naturally suppress HIV-1 to undetectable levels.5 We demonstrated that the NVS had a much lower proviral copy number than controls (including LTNPs), thus confirming our case definition of this cohort. In the past few years, there has been a steady increase in the number of publications involving HIV infected cohorts similar to the NVS;6–14 however, there has been little focus on the natural history and epidemiologic characteristics of these patients. Because such cohorts represent a successful model of HIV suppression and are more homogenous than previously studied cohorts, detailed epidemiologic studies on such cohorts are important in that they might provide insight into the factors related to viral control. In this study, we detail the epidemiological characteristics, natural history, and rate of progression of the NVS cohort.
METHODS
After informed consent was obtained, NVS patients had to be confirmed HIV-1 positive by Western Blot and proviral DNA, and have demonstrated viral loads <400 copies/ml for a 2 year time period without the use of antiretroviral therapy (one viral load > 400 copies/ml in a 2 year period was allowed), as previously described.5 Patients were recruited from the Institute of Human Virology’s clinics in Baltimore (Evelyn Jordan Center, Baltimore Veterans Administration (VA), and Maryland General Hospital), as well as referrals from other local affiliated clinics.
HIV-1 viral loads were assessed using the Versant 3.0 assay, having a limit of detection of 75 HIV-1 copies/ml, or the Roche 1.5 assay with a limit of detection of 50 or 400 copies/ml (Bayer, Tarrytown, NY; Roche, Nutley, New Jersey). High input PCR for Proviral DNA was performed as previously described.1 HLA typing was done with PCR of DNA using the Micro SSP™ HLA DNA Typing Trays (One Lambda, Canoga Park, CA), and DNA was extracted from PBMCs by use of the QIAgen mini-blood kit (Qiagen, Valencia, CA). PCR for the CCR5 delta-32 mutation was carried out as described elsewhere.15
The prevalence and incidence of NVS were determined by performing a chart review using the Clinical Case Registry (CCR) database at the Baltimore VA. The medical records of all HIV-1 patients in the CCR database between 10/17/1997 and 10/17/2007 were reviewed. For the purposes of incidence and prevalence calculation, a second confirmatory HIV-1 test was allowed instead of a positive HIV-1 proviral DNA. Prevalence of NVS was calculated by dividing the number of NVS identified during the 10 year time period by total number of evaluable patients seen in the 10 year time period. Cumulative incidence of NVS from the time of HIV diagnosis was calculated by dividing the number of NVS identified among all new HIV-1 cases in the first 8 years by the total number of new HIV-1 cases identified in the first 8 years of the chart review (allowing for 2 years follow-up required by the NVS definition).
A member of the NVS cohort was termed a “progressor” if during follow-up they no longer met the case definition of NVS. The Kaplan-Meier product limit method was then used to estimate the cumulative probability of progression-free survival. For each of the 40 individuals, we determined survival times through 3/15/08. Those individuals alive at the end of the follow-up contributed to censored observations to the survival analysis of time to progression. CD4 and CD4% slopes were calculated from time of initial HIV diagnosis with linear regression, and between groups analysis was performed using the t test (unpaired). All data analysis was done with GraphPad Prism software (San Diego, CA).
RESULTS
Forty patients met the case definition as NVS and were enrolled from Baltimore and the surrounding metropolitan area (Evelyn Jordan Center (n=21), Baltimore VA (n=13), Maryland General Hospital (n=2), as well as referrals from other local affiliated clinics (n=4). The median age for the NVS cohort was 50 years old (25th – 75th percentile: 44–55) and the median year of diagnosis was 1994. Twenty two individuals (55%) had a history of injection drug abuse (IDU) with needle sharing, and eighteen individuals (45%) had a history of unprotected sex and likely acquired HIV sexually (2 of which were men who have sex with men). All 40 patients were African-American, with 52% being male. Laboratory analysis indicated a median CD4 count of 795 cells/ul, and proviral DNA testing detected DNA in the range of 1 to 118 DNA copies/106 PBMCs. Twelve of 27 patients (44%) showed a B57 pattern by HLA testing, and only 1 of 28 patients (4%) was heterozygous for the CCR5 delta-32 mutation. A summary of the demographic data can be found in Table 1, and Figure 1 shows representative patterns of CD4 slopes and viral suppression seen in this cohort. A comparison of the demographic data of the NVS recruited from the EJC and VA with the general HIV clinic population of those clinics is shown in Table 2.
Table 1.
Demographics of the NVS Cohort
| Patient | Age | Sex | Race | HIV risk Factor |
Year of HIV Diagnosis |
Years of known HIV suppression |
Number of HIV viral loads tested |
Last CD4 (cells/ul) |
Last CD4/CD8 ratio |
Proviral copy number per 106 PBMCs |
CCR5 Δ32 genotype |
HLA B57 Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NVS 1 | 51 | M | AA | HS | 2003 | 2.33 | 6 | 1306 | 1.53 | 10 | WT | + |
| NVS 2 | 53 | F | AA | IDU | 1987 | 4.67 | 8 | 932 | 1.12 | 10 | WT | + |
| NVS 3 | 62 | M | AA | IDU | 1991 | 5.75 | 14 | 956 | 0.44 | 46 | WT | − |
| NVS 4 | 48 | F | AA | HS | 1993 | 11.08 | 29 | 845 | 2.34 | 2 | WT | + |
| NVS 5 | 50 | M | AA | HS | 1991 | 9 | 22 | 668 | 0.34 | 24 | HT | − |
| NVS 6 | 48 | F | AA | HS | 1992 | 11.16 | 25 | 1422 | 2.31 | 1 | WT | + |
| NVS 7 | 60 | M | AA | IDU | 1994 | 9.83 | 17 | 1323 | 1.25 | 5 | WT | − |
| NVS 8 | 58 | M | AA | IDU | 1989 | 9.08 | 27 | 722 | 1.07 | 25 | WT | + |
| NVS 9 | 53 | M | AA | IDU | 2003 | 4.25 | 18 | 981 | .90 | 1 | WT | + |
| NVS10 | 57 | F | AA | HS | 1995 | 7.12 | 14 | 990 | 2.11 | 2 | WT | + |
| NVS11 | 60 | M | AA | IDU | 1997 | 9.84 | 17 | 1163 | 1.38 | 1 | WT | + |
| NVS12 | 40 | M | AA | IDU | 1997 | 5.5 | 16 | 1245 | ND | 54 | WT | − |
| NVS13 | 54 | M | AA | IDU | 1993 | 9.66 | 20 | 821 | 4.59 | 4 | WT | − |
| NVS14 | 36 | F | AA | HS | 2002 | 3.75 | 17 | 1533 | ND | 10 | WT | − |
| NVS15 | 44 | F | AA | HS | 1988 | 10.75 | 23 | 1305 | 1.80 | 1 | WT | + |
| NVS16 | 58 | M | AA | HS | 1997 | 8.25 | 24 | 696 | 2.91 | 17 | WT | − |
| NVS17 | 54 | F | AA | IDU | 1995 | 5.75 | 12 | 517 | 1.09 | 69 | WT | − |
| NVS18 | 60 | M | AA | HS | 1994 | 6.16 | 7 | 630 | 0.44 | 34 | WT | − |
| NVS19 | 36 | F | AA | HS | 1995 | 4.84 | 10 | 990 | 2.04 | 14 | WT | − |
| NVS20 | 55 | M | AA | IDU | 1989 | 6.92 | 12 | 459 | 0.59 | 10 | WT | + |
| NVS21 | 55 | M | AA | IDU | 2000 | 7.86 | 9 | 638 | 0.91 | 20 | WT | − |
| NVS22 | 31 | F | AA | HS | 2005 | 2 | 5 | 771 | 1.9 | 11 | WT | ND |
| NVS23 | 49 | M | AA | MSM | 1992 | 11 | 11 | 696 | .63 | 14 | WT | − |
| NVS24 | 50 | F | AA | IDU | 2000 | 6.75 | 12 | 555 | 0.81 | 21 | WT | − |
| NVS23 | 53 | M | AA | IDU | 1997 | 7.75 | 18 | 562 | 0.71 | 63 | WT | − |
| NVS26 | 30 | F | AA | HS | 1995 | 10.25 | 23 | 1210 | 1.34 | 2 | WT | + |
| NVS27 | 35 | F | AA | IDU | 1995 | 8.33 | 11 | 1232 | 0.85 | 4 | ND | − |
| NVS28 | 55 | M | AA | IDU | 2006 | 2 | 5 | 663 | 1.55 | 4 | ND | ND |
| NVS29 | 57 | M | AA | IDU | 1990 | 6 | 18 | 647 | 0.82 | 1 | ND | ND |
| NVS30 | 45 | F | AA | IDU | 2004 | 3.66 | 10 | 307 | .34 | 35 | ND | ND |
| NVS31 | 37 | F | AA | HS | 1996 | 9.92 | 8 | 701 | 1.13 | 11 | ND | ND |
| NVS32 | 44 | F | AA | HS | 1986 | 2.66 | 23 | 825 | .81 | 5 | ND | ND |
| NVS33 | 48 | M | AA | IDU | 1992 | 7.5 | 14 | 471 | .85 | 1 | ND | ND |
| NVS34 | 52 | F | AA | IDU | 1986 | 5.5 | 15 | 799 | 1 | 4 | ND | ND |
| NVS35 | 57 | F | AA | HS | 2004 | 3.66 | 5 | 1373 | 2.71 | 1 | ND | ND |
| NVS36 | 49 | M | AA | IDU | 1994 | 2.42 | 5 | 790 | .81 | 9 | ND | ND |
| NVS37 | 48 | F | AA | IDU | 1991 | 2.58 | 4 | 753 | 1.98 | 1 | ND | ND |
| NVS38 | 45 | M | AA | IDU | 1990 | 6.42 | 4 | 640 | 1.6 | 7 | WT | ND |
| NVS39 | 48 | F | AA | HS | 1991 | 8.16 | 5 | 1929 | 2.29 | 4 | ND | ND |
| NVS40 | 43 | M | AA | MSM | 1990 | 5.16 | 5 | 360 | 0.23 | 118 | WT | + |
| Patient Summary |
Median 50 |
52% M 48% F |
100% AA |
IDU-55% HS-40% MSM-5% |
Median 1994 |
Median 6.67 |
Median 13 |
Median 795 |
Median 1.11 |
Median 9.5 |
4% HT 96% WT |
44% B57+ 56% B57 − |
M=male, F=female, AA= African-American, HS=heterosexual, IDU=injection drug user, MSM=men who have sex with men, WT=wild type, HT= heterozygous, ND= not done
Figure 1.
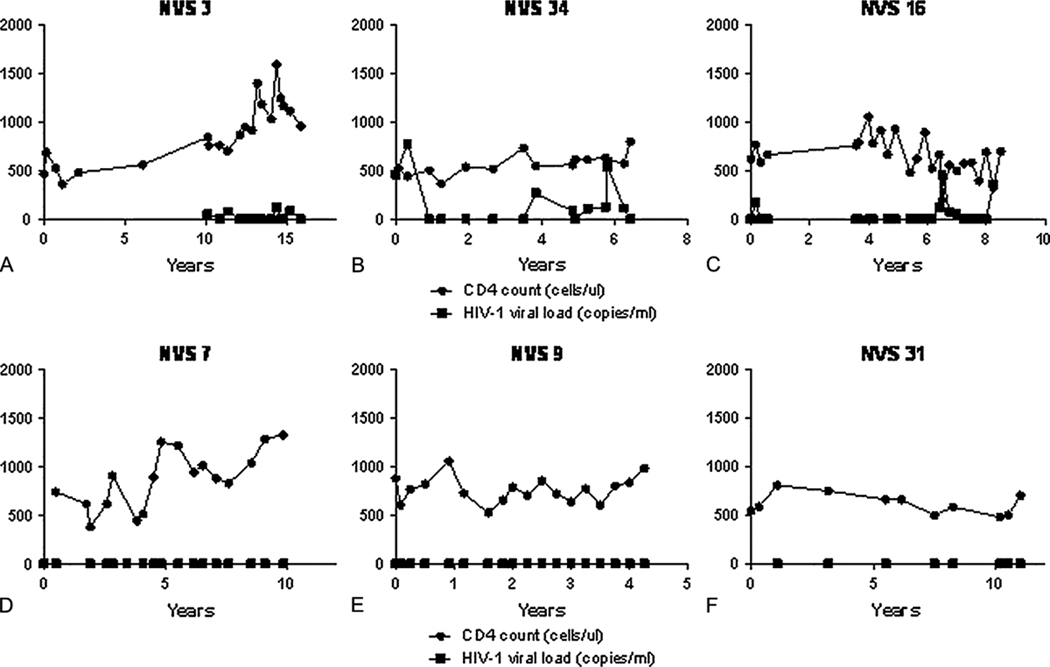
Representative graphs of the different patterns of CD4 slope and viral control seen in the NVS. The Y axis represents CD4 cells/ul and HIV-1 copy number/ml (a value of 0 corresponds to a value below the sensitivity of the assay). As a group the CD4 slope ranged from −66 to + 205 cells/ul per year and CD4 % slope ranged from −3 to +4.44% per year (not shown). Figures A- intermittent detectable viremia in the presence of a rising CD4 slope (+74). Figure B- intermittent detectable viremia in the presence of a stable CD4 slope (−1) Figure C- intermittent detectable viremia in the presence of a falling CD4 slope (−26). Figure D- absence of viremia in the presence of a rising CD4 slope (+75). Figure E- absence of viremia in the presence of a stable CD4 slope (+6). Figure F- absence of viremia in the presence of a falling CD4 slope (−9).
Table 2.
Comparison of NVS and HIV clinic population at the EJC and VA
| EJC + VA | NVS at the EJC + VA | P value* | |
| Median Age | 46 (EJC),51 (VA) | 50 | ND |
| Race | 85% AA, 15% W | 100% AA | p=.007 |
| Sex | 65% M, 35% F | 53% M, 47% F | p=.13 |
| Risk Factor for HIV | 60% IDU, 35% HS, 6% MSM | 59% IDU, 35% HS, 6% MSM | p=.99 |
N=2484 total HIV-1 patients at the EJC clinics. N= 34 NVS at the EJC and VA clinics (there were six other NVS in the study recruited from other clinics, but their demographics were not significantly different from the other NVS and not shown here). AA= african-american, W=white, M=male, F=female, IDU= injection drug use, HS= heterosexual, MSM=men who have sex with men, ND=not done.
P value derived from chi-square or Fisher’s exact test.
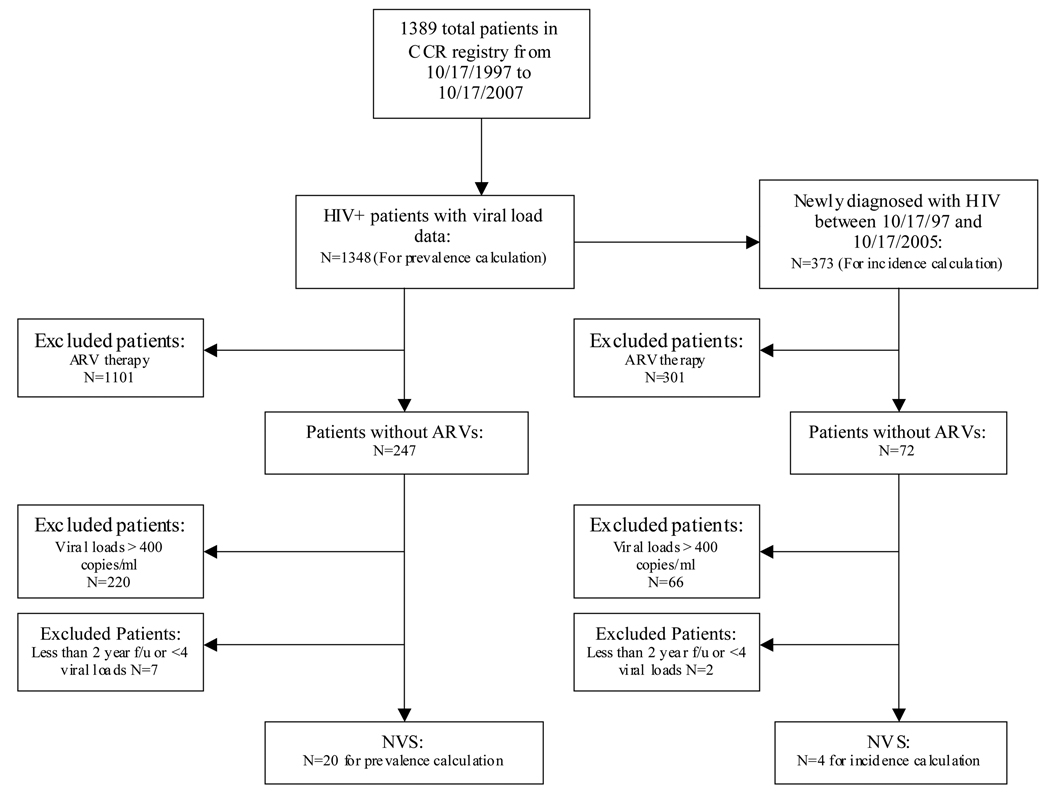
Of 1348 patients identified in the Baltimore VA population in the years 1997–2007, 20 NVS were identified giving a prevalence estimate of 1.5% (95% CI, 0.8–2.1). Thirteen of the 20 NVS identified are part of the larger cohort of 40 NVS followed in this analysis. The calculated incidence of NVS in the cohort of patients who were initially diagnosed with HIV-1 between 1997 and 2005 was 1.1% (95%CI, 0.0–2.1) (4 NVS out of 373 patients). The identification of the NVS at the Baltimore VA for the incidence and prevalence calculations can be seen in the flow chart in Figure 2.
Figure 2.
Flow Chart of NVS cases identified for incidence and prevalence at the Baltimore VA using the CCR database.
For the entire 40-person NVS cohort, in the 184 patient-years since meeting the criteria for NVS, one patient has progressed. This patient, NVS 40, was initially diagnosed in 1990. Approximately one year prior to enrollment in the NVS study, his CD4 count was 1058 cells/ul and viral load was <75 copies/ml. In 2005, upon enrollment into the NVS study and after 5 years of documented natural viral suppression, his viral load was 17,100 copies/ml and his CD4 count was 710 cells/ul. Within three months, his CD4 count dropped to 396 cells/ul and viral load was 19,100 copies/ml. The patient’s viral load rebound and CD4 count decline occurred within one year of relapse in drug use and male prostitution. Two years after this initial increase in viral load and drop in CD4 count, both values had remained stable, with a CD4 count of 360 cells/ul and viral load of 24,100 copies/ml, and he had not been started on antiretrovirals. Sequencing of the proviral DNA from the time after the viral rebound has demonstrated 2 genetically distinct quasi-species of HIV-1 by bootstrap analysis; however, there were no prior samples to confirm this finding.
Figure 3 shows the Kaplan-Meier estimate of the cumulative probability of progression-free survival for the 40-person NVS cohort. Within the first 10 years for follow-up having met the definition of NVS, 95.1% (95% CI 86.5%–100%) of the NVS continued to control their viral loads to undetectable levels. Low grade detectable viremia (>50 copies/ml) in the past two years did occur in 17 of 40 patients in the cohort without significantly being associated with the latest CD4 count, CD4/CD8 ratio, CD4 slope, CD4% slope, or HIV proviral load (data not shown). However, those patients with both a negative CD4 and CD4% slope had a significantly lower absolute CD4 count (p=.004), a lower CD4/CD8 ratio (p=.004), and were more likely to have detectable viremia in the past two years (p=.04).
Figure 3.
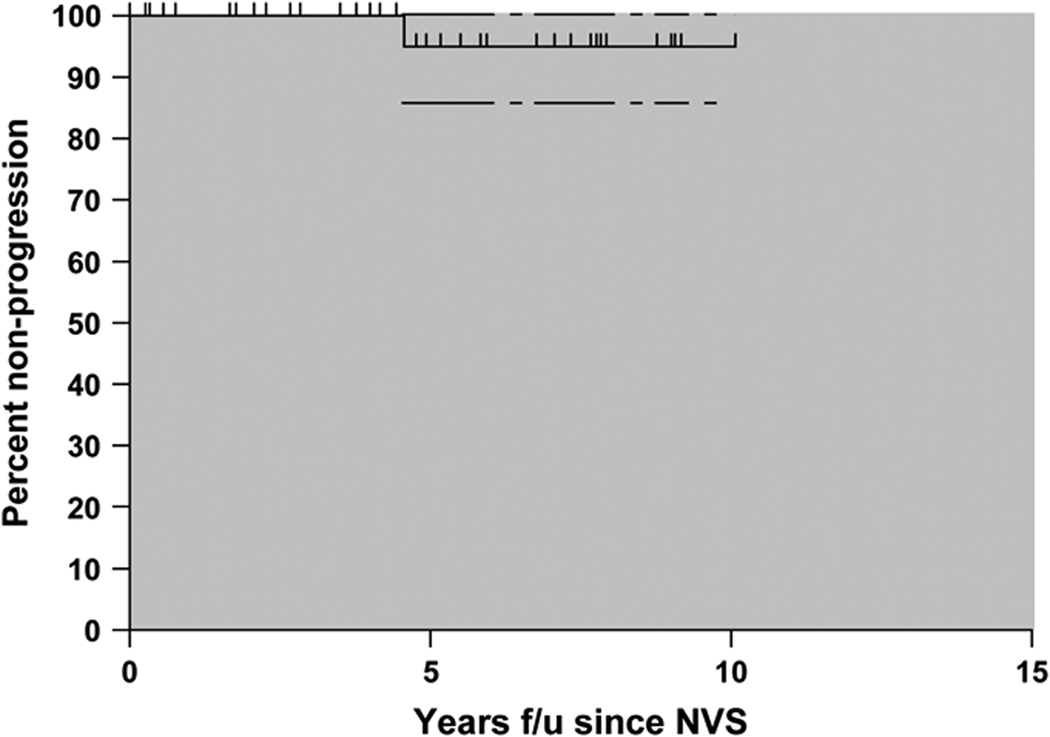
Kaplan Meir curve for progression demonstrating 95.1% (95% CI 86.5%–100%) progression-free status at 10 years from the time of meeting NVS definition. 184 patient-years of follow-up.
DISCUSSION
Natural Viral Suppressors represent individuals who are able to suppress HIV-1 viral replication to extremely low levels.5 During the past several years similar cohorts have been described (referred to as elite suppressors, elite controllers, natural controllers)6–9 ; however, other than two small cases series (4 and 15 patients each),6,7 there has been little focus on the natural history and epidemiologic characteristics of these patients. The presentation of the 40 cases in this report represents the largest epidemiological description of such a cohort to date.
The epidemiological characteristics of the NVS cohort closely mirror that found in our HIV clinic population with one exception. The most notable difference is the racial makeup, where 100% of the persons in the NVS cohort are African American, compared with 85% of the population in the clinics (p=.007). The significance of this finding is unknown and warrants further study.
The 40 individuals in this cohort have been infected with HIV-1 for a median of 14 years and have a median CD4 count of 795 cells/ul. The median duration of viral suppression is over 6 years. As noted in prior studies,8,16 an overrepresentation of persons with the HLAB57 allele is found in the NVS cohort, with 48% seen in the NVS versus 5.7% in African Americans.17 Another known protective factor, the CCR5 heterozygous state, does not appear to be important in this cohort: only 4% of those tested were heterozygous for CCR5 compared to the 2% seen in the African American population.18
We estimate the incidence of NVS to be 1.1% and the prevalence to be 1.5%. These numbers are comparable to the 0.6% prevalence of “HIV Controllers” described by Lambotte et al.7 The lower number in that study is likely due the requirement of 10 years of HIV-1 infection (although they did not exclude survival bias in their calculations). We excluded survival bias by calculating the incidence based on inclusion of only those with a new diagnosis of HIV-1 within an 8 year time period. Survival bias likely accounts for the discrepancy between the 1.1% incidence of NVS and the higher prevalence of 1.5%. Given the high median number of years since diagnosis of HIV-1 this is not surprising.
The use of the CCR database and VA electronic medical records gave strength to our study in identifying NVS by giving us the ability to capture patients’ full clinical profile. We had access to patients outside of the usual clinic setting, and captured patients who were not under routine care (ER visits, substance abuse treatment, etc.). Additionally, for those in the Baltimore CCR database who were also seen at other VA sites, we could then access the broader nationwide VA database. The comprehensiveness of the CCR database allows generalizability of our findings to similar patient populations (African-American with a high percentage of IDUs).
Although we attempted to eliminate potential sources of bias in the incidence and prevalence calculations, our methods were limited by their retrospective nature. Because there were various viral load assays used during this study, we chose a viral load of <400 copies/ml, rather than 50 or 75, so the results of all three assays could be included to increase the number of viral loads for interpretation. The incidence and prevalence results presented may actually be a slight underestimation because at least 4 viral load measurements were required. Finally, although the definition of NVS for the prevalence calculations was slightly different because of the inability to perform an HIV-1 proviral DNA test on those who did not enroll in the study; however, in our experience a second confirmatory HIV-1 test rules out a false positive HIV-1 Western Blot just as well as our HIV-1 proviral DNA test (unpublished data).
All of the patients in the NVS cohort, except for one, showed remarkably stable viral loads. For the NVS cohort, the Kaplan-Meier curve demonstrated a rate of progression (no longer meeting the NVS case definition) of 4.9% from the time of meeting the definition of NVS. One patient out of 40, or 2.5% of the patients has progressed using our definition. Although we could not find any other studies addressing progression in patients similar the NVS, there are some studies in LTNPs that look at progression (no longer meeting LTNP definition). Although the methods, duration of follow-up, and definition of LTNP were different in the 5 studies that addressed this, it is noteworthy that the rates of progression were high in the five studies, ranging from 23% to 50% (an average of 28%).1,19–22
In the NVS cohort, patients who had viral loads between 50–400 copies/ml could not be distinguished in any way from those who remained less than 50 copies/ml; however, those with both a negative CD4% and CD4 slope did have lower CD4 counts, CD4/CD8 ratios, and viral load blips. These data suggest that with enough time, at least some of these individuals could progress because of loss of viral suppression or continued immune depression. One case of the later was recently described in which a patient similar to the NVS had a persistent CD4 decline to less than 200 cells/ul despite continuing viral suppression.12
The progression of NVS 40 is intriguing in several aspects. The loss of viral control occurred 15 years after the initial diagnosis. Interestingly, almost two years after the initial rise in viral load and drop in CD4 to 397 (15%) was noted, his CD4 count and viral load had remained relatively stable at 360 (16 %) and 24,100 copies/ml, respectively. The increase in viral load most likely represented a super-infection, which is supported by the epidemiological history of resumption of high-risk behavior, as well as the isolation of 2 genetically distinct quasispecies of HIV-1. This would imply that that the successful control HIV-1 virus does not necessarily equate with a protective immune response, which is an important point to consider as these cohorts are studied in the hopes of finding immune correlates of protection. Whatever the cause, this case demonstrates the fragile balance between the host and virus, between an immune response and immunity, even in this cohort of individuals which has successfully demonstrated the ability to suppress HIV-1 replication without antiretroviral therapy.
CONCLUSION
In this study, NVS had an incidence of 1.1% and prevalence of 1.5% in an HIV-1 infected population. In all, the NVS cohort demonstrated remarkable stability by exhibiting a low rate of progression over many years. Of the 40 patients in the NVS cohort, one has demonstrated progression 15 years after the initial diagnosis. Detailed evaluations of viral-host immune regulatory factors associated with persistent HIV-1 natural viral suppression, as well as loss of such suppression, has the potential to provide important new insight in HIV pathogenesis and future immune regulatory targeted preventive and therapeutic research.
Acknowledgment
This study was supported by a National Institutes of Health K-12 award.
Disclaimers/Sources of funding: M.M.S supported by an NIH K-12 award.
References
- 1.Sajadi MM, Heredia A, Le N, Constantine N, Redfield R. HIV-1 Natural Viral Suppressors: Control of Viral Replication in the Absence of Therapy. AIDS. 2007 Feb 19;21(4):517–519. doi: 10.1097/QAD.0b013e328013d9eb. [DOI] [PubMed] [Google Scholar]
- 2.Candotti D, Costagliola D, Joberty C, Bonduelle O, Rouzioux C, Autran B, et al. French ALT Study Group. Status of long-term asymptomatic HIV-1 infection correlates with viral load but not with virus replication properties and cell tropism. J Med Virol. 1999 Jul;58(3):256–263. [PubMed] [Google Scholar]
- 3.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen OJ, Demarest JF, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995 Jan 26;332(4):209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995 Jan 26;332(4):201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 5.Ashton LJ, Carr A, Cunningham PH, Roggensack M, McLean K, Law, et al. Australian Long-Term Nonprogressor Study Group. Predictors of progression in long-term nonprogressors. AIDS Res Hum Retroviruses. 1998 Jan 20;14(2):117–121. doi: 10.1089/aid.1998.14.117. [DOI] [PubMed] [Google Scholar]
- 6.Kloosterboer N, Groeneveld P, Jansen C, van der Vorst TJ, Koning F, Winkel CN, et al. Natural controlled HIV infection: preserved HIV-specific immunity despite undetectable replication competent virus. Virology. 2005 Aug 15;339(1):70–80. doi: 10.1016/j.virol.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005 Oct 1;41(7):1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- 8.Pereyra F, Addo MM, Kaufmann DE, Lin Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008 Feb 15;197(4):563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 9.Bailey JR, Lassen KG, Yang HC, Quinn TC, Ray SC, Blankson JN, et al. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006 May;80(10):4758–4770. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J Exp Med. 2006 May 15;203(5):1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei M, Hong K, Huang H, Tang H, Qin G, et al. Biased G-to-A hypermutation in HIV-1 proviral DNA from a long-term non-progressor. AIDS. 2004 Sep 3;18(13):1863–1865. doi: 10.1097/00002030-200409030-00023. [DOI] [PubMed] [Google Scholar]
- 12.Andrade A, Bailey JR, Xu J, Philp FH, Quinn TC, Williams TM, et al. CD4+ T Cell Depletion in an Untreated HIV Type 1–Infected Human Leukocyte Antigen–B*5801–Positive Patient with an Undetectable Viral Load. Clinical Infectious Diseases. 2008;46:e78–e82. doi: 10.1086/529387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blankson JN, Bailey JR, Thayol S, Yang HC, Lassen K, Lai J, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007 Mar;81(5):2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Y, Lai J, Barditch-Crovo P, Gallant JE, Williams TM, Siliciano RF, et al. The role of protective HCP5 and HLA-C associated polymorphisms in the control of HIV-1 replication in a subset of elite suppressors. AIDS. 2008 Feb 19;22(4):541–544. doi: 10.1097/QAD.0b013e3282f470e4. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Paxton W, Kassam N, Ruffing N, Rottman JB, Sullivan N, et al. CCR5 levels and Expression Pattern Correlate with Infectability by Macrophage-tropic HIV-1 In Vitro. J Exp Med 185. 1997 May;9:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori M, Beatty PG, Graves M, Boucher KM, Milford EL. HLA gene and haplotype frequencies in the North American population: the National Marrow Donor Program Donor Registry. Transplantation. 1997 Oct 15;64(7):1017–1027. doi: 10.1097/00007890-199710150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Mann DL, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 19.Vento S, Lanzafame M, Malena M, Tositti G, Cainelli F, Concia E, et al. Can we really identify HIV-1 long-term nonprogressors? J Acquir Immune Defic Syndr. 2004 Sep 1;37(1):1218–1219. doi: 10.1097/01.qai.0000136723.15758.60. [DOI] [PubMed] [Google Scholar]
- 20.Rodes B, Toro C, Paxinos E, Poveda E, Martinez-Padial M, Benito JM, et al. Differences in disease progression in a cohort of long-term non-progressors after more than 16 years of HIV-1 infection. AIDS. 2004 May 21;18(8):1109–1116. doi: 10.1097/00002030-200405210-00004. [DOI] [PubMed] [Google Scholar]
- 21.Garbuglia AR, Salvi R, Di Caro A, Cappiello G, Montella F, Di Sora F, et al. In vitro activation of HIV RNA expression in peripheral blood lymphocytes as a marker to predict the stability of non-progressive status in long-term survivors. AIDS. 1996 Jan;10(1):17–21. doi: 10.1097/00002030-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Birch MR, Learmont JC, Dyer WB, Deacon NJ, Zaunders JJ, Saksena N, et al. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC) J Clin Virol. 2001 Oct;22(3):263–270. doi: 10.1016/s1386-6532(01)00198-6. [DOI] [PubMed] [Google Scholar]