Abstract
Cbl proteins are multifunctional adaptor molecules that modulate cellular activity by targeting the ubiquitylating system, endocytic complexes, and other effectors to a wide variety of regulatory proteins, especially activated receptor and nonreceptor tyrosine kinases. Cbl and Cbl-b perform unique functions in various cells, in addition to redundant functions that are required for embryonic development. We previously showed that eliminating Cbl impaired osteoclast motility, which modestly delayed embryonic bone development. We now report that Cbl-b−/− mice are osteopenic, because of increased bone resorption with little compensating increase in bone formation. In vitro bone-resorbing activity and differentiation of osteoclast-like cells (OCLs) were increased, as were some RANKL-induced signaling events (activation of NF-κB and the mitogen-activated protein kinases extracellular signal-regulated kinase [ERK] and p38), suggesting that specific RANKL-activated mechanisms contribute to the increased rate of differentiation and bone-resorbing activity. Re-expressing Cbl-b in Cbl-b−/− OCLs normalized the increased bone-resorbing activity and overexpressing Cbl-b in wildtype OCLs inhibited bone resorption. Cbl was without effect in either wildtype or Cbl-b−/− OCLs. Functional tyrosine kinase binding (TKB) and RING finger domains were required for the rescue by Cbl-b. Thus, both Cbl and Cbl-b perform regulatory functions in osteoclasts that are unique to one or the other protein (i.e., functions that cannot be compensated by the other homolog). One of Cbl-b's unique functions in osteoclasts is to downregulate bone resorption.
Key words: Cbl-b, osteoclast, osteopenia, bone resorption, differentiation
INTRODUCTION
The integrated function of complex tissues such as bone involves the coordinated regulation of cellular activity by multiple factors. Such regulation requires the dynamic control of the amplitude and duration of cellular responses, accomplished by modulating both the coupling of receptors to signaling effectors and the activity of those downstream effectors. Numerous proteins have been identified that desensitize or otherwise downregulate the signaling by a wide variety of receptors and signaling effectors, among them the Cbl proteins, which regulate signaling from growth factor and cytokine receptors, Src family tyrosine kinases, and associated effector proteins.(1–3)
The Cbl family includes the mammalian Cbl, Cbl-b, and Cbl-3 proteins, but only Cbl and Cbl-b are abundantly expressed in osteoclasts and other hematopoietic cells. Cbl proteins were originally identified as adaptor molecules and later shown to also function as ubiquitin ligases that target the ubiquitylating system to receptor and nonreceptor tyrosine kinases, including growth factor receptors and antigen receptors.(2–6) They also have been reported to promote the internalization of activated growth factor receptors by coupling the receptors to endocytic complexes.(7–9) Both the RING finger domain-dependent ubiquitin ligase activity,(10–13) and the adapter function(2,3) contribute to the ability of the Cbl proteins to downregulate signaling. Although the C-terminal halves of Cbl and Cbl-b are less homologous, several functionally important binding sites are conserved in the two proteins, including proline-rich motifs that bind the SH3 domains of Grb2,(14) Src,(15,16) and CIN85(7–9) and tyrosine residues that form binding sites for SH2 domain-containing proteins when phosphorylated by various tyrosine kinases after the stimulation of a diverse array of cell surface receptors.(2,3) The functional importance of this high degree of homology is shown by the observation that the loss of both genes results in lethality before embryonic day 10.5,(17) indicating that the two proteins have important redundant functions.
In addition to the as yet uncharacterized redundant functions of Cbl and Cbl-b, deletion of either gene alone shows nonredundant functions in various cell types.(17–21) In addition, it has been suggested that Cbl and Cbl-b play different roles in the coupling of RANK to downstream signaling events and in the downregulation of RANK in dendritic cells and 293HEK cells.(22,23) Structural differences that could contribute to these unique functions include a tyrosine present only in Cbl (Cbl Y731) that acts as a docking site for the SH2 domain of the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3K) when phosphorylated(24–26) and sequence differences in the UBA domains at the C terminals that differ in their abilities to bind polyubiquitin chains and ubiquitylated proteins.(27)
We have shown that Cbl acts downstream of Src activation in a pathway that is required for normal bone resorption. In vitro bone resorption by osteoclast-like cells (OCLs) was reduced by Cbl antisense oligonucleotides(28) and by overexpressing Cbl constructs with disabled binding sites for Src(16) and PI3-K.(29) We also showed that Cbl−/− osteoclasts migrate less in vitro and in vivo, leading to a delay in osteoclast invasion and ossification during skeletal development.(30,31) Overall, however, the skeletal phenotype of Cbl−/− mice is mild and not detectable in adult mice.(31)
We report here that Cbl-b−/− mice have a more pronounced and different skeletal phenotype than the Cbl−/− mice, exhibiting a significant decrease in the bone volume with little change in bone formation. In vitro osteoclast differentiation was accelerated and both in vivo and in vitro bone resorption was increased, indicating a cell-autonomous phenotype. Some, but not all, RANKL-induced signaling events were elevated, suggesting that specific RANKL-activated mechanisms might be responsible for the increased rate of differentiation and bone-resorbing activity. Re-expressing Cbl-b in Cbl-b−/− OCLs completely reversed the increased bone-resorbing activity, and overexpressing Cbl-b in wildtype cells strongly inhibited pit formation, whereas Cbl was without effect in either wildtype or Cbl-b−/− OCLs. Thus, both Cbl and Cbl-b contribute to the regulation of osteoclast differentiation, function, and survival in part by performing functions that are unique to one or the other protein (i.e., functions that cannot be compensated by the other homolog). Downregulation of bone resorption is shown here to be a unique function of Cbl-b.
MATERIALS AND METHODS
Chemicals and antibodies
αMEM, DMEM, and FBS were purchased from Sigma (St. Louis, MO, USA). Collagen gel was obtained from Nitta Gelatin (Osaka, Japan). Bacterial collagenase and dispase were purchased from Calbiochem (San Diego, CA, USA). Antibodies against phospho-p38, p38, phospho-Akt, Akt, phospho-c-Jun N-terminal kinase (JNK), JNK, phospho-extracellular signal-regulated kinase (ERK), and IκB-α antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-ERK2, anti-p65, anti-Cbl-b, anti-HA, and anti-Myc antibodies were purchased from Santa Cruz biotechnology (Santa Cruz, CA, USA). Anti-Cbl antibody was purchased from BD Biosciences (San Jose, CA, USA). Anti-actin antibody was purchased from Chemicon (Temecula, CA, USA). TO-PRO-3 was purchased from Molecular Probes (Eugene, OR, USA).
Histology and histomorphometry
Cbl-b−/− mice were generated and identified as previously described.(20) For histological and histomorphometric analysis, 6-wk-old littermates were killed by halothane inhalation. To measure dynamic bone formation parameters, mice were injected with calcein (30 mg/kg body weight) 10 and 3 days before death. Tibias and vertebrae were dissected out, fixed in 3.7% formaldehyde in PBS, and embedded in methylmethacrylate resin as described previously.(32) Sections (5 μm) were deplasticized and stained with 1% toluidine blue or left unstained for the measurements of the fluorochrome labels. Sections of proximal tibia were stained by the von Kossa procedure as described elsewhere.(33) Histomorphometric analysis of the secondary spongiosa was performed using the Osteomeasure system (Osteometrics, Atlanta, GA, USA) using standard procedures(34) to assess changes in bone structure and remodeling. Tibial sections were measured in the proximal metaphysis beginning 340 μm below the chondro-osseous junction, in a region that corresponds to secondary spongiosa. All animal protocols were approved by the Institutional Animal Care and Use Committees of Yale University and Temple University.
Determination of serum collagen telopeptide
Serum was prepared from blood collected by cardiac puncture. Concentrations of C-telopeptide degradation products of type I collagen in serum of 6- to 8-wk-old mice were determined using the Rat Laps ELISA system (Osteometer BioTech, Herlev, Denmark).
Constructs and gene transduction
HA-tagged constructs of wildtype, tyrosine kinase binding (TKB)-disabled (Cbl-bG298E; TKBm), and RING finger-disabled (Cbl-bC373A; RFm) Cbl-b constructs were obtained from Dr. S. Lipkovitz (NIH). Adenovirus vectors carrying the various forms of Cbl-b were constructed as reported previously.(35) The recombinant adenovirus vector carrying wildtype Cbl was constructed as previously described.(29) Infection of OCLs with adenovirus vectors was carried out as previously described.(29,35)
Cells and cell cultures
OCLs were generated by the co-culture method as previously described.(30) Briefly, mouse primary osteoblastic cells were obtained from 1-day-old mouse calvaria by enzymatic digestion, and bone marrow cells were obtained from tibias and femurs of 6- to 8-wk-old WT, Cbl−/−, or Cbl-b−/− mice. Bone marrow cells (1 × 107 cells/dish) were co-cultured with calvarial cells (5 × 105 cells/dish) on 10-cm tissue culture dishes or collagen gel–coated dishes in the presence of 10 nM 1,25-dihydroxy vitamin D3 and 1 μM prostaglandin E2 (Sigma). For biochemical assays, OCLs were purified as reported previously.(30) For differentiation experiments, bone marrow cells suspended in αMEM with 10% FBS were plated on tissue culture plastic to remove the adherent stromal cells. Nonadherent marrow cells were cultured in αMEM with 10% FBS and 20 ng/ml macrophage-colony stimulating factor (M-CSF; R&D Systems, Minneapolis, MN, USA) for 2 days. Cells were further cultured in the presence of 100 ng/ml RANKL (Peprotech, Rocky Hill, NJ, USA) and 20 ng/ml M-CSF for an additional 6 days.
RT-PCR analysis
The levels of osteoclast markers were analyzed by semiquantitative RT-PCR. Total RNA was isolated from OCL cultures at various times using RNeasy mini kit (Qiagen, Valencia, CA, USA). One microgram of total cellular RNA from each sample was reverse transcribed to cDNAs using SuperScript II (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The cDNA samples were equally divided, and cDNAs that encoded nuclear factor of activated T cells 1 (NFATc1), c-fos, matrix metalloproteinase 9 (MMP9), TRACP, calcitonin receptor (CalcR), and GAPDH were amplified by PCR using the following murine-specific primers—CalcR sense: 5′-AGCCACAGCCTATCAGCACT3′, antisense: 5′GACCCACAAGAGCCAGGTAA-3′; CTSK sense: 5′-CAGCTTCCCCAAGATGTGAT-3′, antisense: 5′-AAAAATGCCCTGTTGTGTCC-3′; c-fos sense: 5′-CCAGTCAAGAGCATCAGCAA-3′, antisense: 5′-AAGTAGTGCAGCCCGGAGTA-3′; GAPDH sense: 5′-TGTCTTCACCACCATGGAGAAG-3′, antisense: 5′-GTGGATGCAGGGATGATGTTCTG-3′; MMP9 sense: 5′-TGAATCAGCTGGCTTTTGTG-3′, antisense: 5′-GTGGATAGCTCGGTGGTGTT-3′; NFATc1 sense: 5′-GCCCACTGGATCAAAACACT-3′, antisense: 5′-TAGGGCAGCCAGAAAAGCTA-3′; PU.1 sense: 5′-GGCAGCAAGAAAAAGATTCG-3′; antisense: 5′-TTTCTTCACCTCGCCTGTCT-3′; RANK sense: 5′-AAACCTTGGACCAACTGCAC-3′, antisense: 5′-TCATTGACCCAATTCCACAA-3′; Src sense: 5′-GAAGGCAGGCACCAAACTCAGC-3′, antisense: 5′-CCCCGGTAATCCCCCAGCATCA-3′; TRACP sense: 5′-TCCTGGCTCAAAAAGCAGTT-3′, antisense: 5′-ACATAGCCCACACCGTTCTC-3′.
Immunofluorescence microscopy
Cells were plated on sterile FBS-coated glass coverslips. OCLs were fixed in PBS containing 3.7% formaldehyde for 10 min and permeabilized with ice-cold acetone for 5 min. Coverslips for actin labeling were incubated in a 1:40 dilution (in PBS) of rhodamine phalloidin stock solution (Molecular Probes) for 20 min. Cells were sequentially stained with appropriate primary antibody followed by fluorescein-conjugated secondary antibody as previously reported.(30) Cells were examined using a confocal imaging system (Zeiss LSM 510 Meta System).
Bone resorption assay
In vitro bone-resorbing activity was assayed as previously described.(35) Briefly, OCLs were generated in co-culture as described above in plates coated with collagen gel. Collagen was removed by gentle digestion with 0.1% collagenase, and cells were seeded onto sterile dentine slices in 96-well plates. Forty-eight hours later, slices were immersed in 1 M ammonium hydroxide for 5 min, sonicated for 10 s, stained for 4 min with 1% toluidine blue/1% sodium borate (Sigma), and briefly washed in water. Some pit assays were performed for 48 h in the presence of 100 ng/ml RANKL (Peprotech, Rocky Hill, NJ, USA). Pit area was quantified with the Osteomeasure system and was normalized to the number of osteoclasts actually present in each sample, determined by counting OCLs present in a separate aliquot of OCL suspension.
Western blot analysis
Typically, 30–40 μg of total cell lysate protein was electrophoresed on 4–12% NuPAGE gels (Invitrogen). Proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Waltham, MA, USA). Transferred proteins were visualized by staining the membrane with 0.2% Ponceau S in 3% trichloroacetic acid (Sigma). To block nonspecific binding, the membranes were incubated for 2 h at room temperature in 5% milk, TBST buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20). Antigens were visualized by immunoblotting with the appropriate primary antibody (1:1000 dilution), followed by a horseradish peroxidase–conjugated anti-mouse IgG or anti-rabbit IgG antibody. All blots were developed using enhanced chemiluminescence reagents from Amersham Pharmacia Biotech (Piscataway, NJ, USA). To quantify changes in phosphorylation, membranes blotted with phospho-specific antibodies were stripped and reprobed with antibodies against the protein. Densitometry of the phosphorylated and total protein bands was performed using the Scion Image 1.62 program. The values for the phosphorylated band were normalized to the density of the corresponding total protein band in the reprobed blot.
In vitro Src kinase assay
Src was immunoprecipitated, and the kinase activity was assayed and normalized to the amount of Src protein in the immune complexes as previously described.(30)
Statistical analysis
Each experiment was repeated at least three times. The results obtained from a typical experiment were expressed as the means ± SD. Significant differences were determined using ANOVA. p < 0.05 was considered significant.
RESULTS
Cbl-b−/− mice are osteopenic because of increased bone resorption
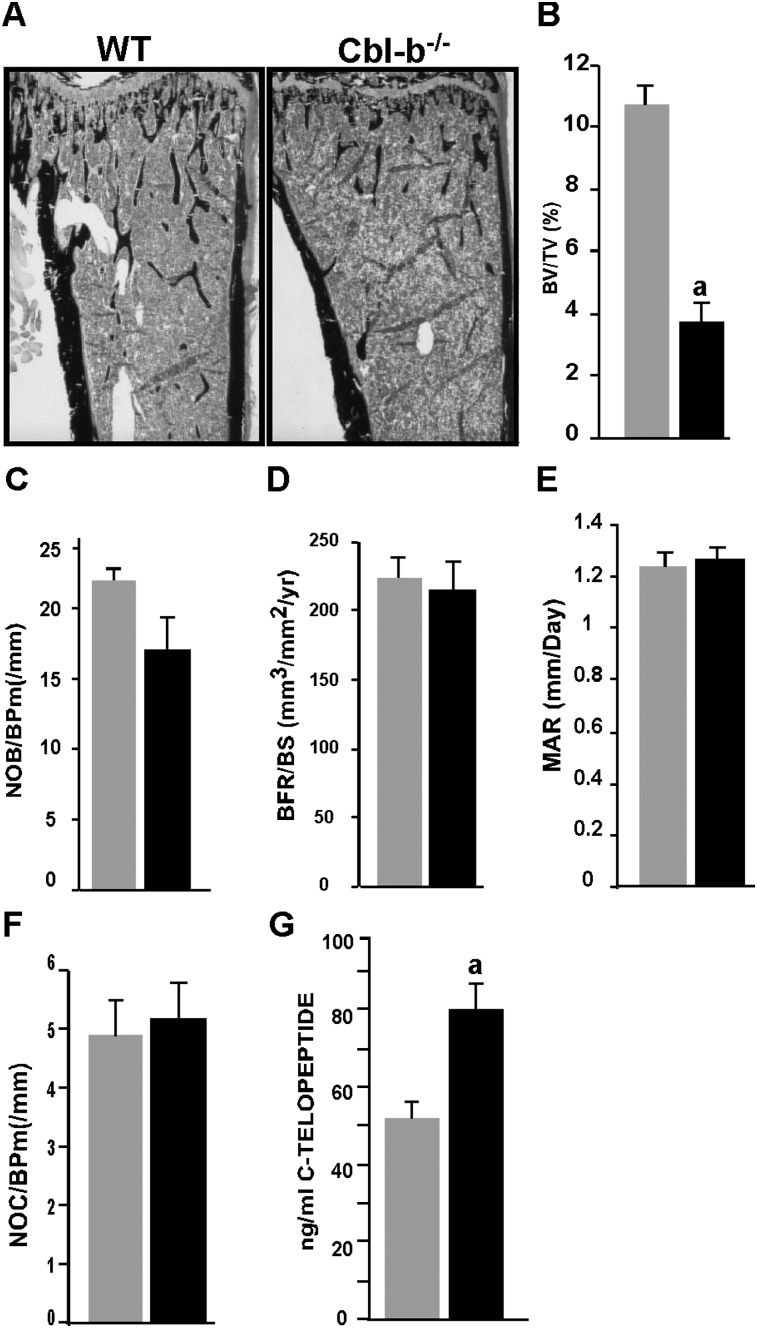
Histological examination of tibias and vertebrae from 6-wk-old Cbl-b−/− mice showed that the amount of cancellous bone was decreased relative to the age-matched control samples (Fig. 1A and data not shown). Histomorphometric analysis (Fig. 1; Table 1) showed that bone volume in the Cbl-b−/− mice was less than one half the bone volume in the control mice (Fig. 1B). Trabecular number was decreased and trabecular separation was increased in the Cbl-b−/− bones. No difference was observed in the cortical bone thickness. There were no significant differences in either static bone formation parameters (osteoblast surface and number, osteoid surface, and thickness; Fig. 1C; Table 1) or dynamic bone formation parameters (mineral apposition rate and bone formation rate; Figs. 1D and 1E). Thus, trabecular bone mass was decreased, despite an unaltered bone formation rate. The number of osteoclasts was not significantly different (Fig. 1F; Table 1), but the serum level of C-terminal collagen telopeptide, a specific product of bone degradation by osteoclasts, was increased by 44% in the Cbl-b−/− mice (Fig. 1G), indicating that the bone-resorbing activity of the Cbl-b−/− osteoclasts was increased. Cumulatively, the analysis of the Cbl-b−/− mice suggested that the absence of Cbl-b results in osteopenia caused primarily by the hyperactivity of individual osteoclasts.
FIG. 1.
Cbl-b−/− mice are osteopenic. (A) Photomicrographs of von Kossa--stained sagittal sections of undecalcified proximal tibias from 6-wk-old control (left) and Cbl-b−/− (right) mice. The bone in the secondary spongiosa of the metaphysis of the Cbl-b−/− mice was markedly decreased. (B–F) Histomorphometric analysis of the cancellous region of the tibial metaphysis of the 6-wk-old control and Cbl-b−/− mice. (B) Trabecular bone volume expressed as the percentage of tissue volume (BV/TV). (C) Osteoblast number per unit bone surface (NOb/BPm). (D) Bone formation rate (BFR/BS). (E) Mineral apposition rate (MAR). (F) Osteoclast number per unit bone surface (NOc/BPm). (G) Serum collagen telopeptide concentrations. In the Cbl-b−/− mice, the trabecular bone volume was significantly decreased. Bone formation parameters (number of osteoblasts, mineral apposition rate, and bone formation rate) were not significantly different. The number of osteoclasts was not changed. Serum concentrations of collagen telopeptide, determined in serum by radioimmunoassay, increased. Data are presented as mean ± SE (n = 6). a p < 0.01 compared with control mice.
Table 1.
Static and Dynamic Histomorphometry of 6-wk-old WT and Cbl-b−/− mice
| WT L4 | Cbl-b−/− L4 | WT tibias | Cbl-b−/− tibias | |
| BV/TV (%) | 21.9 ± 2.1 | 15.9 ± 2.8* | 11.0 ± 1.5 | 3.8 ± 1.2† |
| TbTh (μm) | 30.9 ± 4.0 | 32.8 ± 5.5 | 26.6 ± 3.5 | 21.0 ± 3.3 |
| TbN (no./mm) | 7.3 ± 0.7 | 4.9 ± 0.4 | 4.2 ± 0.8 | 1.8 ± 0.3† |
| OV/BV (%) | 0.3 ± 0.2 | 0.4 ± 0.3 | 1.4 ± 0.5 | 1.1 ± 0.4 |
| ObS/BS (%) | 22.4 ± 7.5 | 22.3 ± 7.6 | 26.1 ± 1.4 | 19.2 ± 4.2 |
| OcS/BS (%) | 6.8 ± 1.4 | 7.3 ± 2.6 | 16.4 ± 1.5 | 16.2 ± 2.7 |
| NOb/BPm (/mm) | 18.9 ± 6.7 | 18.2 ± 5.5 | 22.6 ± 2.0 | 17.0 ± 3.8 |
| NOc/BPm (/mm) | 2.5 ± 0.5 | 2.4 ± 0.9 | 4.8 ± 0.5 | 5.3 ± 1.2 |
| MS/BS (%) | 55.9 ± 9.4 | 52.3 ± 7.5 | 62.9 ± 2.0 | 55.8 ± 3.1 |
| MAR (μm/d) | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.6 ± 0.2 | 1.6 ± 0.0 |
| BFR/BS (μm3/μm2/d) | 209.1 ± 39.6 | 205.5 ± 43.8 | 355.4 ± 24.5 | 328.8 ± 23.2 |
Histomorphometric analysis was performed on L4 vertebrae and tibias. Values are mean ± SE; n = 6.
* p < 0.01 vs. WT.
† p < 0.001 vs. WT.
BV, bone volume; TV, total volume; TbTh, trabecular thickness; TbN, trabecular number; OV, osteoid volume; BS, bone surface; ObS osteoblast surface; OcS, osteoclast surface; NOb, number of osteoblasts; NOc, number of osteoclasts; BPm, bone perimeter; MS, mineralizing surface; MAR, mineral apposition rate; BFR, bone formation rate.
In vitro differentiation and bone-resorbing activity of Cbl-b−/− OCLs is increased
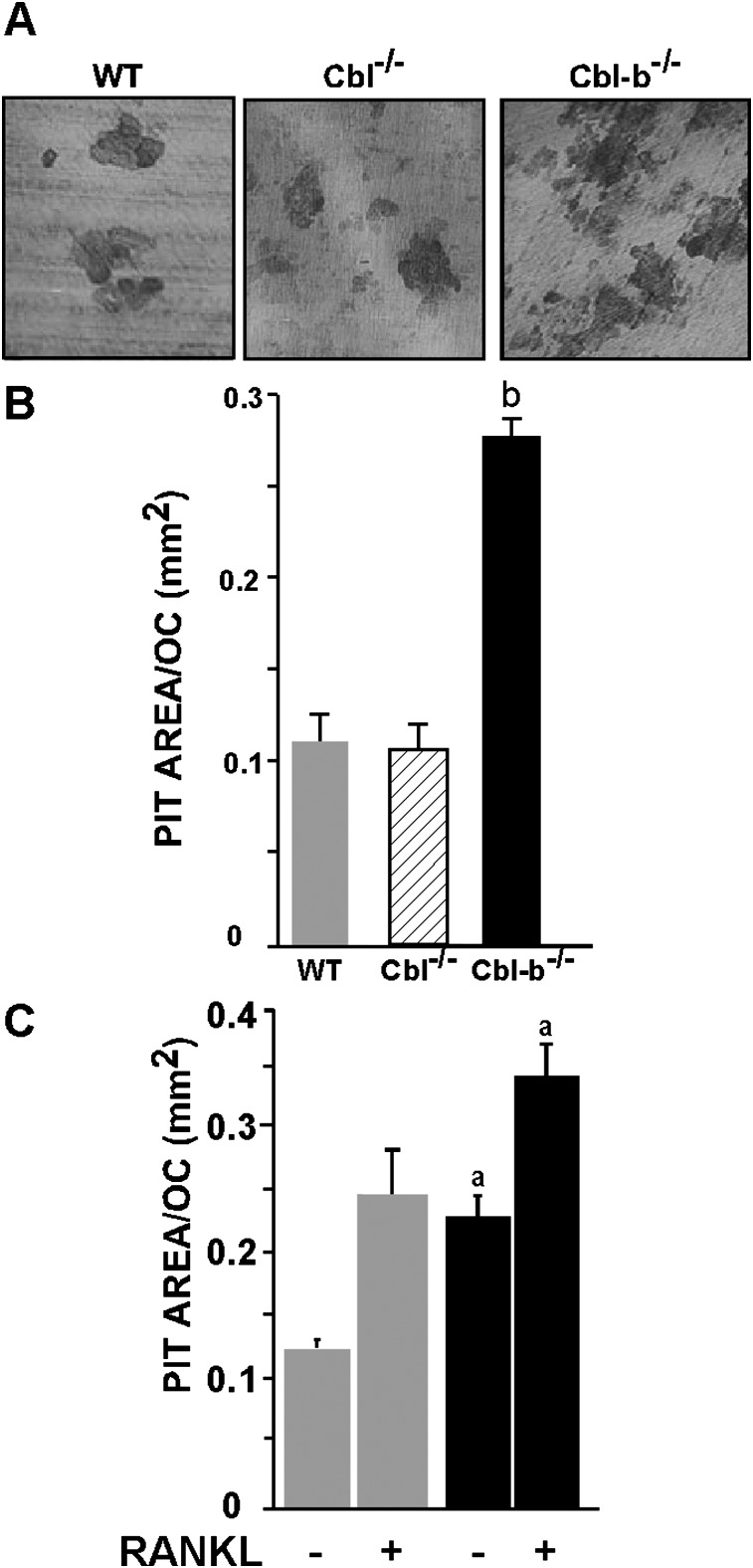
To determine whether the bone-resorbing activity of individual Cbl-b−/− osteoclasts was indeed increased, we quantified in vitro osteoclast bone resorption. Cbl-b−/− and wildtype OCLs were generated, a portion of the crude OCL preparation was cultured on dentine slices for 48 h, and the resorbed area was quantified and normalized to the number of OCLs as described in the Materials and Methods section. The Cbl-b−/− OCLs resorbed 2.5-fold more dentin surface area/cell than the wildtype OCLs (Figs. 2A and 2B), providing additional evidence that the absence of Cbl-b leads to an increased intrinsic bone-resorbing activity. In contrast, the areas resorbed by the Cbl−/− and wildtype OCLs were similar. Treatment with RANKL, which stimulates the bone-resorbing activity of mature osteoclasts,(36,37) increased the bone-resorbing activity of wildtype OCLs to the level of untreated Cbl-b−/− OCLs, as expected, and further increased the bone-resorbing activity of the Cbl-b−/− OCLs (Fig. 2C).
FIG. 2.
Cbl-b−/− osteoclasts resorb more bone that WT and Cbl−/− osteoclasts. Dentine-resorbing activity of WT, Cbl−/−, and Cbl-b−/− OCLs was determined. (A) Photomicrographs of the resorption pits. (B) Quantification of resorbed area on the dentine slices after 48 h, normalized for the number of OCLs. b p < 0.001 compared with WT OCLs. (C) Response of WT and Cbl-b−/− OCLs to RANKL (100 ng/ml). a p < 0.05 compared with WT OCLs. Data are presented as mean ± SD (n = 9).
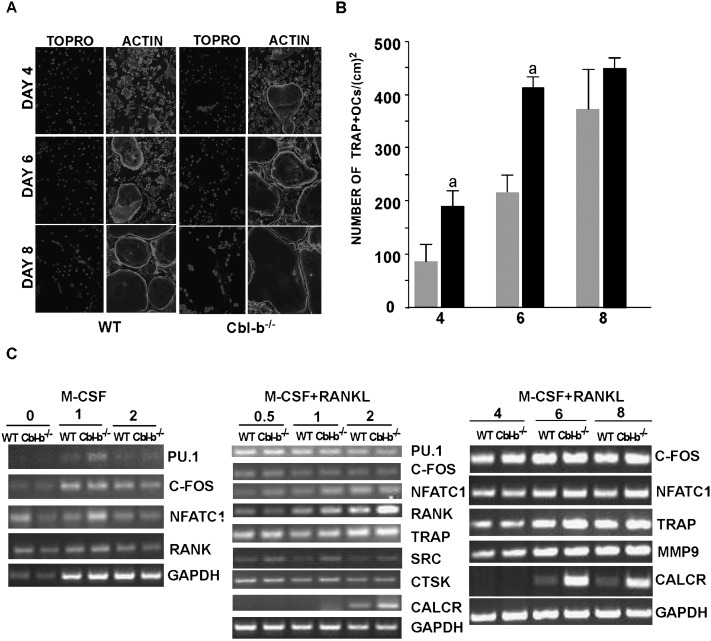
To analyze how the absence of Cbl-b affects osteoclast formation, bone marrow osteoclast precursor cells from control and Cbl-b−/− mice were induced to differentiate by culturing them with M-CSF and RANKL as described in the Materials and Methods section, and the numbers of TRACP+ MNCs present at 2, 4, and 6 days after introducing RANKL (4, 6, and 8 days after the initial plating) were determined. TRACP+ MNCs with typical peripheral podosome belts appeared in the Cbl-b−/− bone marrow cultures at days 4 and 5 of culture compared with the appearance at 6 days in WT cultures, indicating that the rate of osteoclast maturation was increased, although the total number of osteoclasts present in the cultures at day 8 were not significantly different (Figs. 3A and 3B). Similar results were obtained when spleen was used as a source of hematopoietic precursor cells and when the bone marrow precursor cells were co-cultured with wildtype osteoblasts in the presence of vitamin D3 and prostaglandin E2 (data not shown).
FIG. 3.
The generation of mature multinucleated osteoclasts in cultures of bone marrow cells derived from Cbl-b−/− mice is accelerated. Bone marrow cells were cultured for 2 days with M-CSF and for an additional 6 days with M-CSF and RANKL. (A) At days 4, 6, and 8 after the initial plating of the cells (i.e., 2, 4, and 6 days after the addition of RANKL), OCLs were stained for F-actin using rhodamine phalloidin. To determine multinucleation, nuclei were stained with TO-PRO-3. Multinucleated cells with actin rings were observed 2 days earlier in the Cbl-b−/− cultures. (B) The numbers of TRACP+ MNCs in the Cbl-b−/− cultures (black bars) were significantly greater than in WT cultures (gray bars) at days 4 and 6. Data are presented as mean ± SD (n = 3). a p < 0.05. (C) The expression of osteoclast markers (PU.1, c-fos, NFATc1, RANK, TRACP, Src, cathepsin K [CTSK] matrix metalloproteinase 9 [MMP9], and calcitonin receptor [Calt R]; GAPDH as input control) were measured at various times during the 2 days of incubation in M-CSF (left) and the 6 days of incubation with M-CSF + RANKL (middle and right). Slight transient increases in PU.1 and NFATc1 occurred in the Cbl-b−/− cells after 1 day of culture with M-CSF. NFATc1, RANK, TRACP Src, and calcitonin receptor (CalcR) mRNAs were somewhat increased in the Cbl-b−/− cells at the earlier times of incubation with M-CSF + RANKL, whereas only the CalcR was increased in the Cbl-b−/− OCLs at days 4 and 6. (The appearance of CalcR mRNA at day 2 in the middle panel but not in the right panel is because of differences in exposure of the films.)
The expression of marker genes by osteoclast precursors and differentiating OCLs treated with M-CSF or M-CSF plus RANKL was determined by PCR (Fig. 3C). Bone marrow cells from Cbl-b−/− mice and control littermates were cultured for 24 or 48 h with M-CSF as described in the Materials and Methods section, and the levels of mRNAs encoding PU.1, c-fos, NFATc1, and RANK were determined. Slight transient increases in PU.1 and NFATc1 mRNAs, which promote osteoclast differentiation,(38,39) occurred in the Cbl-b−/− cells at 24 h. There was little difference in c-fos and RANK mRNAs.
After 2 days of treatment with M-CSF, the cultures were continued with both M-CSF and RANKL present, and marker mRNAs (PU.1, c-fos, NFATc1, RANK, TRACP, calcitonin receptor, cathepsin K, MMP9, and Src) were analyzed at 0.5, 1, 2, 3, and 4 days after introducing RANKL. NFATc1, RANK, TRACP Src, and the calcitonin receptor (CalcR) mRNAs were somewhat increased in the Cbl-b−/− cells at the earlier times, whereas little or no difference was seen in PU.1, c-fos, and cathepsin K. At 72 and 96 h, only the CalcR was increased in the Cbl-b−/− OCLs.
Some RANKL-induced signaling pathways are upregulated in Cbl-b−/− OCLs
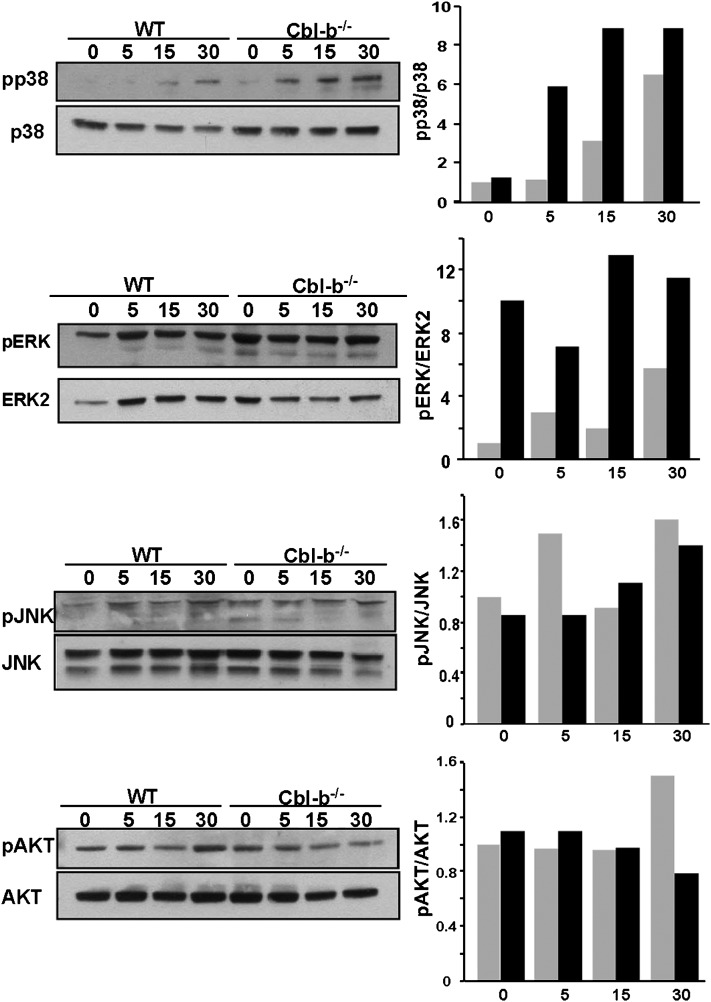
Because osteoclastogenesis is critically dependent on the activation of RANK by RANKL,(40) and Cbl proteins have been reported to be phosphorylated downstream of activated RANK(22) and to modulate RANK expression and signaling,(23) the increased rate of OCL differentiation in the presence of RANKL suggested that RANKL-induced signaling might be increased in the Cbl-b−/− OCLs. Stimulation of RANK leads to the activation of multiple signaling protein kinases including IKK, JNK, p38, ERK, and Src and activates the NF-κB transcription factor.(41,42) We therefore compared the RANKL-induced activities of these signaling effectors in WT and Cbl-b−/− OCLs. Western blot analysis (Fig. 4) showed that ERK phosphorylation was higher in Cbl-b−/− OCLs than in WT cells, both before and after stimulation with RANKL. Similarly, RANKL-induced p38 phosphorylation was significantly increased in the Cbl-b−/− OCLs relative to the WT counterparts, and the elevated levels in the Cbl-b−/− OCLs were sustained as long as 30 min. In contrast, and consistent with previously published studies,(23) RANK-mediated AKT activation was not significantly different in Cbl-b−/− OCLs, and no changes were seen in JNK activation. Src activity, like Akt and JNK activity, was not affected by the absence of Cbl-b (data not shown).
FIG. 4.
The activation of some RANKL-induced signaling pathways is increased in Cbl-b−/− osteoclasts. WT and Cbl-b−/− OCLs were serum-starved for 30 min and treated with RANKL (1 μg/ml) for 0, 5, 15, or 30 min. Cell lysates were processed for Western blot analysis. After blotting with the indicated phospho-specific antibodies (top panels), the membranes were stripped and reprobed with antibodies to the respective proteins to determine loading (bottom). To correct for experimental variability in the amounts of the total proteins in the individual lanes, bands were quantified by scanning the blots using Scion Image 1.62 C program, and the ratios of the phosphorylated protein to the total protein calculated (WT, gray bars; Cbl-b−/−, black bars). A representative experiment of at least three repetitions is shown.
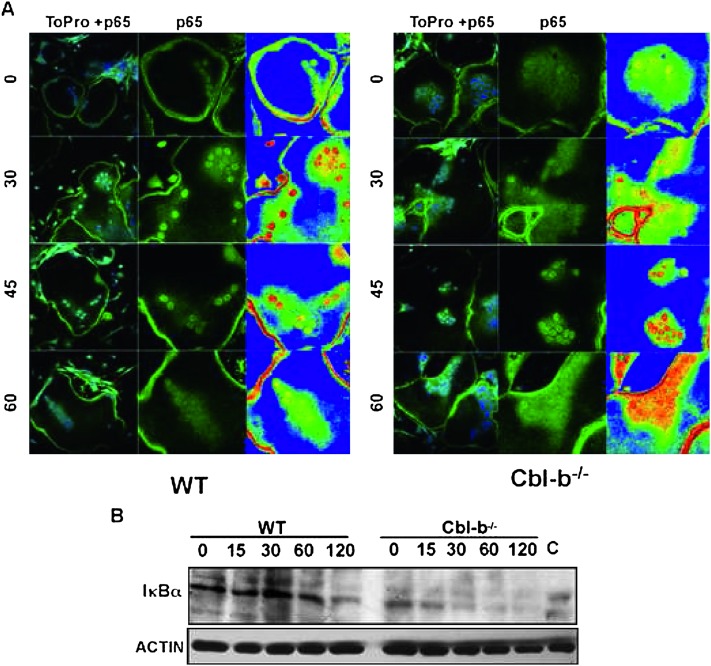
NF-κB is maintained in an inactive state in the cytosol in a complex with the inhibitory IκB proteins. Activation of RANK promotes the degradation of IκB-α and the consequent release and nuclear translocation of active NF-κB. The localization of NF-κB in WT and Cbl-b−/− OCLs treated with RANKL for different times was examined by immunofluorescence microscopy using specific antibodies against the p65 subunit of NF-κB. Under basal conditions, p65 was diffusely localized in the cytosol in both control and Cbl-b−/− OCLs. After 30 min of RANKL stimulation, p65 was detected in the nuclei of most cells in both control and Cbl-b−/− OCLs. By 60 min, the nuclear staining of p65 in the control had returned to pretreatment levels, whereas Cbl-b−/− OCLs continued to exhibit sustained nuclear immunostaining of p65 (Fig. 5A). Interestingly, the levels of IκB-α were significantly lower in Cbl-b−/− OCLs than in control cells, even before the initiation of the time course (Fig. 5B), which might contribute to the prolonged nuclear localization of p65. Thus, the absence of Cbl-b resulted in increased basal and RANKL-induced activity of some but not all signaling events downstream of RANK.
FIG. 5.
NF-κB activity is significantly altered in Cbl-b−/− OCLs. (A) Translocation of p65, a subunit of NF-κB, into the nuclei of OCLs in response to RANKL is prolonged. WT and Cbl-b−/− OCLs were treated with RANKL (1 μg/ml) for the indicated periods of time. Cells were fixed and stained for p65 and the subcellular localization of p65 was determined by fluorescence microscopy. Nuclei were stained with TO-PRO-3. Before stimulation, p65 was localized in the cytoplasm, especially around the nucleus. After 30 min of RANKL stimulation, most of the p65 accumulated in the nuclei in both WT and Cbl-b−/− OCLs. By 60 min after adding RANKL, nuclear p65 disappeared from WT OCLs, in contrast to the sustained nuclear localization of p65 observed in Cbl-b−/− OCLs. Pseudocolorization as a function of p65 staining intensity (right) was performed using the Zeiss system software. (B) Levels of IκB-α are reduced in Cbl-b−/− OCLs. WT and Cbl-b−/− OCLs were treated with RANKL (1 μg/ml) for the indicated time periods. Cell lysates were processed for Western blot analysis, and the levels of IκBα were determined by Western blot (top). Equal loading of protein was verified by stripping and reprobing the membrane with anti-actin antibodies. Platelet lysate was used as a positive control for IκB-α (C).
Downregulation of bone resorption by Cbl-b requires both the TKB and the RING finger domains
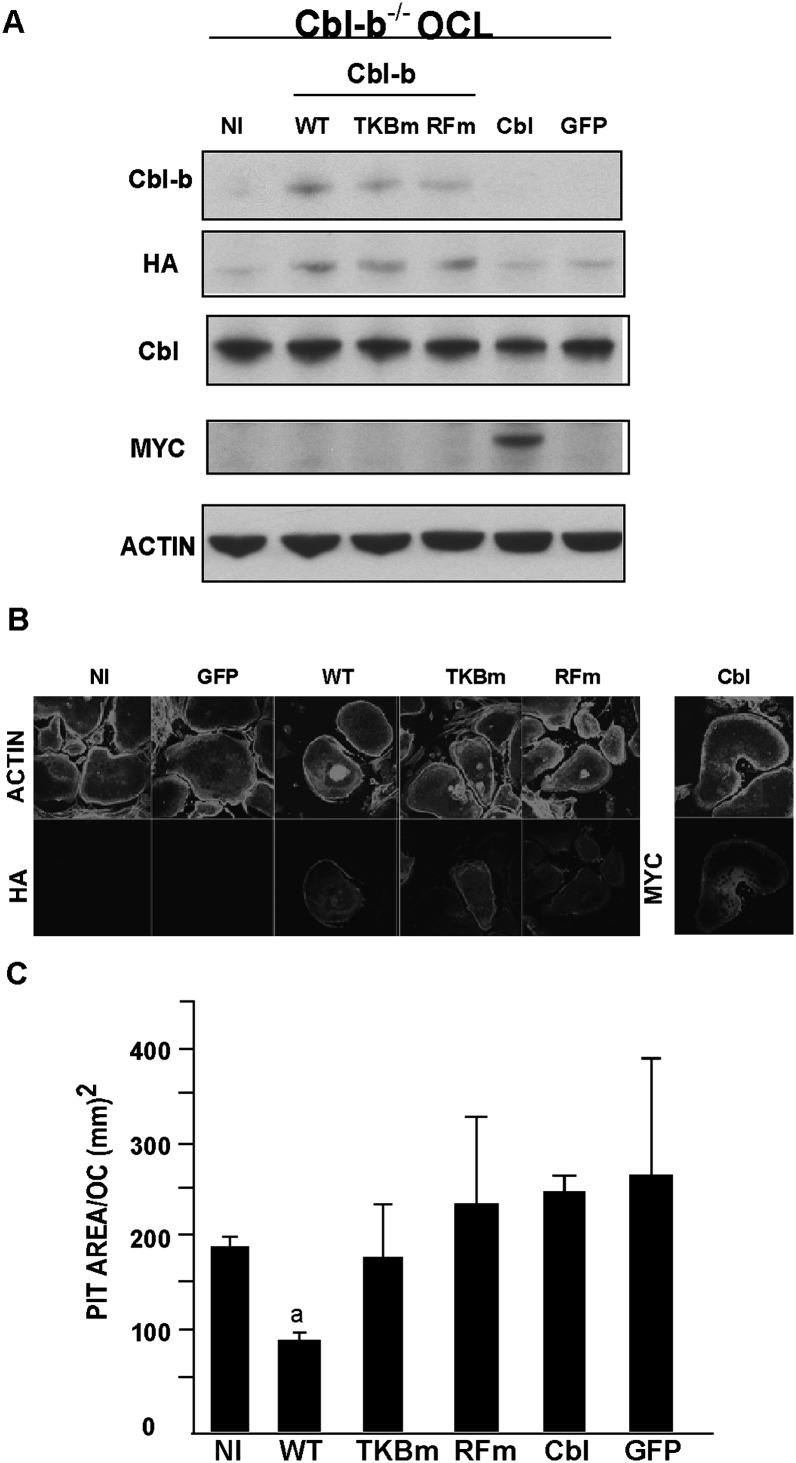
To confirm that the change in osteoclast bone-resorbing activity of Cbl-b−/− OCLs was caused by the absence of Cbl-b, we re-expressed wildtype Cbl-b in the Cbl-b−/− OCLs using an adenovirus encoding HA-tagged wildtype Cbl-b. Three days after infection, exogenous Cbl-b expression was confirmed by Western blot analysis using anti-HA antibodies or anti-Cbl-b antibodies (Fig. 6A). Infection with the adenovirus had no effect on the formation of the peripheral podosome belts (Fig. 6B).
FIG. 6.
Re-expression of Cbl-b restores bone-resorbing activity of Cbl-b−/− OCLs to normal. (A) Cbl-b−/− OCLs were infected at a multiplicity of infection (m.o.i.) of 50 with adenovirus vector encoding wildtype Cbl-b (WT), mutant Cbl-b proteins (TKB domain mutant TKBm; and RING finger domain mutant RFm), Cbl, or green fluorescence protein (GFP; NI, not infected). The expression of exogenous HA-tagged Cbl-b proteins in total cell lysates was analyzed by Western blot with anti-HA antibodies, the membrane was stripped and reprobed with anti-Cbl-b antibodies. Expression of exogenous Cbl protein was evaluated by Western blot with anti-Myc antibodies. The blot was stripped and reprobed with anti-Cbl antibodies to determine the levels of endogenous Cbl protein. To verify equal loading, the blot was stripped and reprobed with anti-actin antibodies. (B) Immunofluorescence of Cbl-b−/− OCLs expressing Cbl-b and Cbl proteins. Double immunofluoresence of F-actin and HA-tagged Cbl-b proteins or myc-tagged Cbl proteins at an m.o.i. of 50. (C) Bone-resorbing activity of Cbl-b−/− OCLs infected with adenovirus encoding wildtype Cbl-b, Cbl-b mutants, or Cbl. OCLs generated in co-culture were transferred onto dentine and further cultured for 48 h. Data are presented as mean ± SD (n = 9). a p < 0.01 compared with uninfected (NI) OCLs.
The re-expression of Cbl-b in Cbl-b−/− OCLs reduced bone-resorbing activity by about one half to a level comparable to that of wildtype OCLs (Fig. 6C). Expression of recombinant Cbl in Cbl-b−/− OCLs had no effect on their pit-forming activity, further showing that Cbl is not able to compensate for the absence of Cbl-b in downregulating bone resorption and indicating that, whereas the two proteins have some redundant functions in osteoclasts, including the microtubule-dependent organization of the podosome belt,(43) they also have distinct roles in osteoclast biology. Specifically, Cbl-b seems to downregulate bone resorption, at least in part by modulating signaling mechanisms downstream of RANK.
Cbl proteins downregulate the signaling activity of a variety of receptors by binding to phosphotyrosines on the activated receptors and associated signaling effectors through the Cbl proteins' TKB domain and subsequently ubiquitylating the targeted proteins through a mechanism that requires the Cbl RING finger domain.(2,3) We therefore determined whether the TKB and/or RING finger domains of Cbl-b were required to reduce bone-resorbing activity to normal. Cbl-b−/− OCLs were infected with adenoviruses encoding Cbl-b proteins with disabling mutations within the TKB domain (Cbl-b G298E; TKBm) or the RING finger domain (Cbl-b C372A; RFm). The mutated Cbl-b proteins were appropriately expressed in the infected OCLs (Figs. 6A and 6B). However, in contrast to the effect of wildtype Cbl-b, neither Cbl-b mutant reduced the bone-resorbing activity of the Cbl-b−/− OCLs to normal levels (Fig. 6C), indicating that both the TKB and the RING finger domains of Cbl-b are required for the normal regulation of osteoclastic bone resorption by Cbl-b.
Overexpression of Cbl-b in wildtype osteoclasts inhibits bone resorption
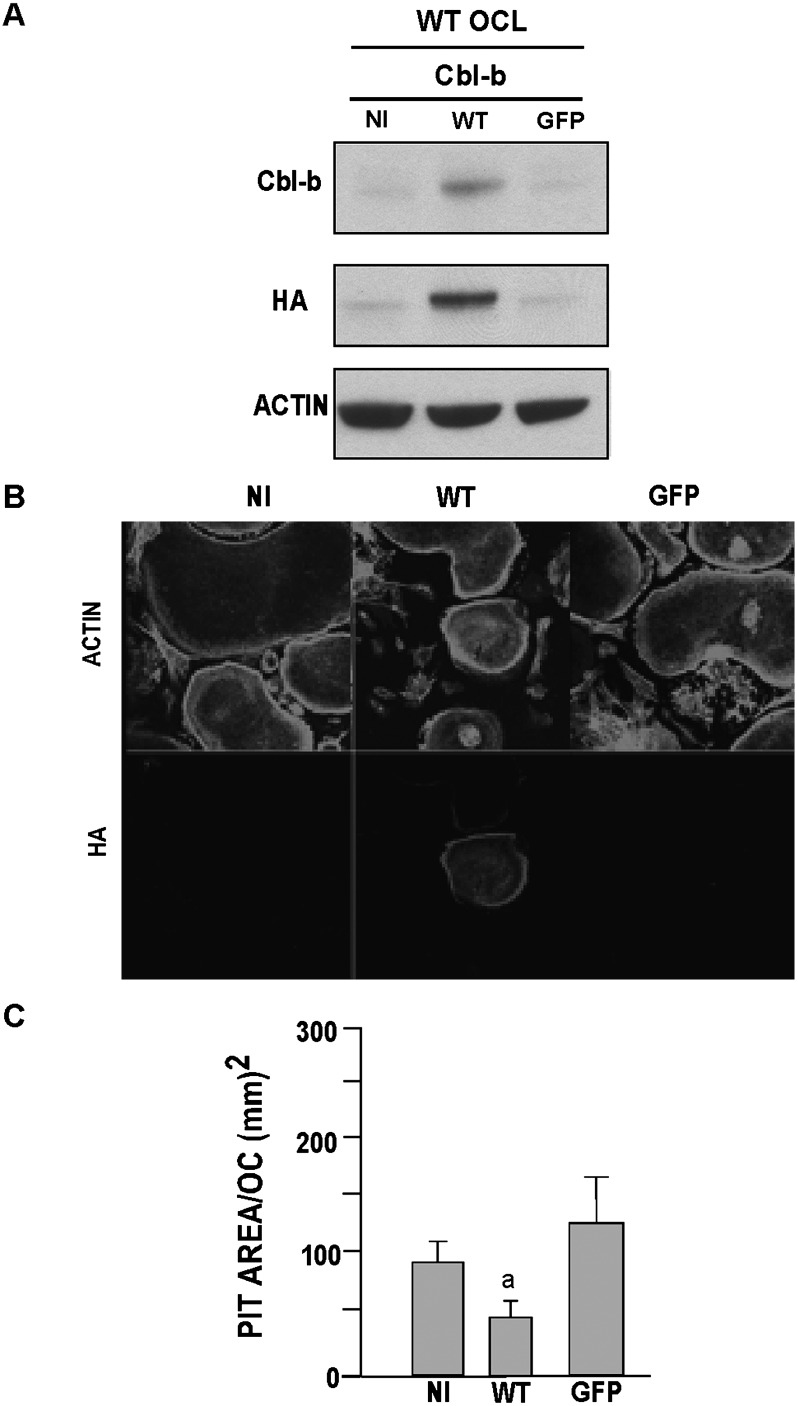
Because WT Cbl-b reversed the hyperactivity of Cbl-b−/− OCLs, we asked whether overexpressing Cbl-b would reduce the bone-resorbing activity of WT OCLs. We found that the overexpression of Cbl-b in wildtype osteoclasts inhibited pit formation by 60–70% (Fig. 7), in contrast to our previously reported finding that overexpressing Cbl in wildtype OCLs had no effect on in vitro pit formation.(16,29) This result, together with the increased bone-resorbing activity of the Cbl-b−/− OCLs, shows that Cbl-b actively downregulates bone resorption.
FIG. 7.
Overexpression of Cbl-b in wildtype OCLs reduces bone resorption. Wildtype OCLs were either not infected (NI) or infected with adenovirus vector encoding GFP or wildtype (WT) Cbl-b at an m.o.i. of 50. (A) Recombinant protein expression was analyzed by Western blot as described in Fig. 6. (B) Immunoflorescence of WT OCLs expressing Cbl-b protein. Immunofluoresence of F-actin and HA-tagged Cbl-b. (C) Bone-resorbing activity of uninfected (NI), Cbl-b-infected, and GFP-infected wildtype OCLs (m.o.i. of 50) was determined. Data are presented as mean ± SD (n = 9). a p < 0.01 compared with WT OCLs.
DISCUSSION
As noted earlier, Cbl and Cbl-b are highly homologous in both domain organization and sequence. The embryonic lethality that occurs in the absence of both proteins(17) (unpublished observations) indicates that these two homologous proteins play critically important redundant functions in a variety of cell types, including in osteoclasts.(43) In addition to these redundant functions, however, it is also apparent that the two proteins perform additional functions that are unique to each, as previously described in T cells(17–20) and in bone marrow–derived mast cells.(21)
We have now shown that Cbl and Cbl-b also perform different specific functions in osteoclasts (this study).(31) Thus, we previously reported that the deletion of the c-cbl gene decreases osteoclast migration both in vivo and in vitro.(30,31) As a consequence, there is a slight delay in the resorption and ossification of cartilage during the development of long bones, although this defect is corrected over time, and the adult Cbl−/− mice do not exhibit any overt skeletal phenotype.(31) We and other investigators have provided evidence that Cbl acts downstream of αvβ3 integrin, Pyk2, and Src in promoting osteoclast motility.(29,30,44)
Our observation that the Cbl-b−/− mice are osteopenic shows that the absence of Cbl-b has distinctly different consequences in bone. Histomorphometric analysis and increased serum collagen telopeptide levels (Fig. 1) indicated that the decreased bone volume was for the most part a consequence of increased bone resorption, not reduced bone formation, and the in vitro bone resorption assay (Fig. 2A) confirmed that the absence of Cbl-b increased osteoclast bone-resorbing activity, notwithstanding the presence of normal amounts of Cbl. The increased in vitro bone resorption was reversed by re-expressing Cbl-b, but not by overexpressing Cbl (Fig. 6C), and overexpression of Cbl-b in wildtype OCLs significantly reduced their bone-resorbing capacity (Fig. 7C). These observations show that Cbl-b performs a unique function that negatively regulates osteoclast bone-resorbing activity.
Our data suggest that this function of Cbl-b may be related to the downregulation of some signaling events downstream of RANK, which is known to stimulate bone resorption by mature osteoclasts.(36,37) Cbl proteins downregulate signaling by receptors and downstream signaling effectors, including nonreceptor tyrosine kinases, by two known mechanisms: ubiquitylation, leading to degradation by the proteosome, and coupling the receptors to endocytic complexes to promote internalization.(1–3) Arron et al.(23) have reported that Cbl-b, but not Cbl, promotes the proteosome-mediated degradation of RANK when the proteins are overexpressed in HEK293 cells, although we observed the opposite effects when the proteins are expressed in HEK293 cells that stably overexpress the vitronectin receptor (αvβ3 integrin) (unpublished observations).
Our results suggest that Cbl-b modulates a subset of RANK-coupled signaling mechanisms to a greater degree than the expression or activity of RANK itself, although we cannot rule out a subtle direct effect on RANK. Thus, the absence of Cbl-b resulted in increased phosphorylation of ERK and p38 but had little effect on JNK, Akt, or Src activity. The nuclear localization of NF-κB p65 was more prolonged, a likely consequence of the reduced level of IκB-α that we consistently observed in the Cbl-b−/− OCLs. This reduction in IκB-α in the mature OCLs, whose generation requires RANKL-induced activation of NF-κB, suggests that one of the roles of Cbl-b in osteoclasts is to downregulate the coupling of RANK to NF-κB upstream of IκB-α. Detailed characterization of how Cbl-b regulates the coupling of RANK to NF-κB and other RANK-activated signaling mechanisms will be the focus of future research.
Cbl and Cbl-b have also been reported to differ in the way they downregulate signaling effectors, including Syk and PLCγ,(21,45,46) and one or more of these differences could also contribute to the different Cbl−/− and Cbl-b−/− osteoclast phenotypes. In addition, although Cbl and Cbl-b seem to have equal capacity to act as ubiquitin ligases toward a range of different proteins in in vitro ubiquitylating assays, the fate of their ubiquitylated substrates are sometimes different. For example, loss of Cbl-b does not alter the amount of p85 or Vav in T cells, but it affects activation, localization, and association of these proteins with other signaling molecules.(19,20,47) Any of these structural and functional differences could underlie the differences in the osteoclast and bone phenotypes of the Cbl−/− and Cbl-b−/− mice.
We sought to identify the specific structural features that are required for the unique function of Cbl-b. Our results show that disabling either the TKB domain or the RING finger domain of Cbl-b prevents the negative regulation of resorbing activity (Fig. 6C), indicating that the binding of Cbl-b to a tyrosine-phosphorylated signaling effector and the effector's ubiquitylation are part of the mechanism by which Cbl-b downregulates the bone-resorbing activity of osteoclasts. However, the near-identity of the TKB and RING finger domains (TKB = 84%, RING = 98%) and the abilities of the homologous Cbl domains to perform the same functions raise the question of how these domains could impart the specificity of Cbl-b that we showed here. Although we do not rule out the possibility that one or more of the few Cbl-b–specific residues of the TKB domain could impart unique targeting specificity to Cbl-b, differences in the more divergent C-terminal halves of the proteins (<50% identical) are perhaps more likely to be responsible. Two functionally important differences that must be considered are the presence of an inducible binding site for the p85 subunit of PI3K at Y731 in Cbl but not in Cbl-b, which enables Cbl to recruit PI3K to cell membranes,(24–26) and differences in the C-terminal UBA domains of the two proteins that confer different abilities to bind multiubiquitin and other proteins.(27) Overexpressing the CblY731F mutant strongly reduces in vitro bone resorption,(29) similar to the effect of overexpressing the wildtype Cbl-b. Further study will be required to determine whether disabling the Src-inducible recruitment of PI3K to Cbl confers a Cbl-b-like activity or whether it interferes with some function of Cbl that is required for normal bone-resorbing activity.
In conclusion, deleting the cbl-b gene results in osteopenia that is largely the consequence of increased bone resorption. The Cbl-b−/− OCLs differentiate more rapidly and resorb bone more actively. The increased bone-resorbing activity is reduced by re-expressing Cbl-b but not by overexpressing Cbl, and this unique function of Cbl-b requires functional TKB and RING finger domains. A subset of RANK-coupled signaling effectors (NFκB, ERKs, p38) are more active in the Cbl-b−/− OCLs, suggesting that these effectors contribute to the observed phenotype. Thus, this study shows that Cbl and Cbl-b play unique roles in osteoclast functions in addition to their redundant functions and that a unique and important function of Cbl-b is to downregulate bone resorption, a function that cannot be performed by Cbl.
ACKNOWLEDGMENTS
The Cbl-b−/− mice were generously provided by Dr. Hua Gu, Columbia University. cDNAs for wildtype and mutated human Cbl-b were generously provided by Dr. Stanley Lipkowitz, National Institutes of Health. This work was supported in part by a Pilot and Feasibility Project grant (to A.S.) from the Yale Core Center for Musculoskeletal Diseases (2P30 AR 46032) and by grants AR-49879 (to W.C.H.) and DE-04724 (to R.B.) from the National Institutes of Health.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Horne WC, Sanjay A, Bruzzaniti A, Baron R. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev. 2005;208:106–125. doi: 10.1111/j.0105-2896.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 2.Thien CBF, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: Substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–166. doi: 10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaminathan G, Tsygankov AY. The Cbl family proteins: Ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- 4.Sanjay A, Horne WC, Baron R. The Cbl family: Ubiquitin ligases regulating signaling by tyrosine kinases. Sci STKE. 2001. http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2001/110/pe40. Accessed November 27, 2001. [DOI] [PubMed]
- 5.Thien CBF, Langdon WY. Cbl: Many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–305. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 6.Dikic I, Szymkiewicz I, Soubeyran P. Cbl signaling networks in the regulation of cell function. Cell Mol Life Sci. 2003;60:1805–1827. doi: 10.1007/s00018-003-3029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416:187–190. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- 8.Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- 9.Szymkiewicz I, Kowanetz K, Soubeyran P, Dinarina A, Lipkowitz S, Dikic I. CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases. J Biol Chem. 2002;277:39666–39672. doi: 10.1074/jbc.M205535200. [DOI] [PubMed] [Google Scholar]
- 10.Yokouchi M, Kondo T, Houghton A, Bartkiewicz M, Horne WC, Zhang H, Yoshimura A, Baron R. Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J Biol Chem. 1999;274:31707–31712. doi: 10.1074/jbc.274.44.31707. [DOI] [PubMed] [Google Scholar]
- 11.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 12.Joazeiro CAP, Wing SS, Huang H-K, Leverson JD, Hunter T, Liu Y-C. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2- dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 13.Yokouchi M, Kondo T, Sanjay A, Houghton A, Yoshimura A, Komiya S, Zhang H, Baron R. Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J Biol Chem. 2001;276:31185–31193. doi: 10.1074/jbc.M102219200. [DOI] [PubMed] [Google Scholar]
- 14.Donovan JA, Ota Y, Langdon WY, Samelson LE. Regulation of the association of p120cbl with Grb2 in Jurkat T cells. J Biol Chem. 1996;271:26369–26374. doi: 10.1074/jbc.271.42.26369. [DOI] [PubMed] [Google Scholar]
- 15.Szymkiewicz I, Destaing O, Jurdic P, Dikic I. SH3P2 in complex with Cbl and Src. FEBS Lett. 2004;565:33–38. doi: 10.1016/j.febslet.2004.03.100. [DOI] [PubMed] [Google Scholar]
- 16.Sanjay A, Miyazaki T, Itzstein C, Purev E, Horne WC, Baron R. Identification and functional characterization of an Src homology domain 3 domain-binding site on Cbl. FEBS J. 2006;273:5442–5456. doi: 10.1111/j.1742-4658.2006.05535.x. [DOI] [PubMed] [Google Scholar]
- 17.Naramura M, Jang I-K, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 18.Naramura M, Kole HK, Hu RJ, Gu H. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc Natl Acad Sci USA. 1998;95:15547–15552. doi: 10.1073/pnas.95.26.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachmaier K, Krawczyk C, Kozieradzki I, Kong Y-Y, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 20.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu R-J, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Chiang YJ, Hodes RJ, Siraganian RP. Inactivation of c-Cbl or Cbl-b differentially affects signaling from the high affinity IgE receptor. J Immunol. 2004;173:1811–1818. doi: 10.4049/jimmunol.173.3.1811. [DOI] [PubMed] [Google Scholar]
- 22.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 23.Arron JR, Vologodskaia M, Wong BR, Naramura M, Kim N, Gu H, Choi Y. A positive regulatory role for Cbl family proteins in tumor necrosis factor-related activation-induced cytokine (TRANCE) and CD40L-mediated akt activation. J Biol Chem. 2001;276:30011–30017. doi: 10.1074/jbc.M100414200. [DOI] [PubMed] [Google Scholar]
- 24.Ueno H, Sasaki K, Honda H, Nakamoto T, Yamagata T, Miyagawa K, Mitani K, Yazaki Y, Hirai H. c-Cbl is tyrosine-phosphorylated by interleukin-4 and enhances mitogenic and survival signals of interleukin-4 receptor by linking with the phosphatidylinositol 3′-kinase pathway. Blood. 1998;91:46–53. [PubMed] [Google Scholar]
- 25.Hunter S, Burton EA, Wu SC, Anderson SM. Fyn associates with Cbl and phosphorylates tyrosine 731 in Cbl, a binding site for phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:2097–2106. doi: 10.1074/jbc.274.4.2097. [DOI] [PubMed] [Google Scholar]
- 26.Lazaar AL, Krymskaya VP, Das SKP. VCAM-1 activates phosphatidylinositol 3-kinase and induces p120Cbl phosphorylation in human airway smooth muscle cells. J Immunol. 2001;166:155–161. doi: 10.4049/jimmunol.166.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Davies GC, Ettenberg SA, Coats AO, Mussante M, Ravichandran S, Collins J, Nau MM, Lipkowitz S. Cbl-b interacts with ubiquitinated proteins; differential functions of the UBA domains of c-Cbl and Cbl-b. Oncogene. 2004;23:7104–7115. doi: 10.1038/sj.onc.1207952. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka S, Amling M, Neff L, Peyman A, Uhlmann E, Levy JB, Baron R. c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki T, Sanjay A, Neff L, Tanaka S, Horne WC, Baron R. Src kinase activity is essential for osteoclast function. J Biol Chem. 2004;279:17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- 30.Sanjay A, Houghton A, Neff L, Didomenico E, Bardelay C, Antoine E, Levy J, Gailit J, Bowtell D, Horne WC, Baron R. Cbl associates with Pyk2 and Src to regulate Src kinase activity, αvβ3 integrin-mediated signaling, cell adhesion, and osteoclast motility. J Cell Biol. 2001;152:181–195. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiusaroli R, Sanjay A, Henriksen K, Engsig MT, Horne WC, Gu H, Baron R. Deletion of the gene encoding c-Cbl alters the ability of osteoclasts to migrate, delaying resorption and ossification of cartilage during the development of long bones. Dev Biol. 2003;261:537–547. doi: 10.1016/s0012-1606(03)00299-9. [DOI] [PubMed] [Google Scholar]
- 32.Sims NA, Clement-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest. 2000;106:1095–1103. doi: 10.1172/JCI10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baron R, Vignery A, Neff L, Silverglate A, Santa Maria A. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. vol. 1. Boca Raton, FL, USA: CRC Press; 1983. pp. 13–35. [Google Scholar]
- 34.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol. 2003;160:709–718. doi: 10.1083/jcb.200209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgess TL, Qian Y-X, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145:527–538. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aoki K, Saito H, Itzstein C, Ishiguro M, Shibata T, Blanque R, Mian AH, Takahashi M, Suzuki Y, Yoshimatsu M, Yamaguchi A, Deprez P, Mollat P, Murali R, Ohya K, Horne WC, Baron R. A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. J Clin Invest. 2006;116:1525–1534. doi: 10.1172/JCI22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, Teitelbaum SL. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature. 1997;386:81–84. doi: 10.1038/386081a0. [DOI] [PubMed] [Google Scholar]
- 39.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 40.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka S. Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am J Nephrol. 2007;27:466–478. doi: 10.1159/000106484. [DOI] [PubMed] [Google Scholar]
- 43.Purev E, Neff L, Horne W, Baron R. Depletion of both Cbl and Cbl-b in osteoclasts leads to microtubule-dependent cytoskeletal alterations and apoptosis. Bone. 2007;40(Suppl 2):S150. [Google Scholar]
- 44.Faccio R, Novack DV, Zallone A, Ross FP, Teitelbaum SL. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by β3 integrin. J Cell Biol. 2003;162:499–509. doi: 10.1083/jcb.200212082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasuda T, Maeda A, Kurosaki M, Tezuka T, Hironaka K, Yamamoto T, Kurosaki T. Cbl suppresses B cell receptor-mediated phospholipase C (PLC)-γ2 activation by regulating B cell linker protein-PLC-γ2 binding. J Exp Med. 2000;191:641–650. doi: 10.1084/jem.191.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuda T, Tezuka T, Maeda A, Inazu T, Yamanashi Y, Gu H, Kurosaki T, Yamamoto T. Cbl-b positively regulates Btk-mediated activation of phospholipase C-γ2 in B cells. J Exp Med. 2002;196:51–63. doi: 10.1084/jem.20020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang D, Liu Y-C. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]