Abstract
An effective, asymmetric total synthesis of the antitumor antibiotic (-)-okilactomycin (1) and assignment of the absolute configuration, has been achieved exploiting a convergent strategy. Highlights of the synthesis include: a diastereoselective oxy-Cope rearrangement/oxidation sequence to install the C(1) and C(13) stereogenic centers; a Petasis-Ferrier union/rearrangement to construct the highly functionalized tetrahydropyranone inscribed within the thirteen membered macrocycle ring, employing for the first time a sterically demanding acetal; an intramolecular chemoselective acylation to access an embedded bicyclic lactone; and an efficient ring closing metathesis (RCM) reaction to generate the macrocyclic ring.
Introduction
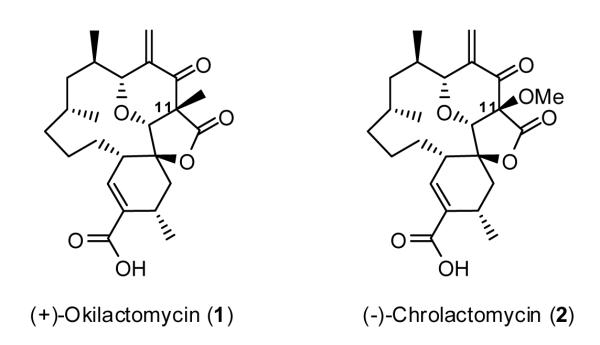
In 1987 Imai and coworkers disclosed the isolation and structural elucidation of (+)-okilactomycin (1), an architecturally complex polyketide antitumor antibiotic derived from a bioactive filtrate produced by the actinomycetes, Streptomyces griseoflavus, obtained from a soil sample on the island of Zamami, Okinawa Japan (Figure 1).1 The structure, initially assigned via a combination of spectroscopic methods, including FAB-MS, EI-MS, 1H and 13C NMR, and 2D NMR experiments,2 comprises a highly functionalized cyclohexene ring, complete with a C(13) spirocenter, a 2,6-cis-tetrahydropyranone moiety, and a five-member lactone all inscribed within a 13-membered ring. Single crystal X-ray analysis confirmed the connectivity and relative stereochemistry;3 the absolute configuration however remained undefined. From the biological perspective, (+)-okilactomycin (1) exhibits significant in vitro cytotoxicity when assayed against a number of human cancer cell lines, including lymphoid leukemia L1210 and leukemia P388 (IC50 = 0.09 and 0.037 μg/mL respectively), as well as in vivo activity against Ehrlich ascites carcinoma.
Figure 1.
More recently (2001), a related congener, (-)-chrolactomycin (2), was isolated by Yamashita and coworkers4 endowed with a C(11) methoxy group instead of a methyl substituent. Chrolactomycin [(-)-2] also displayed significant cytotoxicity in vitro against several human cell lines, including ACHN, A431, MCF-7, and T24 (IC50 = 1.2, 1.6, 0.69, and 0.45 μg/mL, respectively).
The unique architecture of this class of antitumor antibiotics, in conjunction with the biological profiles, prompted early interest by the Takeda and Paquette laboratories.5 Equally intrigued with these antitumor antibiotics, we initiated a synthetic program in 2003. From the outset, we saw an opportunity to exploit the Petasis-Ferrier union/rearrangement6 developed in our laboratory to construct the tetrahydropyranone embedded in the tetracyclic skeleton.7 Herein, we describe a full account of the evolution of the first total synthesis of (-)-okilactomycin (1), which not only would provide access to natural (+)-okilactomycin (1) as well as related congeners, but also permits assignment of the absolute configuration.8
Results and Discussions
Synthetic Analysis
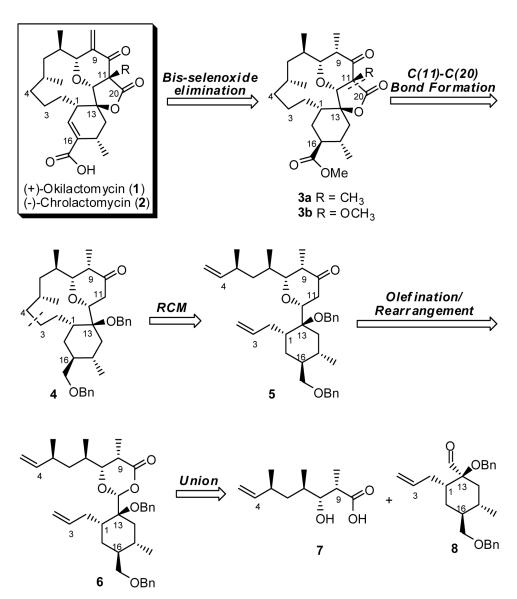
Given the close structural similarity between (+)-okilactomycin (1) and (-)-chrolactomycin (2), the development of a unified synthetic strategy was central to our initial design. Towards this end, we envisioned an end game involving bis-selenation of ketolactone 3, carrying either a methyl or methoxy substituent at C(11), followed by oxidative-elimination to introduce the resident olefins. Construction of the macrocycle ring would, not surprisingly, call upon ring-closing metathesis (RCM),9 a tactic that has revolutionized complex molecule synthesis in the late 20th century. Two central questions however remained: when to elaborate the five membered ring lactone, complete with introduction of either the quaternary C(11) methyl or methoxy substituents for (+)-okilactomycin (1) and (-)-chrolactomycin (2), respectively, and should these operations occur prior to, or after the macrocyclization by ring-closing metathesis? We selected macrocyclization prior to elaboration of the embedded lactone at the outset to avoid an excessive orthogonal protecting group strategy (vide infra). The envisioned Petasis-Ferrier Union/Rearrangement in turn would call for the union of β-hydroxyacid 7 with a sterically encumbered aldehyde or acetal (cf. 8) to construct the cis-2,6-disubstituted tetrahydropyranone ring resident in the RCM substrate (5). At the time however, encumbered aldehydes or the corresponding acetals had not served as Petasis-Ferrier substrates. Notwithstanding this issue, construction of the embedded five membered lactone would take advantage of the kinetic enolate derived from 4 to introduce a CO2 equivalent, followed by introduction of either the methyl or methoxy substitutent, thus achieving a unified strategy.
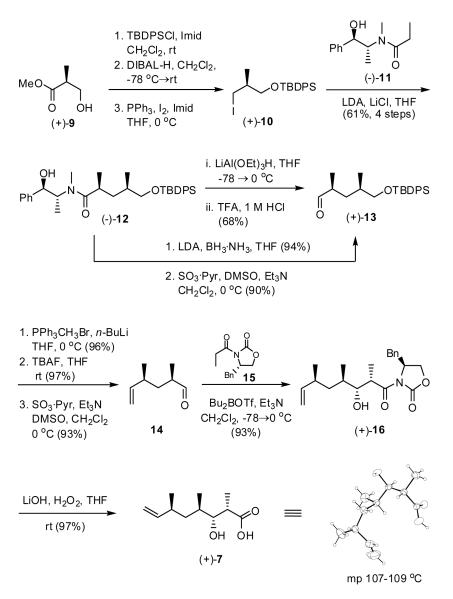
Construction of β-Hydroxy Acid 7
We began the synthesis of β-hydroxy acid 7 with commercially available (S)-(+)-Roche ester (9). Protection of (+)-9 as the TBDPS ether, followed by DIBAL-H reduction and conversion of the resultant alcohol to the iodide with iodine and triphenylphosphine furnished known iodide (+)-1010 (Scheme 2). Diastereoselective alkylation employing the Myers pseudoephedrine amide (-)-1111 then led to (-)-12 as a single diastereomer, existing as a mixture of rotamers (4.5:1 by NMR). This four-step sequence could be conveniently conducted on >75 g scale, requiring only one purification after the alkylation. Reduction of (-)-12 to aldehyde (+)-13 was next achieved with LiAl(OEt)3H;11 the yield however was modest. A two-step process involving reduction to the primary alcohol employing BH3·NH3,11 followed by Parikh-Doering oxidation12 proved considerably more effective on larger scale (ca. 90% on 34 gram scale). The resultant aldehyde was then subjected to Wittig methylenation, followed by removal of the TBDPS group with tetrabutylammonium flouride (TBAF) to generate the primary alcohol; Parikh-Doering oxidation produced aldehyde (+)-14. The requisite β-hydroxy acid (+)-7, a crystalline solid, was then available via a syn diastereoselective aldol condensation13 between the Evans oxazolidinone 1514 and aldehyde (+)-14, followed by hydrolytic removal of the auxiliary. Single crystal X-ray analysis confirmed the connectivity and relative stereochemistry. The overall sequence proceeded in 10 steps and in 23% overall yield from Roche ester (+)-9.
Scheme 2.
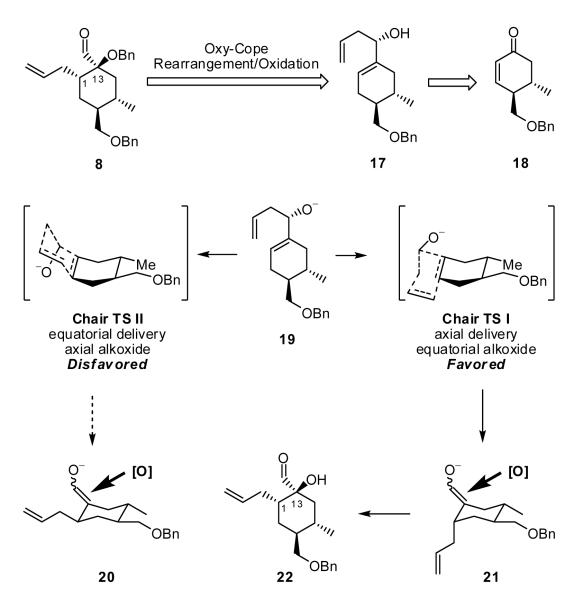
Construction of Hydroxy Aldehyde 8
We envisioned that aldehyde 8, complete with the required stereogenicity at C(1) and C(13), could be generated from allylic alcohol 17 via a reaction sequence involving a stereochemical relay. A two-step protocol involving an anionic oxy-Cope rearrangement/oxidation sequence15 appeared ideal (Scheme 3). The R configuration of alcohol 17 was selected to initiate the relay. For this transformation, we envision a chair transition state (TS I) involving alkoxide 19 (i.e., matched case), with allyl group delivery anticipated to occur in an axial fashion, to furnish the enolate (21) of the desired aldehyde. The alternative transition state (TS II), with the alkoxide residing in the pseudoaxial conformation, would suggest allyl delivery from the equatorial direction to furnish the undesired diastereomer (20). In the absence of significant steric considerations, the literature teaches a strong stereoelectronic bias (ca. > 85:15) towards axial delivery.16 Subsequent approach of an oxidant would then be expected to occur opposite to the axial allyl group to deliver the desired α-hydroxy aldehyde 22. Continuing with this analysis, allylic alcohol 17 would derive from enone 18 via 1,4-reduction, capture of the resultant enolate as an enol triflate, carbonylation to the corresponding aldehyde, and asymmetric introduction of the allyl group. Finally, construction of cyclohexenone 18, would call upon a Rawal diastereoselective Diels-Alder reaction17 to set the stereocenters at C(15) and C(16).
Scheme 3.
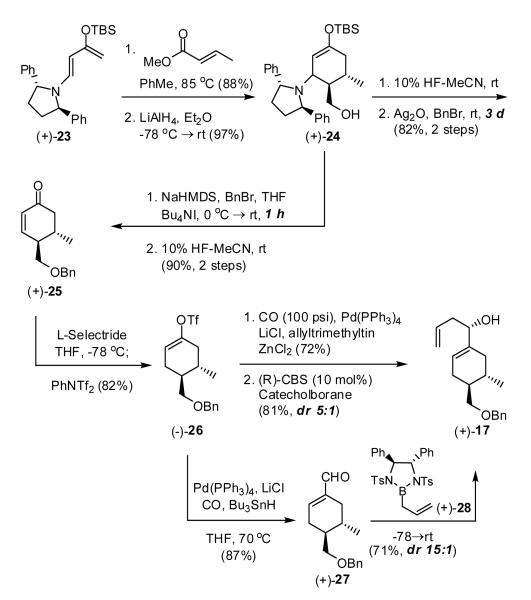
We began with the Rawal diastereoselective Diels-Alder reaction employing diene (+)-2317 and methyl crotonate to deliver an intermediate ester, which upon reduction with LiAlH4 furnished primary alcohol (+)-24 as a mixture (8:1) of exo and endo diastereomers (Scheme 4). Removal of the pyrrolidine auxiliary mediated by HF in acetonitrile, followed by protection of the hydroxyl group as a benzyl ether furnished (+)-25. To circumvent both the use of expensive Ag2O and long reaction time (ca. 3 days), an improved protocol was developed by inverting the order of the steps and using NaHMDS as the base for benzylation, over a period of 1 h. Low temperature 1,4-reduction of (+)-25 with L-Selectride, followed by capture of the resulting enolate with N-phenyltriflimide then generated vinyl triflate (-)-26.18
Scheme 4.
To arrive at allylic alcohol 17, we initially examined carbonylation via a Stille protocol employing allyltrimethyltin,19 followed by CBS reduction.20 Although the carbonylation proceeded in good yield (72%), subsequent reduction with the (R)-CBS catalyst resulted only in moderate diastereoselectivity (5:1). We therefore turned to an alternative two-step formylation/allylation sequence21 employing triflate (-)-26; again the formylation proceed in excellent yield. Diastereoselective allylation of the resultant aldehyde (+)-27 with Corey reagent (+)-2822 furnished alcohol (+)-17 in 71% yield, now with excellent diasteroselectivity (15:1).
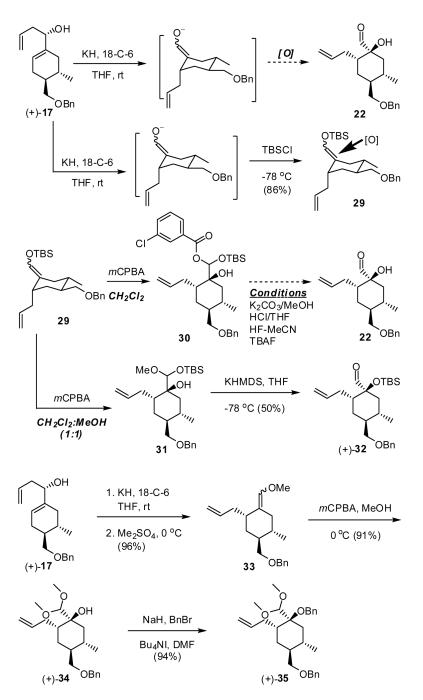
The Oxy-Cope/Oxidation Sequence
The oxy-Cope rearrangement of (+)-17, initially employing KHMDS both with and without 18-C-6, followed by attempted capture of the anticipated enolate with TBSCl, led to only decomposition. However, employing KH as base with an equimolar amount of 18-C-6, followed by treatment with TBSCl furnished the desired enol ether 29 as a mixture of diastereomers. To optimize and extend this process, we examined a tandem Oxy-Cope anion rearrangement/oxidation sequence (Scheme 5), replacing TBSCl with a variety of oxygen electrophiles (cf. the Davis oxaziridine,23 oxygen, and dibenzylcarbonylperoxide24) to capture the resultant enolate. Only decomposition or formation of side products resulted. We therefore turned to a two-step sequence exploiting Rubottom oxidation25 as the second step. In this case, the initially derived aldehyde enolate was first captured as the TBS silyl enol ether 29; treatment with m-CPBA however unexpectedly led to a mixture of silyl acetals (30) instead of the anticipated α-siloxy aldehyde. Presumably the acetals were formed via ring opening of the intermediate epoxide by the m-chlorobenzoic acid byproduct (Scheme 5).25 Attempts to transform the acetals to the desired α-hydroxyaldehyde (+)-22, employing a variety of acidic and basic conditions, proved unsuccessful. We reasoned that we could take advantage of the observed formation of the silyl acetal by employing a “non-transferable” group to achieve opening of the reactive epoxide, thereby permitting selective transfer of the silyl group to the tertiary hydroxyl. Treatment of silyl enol ether 29 with m-CPBA in the presence of MeOH produced a mixed methylsilyl acetal (31), which upon treatment with KHMDS furnished the desired α-siloxyaldehyde (+)-32 in moderate yield. To improve the overall yield, we next explored a sequence involving initial alkylation of the enolate with a “non-transferable” group, followed by protection of the tertiary hydroxyl group as the benzyl ether. We employed Me2SO4, which furnished enol ether 33 (E/Z 2:1). Subsequent exposure to m-CPBA in MeOH led via a Rubottom-like oxidation to α-hydroxy dimethylacetal (+)-34 in high yield. The hydroxyl group was then protected as the benzyl ether to provide the desired α-benzyloxy dimethylacetal (+)-35. Pleasingly, this optimized and scalable three-step protocol now proceeds with an overall yield of 82%.
Scheme 5.
Construction of Tetrahydropyranone 5: Application of the Petasis-Ferrier Union/Rearrangement
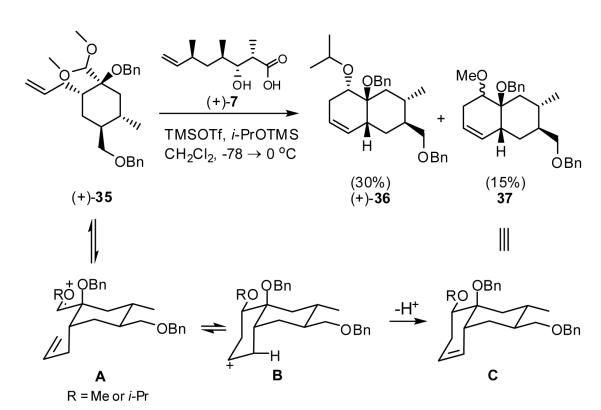
Having secured ample quantities of both β-hydroxy acid (+)-7 and acetal (+)-35, we turned to the Petasis-Ferrier union/rearrangement6 to elaborate tetrahydropyranone 5 (Scheme 6). Utilizing the modified Noyori conditions26 developed by Kurihara and coworkers,27 we attempted the union of acid (+)-7 with the sterically encumbered acetal (+)-35 in the presence of TMSOTf and i-PrOTMS.24 The anticipated dioxanone was not obtained; instead a mixture of isopropyl and methyl homoallylic ethers, assigned as (+)-36 and (+)-37 predominated. This result was quickly recognized as a Prins cyclization28 involving capture of oxonium ion A (R=Me or iPr) by the terminal olefin (Scheme 6). We were therefore forced to consider masking the alkene in (+)-35 prior to moving forward.
Scheme 6.
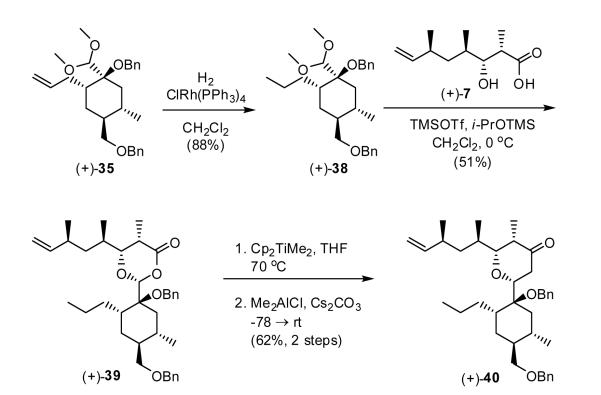
However, to verify the feasibility of employing a relatively hindered aldehyde and/or acetal in the proposed Petasis/Ferrier union/rearrangement, we prepared acetal (+)-38 as a model system via hydrogentation of (+)-35 (Scheme 7). Condensation with β-hydroxy acid (+)-7 proceeded smoothly to provide dioxanone (+)-39; the yield however was modest. Execution of the Petasis-Ferrier sequence employing the Petasis-Tebbe methylenation protocol29 to furnish the requisite enol ether, followed without purification by rearrangement induced by Me2AlCl led to tetrahydropyranone (+)-40 in an unoptimized yield of 62% for the two steps. Encouraged by these results, we turned to devise a suitable mask for the alkene in (+)-35. A key consideration would be facile regeneration of the terminal olefin after the Petasis-Ferrier rearrangement.
Scheme 7.
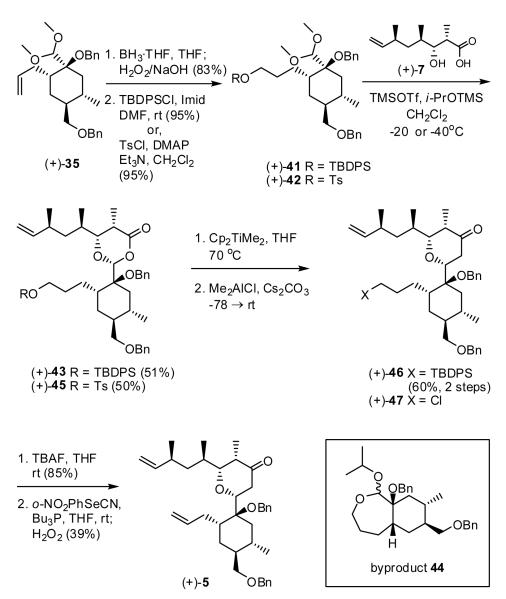
Towards this end, we prepared the TBDPS ether (+)-41 and tosylate (+)-42, the TBDPS ether and tosyl via hydroboration of (+)-35, followed respectively by silylation or tosylation of the resulting alcohol (Scheme 8). Both masks were expected to permit ready regeneration of the alkene moiety.
Scheme 8.
Gratifyingly, union of acid (+)-7 with acetal (+)-41 via the now standard Kurihara protocol (i.e., TMSOTf, iPrOTMS, CH2Cl2) led to the desired dioxanone (+)-43. Again the yield was moderate, in this case due to formation of a seven-membered cyclic acetal (44), the result of an intramolecular capture of the derived oxocarbenium ion, followed by desilylation and reaction with isopropyl alcohol. Methylenation of (+)-43 with the Petasis-Tebbe reagent, followed by Me2AlCl-promoted rearrangement furnished the desired tetrahydropyranone (+)-46 in 58% for the two steps. Notwithstanding the modest yields, this reaction sequence constitutes the first example of a Petasis-Ferrier union/rearrangement involving a sterically encumbered acetal. As anticipated, unmasking the terminal alkene was readily achieved by removal of the TBDPS group, followed by Grieco-Nishizawa elimination30 to provide (+)-5.
The low efficiency of both the union and olefin regeneration prompted us to examine the more electron deficient tosylate (+)-42. Condensation of (+)-42 with acid (+)-7, promoted by a stoichiometric amount of TMSOTf necessary in this case due to the Lewis basicity of the tosylate group, furnished dioxanone (+)-45 again in modest yield (Scheme 8). Petasis-Ferrier rearrangement as expected led to tetrahydropyranone 47, albeit now furnishing the chloride in place of the tosylate group, a direct result of the Me2AlCl promoter. Although the chloride could readily be converted to an alkene after the Petasis-Ferrier sequence, we turned instead to use of a halogen to mask the olefin in acetal (+)-35.
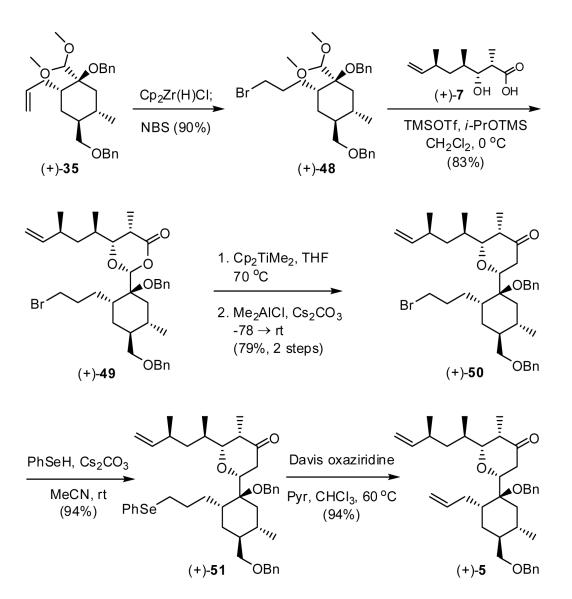
Acetal (+)-48 carrying a bromine was readily prepared from (+)-35 via hydrozirconation, followed by bromination with N-bromosuccimide (NBS).31 Execution of the Petasis-Ferrier union/rearrangement delivered tetrahydropyranone (+)-50 (Scheme 9) now in good yield for the three steps. During optimization and scale up of the Petasis-Ferrier rearrangement, we discovered that Cs2CO3, previously employed for small-scale reactions was not required.7e Regeneration of the alkene was then achieved via a two-step sequence involving conversion to selenide (+)-51,32 followed by oxidative elimination to furnish (+)-5.33 The yield for the optimized 5-step sequence from (+)-35 to (+)-5 was 52%.
Scheme 9.
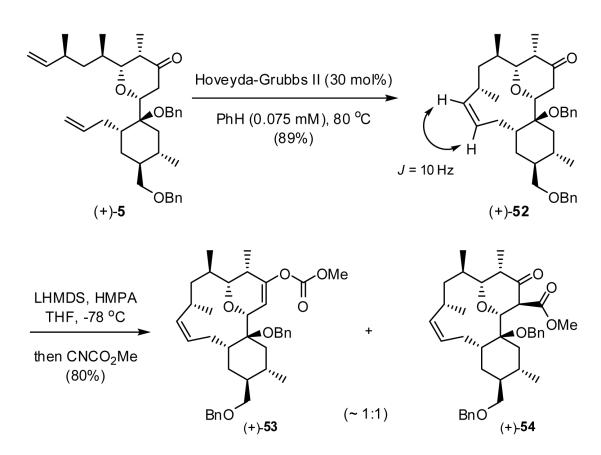
Having arrived at tetrahydropyranone (+)-5, we now considered our alternatives: macrocyclization via ring-closing metathesis (RCM), followed by elaboration of the embedded lactone, or the reverse sequence. Pleasingly, ring-closing metathesis employing the second generation Hoveyda-Grubbs catalyst,34 under high dilution (ca. 0.075 mM), furnished exclusively the cis-olefin (+)-52 in 89% yield (Scheme 10).
Scheme 10.
Construction of the embeded lactone, the next operation, proved more difficult. Kinetic enolization of ketone (+)-52 with LHMDS/HMPA in THF, followed by treatment with Mander’s reagent35a led to a mixture (ca. 1:1) of (+)-53 and (+)-54, respectively in spite of the propensity for the Mander’s reagent to furnish predominantly C-over O-acylation. Although vinyl carbonate (+)-53 could be recycled and resubjected to the reaction conditions, this tactic did not lead to efficient material advancement. Presumably steric hindrance at the α-carbon by the benzyloxy group, in conjunction with the rigidity of the tricyclic system (vide infra) conspire to increase O-acylation. As will be seen, this result proved to be a harbinger of events to come.
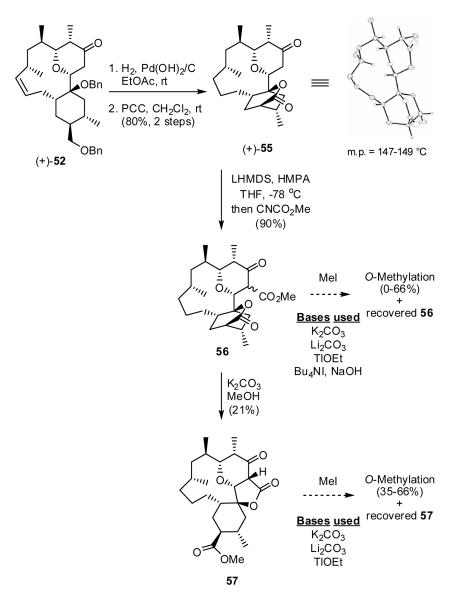
To relieve both the steric issue at the α-carbon, as well as to adjust the C(16) carbon to the requisite acid prior to introduction of the lactone, (+)-52 was subjected to hydrogenation, followed by oxidation (PCC) to furnish lactone (+)-55, the result of in situ intramolecular cyclization (Scheme 11). Single crystal X-ray analysis confirmed the connectivity and relative stereochemistry of (+)-55.
Scheme 11.
Carboxymethylation of (+)-55 with the Mander’s reagent35a next proceeded in excellent yield at carbon to furnish the desired β-ketoester 56. However, upon attempted methylation with MeI, employing a variety of bases, only O-methylation and/or recovery of starting material resulted.35b To reduce the possible rigidity of the system, we subjected lactone 56 to methanolysis to open the bicyclic lactone; in situ cyclization of the derived tertiary hydroxyl occurred to furnish β-ketolactone 57, albeit in low yield. All attempts to introduce the C(11) methyl group again only resulted in O-methylation. Unable to achieve alkylation at carbon, we were forced to explore a second-generation strategy that would require construction of the tricyclic skeleton, including installation of the C(11) quaternary center, prior to ring-closing metathesis.
A Second-Generation Synthetic Strategy: Construction of Tetrahydropyranone (+)-62 and Elaboration of Ketolactone 67
The second-generation strategy, not surprisingly, would rely on the original end game (Scheme 1), but now called for advance intermediate 62 (Scheme 12), differently functionalized at C(13) to permit elaboration of the embedded 5-membered ring lactone. We selected the 2-napthylmethyl (Nap)36 protecting group for the C(13) tertiary hydroxyl, as removal should be possible in the presence of the benzyl group under oxidative condition.37
Scheme 1.
Scheme 12.
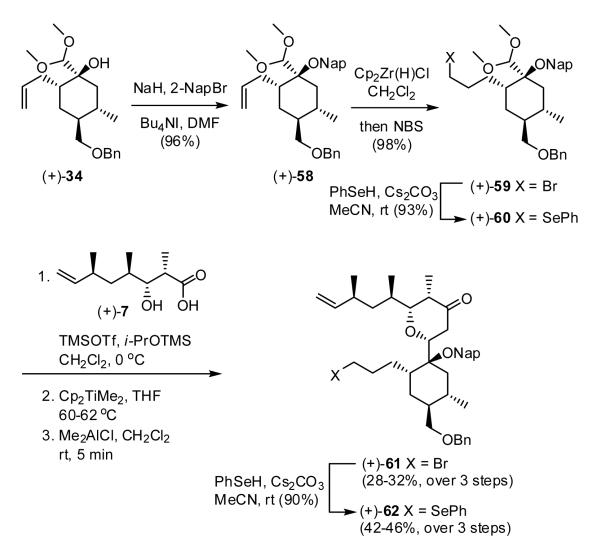
Towards this end, protection of (+)-34 with 2-napthylmethyl bromide (NapBr), followed by hydrozirconation of the olefin and treatment with N-bromosuccinimide (NBS) as described in our first generation synthesis, furnished acetal (+)-59 (X = Br) (Scheme 12). Condensation with acid (+)-7 to furnish the corresponding dioxanone and execution of the Petasis-Ferrier rearrangement proceeded as anticipated to the desired tetrahydropyranone (+)-61. The yield in this case was modest, 28-32% for the two steps, as the bromide underwent competitive elimination during the Petasis olefination, a transformation found to be dependent upon the amount of Me2AlCl employed. We subsequently discovered that selenide (+)-60, prepared by displacement of bromide (+)-59 with PhSeH, was the preferred Petasis-Ferrier substrate, furnishing pyranone (+)-62 as a single diastereomer in 42-46% for the three steps.
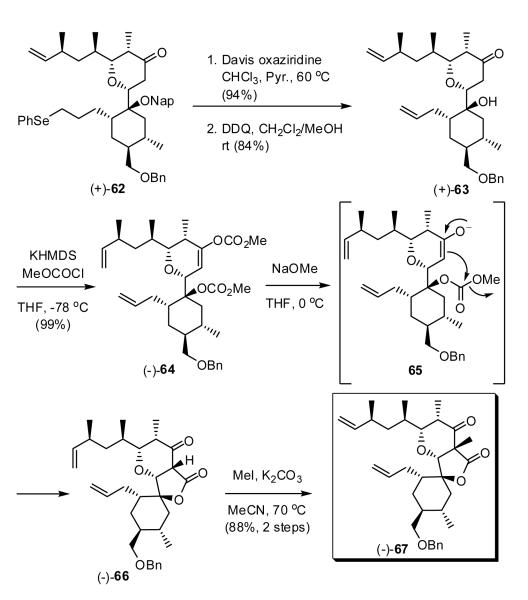
Having secured a viable route to ample quantities of tetrahydropyranone (+)-62, we next focused on construction of the spirolactone moiety (Scheme 13). Oxidative elimination of the phenyl selenide in (+)-62 to reveal the terminal olefin, followed by treatment with DDQ in methanol37 cleanly led to removal of the 2-naphthylmethyl group to furnish carbinol (+)-63, the pivotal intermediate envisioned for construction of tricycle 67. From the outset of the second-generation venture, we had planned to introduce the lactone moiety by formation of a methyl carbonate at the tertiary hydroxyl in (+)-63, followed by regioselective enolization of the tetrahydropyranone and cyclization. However, upon treatment of (+)-63 with one equivalent of KHMDS and methylchloroformate, the C(10) vinyl carbonate was formed exclusively, while the use of excess methylchloroformate and KHMDS led to the bis-carbonate (-)-64. At this stage differentiation of the two carbonates on steric grounds proved possible. Reaction with sodium methoxide at 0 °C led in a chemoselective fashion to methanolysis of the enol carbonate, and in turn cyclization with the tertiary carbonate to furnish lactone (-)-66 as a single diastereomer.38 With construction of the five member lactone complete, we turned to the methylation at C(11), albeit with some trepidation (vide infra). However, unlike the first generation synthesis, exclusive C-methylation (K2CO3/MeI)39 pleasingly occurred to furnish the desired RCM precursor (-)-67. The optimal yield for the three-step sequence [(+)-63 → (-)-67] was 87%.
Scheme 13.
Ring Closing Metathesis (RCM) as Prelude to Final Introduction of Unsaturation
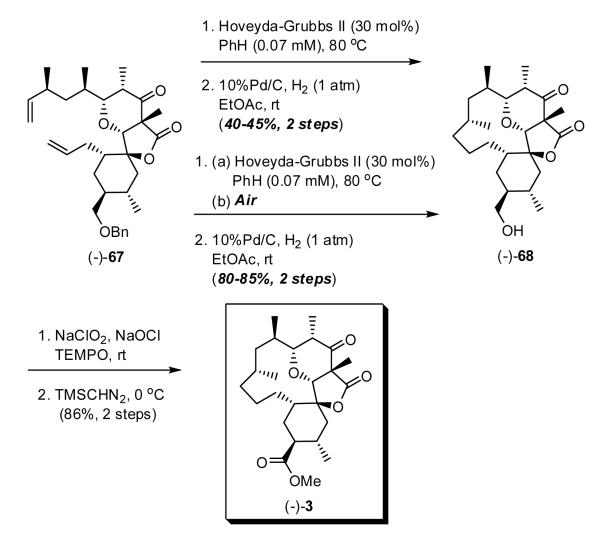
As anticipated from our first-generation approach, treatment of (-)-67 with the second-generation Hoveyda-Grubbs catalyst34 smoothly led to ring closure to furnish exclusively a macrocycle (Scheme 14), again possessing the cis-alkene as determined by H1 NMR (J = 10 Hz). Best results were obtained at high dilution (0.07 mM) to diminish competitive polymerization. The RCM product however proved difficult to separate from the catalyst. We eventually discovered that exposure of the RCM product mixture first to air to destroy the catalyst and then to hydrogen employing 10% Pd on carbon to remove the benzyl group permitted isolation of (-)-68 in 80-85% yield after silica gel chromatography. Oxidation of the corresponding alcohol (TEMPO), followed by esterification (TMSCHN2) of the resulting acid then furnished (-)-3, the requisite precursor for bis-selenation and oxidative elimination to complete construction of okilactomycin (1).
Scheme 14.
Introduction of the Unsaturation at C(9) and C(16): A Non-Trivial Task
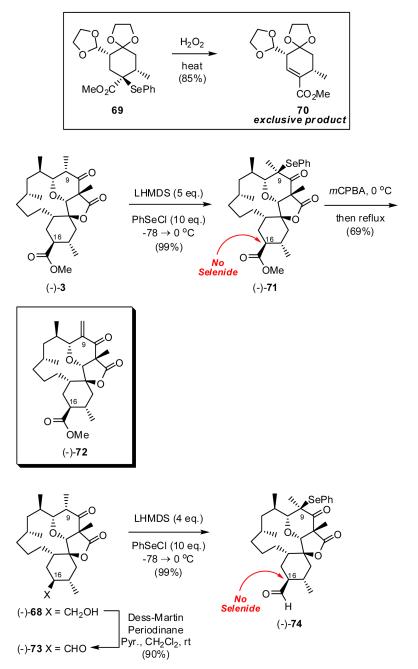
Ample precedent for both selenation and oxidative elimination exists in the literature. Hirenken and coworkers for example reported that selenide 69, efficiently leads to trisubstituted alkene 70 in 85% upon oxidative elimination (Scheme 15).40 Equally important, oxidative eliminations of selenides such as at C(9), away from the C(8) oxygen are also well known.41 Surprisingly, initial attempts to achieve bis-selenation of (-)-3 with LHMDS and benzeneselenyl chloride surprisingly resulted only in the C(9) mono-selenide (-)-71, albeit in near quantitative yield (Scheme 15). The stereochemical assignment at C(9) in (-)-71 and related selenations (vide infra) is tentative and rests on the anticipated convex approach of phenylselenyl chloride to the enolate derived from (-)-3. Resubjection of (-)-71 to a variety of conditions, including stronger amide bases (cf. LDA and lithium pyrrolidine), employing various temperatures and solvent regimes (toluene and THF), resulted only in the recovery of starting material and/or decomposition. Interestingly, no epimerization was observed at C(16), as revealed by 1H-NMR, suggesting our inability to access the C(16) enolate. Two possibilities exist to explain this observation; significant steric crowding and/or poor alignment between the -CO2Me carboxyl group and the α-hydrogen, as required for α-proton acidity. The corresponding aldehyde (-)-73, prepared by oxidation of alcohol (-)-68, also proved resistant to selenation.42
Scheme 15.
With mono-selenide (-)-71 in hand, we took the opportunity to explore the oxidative elimination process (Scheme 15). As expected, treatment of (-)-71 with m-CPBA, followed by heating at reflux led exclusively to formation of the desired exocyclic alkene at C(9) to furnish (-)-72, the methyl ester of dihydro okilactomycin, in 69% yield.
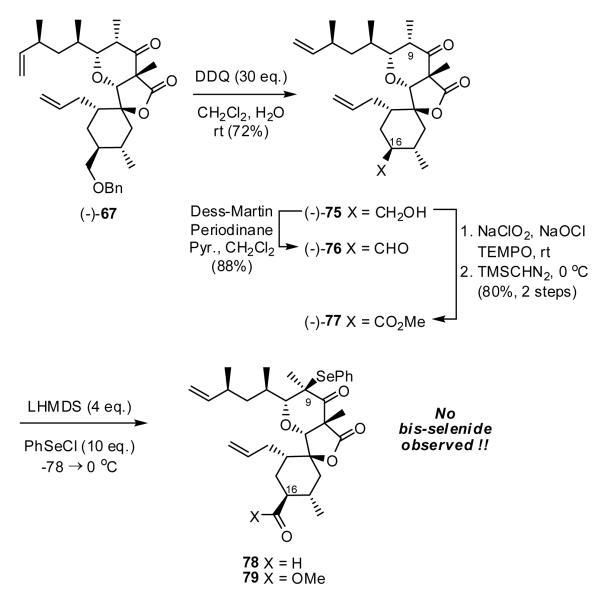
At this juncture we turned to possible bis-selenation prior to the RCM event. Treatment of (-)-67 with DDQ provided primary alcohol (-)-75,43 which was oxidized to aldehyde (-)-76 as well as ester (-)-77. Again, we were disappointed to learn that treatment of either (-)-76 or (-)-77 with excess of LHMDS and PhSeCl led only to mono-selenides 78 and 79 with no noticeable epimerization at C(16).
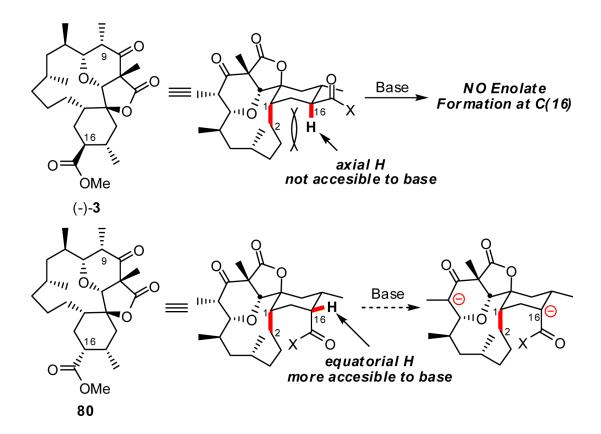
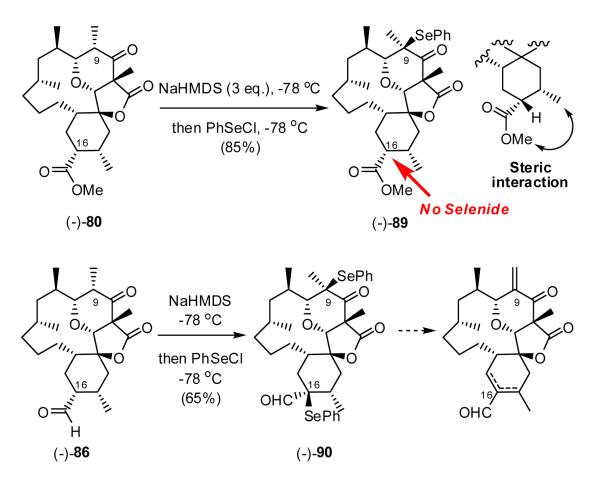
Taken together these results indicate that removal of the hydrogen at C(16) is simply not possible, either due to the steric hinderance or stereoelectronic considerations (vide infra). Inversion of the stereochemistry at C(16) [ca. (-)-3 → 80] on the other hand would place the C(16) hydrogen in what would appear to be a sterically more accessible position, and thus hold the promise for successful deprotonation (Scheme 17).
Scheme 17.
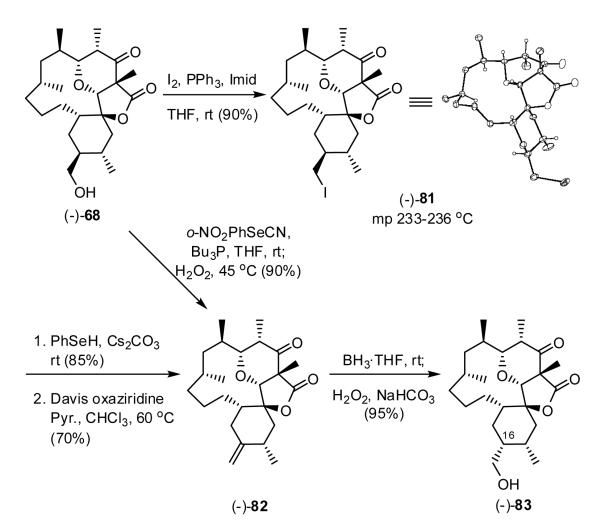
To access 80, alcohol (-)-68 was converted to iodide (-)-81 (Scheme 18). Single crystal X-ray analysis confirmed the structure and relative stereochemistry. Subsequent treatment with PhSeH and Cs2CO3 produced the corresponding selenide, which upon oxidation with the Davis oxaziridine readily underwent elimination to furnish olefin (-)-82 in 70% yield. Alcohol (-)-68 could also be converted directly to olefin (-)-82 in one step employing the Grieco-Nishizawa protocol.30 Hydroboration of (-)-82 with BH3 in THF occurred as anticipated from the more accessible β-face to provide primary alcohol (-)-83 after oxidation (H2O2).
Scheme 18.
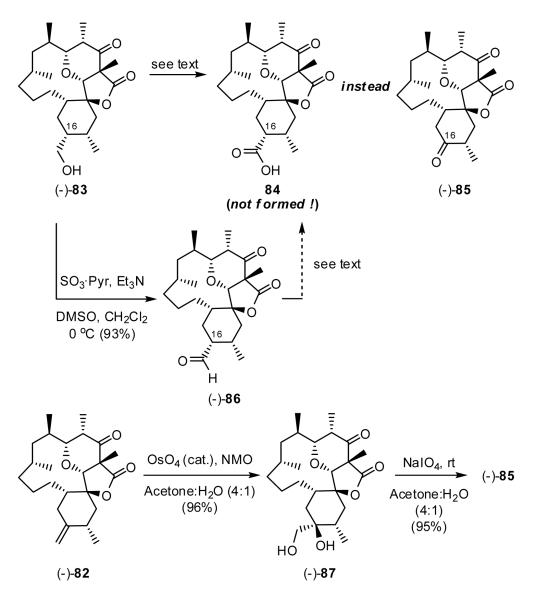
Intervention of an Unusual Oxidation
With alcohol (-)-83 in hand, oxidation to the corresponding carboxylic acid 84 appeared straightforward. This however was not the case (Scheme 19). All conditions explored for the direct conversion of (-)-83 to acid 84 led only to bis-ketone (-)-85! The conditions included: (a) KMnO4, H2SO4, (b) NaClO2, TEMPO, bleach, (c) PDC, DMF. Milder conditions (cf. PtO2, O2, acetone, H2O, 45 °C)44 also proved ineffective resulting only in incomplete conversion to the intermediate aldehyde (-)-86, with no evidence of the carboxylic acid. The structure of (-)-85 was assigned via chemical correlation, involving dihydroxylation of alkene (-)-82 with OsO4,45 followed by NaIO4-mediated oxidative cleavage46 to produce a diketone, identical in all aspects with (-)-85 (cf. 1H, 13C, IR and optical rotation). Precedent for this unexpected transformation can be found in the early work of Barton47 and more recently Kumar.48
Scheme 19.
Efforts were next directed towards the stepwise generation of acid 84 via the aldehyde (-)-86. While alcohol (-)-83 could be oxidized to (-)-86 without complication, subsequent oxidation to the acid again was not possible. In this case, treatment of (-)-86 with NaClO2 and NaH2PO4 in a mixture of t-BuOH and water led only to complete recovery of starting material with no detectable formation of acid 84.
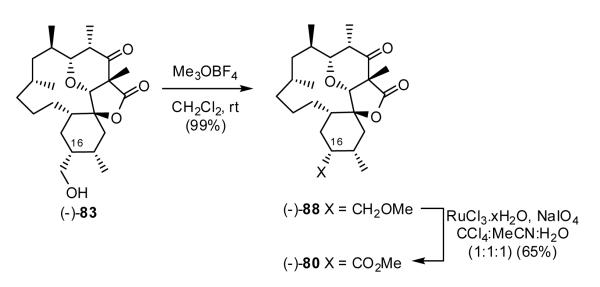
Forced to seek an alternative (i.e., unconventional) route to access a carboxyl group at C(16), we turned to the Sharpless oxidation49 of aliphatic ethers. Treatment of (-)-83 with Me3OBF450 (Scheme 20) produced methyl ether (-)-88, which upon exposure to ruthenium trichloride and sodium periodate (RuCl3·xH2O, NaIO4)49 furnished methyl ester (-)-80 in 65% yield.
Scheme 20.
With the axial methyl ester finally in hand, we again turn to production of the bis-selenide; however, only mono-selenide (-)-89 was obtained employing excess NaHMDS, followed by treatment with PhSeCl (Scheme 21). Given these results, we now favor a stereoelectronic argument over steric encumberance at C(16). That is, to generate an enolate at C(16) the C-H bond must approximate a perpendicular orientation to the carboxyl group. Such a conformation, in what appears to be a highly rigid system, would create significant steric interaction between the -OMe of the ester and the C(15) methyl group. Reasoning that the aldehydic proton would experience less steric interaction with the C(15) methyl group, we turned to (-)-86. Treatment with NaHMDS at -78 °C, followed by capture with PhSeCl in fact led to formation of the desired bis-selenide (-)-90 in 65% yield. The success of this reaction was critically dependent on the reaction time and temperature. Enolate formation at both C(9) and C(16) was complete within 5 minutes upon addition of NaHMDS; longer reactions times resulted in near complete decomposition. Attempted oxidative elimination however proved unrewarding! Analysis of the unpurified product by 1H-NMR clearly revealed formation of an exocyclic olefin at C(9); however, there was no evidence for the formation of an olefin at C(16). Further studies along these lines were thus abandoned.
Scheme 21.
Allylic Rearrangement: A Viable Tactic to Introduce the Requisite C(16,17) Olefin
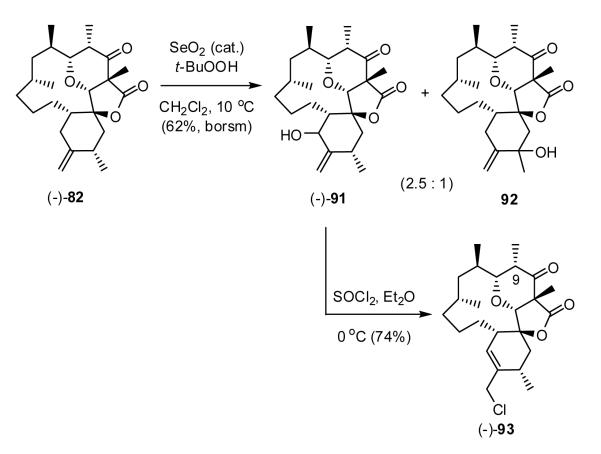
Unable to access C(16,17) unsaturation via either α- or β- esters (-)-3 or (-)-80, we returned to olefin (-)-82, reasoning that allylic oxidation, followed by rearrangement would permit introduction of the required C(16,17) olefin. Toward this end, selenium dioxide mediated allylic oxidation51 of (-)-82 produced the desired allylic alcohol (-)-91 (Scheme 22), albeit in low yield (20-30%), along with the undesired alcohol 92 in 40-50% yield. Attempts to optimize the regioselectivity in favor of (-)-91 by varying the reaction time, concentration, and/or solvent system (cf. methylene chloride, 1,4-dioxane, toluene) were not initially successful. However, by lowering the temperature from 23°C to 10 °C, alcohols (-)-91 and 92 could be obtained as a 1:1 mixture after 36 hours. Shortening the reaction time from 36 to 24 hours resulted in incomplete conversion, furnishing a 2.5:1 mixture of (-)-91 and 92, which could be separated by chromatograph to provide (-)-91 in 62% yield based on recovered starting material. Pleasingly, treatment of (-)-91 with SOCl2 at 0 °C furnished allylic chloride (-)-93 in 74% yield,52 finally securing the requisite C(16, 17)-trisubstituted olefin required for okilactomycin (1).
Scheme 22.
Final Elaboration of (-)-Okilactomycin (1)
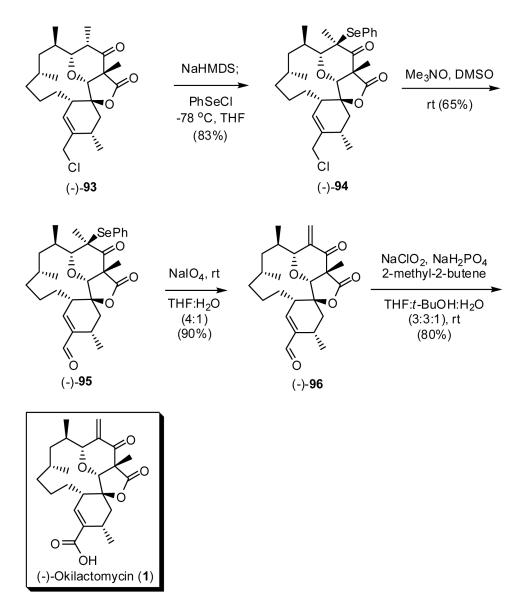
With (-)-93 in hand, treatment with NaHMDS and PhSeCl at -78 °C led to the formation of allyl chloride (-)-94 as a single diasteromer possessing a C(9)-selenide (Scheme 23). We next took advantage of an observation from the Ganem laboratory53 that an allylic chloride can be smoothly converted to the corresponding unsaturated aldehyde by treatment with Me3NO in DMSO at room temperature. Execution of this protocol furnished aldehyde (-)-95 in 65% yield. Subsequent NaIO4 oxidation then led, via elimination of the C(9) selenoxide, to alkene (-)-96, which upon Pinnick oxidation54 completed construction of (-)-okilactomycin (1), identical in all respects (cf. 1H, 13C, HRMS) to an authentic sample obtained from the natural source,1 with the exception of chiroptic properties {[α]D20 = -37 (c = 0.03, MeOH); lit.1 [α]D20 = +34 (c = 1, MeOH)}. The total synthesis of the unnatural enantiomer had thus been achieved. The overall yield for the final four-step sequence was 39%.
Scheme 23.
Summary
The first total synthesis of (-)-okilactomycin (1) has been achieved. Key features of this synthetic venture include a highly diastereoselective oxy-Cope rearrangement/oxidation sequence to secure the C(1) and C(13) stereogenic centers, a Petasis-Ferrier union/rearrangement employed in a sterically encumbered setting to construct the highly congested 2,6-cis-tetrahydropyranone ring, innovative elaboration of the embedded bicyclic lactone, unconventional access to an ester, and an efficient ring closing metathesis reaction to construct the 13-membered macrocyclic ring. Importantly, the synthetic venture not only provides a viable route to this architecturally complex antitumor antibiotic, as well as potential analogs thereof, but also potential access to (-)-chrolactomycin (2) employing the unified strategy. Finally, the absolute stereochemistry of natural (+)-okilactomycin (1) has been established.
Supplementary Material
Scheme 16.
Acknowledgement
Support was provided by the National Institutes of Health (National Cancer Institute) through Grant CA-19033, and by a postdoctoral fellowship (CA-101532) to T.B. We also thank Drs. G. Furst, R. K. Kohli and P. Carroll for assistance in obtaining NMR spectra, high resolution mass spectra, and X-ray crystallographic data, respectively. We also appreciate the assistance of Dr. Jim Hall from AstraZeneca to obtain NMR spectra. Finally we thank the Yamanouchi Pharmaceutical Co., Ltd. (now Astellas Pharmaceutical Co.) for an authentic sample of (+)-okilactomycin.
Footnotes
Supporting Information Available: Experimental procedures, crystallographic information files, complete spectroscopic and analytical data for all new compounds. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Imai H, Suzuki K, Morioka M, Numasaki Y, Kadota S, Nagai K, Sato T, Iwanami M, Saito T. J. Antibiotics. 1987;40:1475. doi: 10.7164/antibiotics.40.1475. [DOI] [PubMed] [Google Scholar]
- 2.Imai H, Nakagawa A, Ōmura S. J. Antibiotics. 1989;42:1321. doi: 10.7164/antibiotics.42.1321. [DOI] [PubMed] [Google Scholar]
- 3.Imai H, Kaniwa H, Tokunaga T, Fujita S, Furuya T, Matsumoto H, Shimizu M. J. Antibiotics. 1987;40:1483. doi: 10.7164/antibiotics.40.1483. [DOI] [PubMed] [Google Scholar]
- 4.Nakai R, Kakita S, Asai A, Chiba S, Akinaga S, Mizukami T, Yamashita Y. J. Antibiot. 2001;54:836. doi: 10.7164/antibiotics.54.836. [DOI] [PubMed] [Google Scholar]
- 5(a).Paquette LA, Boulet SL. Synthesis. 2002:888. [Google Scholar]; (b) Paquette LA, Boulet SL. Synthesis. 2002:895. [Google Scholar]; (c) Takeda K, Shimotani A, Yoshii E. Heterocycles. 1992;34:2259. [Google Scholar]
- 6(a).Petasis NA, Lu SP. Tetrahedron Lett. 1996;37:141.Ferrier RJ, Middleton S. Chem. Rev. 1993;93:2779.For an extension of this methodology to the conversion of cyclopropyl diols to oxepanes, see: O’Neil KE, Kingree SV, Minbiole KPC. Org. Lett. 2005;7:515. doi: 10.1021/ol047426t.
- 7(a).Smith AB, III, Verhoest PR, Minbiole KP, Lim JJ. Org. Lett. 1999;1:909. doi: 10.1021/ol990830l. [DOI] [PubMed] [Google Scholar]; (b) Smith AB, III, Minbiole KP, Verhoest PR, Beauchamp TJ. Org. Lett. 1999;1:913. doi: 10.1021/ol990829m. [DOI] [PubMed] [Google Scholar]; (c) Smith AB, III, Minbiole KP, Verhoest PR, Schelhass M. J. Am. Chem. Soc. 2001;123:10942. doi: 10.1021/ja011604l. [DOI] [PubMed] [Google Scholar]; (d) Smith AB, III, Safonov IG, Corbett RM. J. Am. Chem. Soc. 2002;124:11102. doi: 10.1021/ja020635t. [DOI] [PubMed] [Google Scholar]; (e) Smith AB, III, Sfouggatakis C, Gotchev DB, Shirakami S, Bauer D, Zhu W, Doughty VA. Org. Lett. 2004;6:3637. doi: 10.1021/ol048418f. [DOI] [PubMed] [Google Scholar]; (f) Smith AB, III, Mesaros EF, Meyer EA. J. Am. Chem. Soc. 2005;127:6948. doi: 10.1021/ja051420x. [DOI] [PubMed] [Google Scholar]; (g) Smith AB, III, Simov V. Org. Lett. 2006;8:3315. doi: 10.1021/ol0611752. [DOI] [PubMed] [Google Scholar]; (h) Smith AB, III, Fox RJ, Razler TM. Acc. Chem. Res. 2008;41:675. doi: 10.1021/ar700234r. [DOI] [PubMed] [Google Scholar]
- 8.Smith AB, III, Basu K, Bosanac T. J. Am. Chem. Soc. 2007;129:14872. doi: 10.1021/ja077569l. [DOI] [PubMed] [Google Scholar]
- 9(a).For recent reviews on the utility of the RCM for macrocycle construction, see: Gradillas A, Pérez-Castells J. Angew. Chem., Int. Ed. 2006;45:6086. doi: 10.1002/anie.200600641.Deiters A, Martin SF. Chem. Rev. 2004;126:14720. doi: 10.1021/cr0200872.
- 10.Yao G, Steliou K. Org. Lett. 2002;4:485. doi: 10.1021/ol016943y. [DOI] [PubMed] [Google Scholar]
- 11.Myers AG, Yang BH, Chen H, Mckinstry L, Kopecky DJ, Gleason JL. J. Am. Chem. Soc. 1997;119:6496. [Google Scholar]
- 12.Parikh JR, Doering WVE. J. Am. Chem. Soc. 1967;89:5505. [Google Scholar]
- 13.Evans DA, Bartroli J, Shih TL. J. Am. Chem. Soc. 1981;103:2127. [Google Scholar]
- 14.Gage JR, Evans DA. Org. Synth. 1990;68:83. [Google Scholar]
- 15(a).Evans DA, Golob AM. J. Am. Chem. Soc. 1975;97:4765.For a review on anionic oxy-Cope, see: Paquette LA. Tetrahedron. 1997;53:13971.
- 16.Ireland RE, Varney MD. J. Org. Chem. 1983;48:1829. [Google Scholar]
- 17(a).Kozmin SA, Rawal VH. J. Am. Chem. Soc. 1997;119:7165.Kozmin SA, Rawal VH. J. Org. Chem. 1997;62:5252.Kozmin SA, Rawal VH. J. Am. Chem. Soc. 1999;121:9562.For an early racemic version of this Diels-Alder tactic, see: Smith AB, III, Wexler BA, Tu C-Y, Konopelski JP. J. Am. Chem. Soc. 1985;107:1308.
- 18.Crisp GT, Scott WJ. Synthesis. 1985:335. [Google Scholar]
- 19.Crisp GT, Scott WJ, Stille JK. J. Am. Chem. Soc. 1984;106:7500. [Google Scholar]
- 20(a).Corey EJ, Helal CJ. Angew. Chem. Int. Ed. 1998;37:1986. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]; (b) Cho BT. Aldrichimica Acta. 2002;35:3. [Google Scholar]
- 21(a).Baillargeon VP, Stille JK. J. Am. Chem. Soc. 1983;105:7175. doi: 10.1021/ja00263a015. [DOI] [PubMed] [Google Scholar]; (b) Baillargeon VP, Stille JK. J. Am. Chem. Soc. 1986;108:452. doi: 10.1021/ja00263a015. [DOI] [PubMed] [Google Scholar]
- 22.Corey EJ, Imwinkelried R, Pikul S, Xiang YB. J. Am. Chem. Soc. 1989;111:5493. [Google Scholar]
- 23.Davis FA, Chen BC. Chem. Rev. 1992;92:919. [Google Scholar]
- 24.Gore MP, Vederas JC. J. Org. Chem. 1986;51:3700. [Google Scholar]
- 25(a).Rubottom GM, Vazquez MA, Pelegrina DR. Tetrahedron Lett. 1974;15:4319. [Google Scholar]; (b) Hassner A, Reuss RH, Pinnick HW. J. Org. Chem. 1975;40:3427. [Google Scholar]
- 26.Tsunoda T, Suzuki M, Noyori R. Tetrahedron Lett. 1980;21:1357. [Google Scholar]
- 27.Kurihara M, Hakamata W. J. Org. Chem. 2003;68:3413. doi: 10.1021/jo020471z. [DOI] [PubMed] [Google Scholar]
- 28(a).Wölfling J, Frank É, Mernyák E, Bunkóczi G, Seijo JAC, Schneider G. Tetrahedron. 2002;58:6851.For a recent review on Prins-cyclization, see: Pastor IM, Yus M. Curr. Org. Chem. 2007;11:925.
- 29.Petasis NA, Bzowej EI. J. Am. Chem. Soc. 1990;112:6392. [Google Scholar]
- 30.Grieco PA, Nishizawa M. J. Org. Chem. 1977;42:1717. [Google Scholar]
- 31.Hart DW, Schwartz J. J. Am. Chem. Soc. 1974;96:8115. [Google Scholar]
- 32(a).Matsuda F, Tomiyoshi N, Yanagiya M, Matsumoto T. Tetrahedron. 1988;44:7063. [Google Scholar]; (b) Bogdan S, Joan H, Martin K, Andre —S,D. Tetrahedron. 1987;43:4875. [Google Scholar]; (c) Reich HJ, Clark MC, Willis WW., Jr. J. Org. Chem. 1982;47:1618. [Google Scholar]
- 33.Davis FA, Stringer OD, Billmers JM. Tetrahedron Lett. 1983;24:1213.Other oxidants (e.g. H2O2, m-CPBA, NaIO4) gave poor yields.
- 34.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168. [Google Scholar]
- 35(a).Mander LN, Sethi SP. Tetrahedron Lett. 1983;24:5425.For an example of similar difficulty in C-alkylation see: Rivas F, Ghosh S, Theodorakis EA. Tetrahedron Lett. 2005;46:5281.
- 36.Gaunt MJ, Yu J, Spencer JB. J. Org. Chem. 1998;63:4172. [Google Scholar]
- 37.Xia J, Abbas SA, Locke RD, Piskorz CF, Alderfer JL, Matta KL. Tetrahedron Lett. 2000;41:169. [Google Scholar]
- 38.Goldsmith DJ, John TK, Van Middlesworth F. Synth. Commun. 1980;10:551. [Google Scholar]
- 39.Barco A, Benetti S, Pollini GP. Synthesis. 1973:316. [Google Scholar]
- 40.Hirsenkorn R, Schmidt RR. Leibigs Ann. Chem. 1990:883. [Google Scholar]
- 41(a).Reich HJ, Wollowitz S. In: Organic Reactions. Paquette LA, editor. John Wiley & Sons; New York: 1993. p. 1. [Google Scholar]; (b) Clive DLJ. Tetrahedron. 1978;34:1049. [Google Scholar]; (c) Curtis NR, Holmes AB, Looney MG. Tetrahedron. 1991;47:7171. [Google Scholar]
- 42(a).For selenoxide elimination α to aldehyde, see: Ramachandran SA, Kharul RK, Marque S, Soucy P, Jacques F, Chenevert R, Deslongchamps P. J. Org. Chem. 2006;71:6149. doi: 10.1021/jo0608725.Buynak JD, Ghadachanda VR, Vogeti L, Zhang H, Chen H. J. Org. Chem. 2005;70:4510. doi: 10.1021/jo050004s.Yu J, Wang T, Liu X, Deschamps J, Flippen -A,J, Liao X, Cook JM. J. Org. Chem. 2003;68:7565. doi: 10.1021/jo030006h.Huot JF, Outurquin F, Paulmier C. Chem. Lett. 1991;11:1957.
- 43.Ikemoto N, Schreiber SL. J. Am. Chem. Soc. 1992;114:2524. [Google Scholar]
- 44.Fried J, Sih JC. Tetrahedron Lett. 1973;14:3899. [Google Scholar]
- 45.Sharpless KB, Lauer RF. J. Am. Chem. Soc. 1972;94:7154. [Google Scholar]
- 46.Steiger M, Reichstein T. Helv. Chim. Acta. 1938;21:161. [Google Scholar]
- 47.Baldwin JE, Barton DHR, Faulkner DJ, Templeton JF. J. Chem. Soc. 1962:4743. [Google Scholar]
- 48.Fernandes RA, Kumar P. Tetrahedron Lett. 2003;44:1275. [Google Scholar]
- 49.Carlsen PHJ, Katsuki T, Martin VS, Sharpless KB. J. Org. Chem. 1981;46:3936.Also see: Smith AB, III, Scarborough RM., Jr. Synth. Commun. 1980;10:205.
- 50(a).Meerwein H, Hinz G, Hofmann G, Kroning E, Pfeil E. J. Prakt. Chem. 1937;147:257. [Google Scholar]; (b) Stefan P. Synlett. 2004:195. [Google Scholar]
- 51.Umbreit MA, Sharpless KB. J. Am. Chem. Soc. 1977;99:5526. [Google Scholar]
- 52(a).For representative examples, see: Young WG, Caserio F, Brandon D. Science. 1953;117:473.Young WG, Caserio F, Dennis GE, DeWolfe RH. J. Am. Chem. Soc. 1955;77:4182.Ireland RE, Wrigley TI, Young WG. J. Am. Chem. Soc. 1958;80:4604.Wender PA, Jesudason CD, Nakahira H, Tamura N, Tebbe AL, Ueno Y. J. Am. Chem. Soc. 1997;119:12976.
- 53.Godfrey AG, Ganem B. Tetrahedron Lett. 1990;31:4825.For examples of this rearrangement in synthesis, see: Griffith WP, Jolliffe JM, Ley SV, Springhorn KF, Tiffin PD. Synth. Commun. 1992;22:1967.Paquette WD, Taylor RE. Org. Lett. 2004;6:103. doi: 10.1021/ol0361397.
- 54.Bal BS, Childers WE, Jr., Pinnick HW. Tetrahedron. 1981;37:2091. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.