Abstract
Background and purpose:
Thyroid hormone receptor (TR) agonists are in clinical trials for the treatment of hypercholesterolaemia. As statins are the standard of clinical care, any new therapies must have adjunctive activity, when given in combination with statins. As already known for the statins, the cholesterol lowering effect of TR activation involves increased expression of the low-density lipoprotein receptor. Using animal models, we tested whether TR activation would have additive cholesterol lowering activity in the presence of effective doses of a statin.
Experimental approach:
We evaluated the activity of a liver-targeted prodrug, MB07811, of a novel TH receptor β agonist, MB07344, as monotherapy and in combination with atorvastatin in rabbits, dogs and monkeys.
Key results:
In rabbits, MB07344 (i.v.) decreased total plasma cholesterol (TPC) comparable to that achieved with a maximally effective dose of atorvastatin (p.o.). The addition of MB07344 to atorvastatin resulted in a further decrease in TPC. Similarly, the addition of MB07811 (p.o.) to atorvastatin treatment decreased TPC beyond the level achieved with either agent as monotherapy. In dogs and monkeys, atorvastatin and MB07811 were administered as monotherapy or in combination. Consistent with the rabbit studies, the combination treatment caused a greater decrease in TPC than either MB07811 or atorvastatin administered as monotherapy.
Conclusions and implications:
We conclude that the effects of MB07811 and atorvastatin in lowering cholesterol are additive in animals. These results would encourage and support the demonstration of similarly improved efficacy of combination versus monotherapy with such agents in the clinic.
Keywords: LDL cholesterol, hyperlipidaemia, statins, thyroid receptor agonists
Introduction
According to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines, statins [3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors] should be the first-line therapy to decrease low-density lipoprotein cholesterol (LDL-C) levels in patients with dyslipidaemia (National Cholesterol Education Panel. NHLBI NIoH, 2002). However, despite the overwhelming evidence supporting the efficacy of statins, a high percentage of patients are not reaching target LDL-C goals (Pearson et al., 2000). The 2004 revision of the NCEP recommendations for even lower LDL-C target levels, <1.81 mmol·L−1 (70 mg per 100 mL) for high risk patients (Grundy et al., 2004), and the recent clinical trials demonstrating additional cardiovascular risk reduction with aggressive cholesterol lowering have stimulated interest in novel cholesterol lowering strategies, particularly those that have adjunctive activity when administered in combination with a statin (Denke, 2004).
One cholesterol lowering approach has focused on compounds that activate thyroid hormone nuclear receptors (TRs). Thyroid hormones (TH) (T3 and T4) are known to be important modulators of many aspects of lipid homeostasis and metabolism via activation of specific TR isoforms, which are differentially expressed among tissue types (Yen, 2001). Compounds that activate TRs, in particular the TH receptor β (TRβ) iso form, the predominant liver TH receptor, may have potential for the treatment of hypercholesterolaemia. Support comes from both animal and clinical data in which enhanced TR activation has been associated with a reduction in LDL-C (Abrams and Grundy, 1981; Gullberg et al., 2002; Grover et al., 2003; Morkin et al., 2004; Johansson et al., 2005; Ratliff et al., 2006; Erion et al., 2007; Berkenstam et al., 2008). However, the clinical development of a TR agonist to treat hypercholesterolaemia has been hindered due to dose-limiting cardiotoxicity and adverse effects on the TH axis (Ness et al., 1998; Grover et al., 2003; Morkin et al., 2004). Of particular interest is a recent report that the TRβ agonist, KB2115, has activity to lower cholesterol in the absence of cardiac effects and moderate activity on the thyroid axis in man (Berkenstam et al., 2008).
Although the cholesterol lowering action of a number of TRβ agonists is well described, it is currently unknown whether this activity would be preserved in the presence of an HMG-CoA reductase inhibitor, such as atorvastatin. The action of atorvastatin is mediated via up-regulation of hepatic low-density lipoprotein receptors (LDL-R) resulting from inhibition of cholesterol biosynthesis in the liver (Goldstein and Brown, 1990). Notably, a key pathway responsible for the cholesterol lowering effect of TR agonists is also postulated to be increased LDL-R gene expression, although via a mechanism of direct up-regulation of gene transcription (Lopez et al., 2007). Thus, there is a possibility that TR activation added to a statin would fail to result in a further reduction in cholesterol which would limit its clinical utility. However, since the mechanisms of LDL-R gene regulation are different between the two types of agents, it is also possible there could be additive efficacy provided by combination treatment.
The primary objective of the studies described herein was to determine whether TR activation would have additive activity with respect to cholesterol lowering when administered in combination with an efficacious dose of a statin in the rabbit, dog and monkey models. In the current studies, TR activation was achieved utilizing the clinical candidate MB07811 (Erion et al., 2007). MB07811 is the liver-targeted prodrug of the novel TR agonist 3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)-phenoxy methylphosphonic acid (MB07344) and has been shown to have oral cholesterol lowering activity in a variety of animal models (Erion et al., 2007). MB07344 exhibits a TRβ binding affinity Ki of 2.17 ± 0.03 nmol·L−1, and a Ki TRα/Ki TRβ ratio of 15.8, whereas MB07811 has low TR affinity (>12 µmol·L−1). We report that MB07811/MB07344 had adjunctive activity when given in combination with atorvastatin in all three species. These results are supportive of the potential for TR agonists, such as MB07811, to have clinical utility as a treatment to further lower cholesterol in those patients who do not successfully achieve cholesterol goals with statin treatment alone.
Methods
Animals
All animal studies were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. Animal use protocols for the rabbit and dog studies were approved by the Metabasis Institutional Animal Use and Care Committee (IACUC). Monkey studies were approved by the MPI Research IACUC. Male New Zealand White (NZW) rabbits (2.5–3 kg) (Rabbit Source, Ramona, CA) were maintained on a 7 am–7 pm light schedule with an ad libitum diet of standard lab chow (Purina #5321) or lab chow formulated with 0.2% cholesterol (Research Diets, New Brunswick, NJ). Male and female purpose-bred class A beagle dogs (8–12 kg) (Harlan, IN and Marshall Farms, NY) were maintained on a 7 am–7 pm light schedule and a dog chow diet (Premium Edge, MO) with free access to water. Cynomolgus monkey studies were conducted under contract with MPI Research Inc. (Mattawan, Michigan). Monkeys (male: 4.1–7.5 kg; female: 2.6–3.9 kg) were maintained on a normal chow diet (Lab Diet #5048). Blood samples for cholesterol measurements were obtained from conscious animals via a superficial peripheral vein.
Experimental procedures
Activity of atorvastatin in normocholesterolemic and hypercholesterolaemic rabbits
These studies were designed to determine the dose-response activity of atorvastatin in normocholesterolaemic and diet-induced hypercholesterolaemic rabbits. In the normocholesterolaemic study, NZW rabbits (n = 25) were acclimatized to the facility (>2 weeks) and maintained on a standard Purina #5321 chow diet prior to study initiation. Following baseline cholesterol measurements taken over a 2 week period, animals were assigned (five per group) to the control or atorvastatin treated groups (0.1, 0.3, 1 and 3 mg·kg−1·day−1) such that baseline cholesterol levels were similar among the groups. Total plasma cholesterol (TPC) measurements were repeated at weekly intervals for a total of 3 weeks. In the hypercholesterolaemic study, rabbits were acclimatized on a normal chow diet and baseline TPC measurements made prior to introduction of the 0.2% cholesterol diet. This diet was maintained for approximately 5 weeks with weekly TPC determinations prior to study enrolment. After 5 weeks, animals were separated into four experimental groups (control, atorvastatin 1 mg·kg−1·day−1, atorvastatin 3 mg·kg−1·day−1, and atorvastatin 6 mg·kg−1·day−1) such that average cholesterol levels were similar among the groups. Except for the 6 mg·kg−1·day−1 group, animals were maintained on their respective atorvastatin (AT)-containing diets for an additional 6 weeks. In this latter group (120 p.p.m. atorvastatin in diet), severe reductions in food intake were observed in several animals over 5 days. Consequently, all animals in this group were switched to the 1 mg·kg−1·day−1 atorvastatin diet for days 6–11, and then to the 3 mg·kg−1·day−1 level from day 11 to the end of the study. Body weight and food intake were measured daily in all animals.
Activity of MB07344 as monotherapy and in combination with atorvastatin in normal rabbits
The focus of this experiment was to determine whether parenteral administration of the active TR agonist, MB07344, would have adjunctive activity to atorvastatin in the normal rabbit model. In this study, 32 rabbits (2.6–3.7 kg) were acclimatized for 29 days prior to entrance into the protocol. Baseline daily food intake was monitored and blood samples were collected via a superficial ear vein into potassium EDTA tubes, once on day −7, and again on day 0 to obtain two points for measurement of baseline TPC. Rabbits were randomized into four treatment groups (n = 8 per group) such that the average TPC were similar among the groups. The groups were non-drug control, atorvastatin alone, atorvastatin + MB07344, and MB07344 alone. The duration of the experiment was 5 weeks with blood samples taken weekly from each animal. Animals in the control group were maintained on the normal chow diet for the entire 5 week period with vehicle administration (saline) for the last 3 weeks on the same schedule as the MB07344 treated animals. Animals in the atorvastatin alone group were switched to the atorvastatin containing diet on day 0 and maintained on the diet for the entire 5 week period. These animals received vehicle for the last 3 weeks. Animals in the atorvastatin + MB07344 group were also maintained on the atorvastatin diet from day 0 for 5 weeks, but received MB07344 (0.05 mg·kg−1) via an ear vein 3 times per week for the last 3 weeks. Animals in the MB07344 alone group were maintained on the normal chow diet for the entire 5 week period but were treated with MB07344 (0.05 mg·kg−1) 3 times per week for the last 3 weeks. Body weight and food intake were monitored daily for all animals. There were no significant effects on either body weight or food intake (measured daily) among the experimental groups.
Activity of MB07811 in normal and hypercholesterolaemic rabbits
These experiments were conducted to determine the oral efficacy of MB07811 in the normal and hypercholesterolaemic rabbit models. For the evaluation in normal rabbits, following baseline TPC measurements, animals were randomized into control (n = 6) and MB07811 groups (10 mg·kg−1·day−1, n = 6). Drug treatment (MB07811, 200 p.p.m. in diet) was instituted for 4 weeks with daily monitoring of food intake and body weight. TPC measurements were obtained weekly as previously described. In hypercholesterolaemic rabbits, a separate cohort of rabbits was placed on a diet containing 0.2% cholesterol. After 4 weeks, when TPC levels had increased to 13.0–15.5 mmol·L−1, animals were randomized to control (n = 7) and MB07811-treated (n = 8) experimental groups. MB07811 animals were treated with MB07811 (added to 0.2% cholesterol diet) at a dose of 5 mg·kg−1·day−1 for 2 weeks followed by 10 mg·kg−1·day−1 for an additional 5 weeks. TPC, body weight and food intake measurements were obtained weekly.
Adjunctive activity of MB07811 in combination with atorvastatin in normocholesterolaemic rabbits
To evaluate the oral activity of MB07811 when administered as adjunctive treatment to atorvastatin, an experimental design similar to the MB07344 ± atorvastatin study described above was employed. The experimental groups (n = 6/group) were non-drug control, atorvastatin alone (3 mg·kg−1·day−1), MB07811 alone (10 mg·kg−1·day−1) and atorvastatin (3 mg·kg−1·day−1) + MB07811 (10 mg·kg−1·day−1). The duration of atorvastatin exposure prior to initiation of MB07811 treatment was increased to 3 weeks to ensure that TPC levels were stable prior to administration of MB07811. All other aspects of the protocol were identical to the MB07344 ± atorvastatin study. As with the first combination study, there were no significant effects on either body weight or food intake among the experimental groups.
MB07811 and atorvastatin monotherapy/combination efficacy in dogs
A study was conducted in six male and six female dogs utilizing a three-way crossover design consisting of two male and two female dogs/group, three treatment groups/cycle, and three treatment cycles. The experimental groups were MB07811 (30 mg·kg−1·day−1), atorvastatin (10 mg·kg−1·day−1) or a combination of MB07811 (30 mg·kg−1·day−1) and atorvastatin (10 mg·kg−1·day−1) administered by oral gavage daily for seven consecutive days. The dose of atorvastatin was chosen as 10 mg·kg−1·day−1 as this has been determined to be the no-adverse-effect level in chronic studies in dogs (C. Wilker, pers. comm.). A washout period of a minimum of 14 days was imposed between each cycle. With this experimental design, each dog received all three treatment regimens over the course of the study. Venous blood samples for blood cholesterol measurements were obtained in each animal at t = 0 h before the first dose (pre-dose) and then 24 h after the 7th dose for each treatment cycle. Both MB07811 and atorvastatin were formulated as uniform suspensions in 0.5% carboxymethyl cellulose, and 1% Lutrol in water and administered by oral gavage at 2 mL·kg−1.
MB07811 and atorvastatin monotherapy efficacy in monkeys
Six male and six female cynomolgus monkeys were instrumented with electrocardiogram leads, pressure transducers, and telemetry units and allowed to recover for at least 1 week prior to study initiation. MB07811 (0.03–30 mg·kg−1·day−1 in half-log increments), atorvastatin (0.3–30 mg·kg−1·day−1 in half-log increments) or vehicle (polyethylene glycol 400) was administered orally to groups of three male and three female monkeys on a daily basis for 7 days. Blood was drawn prior to dosing on day 1 (pre-dose) and approximately 24 h after the final dose on day 8 to assess serum cholesterol and clinical chemistry parameters. Treatment stopped on day 8 and the animals were subjected to a washout period of at least 7 days prior to the next treatment. Animals were cycled through 6–8 treatments over the course of the study. Vehicle was administered on two separate cycles, so all animals received vehicle.
MB07811 and atorvastatin monotherapy/combination efficacy and pharmacokinetics in monkey
A study was conducted to evaluate both the cholesterol lowering activity and pharmacokinetics of MB07811 and atorvastatin alone and in combination in cynomolgus monkeys. A total of six male and six female non-naïve cynomolgus monkeys were assigned to the study. Groups of four monkeys (two males and two females) were placed under one of three oral treatment regimens: (i) 3 mg·kg−1·day−1 of MB07811 for 8 days; (ii) 3 mg·kg−1·day−1 of atorvastatin for 8 days; and (iii) a combination of 3 mg·kg−1·day−1 of MB07811 and 3 mg·kg−1·day−1 of atorvastatin for 8 days. The vehicle for all treatments was polyethylene glycol-400. Prior to treatment and at 24 h following the 7th dose, blood samples were collected from the femoral vein or artery and serum prepared for analysis of total cholesterol. Blood was also collected on the final day (day 8) of compound administration at 0 (pre-dose), 0.5, 1, 2, 4, 8, 12 and 24 h post last dose (day 9) for each regimen for pharmacokinetic evaluation. For this latter purpose, plasma proteins from plasma samples (50 µL) were precipitated by addition of methanol (75 µL). After 20 min of centrifugation (Eppendorf microfuge) at 1500× g and room temperature, the resulting supernatant was collected and concentrations of MB07811, AT and their respective active metabolites MB07344 and hydroxy-atorvastatin were measured by liquid chromatography-tandem mass spectroscopy (LC-MS/MS) as described below. Non-compartmental pharmacokinetic analysis was performed on the resulting plasma concentration-time profiles for each analyte using WinNonLin (ver. 1.1; Scientific Consultants, Cary, NC). The pharmacokinetic variables measured were defined as follows: AUClast, the area under the curve from the time of dosing (t = 0) to the last measurable concentration (24 h); Cmax, maximum observed concentration; MRT, mean residence time from the time of dosing to the time of the last measurable concentration and t1/2, terminal half-life estimated via linear regression of the time versus log concentration plot.
Following washout periods of 2 weeks, the monkeys were crossed over (three-way crossover design) into different treatment groups such that each group of four animals received all three dosing regimens.
LC-MS/MS analysis
A 10 µL plasma extract aliquot was injected onto a Gemini C18 column (5 µm, 2 × 50 mm, Phenomenex) fitted with a Gemini C18 guard column (5 µm, 4.0 × 3.0 mm, Phenomenex, Torrance, CA) and eluted with a gradient consisting of mobile phase A (20 mmol·L−1 N,N-dimethylhexylamine and 10 mmol·L−1 propionic acid in 20% methanol) and B (20 mmol·L−1 N,N-dimethylhexylamine and 10 mmol·L−1 propionic acid in 80% methanol) at a flow rate of 0.4 mL·min−1 (0 min, 60% B; 0–0.5 min, 60–100% B; 0.5–6 min, 100% B; 6–6.1 min, 100–60% B; 6.1–9 min, 60% B). The injector temperature was set at 10°C. Elution times for MB07344, MB07811, atorvastatin and hydroxyatorvastatin were approximately 2.7, 4.9, 2.3 and 2.2 min respectively. MB07811, MB07734, AT and hydroxyatorvastatin were detected using the MS/MS mode (513/63.1 for MB07811, 363.3/63.1 for MB07344, 557.5/278.4 for AT and 573.5/278.4 for hydroxyatorvastatin) and quantified by comparison of peak areas to standard curves obtained by adding known concentrations of the analytes to blank monkey plasma. Calibration curves ranging from 1 to 3000 ng·mL−1 for MB07344 and MB07811 (LOQ of 1 ng·mL−1) and from 0.1 to 3000 ng·mL−1 for atorvastatin (LOQ of 0.1 ng·mL−1) were generated. Although there is more than one possible isomer of hydroxyatorvastatin, this assay could not distinguish between them. As hydroxyatorvastatin standards were not available, plasma concentrations of this analyte were estimated using the atorvastatin calibration curve.
Data analysis
Statistical analyses
The data are presented as mean with the standard error of the mean (SEM) or standard deviations (SD) as indicated. To determine treatment effects, statistical analysis was performed in two ways: paired t-test, animal-by-animal comparison in which measurements were made in each animal before and after each intervention (i.e. before and after MB07811 or atorvastatin); or multiple-group comparison with one-way analysis of variance (anova) applied to three or more treatment groups. P-values of less than 0.05 were considered statistically significant and are indicated by asterisks in the graphs.
Materials
MB07344 was synthesized (Erion et al., 2004) at Metabasis Therapeutics, formulated in saline and sterile filtered for intravenous administration. Atorvastatin was purchased from ChemPacific Corporation (Baltimore, MD) and formulated at 2, 6, 20, 60 and 120 p.p.m. in either Purina #5321 normal rabbit chow or Purina #5321 supplemented with 0.2% cholesterol (Research Diets, New Brunswick, NJ). These concentrations were calculated to give an atorvastatin exposure of 0.1–6 mg·kg−1·day−1 based on rabbit food consumption of 50 g·kg−1·day−1. Diet concentrations of atorvastatin of 3 mg·kg−1·day−1 (60 p.p.m.) and lower were well tolerated with minor effects (<10% decrease) on food consumption. MB07811 was synthesized (Erion et al., 2004) at Metabasis Therapeutics and formulated at 1, 3, 10 mg·kg−1·day−1 (20, 60 and 200 p.p.m.) in Purina #5321 or 0.2% cholesterol supplemented rabbit chow (Research Diets, New Brunswick, NJ). These concentrations were also well tolerated with minor effects on food consumption (<10% decrease) and no effects on body weight. Infinity Cholesterol Liquid Stable Reagent Kit (Lot: D04362) and cholesterol standard for measurement of TPC were purchased from ThermoDNA (Arlington, TX) and used for all rabbit studies. Measurements of cholesterol for the dog studies were conducted by a commercial diagnostic laboratory (IDEXX, Sacramento, CA). Cholesterol measurements for the monkey studies were determined with an automated analyser (Olympus AU600) at MPI Research.
Results
Rabbit studies
Activity of atorvastatin in normocholesterolaemic rabbits
As shown in Figure 1, oral treatment of normocholesterolaemic animals with atorvastatin from 0.1 to 3 mg·kg−1·day−1 resulted in a dose-dependent reduction in TPC, with equivalent effects in the dose range of 0.3–3 mg·kg−1·day−1. At the dose of 3 mg·kg−1·day−1, TPC fell by 31 ± 9% compared with pre-treatment baseline values which is similar in magnitude to that reported in published studies evaluating atorvastatin in the normolipidaemic rabbit (Alegret et al., 1998; Rashid et al., 2002). In a pilot study (data not shown) a dose of 10 mg·kg−1·day−1 of atorvastatin was not well tolerated and resulted in a large (>75%) reduction in food intake and clinical signs of hepatic toxicity. Based on these findings, the dose level of 3 mg·kg−1·day−1 was chosen as the maximally efficacious and tolerated dose of atorvastatin to be used in subsequent studies.
Figure 1.
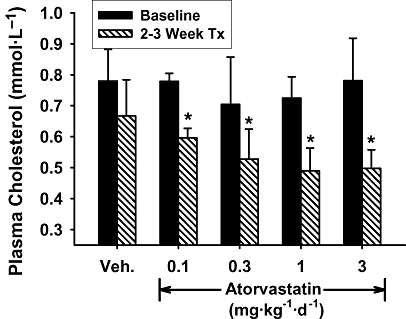
Dose-dependent effect of oral atorvastatin (AT) in normal rabbits. (A) Rabbits (n = 5 per group) on a normal chow (NC) diet. Shown are average total plasma cholesterol (TPC) values at baseline and following 2–3 weeks of treatment. The decrease in TPC produced by atorvastatin was similar at doses of 0.3 to 3 mg·kg−1·day−1. Values are mean ± SEM. *P < 0.05 for treatment vs. baseline.
Activity of MB07344 as monotherapy and in combination with atorvastatin in normal rabbits
The objective of this rabbit study was to determine if MB07344 would have adjunctive activity to a maximally efficacious dose of atorvastatin. The four experimental groups (vehicle control, atorvastatin alone, MB07344 alone and MB07344 + atorvastatin combination) and timing of drug treatment are depicted in Figure 2A. As shown in Figure 2B, cholesterol levels in the model were quite stable as TPC in vehicle control animals remained within 10% of baseline levels for the duration of the 5 week protocol. As observed previously, animals treated with atorvastatin alone exhibited a significant (P < 0.05) and stable reduction in TPC of 30–35% compared with baseline levels. The animals in the combination group, prior to MB07344 administration (weeks 1–2), exhibited a similar decrease in cholesterol. Importantly, MB07344 treatment administered as an adjunct to atorvastatin (starting at week 2) in the combination group was associated with a further significant reduction in TPC to 55 ± 8% compared with baseline. As shown in Figure 2C, MB07344 treatment as monotherapy starting at week 2 in the MB07344-alone group resulted in a decrease of 33–36% compared with baseline, which was comparable to that achieved with atorvastatin alone (Figure 2B).
Figure 2.
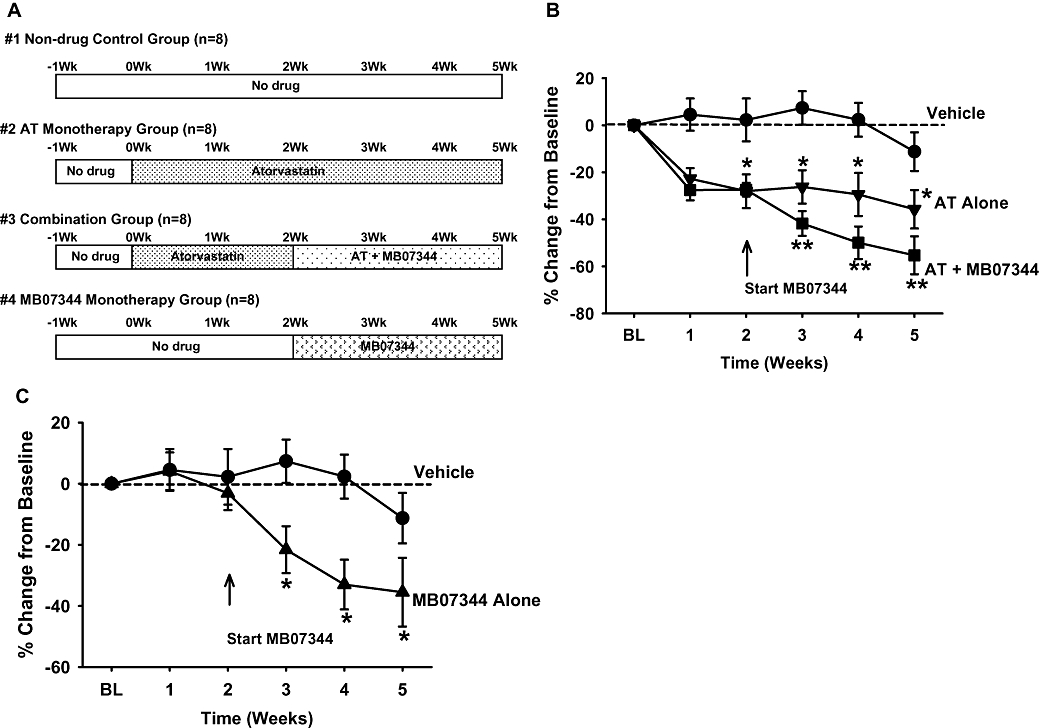
Evaluation of activity of MB07344 as monotherapy and in combination with atorvastatin in normal rabbits. (A) Protocol scheme: Figure depicts the treatment timeline for the four experimental groups. In the combination group, adjunctive MB07344 treatment was added to atorvastatin after 2 weeks of atorvastatin treatment. (B) Average TPC in the vehicle control, atorvastatin alone and atorvastatin + MB0344 groups over the course of the protocol. Atorvastatin as monotherapy resulted in a significant (*P < 0.05) and stable reduction in TPC. The administration of MB07344 as an adjunct to atorvastatin starting at 2 weeks in the combination group resulted in a significant further decrease in TPC (**P < 0.05 vs. atorvastatin alone). Values are mean ± SEM. (C) Average TPC in the MB07344 monotherapy group: MB07344 treatment was associated with a significant reduction in TPC compared with baseline levels (*P < 0.05). Also depicted are the data for the Vehicle Control group for comparison. Values are mean ± SEM.
Activity of MB07811 in normal rabbits
The studies described above supported the concept that parenteral administration of MB07344 had additive cholesterol lowering activity to a maximal dose of atorvastatin in the rabbit model. However, it is known that this compound has poor oral bioavailability (Erion et al., 2007). Thus, an orally available prodrug of MB07344, MB07811 which is the clinical candidate, was also studied in these rabbit models. As shown in Figure 3, oral administration of MB07811 at 10 mg·kg−1·day−1 to rabbits on a normal chow diet resulted in a marked and significant reduction in TPC of 35–42% within 2 weeks that was sustained to the end of the 4 week evaluation.
Figure 3.
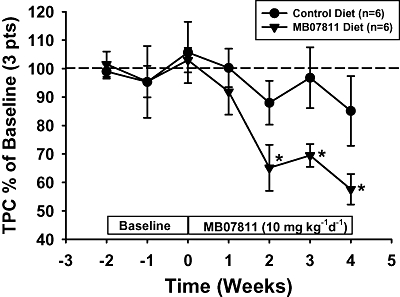
Activity of MB07811 on TPC in normal rabbits: average TPC data (% of baseline) in rabbits on normal chow in the vehicle and MB07811-treated groups. Oral MB07811 (10 mg·kg−1·day−1) resulted in a significant (*P < 0.05) reduction in TPC within 2 weeks of treatment. Values are mean ± SEM.
Adjunctive activity of MB07811 in combination with atorvastatin in normocholesterolaemic rabbits
As MB07811 administered as monotherapy was able to lower TPC in rabbits, a study was performed to evaluate the adjunctive activity of orally administered MB07811 to atorvastatin-treated rabbits. The results from this study are shown in Figure 4. Initial baseline TPC concentrations were similar among the four experimental groups and ranged from 1.11 to 1.24 mmol·L−1. Similar to previous observations, TPC values remained stable in animals in the vehicle control group whereas treatment with atorvastatin as monotherapy at 3 mg·kg−1·day−1 resulted in a significant and stable reduction (≈40%) in TPC over the duration of the 5 week protocol (data not shown). In the atorvastatin + MB07811 combination group, the baseline TPC of 1.24 ± 0.28 mmol·L−1 was significantly decreased to 0.82 ± 0.16 mmol·L−1 with the addition of atorvastatin (32% decrease). Adjunctive treatment with oral MB07811 resulted in a further decrease in TPC to 0.47 ± 0.08 mmol·L−1 which was 59 ± 3% of baseline levels.
Figure 4.
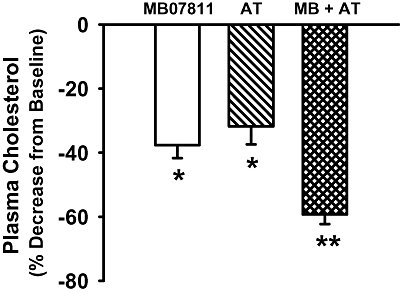
Additive effect of MB07811 in combination with atorvastatin in normal rabbits. Average % decrease in TPC from baseline in the MB07811 monotherapy group, and the atorvastatin ± MB07811 combination group (with atorvastatin alone and with MB07811 added). In the combination group, atorvastatin treatment alone resulted in a significant decrease in TPC (*P < 0.05 vs. baseline) which was further significantly decreased with the addition of MB07811 (**P < 0.05 vs. atorvastatin alone). Values are mean ± SEM.
Activity of atorvastatin and MB07811 in hypercholesterolaemic rabbits
In contrast to the results in normocholesterolaemic rabbits, atorvastatin was without pronounced cholesterol lowering effect in the cholesterol-fed rabbit (Figure 5A). These animals exhibited an average increase in TPC to approximately 13.0–15.5 mmol·L−1 after 5 weeks on the 0.2% cholesterol enriched diet. After an additional 6 weeks, there was a further increase in cholesterol in control animals to over 23.3 mmol·L−1, whereas animals treated with atorvastatin did not increase further. Notably, in these animals atorvastatin treatment was not associated with dose-dependent effects nor pronounced cholesterol lowering activity even at the highest tolerated dose (3 mg·kg−1·day−1) in contrast to what was observed in chow-fed rabbits. This lack of pronounced effect of statins in the cholesterol-fed rabbit is an expected result based on the literature (Bocan et al., 2001; Bolayirli et al., 2007). In a separate group of animals on the high cholesterol diet with TPC values >13 mmol·L−1, the administration of MB07811 at 10 mg·kg−1 was associated with a significant decline of cholesterol below pre-treatment levels and compared with control animals (Figure 5B). However, in contrast to what was observed in normal animals, longer exposure times were required (4–5 weeks).
Figure 5.
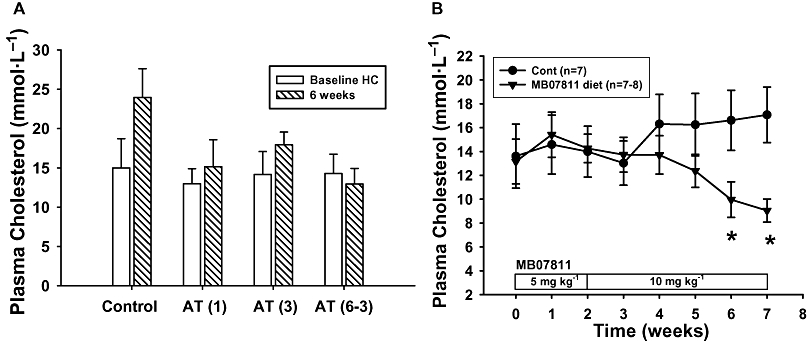
Effect of atorvastatin and MB07811 in hypercholesterolaemic rabbits. (A) Effect of atorvastatin in hypercholesterolaemic rabbits on a 0.2% cholesterol diet. Shown are average TPC values of hypercholesterolaemic (HC) rabbits at baseline and after 6 weeks of treatment with various doses of atorvastatin. Animals in the atorvastatin (6–3) group did not tolerate the 6 mg·kg−1·day−1 dose and were switched to 1 mg·kg−1·day−1 for days 6–11 and then to 3 mg·kg−1·day−1 from day 11 to the end of the study. (B) Effect of MB07811 in hypercholesterolaemic rabbits on a 0.2% cholesterol diet. MB07811 treatment was associated with a significant reduction in TPC (*P < 0.05). Values are mean ± SEM.
Dog studies
In the three-way crossover study of MB07811 and atorvastatin conducted in beagle dogs, the average pre-dose cholesterol levels were not significantly different between groups (MB07811: 4.66 ± 0.23 mmol·L−1; atorvastatin: 4.73 ± 0.34 mmol·L−1; MB + atorvastatin: 4.97 ± 0.36 mmol·L−1). The animal-by-animal responses to the three treatments are shown in Figure 6A. All animals exhibited a clear decrease in cholesterol in response to MB07811 treatment whereas the response to atorvastatin was more modest. Notably, all animals exhibited a marked decrease in cholesterol with combined treatment, irrespective of starting cholesterol levels. The 7 day treatment with either MB07811 or atorvastatin resulted in a significant decrease in serum cholesterol compared with pre-dose levels (P < 0.01) (Figure 6B). Importantly, and in agreement with observations in the rabbit, the combination treatment was associated with a significantly greater decrease in cholesterol compared with either MB07811 or atorvastatin given as monotherapy (P < 0.01).
Figure 6.
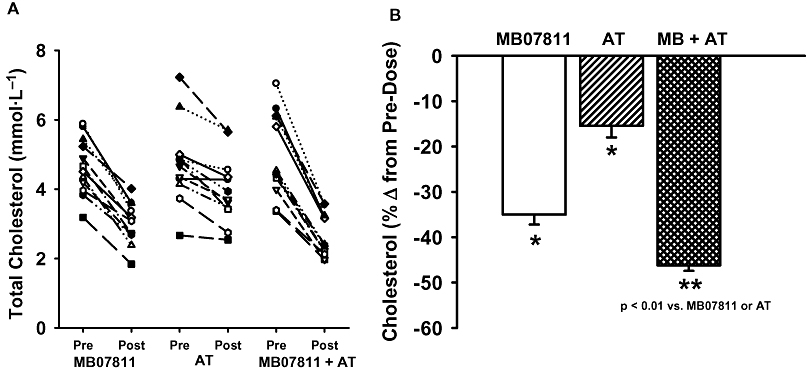
Additive effect of MB07811 in combination with atorvastatin in normal dogs. (A) Animal-by-animal (n = 12) serum cholesterol at pre-dose (pre) and following 7 days of treatment (post) with MB07811, atorvastatin or atorvastatin + MB07811 in combination. Combined treatment resulted in a marked decrease in cholesterol regardless of baseline values in all animals. (B) Data shown are the average (n = 12) % changes in serum cholesterol following 7 days of treatment (post) compared with pre-dose levels taken just prior to the first dose of each cycle. Both MB07811 and atorvastatin given as monotherapy resulted in significant decreases in cholesterol compared with pre-dose levels (*P < 0.01). Notably, the combination treatment resulted in a significantly greater decrease in cholesterol compared with either MB07811 or atorvastatin given alone (**P < 0.01). Values are mean ± SEM.
Monkey studies
MB07811 and atorvastatin monotherapy efficacy in monkey
As shown in Figure 7A, MB07811 lowered total cholesterol in cynomolgus monkeys by 34% after 7 days of treatment with a dose of 30 mg·kg−1·day−1. The effects were dose related with a minimum efficacious dose of 0.1 mg·kg−1·day−1 resulting in a 23% decrease in total cholesterol. Atorvastatin demonstrated similar maximal efficacy (33% decrease in total cholesterol at 30 mg·kg−1·day−1), but had a higher minimal efficacious dose (10 mg·kg−1·day−1) than MB07811 when administered orally. No significant cardiovascular effects were observed with either MB07811 or atorvastatin. Based on these results an intermediate dose of 3 mg·kg−1·day−1 was chosen for a subsequent study evaluation of the efficacy and PK profiles of MB07811 and atorvastatin given as monotherapy and in combination.
Figure 7.
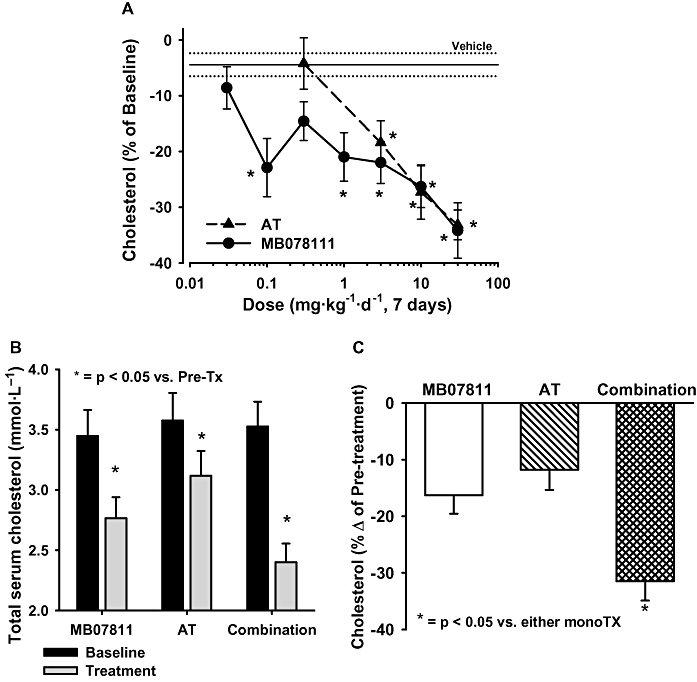
Activity of MB07811 and atorvastatin as monotherapies and in combination in normocholesterolaemic monkeys. (A) Dose-response effects of MB07811 and atorvastatin. Both agents resulted in dose-related decreases in serum cholesterol after 7 days of treatment (*P < 0.05 vs. pre-treatment baseline). (B) Average pre- and post-treatment serum cholesterol in the three experimental groups. For all groups, post-treatment values were significantly decreased versus the pre-treatment level (P < 0.05). (C) Cholesterol levels expressed as % change from baseline. There was a significant decrease in cholesterol in the combination group compared with that achieved with either the MB07811 or atorvastatin monotherapy (*P < 0.05). Values are mean ± SEM.
MB07811 and atorvastatin pharmacokinetics in monkey
The average pharmacokinetic parameters derived from the individual concentration-time profiles of each analyte (MB07811, MB07344, atorvastatin and hydroxyatorvastatin) are shown in Tables 1 and 2. There were no significant differences in the pharmacokinetics of either MB07811 or atorvastatin and their respective metabolites when given in combination compared with each as monotherapy. In addition, the MB07344/MB07811 and hydroxyatorvastatin/atorvastatin ratios of AUClast values observed after monotherapy treatment (i.e. 29 and 1.7 respectively) remained relatively unchanged following combination therapy (i.e. 28 and 1.3 respectively) in male or female animals. These data indicate that the major routes of absorption, metabolism and elimination of MB07811 and atorvastatin were largely unaffected by MB07811 and atorvastatin co-administration.
Table 1.
Pharmacokinetic parameters for MB07811 and MB07344 following MB07811 monotherapy or in combination with atorvastatin (AT) on day 8 in monkeys
| Group | Analyte | AUClast (µg·h·mL−1) | Cmax (µg·mL−1) | MRT (h) | t1/2 (h) |
|---|---|---|---|---|---|
| MB07811 | MB07811 | 0.07 ± 0.06 | 0.012 ± 0.006 | 4.47 ± 2.51 | 4.09 ± 1.35 |
| MB07811 + AT | MB07811 | 0.09 ± 0.10 | 0.021 ± 0.020 | 4.50 ± 1.75 | 5.35 ± 4.10 |
| MB07811 | MB07344 | 2.05 ± 0.81 | 0.19 ± 0.10 | 8.47 ± 1.17 | 7.29 ± 2.20 |
| MB07811 + AT | MB07344 | 2.55 ± 1.22 | 0.25 ± 0.11 | 7.73 ± 1.47 | 6.83 ± 3.23 |
Data shown in Table are means ± SD from 12 animals per group.
AUClast, area under the curve; Cmax, maximum observed concentration; MRT, mean residence time; t1/2, terminal half-life.
Table 2.
Pharmacokinetic parameters for atorvastatin (AT) and hydroxyatorvastatin (AT-OH) following atorvastatin monotherapy or in combination with MB07811 on day 8 in monkeys
| Group | Analyte | AUClast (µg·h·mL−1) | Cmax (µg·mL−1) | MRT (h) | t1/2 (h) |
|---|---|---|---|---|---|
| AT | AT | 0.06 ± 0.02 | 0.009 ± 0.005 | 4.47 ± 2.51 | 8.00 ± 1.47 |
| MB07811 + AT | AT | 0.07 ± 0.04 | 0.013 ± 0.009 | 6.98 ± 1.41 | 7.56 ± 2.92 |
| AT | AT-OH | 0.10 ± 0.07 | 0.007 ± 0.004 | 10.23 ± 1.37 | 14.14 ± 3.57 |
| MB07811 + AT | AT-OH | 0.09 ± 0.06 | 0.008 ± 0.004 | 8.71 ± 1.72 | 10.62 ± 4.35 |
Data shown in Table are means ± SD from 12 animals per group.
AUClast, area under the curve; Cmax, maximum observed concentration; MRT, mean residence time; t1/2, terminal half-life.
Activity of MB07811 as monotherapy and in combination with atorvastatin in normocholesterolaemic monkeys
As shown in Figure 7B, MB07811 (3 mg·kg−1·day−1) or atorvastatin (3 mg·kg−1·day−1) treatment of monkeys for 7 days significantly lowered total serum cholesterol by 0.62 ± 0.10 and 0.41 ± 0.10 mmol·L−1 respectively. Importantly, as was seen in rabbits, the administration of MB07811 and atorvastatin in combination resulted in a significantly more pronounced reduction in total serum cholesterol than that observed with either test compound alone, a 1.01 ± 0.13 mmol·L−1 (32 ± 3%) decrease from baseline values (Figure 7B,C).
Discussion
Statins decrease cholesterol by inhibition of de novo mevalonate synthesis from acetyl CoA via the rate-limiting HMG-CoA reductase enzyme and by up-regulation of LDL-R thereby increasing hepatocyte cholesterol uptake (Slater and MacDonald, 1988; Goldstein and Brown, 1990; Qin et al., 1992). Increased LDL-R expression also plays a role in the cholesterol lowering effects of TH and synthetic TR agonists (Staels et al., 1990; Ness and Lopez, 1995; Bakker et al., 1998; Lopez et al., 2007) that can be mediated directly via a thyroid responsive element on the hepatic LDL-R promoter (Lopez et al., 2007) as well as secondarily through activation of SREBP-2 (Shin and Osborne, 2003).
As nearly all patients with hypercholesterolaemia are prescribed statins (≈95%), with the majority not reaching recommended target LDL-C levels, we evaluated whether TR activation in combination with a statin could produce a further reduction in cholesterol beyond a statin alone. The common mechanism of increased LDL-R involved in the cholesterol lowering action of both statins and TR activation brings into question whether their activity would indeed be additive. To our knowledge, there are no publications demonstrating the additive activity of TR activation in combination with a statin. To evaluate this question in the present studies, MB07344 and MB07811 were tested alone and in combination with atorvastatin in rabbit, dog and monkey animal models. The rabbit is a widely utilized animal model in atherosclerosis research and is responsive to a variety of cholesterol lowering agents including HMG-CoA reductase inhibitors and bile acid-binding resins (Bocan, 1998; Aikawa and Libby, 2000; Rashid et al., 2002). Although chow-fed dogs are not a model of atherosclerosis, there is precedence for their use to evaluate the activity of cholesterol lowering agents in combination with a variety of statins (Davis et al., 2001). Non-human primates are also frequently employed to evaluate the efficacy of potential anti-hyperlipidaemic therapeutics and have been shown to be responsive to statins as well as TR agonists (Bocan, 1998; Grover et al., 2003). Employing these three animal models we report a consistent finding that TR activation with MB07344/MB07811 results in adjunctive and additive activity when given in combination with atorvastatin.
Potential drug–drug interactions
The conversion of atorvastatin and its inactive metabolite, atorvastatin lactone, to their corresponding hydroxy acids by CYP3A4 is their major clearance pathway in vivo (Jacobsen et al., 2000). Similarly, the activation of the MB07811 prodrug to the active TR agonist MB07344 is dependent upon hepatic CYP3A4-mediated cleavage (Erion et al., 2004; 2007). In recognition of this common pathway, a potential drug–drug interaction was assessed early in the development of MB07811. In vitro studies indicated that neither MB07344 nor MB07811 inhibited CYP3A-mediated testosterone-6β-hydroxylation in human liver microsomes (Fujitaki, unpubl. obs.). Additionally, results from the monkey study reported herein showed no change in the plasma AUC or Cmax of either drug or their metabolites when administered in combination for 8 days at therapeutic doses, when compared with each drug administered individually. These results indicate that MB07811 does not suppress or induce CYP3A-mediated metabolism of atorvastatin. This finding is not surprising given that hepatic concentrations of HepDirect prodrugs rise only transiently and remain far below their Km for CYP3A due to efficient conversion to the phosphonic acid.
Normocholesterolaemic rabbits, dogs and monkeys
In the present studies, atorvastatin decreased TPC in normal chow-fed rabbits producing a maximal decrease of approximately 31% at the maximally tolerated dose of 3 mg·kg−1·day−1. Importantly, both MB07344 and MB07811 further reduced cholesterol when given as an adjunctive treatment to this dose of atorvastatin resulting in decreases in cholesterol to 55% and 59% of baseline respectively (Figures 2 and 4). Additional support for this adjunctive activity was provided from complimentary data obtained in the dog as well as the monkey models (Figures 6 and 7). Consistent with data previously obtained in the rodent (Erion et al., 2007), MB07811 treatment of monkeys also resulted in dose-dependent cholesterol lowering activity (Figure 7A). Importantly, the magnitude of this response was similar to that observed with atorvastatin, over the range of tested doses.
There are many results that elucidate the possible mechanisms of this adjunctive activity. Cholesterol homeostasis is maintained by coordinate regulation of three major pathways: (i) de novo synthesis in which HMG-CoA reductase is the rate limiting step; (ii) cholesterol uptake via LDL-R; and (iii) cholesterol elimination via the formation of bile acids. The activity of MB07344/MB07811 is unlikely to be mediated via effects on the de novo synthetic pathway since statins are potent HMG-CoA enzyme inhibitors and the reported effect of T3 or synthetic TR agonists on hepatic HMG-CoA reductase is reported to be up-regulation (Sample et al., 1987; Ness et al., 1998). In contrast, there is considerable literature implicating LDL-R in mediating the cholesterol-modifying effects of TH. Consistent with this concept, we previously reported that MB07811 treatment increases LDL-R mRNA expression in euthyroid SD rats, diet-induced obese mice and thyroidectomized SD rats (Erion et al., 2007). The third cholesterol regulatory pathway is the biliary elimination route. Bile acid synthesis is the major pathway of cholesterol degradation in mammals and approximately 40% of cholesterol removal from the body occurs via degradation to bile acids (Vlahcevic et al., 1991). It is also known that CYP7A is the rate-limiting enzyme in the conversion of cholesterol to bile acids (Chiang, 2004). TH plays an important role in the regulation of CYP7A and positively regulates gene expression in rats and rat hepatocytes (Hylemon et al., 1992; Ness and Lopez, 1995; Pandak et al., 1997). As we showed previously that MB07811 treatment of rats also results in a significant up-regulation of CYP7A in euthyroid animals (Erion et al., 2007), it is plausible that the MB07344/MB07811-induced decrease in cholesterol observed in the present studies could be in part mediated via the up-regulation of CYP7A. Notably, increased bile acid synthesis is associated with administration of the TR agonist KB2115 in man (Berkenstam et al., 2008). Of relevance to the current study, it is known that inhibition of cholesterol synthesis with a statin results in a decrease in CYP7A activity, enzyme mass, steady-state mRNA levels and bile acid synthesis (Pandak et al., 1990; Jones et al., 1993). One can speculate that this may be a limiting factor with respect to statin cholesterol lowering activity, which may be overcome with the addition of a TR agonist such as MB07811.
Activity in hypercholesterolaemic rabbits
Differences in the cholesterol lowering mechanism of MB07344/MB07811 compared with statins are highlighted by findings of others and our studies here showing that atorvastatin does not have a pronounced lowering effect on cholesterol in rabbits fed a high cholesterol diet, even when administered at maximally tolerated doses (Bocan et al., 2001; Bolayirli et al., 2007). The relative lack of effect of HMG-CoA reductase inhibitors in the cholesterol-fed model is presumably due to down-regulation of SREBP-2, LDL-R, and reduced activity of de novo cholesterol synthetic enzymes including HMG-CoA synthase and reductase (Horton et al., 2002). Importantly, it was demonstrated here, that in contrast to atorvastatin, MB07811 caused similar cholesterol lowering in the diet-induced hypercholesterolaemic condition as it did in normocholesterolaemic animals. It should be noted that neither MB07344 nor MB07811 treatment were associated with changes in body weight or food intake commonly observed with hyperthyroidism in animals and man and is consistent with a liver-targeted effect. Limited non-hepatocyte uptake of MB07344, as well as the role of TRα1 regulation of food intake (Pantos et al., 2007), are possible explanations. Although not the focus of the studies described here, the reduced effect of MB07811 on the TH axis compared with KB-141 or T3 has been previously demonstrated (Erion et al., 2007) and is also consistent with a liver-targeted activity.
There is also a body of literature implicating CYP7A in the known exaggerated response to dietary cholesterol in rabbits. Unlike rodents, in which cholesterol feeding increases bile acid synthesis by up-regulating CYP7A mRNA and activity (Jelinek et al., 1990; Jones et al., 1993), cholesterol feeding decreases CYP7A in rabbits (Xu et al., 1995; 1996), monkeys (Rudel et al., 1994) and hamsters (Horton et al., 1995). Further, increasing hepatic CYP7A by bile acid depletion was shown to decrease plasma cholesterol in both NZW and Watanabe heritable hyperlipidemic rabbits on a normal chow diet, although baseline cholesterol levels in these two strains were expectedly quite different (Xu et al., 1996). An important conclusion from this study was that plasma cholesterol could be reduced when CYP7A was stimulated, even in the absence of LDL receptor function. A similar result was recently reported in a LDL-R deficient mouse model, which overexpresses CYP7A (Ratliff et al., 2006). Thus, one can speculate that an increase in cholesterol disposal via TR-mediated up-regulation of CYP7A may be involved in the effects of MB07811 seen in both normal and cholesterol-fed animals.
Benefit
Although statins are the drug of first choice for lowering LDL-C, most moderately high and high-risk patients will require high-dose statin or combination therapy to reach the more aggressive ATP III LDL-C target levels of 1.81–2.59 mmol·L−1 (70–100 mg per 100 mL) (Grundy et al., 2004). Further, it is also known that doubling the dose of a statin achieves only about a 6% decrease in LDL-C, and that increasing the dose is associated with a higher rate of adverse events leading to discontinuation (Davidson and Toth, 2004). Thus, combination therapy by adding a TR agonist to low or moderate dose statin therapy may increase the per cent of patients reaching target cholesterol levels and decrease the statin-related adverse events. Additionally, there is a subpopulation of hypercholesterolaemic patients that may especially benefit from combination therapy with a TR agonist. Cholesterol absorption in humans is highly variable, and patients who have the ‘hyper-absorber’ phenotype respond suboptimally to statins (Gylling and Miettinen, 2002; Hoenig et al., 2007). In these individuals, combination therapy (i.e. statins plus ezetimibe) resulting in inhibition of both cholesterol synthesis and absorption can be efficacious. The results presented here also suggest that a TR agonist such as MB07811 might be useful in these patients.
Limitations
One of the limitations of the results presented in these studies is that complete dose-response evaluations of the cholesterol lowering activity of atorvastatin or TR activation with MB07811/MB07344 were not determined in these pre-clinical models. Although MB07811/MB07344 administration was efficacious in further lowering cholesterol in the presence of a maximally tolerated dose of atorvastatin in the normocholesterolaemic rabbit (Figures 1, 2 and 5), it is unclear whether this would be the case in hypercholesterolaemic models or in man and additional experiments are warranted. What is clear is that the clinical utility of combination therapy for hypercholesterolaemia will be dose-dependent and highly influenced by the tolerability and side-effect profile. Nonetheless, the experiments presented here do support the concept that TR activation with a compound such as MB07811 can provide additional cholesterol lowering in the presence of efficacious doses of a statin.
In summary, these studies demonstrate that selective TRβ activation has adjunctive/additive cholesterol lowering activity when administered in combination with atorvastatin in the rabbit, dog and non-human primate. These results provide the first experimental evidence that TR agonists, such as that provided by the liver-targeted prodrug, MB07811, may be beneficial for combination with statins.
Acknowledgments
We thank Cindy Phan, Ed Chen, Kara Kersjes and Kenny Kim for their technical assistance in the completion of these studies.
Glossary
Abbreviations:
- TH
thyroid hormone
- TPC
total plasma cholesterol
- TR
thyroid hormone receptor
- AT
atorvastatin
Conflicts of interest
The authors are employees of Metabasis Therapeutics Inc. which has a proprietary interest in MB07344 and MB07811.
References
- Abrams JJ, Grundy SM. Cholesterol metabolism in hypothyroidism and hyperthyroidism in man. J Lipid Res. 1981;22:323–338. [PubMed] [Google Scholar]
- Aikawa M, Libby P. Lipid lowering reduces proteolytic and prothrombotic potential in rabbit atheroma. Ann N Y Acad Sci. 2000;902:140–152. doi: 10.1111/j.1749-6632.2000.tb06309.x. [DOI] [PubMed] [Google Scholar]
- Alegret M, Verd JC, Diaz C, Hernandez G, Adzet T, Sanchez RM, et al. Effect of hypolipidemic drugs on key enzyme activities related to lipid metabolism in normolipidemic rabbits. Eur J Pharmacol. 1998;347:283–291. doi: 10.1016/s0014-2999(98)00096-x. [DOI] [PubMed] [Google Scholar]
- Bakker O, Hudig F, Meijssen S, Wiersinga WM. Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem Biophys Res Commun. 1998;249:517–521. doi: 10.1006/bbrc.1998.9174. [DOI] [PubMed] [Google Scholar]
- Berkenstam A, Kristensen J, Mellstrom K, Carlsson B, Malm J, Rehnmark S, et al. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA. 2008;105:663–667. doi: 10.1073/pnas.0705286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocan TM. Animal models of atherosclerosis and interpretation of drug intervention studies. Curr Pharm Des. 1998;4:37–52. [PubMed] [Google Scholar]
- Bocan TM, Krause BR, Rosebury WS, Lu X, Dagle C, Bak Mueller S, et al. The combined effect of inhibiting both ACAT and HMG-CoA reductase may directly induce atherosclerotic lesion regression. Atherosclerosis. 2001;157:97–105. doi: 10.1016/s0021-9150(00)00713-9. [DOI] [PubMed] [Google Scholar]
- Bolayirli IM, Aslan M, Balci H, Altug T, Hacibekiroglu M, Seven A. Effects of atorvastatin therapy on hypercholesterolemic rabbits with respect to oxidative stress, nitric oxide pathway and homocysteine. Life Sci. 2007;81:121–127. doi: 10.1016/j.lfs.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Davidson MH, Toth PP. Comparative effects of lipid-lowering therapies. Prog Cardiovasc Dis. 2004;47:73–104. doi: 10.1016/j.pcad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Davis HR, Jr, Pula KK, Alton KB, Burrier RE, Watkins RW. The synergistic hypocholesterolemic activity of the potent cholesterol absorption inhibitor, ezetimibe, in combination with 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors in dogs. Metabolism. 2001;50:1234–1241. doi: 10.1053/meta.2001.26737. [DOI] [PubMed] [Google Scholar]
- Denke MA. Coadministration of multidrug therapy to achieve lipid goals. J Am Osteopath Assoc. 2004;104:S17–22. [PubMed] [Google Scholar]
- Erion MD, Reddy KR, Boyer SH, Matelich MC, Gomez-Galeno J, Lemus RH, et al. Design, synthesis, and characterization of a series of cytochrome P(450) 3A-activated prodrugs (HepDirect prodrugs) useful for targeting phosph(on)ate-based drugs to the liver. J Am Chem Soc. 2004;126:5154–5163. doi: 10.1021/ja031818y. [DOI] [PubMed] [Google Scholar]
- Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, et al. Targeting thyroid hormone receptor-{beta} agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA. 2007;104:15490–15495. doi: 10.1073/pnas.0702759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Mellstrom K, Ye L, Malm J, Li YL, Bladh LG, et al. Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci USA. 2003;100:10067–10072. doi: 10.1073/pnas.1633737100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- Gullberg H, Rudling M, Salto C, Forrest D, Angelin B, Vennstrom B. Requirement for thyroid hormone receptor beta in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol. 2002;16:1767–1777. doi: 10.1210/me.2002-0009. [DOI] [PubMed] [Google Scholar]
- Gylling H, Miettinen TA. Inheritance of cholesterol metabolism of probands with high or low cholesterol absorption. J Lipid Res. 2002;43:1472–1476. doi: 10.1194/jlr.m200155-jlr200. [DOI] [PubMed] [Google Scholar]
- Hoenig MR, Kostner KM, Read SJ, Walker PJ, Atherton JJ. Implications of the obesity epidemic for statin therapy: shifting cholesterol metabolism to a high synthesis and low dietary absorption state. Endocr Metab Immune Disord Drug Targets. 2007;7:153–166. doi: 10.2174/187153007781662567. [DOI] [PubMed] [Google Scholar]
- Horton JD, Cuthbert JA, Spady DK. Regulation of hepatic 7 alpha-hydroxylase expression and response to dietary cholesterol in the rat and hamster. J Biol Chem. 1995;270:5381–5387. doi: 10.1074/jbc.270.10.5381. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon PB, Gurley EC, Stravitz RT, Litz JS, Pandak WM, Chiang JY, et al. Hormonal regulation of cholesterol 7 alpha-hydroxylase mRNA levels and transcriptional activity in primary rat hepatocyte cultures. J Biol Chem. 1992;267:16866–16871. [PubMed] [Google Scholar]
- Jacobsen W, Kuhn B, Soldner A, Kirchner G, Sewing KF, Kollman PA, et al. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab Dispos. 2000;28:1369–1378. [PubMed] [Google Scholar]
- Jelinek DF, Andersson S, Slaughter CA, Russell DW. Cloning and regulation of cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J Biol Chem. 1990;265:8190–8197. [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J, et al. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA. 2005;102:10297–10302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MP, Pandak WM, Heuman DM, Chiang JY, Hylemon PB, Vlahcevic ZR. Cholesterol 7 alpha-hydroxylase: evidence for transcriptional regulation by cholesterol or metabolic products of cholesterol in the rat. J Lipid Res. 1993;34:885–892. [PubMed] [Google Scholar]
- Lopez D, Abisambra Socarras JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 2007;1771(9):1216–1225. doi: 10.1016/j.bbalip.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Morkin E, Ladenson P, Goldman S, Adamson C. Thyroid hormone analogs for treatment of hypercholesterolemia and heart failure: past, present and future prospects. J Mol Cell Cardiol. 2004;37:1137–1146. doi: 10.1016/j.yjmcc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Panel. NHLBI NIoH. The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circ Res. 2002;106:3413–3421. [PubMed] [Google Scholar]
- Ness GC, Lopez D. Transcriptional regulation of rat hepatic low-density lipoprotein receptor and cholesterol 7 alpha hydroxylase by thyroid hormone. Arch Biochem Biophys. 1995;323:404–408. doi: 10.1006/abbi.1995.0061. [DOI] [PubMed] [Google Scholar]
- Ness GC, Lopez D, Chambers CM, Newsome WP, Cornelius P, Long CA, et al. Effects of L-triiodothyronine and the thyromimetic L-94901 on serum lipoprotein levels and hepatic low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and apo A-I gene expression. Biochem Pharmacol. 1998;56:121–129. doi: 10.1016/s0006-2952(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Pandak WM, Heuman DM, Hylemon PB, Vlahcevic ZR. Regulation of bile acid synthesis. IV. Interrelationship between cholesterol and bile acid biosynthesis pathways. J Lipid Res. 1990;31:79–90. [PubMed] [Google Scholar]
- Pandak WM, Heuman DM, Redford K, Stravitz RT, Chiang JY, Hylemon PB, et al. Hormonal regulation of cholesterol 7alpha-hydroxylase specific activity, mRNA levels, and transcriptional activity in vivo in the rat. J Lipid Res. 1997;38:2483–2491. [PubMed] [Google Scholar]
- Pantos C, Mourouzis I, Paizis I, Malliopoulou V, Xinaris C, Moraitis P, et al. Pharmacological inhibition of TRalpha1 receptor potentiates the thyroxine effect on body weight reduction in rats: potential therapeutic implications in controlling body weight. Diabetes Obes Metab. 2007;9:136–138. doi: 10.1111/j.1463-1326.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med. 2000;160:459–467. doi: 10.1001/archinte.160.4.459. [DOI] [PubMed] [Google Scholar]
- Qin W, Infante J, Wang SR, Infante R. Regulation of HMG-CoA reductase, apoprotein-B and LDL receptor gene expression by the hypocholesterolemic drugs simvastatin and ciprofibrate in Hep G2, human and rat hepatocytes. Biochim Biophys Acta. 1992;1127:57–66. doi: 10.1016/0005-2760(92)90201-6. [DOI] [PubMed] [Google Scholar]
- Rashid S, Uffelman KD, Barrett PH, Lewis GF. Effect of atorvastatin on high-density lipoprotein apolipoprotein A-I production and clearance in the New Zealand white rabbit. Circulation. 2002;106:2955–2960. doi: 10.1161/01.cir.0000038303.84249.4a. [DOI] [PubMed] [Google Scholar]
- Ratliff EP, Gutierrez A, Davis RA. Transgenic expression of CYP7A1 in LDL receptor-deficient mice blocks diet-induced hypercholesterolemia. J Lipid Res. 2006;47:1513–1520. doi: 10.1194/jlr.M600120-JLR200. [DOI] [PubMed] [Google Scholar]
- Rudel L, Deckelman C, Wilson M, Scobey M, Anderson R. Dietary cholesterol and downregulation of cholesterol 7 alpha-hydroxylase and cholesterol absorption in African green monkeys. J Clin Invest. 1994;93:2463–2472. doi: 10.1172/JCI117255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample CE, Pendleton LC, Ness GC. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase mRNA levels by L-triiodothyronine. Biochemistry. 1987;26:727–731. doi: 10.1021/bi00377a011. [DOI] [PubMed] [Google Scholar]
- Shin DJ, Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through Sterol Regulatory Element-Binding Protein-2 (SREBP-2) J Biol Chem. 2003;278:34114–34118. doi: 10.1074/jbc.M305417200. [DOI] [PubMed] [Google Scholar]
- Slater EE, MacDonald JS. Mechanism of action and biological profile of HMG CoA reductase inhibitors. A new therapeutic alternative. Drugs. 1988;36(Suppl.)(3):72–82. doi: 10.2165/00003495-198800363-00016. [DOI] [PubMed] [Google Scholar]
- Staels B, Van Tol A, Chan L, Will H, Verhoeven G, Auwerx J. Alterations in thyroid status modulate apolipoprotein, hepatic triglyceride lipase, and low density lipoprotein receptor in rats. Endocrinology. 1990;127:1144–1152. doi: 10.1210/endo-127-3-1144. [DOI] [PubMed] [Google Scholar]
- Vlahcevic ZR, Heuman DM, Hylemon PB. Regulation of bile acid synthesis. Hepatology. 1991;13:590–600. [PubMed] [Google Scholar]
- Xu G, Salen G, Shefer S, Ness GC, Nguyen LB, Parker TS, et al. Unexpected inhibition of cholesterol 7 alpha-hydroxylase by cholesterol in New Zealand white and Watanabe heritable hyperlipidemic rabbits. J Clin Invest. 1995;95:1497–1504. doi: 10.1172/JCI117821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Salen G, Shefer S, Ness GC, Nguyen LB, Tint GS, et al. Increasing hepatic cholesterol 7alpha-hydroxylase reduces plasma cholesterol concentrations in normocholesterolemic and hypercholesterolemic rabbits. Hepatology. 1996;24:882–887. doi: 10.1002/hep.510240421. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]


