Abstract
Background and purpose:
Artemisinin is an antimalarial drug exerting pleiotropic effects, such as the inhibition of the transcription factor nuclear factor-kappa B and of the sarcoplasmic/endoplasmic reticulum Ca++-ATPase (SERCA) of P. falciparum. As the sesquiterpene lactone thapsigargin, a known inhibitor of mammalian SERCA, enhances the expression of P-glycoprotein (Pgp) by increasing the intracellular Ca++ ([Ca++]i) level, we investigated whether artemisinin and its structural homologue parthenolide could inhibit SERCA in human colon carcinoma HT29 cells and induce a resistance to doxorubicin.
Experimental approach:
HT29 cells were incubated with artemisinin or parthenolide and assessed for SERCA activity, [Ca++]i levels, Pgp expression, doxorubicin accumulation and toxicity, and translocation of the hypoxia-inducible factor, HIF-1α.
Key results:
Artemisinin and parthenolide, like the specific SERCA inhibitors thapsigargin and cyclopiazonic acid, reduced the activity of SERCA. They also increased intracellular calcium concentration ([Ca++]i) and Pgp expression and decreased doxorubicin accumulation and cytotoxicity. The intracellular Ca++ chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, and the inhibitor of calmodulin-dependent kinase II (CaMKII) KN93 prevented these effects. CaMKII is known to promote the phosphorylation and the activation of HIF-1α, which may induce Pgp. In HT29 cells, artemisinin and parthenolide induced the phosphorylation of HIF-1α, which was inhibited by KN93.
Conclusions and implications:
Our results suggest that artemisinin and parthenolide may act as SERCA inhibitors and, like other SERCA inhibitors, induce resistance to doxorubicin in human colon cancer cells, via the CaMKII-dependent activation of HIF-1α and the induction of Pgp.
Keywords: artemisinin, sesquiterpene lactones, doxorubicin, colon cancer cells, calcium, HIF-1α, P-glycoprotein, calmodulin-dependent kinase II
Introduction
The overexpression of the membrane pump P-glycoprotein (Pgp) in cancer cells is one of the main mechanisms of the multidrug resistance (MDR), an intrinsic or acquired cross-resistance towards different chemotherapeutic drugs (Takara et al., 2006). As MDR is the major obstacle to a successful cancer therapy, the mechanism of the transcription of the Pgp gene (mdr1) is the object of intense investigation (Takara et al., 2006). Increased intracellular calcium concentration ([Ca++]i) have been correlated with Pgp expression: in human lung cancer (Calu-3) cells. Ouabain-dependent Pgp expression was blunted by the calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and the sesquiterpene lactone drug thapsigargin, a well-known inhibitor of the mammalian sarcoplasmic/endoplasmic reticulum Ca++-ATPase (SERCA) (Eckstein-Ludwig et al., 2003; Uhleman et al., 2005) enhanced this effect of ouabain (Baudouin-Legros et al., 2003). This means that, by increasing [Ca++]i, thapsigargin may regulate the transcription of the mdr1 gene and enhance the expression of Pgp. An increase of [Ca++]i is known also to activate the transcription factor hypoxia-inducible factor-1 (HIF-1) (Yuan et al., 2005; Hui et al., 2006), which controls several genes involved in cellular growth, glucose and iron metabolism, pH control, angiogenesis and matrix remodelling (O’Donnell et al., 2006), and is also involved in Pgp up-regulation (Comerford et al., 2002). HIF-1 is composed of two subunits: the β subunit is constitutively expressed, whereas the α subunit is rapidly degraded in normoxia, but becomes stable in hypoxia (O'Donnell et al., 2006) and its synthesis increases after stimulation with many growth factors and cytokines (Zhou and Brüne, 2006).
Interestingly, another sesquiterpene lactone, artemisinin, which is widely used in the treatment of drug-resistant malaria (Hien and White, 1993), inhibits the SERCA of Plasmodium falciparum, with a potency comparable to that of thapsigargin (Eckstein-Ludwig et al., 2003; Uhleman et al., 2005). Artemisinin is known to exert pleiotropic effects, and the precise mechanism by which it kills P. falciparum has not been fully clarified (Golenser et al., 2006). For instance, in several cell types artemisinin inhibits the transcription factor nuclear factor-kappa B (NF-kB), a property exhibited also by other sesquiterpene compounds (Aldieri et al., 2003; Li et al., 2006).
In preliminary experiments, we observed that artemisinin, as well as parthenolide, reduced the activity of SERCA and increased [Ca++]i in human colon cancer HT29 cells, making them more resistant to the toxic effects of doxorubicin. Starting from this observation, we decided to investigate whether these sesquiterpene lactones may up-regulate the Pgp expression in HT29 cells by inhibiting SERCA and by increasing [Ca++]i, thus inducing a doxorubicin-resistant phenotype, and whether these events could be related to HIF-1α activation.
Methods
Cells
The human colon cancer cell line (HT29) is sensitive to doxorubicin and cisplatin (Riganti et al., 2005). HT29 cells were cultured in RPMI 1640 supplemented with 10% foetal bovine serum (FBS), 1% penicillin/streptomycin, 1% L-glutamine and maintained in a humidified atmosphere at 37°C, 5% CO2 and 20% O2. To culture them in hypoxic conditions, HT29 cells were grown for 3 h in humidified atmosphere at 37°C, 5% CO2 and 3% O2. Human colon cancer LoVo cells were cultured in HAM's F12 medium, human liver cancer HepG2 cells in RPMI 1640 medium and human breast cancer MCF-7 cells in a 1/1 (v/v) mixture of HAM's F12 and DMEM; each medium was supplemented with 10% FBS, 1% penicillin/streptomycin and 1% L-glutamine.
SERCA activity
Cells were lysed in buffer A (50 mmol·L−1 HEPES, 750 mmol·L−1 KCl, 200 mmol·L−1 sucrose, 10 mmol·L−1 NaHCO3, pH 7.4), supplemented with protease inhibitor cocktail set III (Calbiochem) and centrifuged at 13 000×g for 5 min. Supernatant was collected and centrifuged at 100 000×g for 1 h at 4°C, then the pellet was resuspended in 1 mL of buffer B (20 mmol·L−1 HEPES, 160 mmol·L−1 KCl, 1 mmol·L−1 MgCl2, 1 mmol·L−1 CaCl2, 0.5% TritonX-100, pH 7.4); 100 µg of each sample were immunoprecipitated overnight with the rabbit polyclonal anti-SERCA 1/2/3 antibody (diluted 1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Samples were washed twice with 1 mL of buffer B, supplemented with 2 mmol·L−1 dithiothreitol, then subjected to the following investigations. 10 µg of immunoprecipitated proteins were directly probed with the same antibody (diluted 1:250, in PBS-BSA 1%, Santa Cruz Biotechnology), to measure total SERCA protein, while 50 µg were mixed with 2 mmol·L−1 ATP, 2.5 mmol·L−1 phosphoenolpyruvate, 7.5 U pyruvate kinase, 8.0 U lactate dehydrogenase (LDH), 0.2 mmol·L−1 calmodulin to check SERCA activity, as previously described (Krishna et al., 2001). The reaction was started by adding 0.25 mmol·L−1 NADH and was followed for 10 min, measuring the absorbance at 340 nm with a Lambda 3 spectrophotometer (Perkin Elmer, Waltham, MA, USA). The reaction kinetic was linear throughout the time of measurement. The NADH oxidation rate (expressed as µmol NADH oxidized min−1 mg·protein−1) of each sample was subtracted from that obtained in the absence of SERCA. The ATP hydrolysis rate was calculated stoichiometrically (Krishna et al., 2001) and ATPase activity was expressed as µmol ATP hydrolyzed min−1 mg·protein−1.
[Ca++]i measurement
Cells were grown 24 h on sterile glass coverslips, washed twice with PBS and incubated for 10 min at 37°C in HEPES-Ca buffer (10 mmol·L−1 HEPES, 145 mmol·L−1 NaCl, 1 mmol·L−1 CaCl2, 5 mmol·L−1 KCl, 1 mmol·L−1 MgSO4, 10 mmol·L−1 glucose, pH 7.4), with 10 µmol·L−1 calcium-sensitive fluorescent probe 1-[2-(5-Carboxyoxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2′-amino-5′-methylphenoxy)-ethane-N,N,N′N′-tetraacetic Acid (FURA) acetoxymethylester (AM). After FURA-AM loading, coverslips were washed with HEPES-Ca buffer and firmly positioned in a quartz cuvette (1 cm) containing 1 mL of HEPES-Ca buffer. Either 10 µmol·L−1 parthenolide or 10 µmol·L−1 artemisinin was added when indicated. The cuvette holder was thermostatted at 37°C and the fluorescence of coverslips was measured in a Perkin-Elmer LS-5 spectrofluorimeter (Perkin Elmer). Excitation and emission wavelengths were 490 and 530 nm respectively. Fluorescence was recorded for 1 h, during which the integrity of the monolayer was maintained, as assessed by measuring the extracellular LDH release (see the following paragraphs). Calculation of [Ca++]i levels was performed as previously described (Hallam et al., 1984). The fluorescence of Ca++-saturated dye (Fmax), obtained by treating cells with 10 µmol·L−1 ionomycin in HEPES-Ca buffer, was taken as the maximal emission. 2 mmol·L−1 MnCl2 was then added to displace Ca++ from FURA and to obtain the value of FURA autofluorescence (Fmin) alone. To prevent the increase of [Ca++]i, HT29 cells were pre-incubated for 1 h with 10 µmol·L−1 BAPTA-AM in order to load them with the Ca++ chelator BAPTA, then washed with PBS, and subjected to the same procedures as the other experiments.
Cytochrome c release
Cells were washed twice in ice-cold PBS, then lysed in 0.5 mL buffer A (50 mmol·L−1 Tris, 100 mmol·L−1 KCl, 5 mmol·L−1 MgCl2, 1.8 mmol·L−1 ATP, 1 mmol·L−1 EDTA; pH 7.2), supplemented with protease inhibitor cocktail set III (Calbiochem), 1 mmol·L−1 PMSF and 250 mmol·L−1 NaF. Mitochondrial and cytosolic fractions were separated as described (Wibom et al., 2002). Samples were clarified by centrifuging at 650×g for 3 min at 4°C, and the supernatant was collected and centrifuged at 13 000×g for 5 min at 4°C. The new supernatant (cytosolic fraction) was transferred in other tubes, whereas the pellet (mitochondrial fraction) was rinsed with 0.5 mL buffer A, re-suspended in 0.25 mL buffer B (250 mmol·L−1 sucrose, 15 mmol·L−1 K2HPO4, 2 mmol·L−1 MgCl2, 0.5 mmol·L−1 EDTA, 5% w/v BSA) and sonicated (two bursts of 10 s). 10 µg from each cytosolic or mitochondrial fraction were subjected to 15% SDS-PAGE and probed with an anti-cytochrome c antibody (diluted 1:1000 in PBS-BSA 1%, from Becton Dickinson).
Real-time polymerase chain reaction (RT-PCR)
Total RNA was obtained as previously described (Chomczynski and Sacchi, 1987). 5 µg of RNA were retro-transcribed by 200 U M-MLV reverse transcriptase (Invitrogen, Milan, Italy), in presence of 40 U·µL−1 RNAseOUT (Invitrogen). RT-PCR was carried out using IQ™ SYBR Green Supermix (Biorad), according to the manufacturer's instructions. The same cDNA preparation was used for the quantitation of Pgp and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), used as an housekeeping gene. The sequences of Pgp primers for quantitative RT-PCR were 5′-TGCTGGAGCGGTTCTACG-3′, 5′-ATAGGCAATGTTCTCAGCAATG-3′ (Invitrogen). Cycling for Pgp was: 1 cycle at 94°C for 2 min, followed by 45 cycles at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s. The sequences of GAPDH primers were 5′-GAAGGTGAAGGTCGGAGT-3′, 5′-CATGGTGGAATCATATTGGAA-3′ (Invitrogen). Cycling for GAPDH was: 1 cycle at 94°C for 2 min, followed by 40 cycles at 94°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 30 s. The relative quantitation of each sample was performed comparing the Pgp PCR product with the GAPDH product, using the Biorad Software Gene Expression Quantitation (Biorad).
Western blot analysis
Pgp protein was detected by Western blotting as reported elsewhere (Riganti et al., 2005). The densitometric analysis of the Western blots was performed with the Image J software (http://rsb.info.nih.gov/ij/). To assess HIF-1α phosphorylation, the whole cellular lysate was immunoprecipitated overnight with the mouse monoclonal anti-HIF-1α antibody (diluted 1:250, Santa Cruz Biotechnology) and the immunoprecipitated proteins were separated by SDS-PAGE (10%), transferred to polyvinylidene fluoride membrane sheets (Immobilon-P, Millipore, Bedford, MA, USA) and probed with a biotin-conjugated anti-phosphoserine antibody (diluted 1:1000 in TBS-BSA 3%, Sigma Chemical Co.) for 1 h. The membrane was washed in TBS-Tween 0.1% and subjected for 1 h to a streptavidin- and horseradish peroxidase-conjugated polymer (diluted 1:10 000 in TBS-BSA 3%, Sigma Chemical Co.). The membrane was washed again with TBS-Tween and proteins were detected by enhanced chemiluminescence (Immun-Star, Biorad).
Doxorubicin accumulation
Intracellular doxorubicin accumulation was measured by a fluorimetric assay as described elsewhere (Riganti et al., 2005). Excitation and emission wavelengths were 475 and 553 nm. Fluorescence was converted in ng doxorubicin mg·protein−1, using a calibration curve prepared previously.
Extracellular LDH activity
After a 6 h incubation under different experimental conditions in the presence of 5 µmol·L−1 doxorubicin, LDH activity was measured in the extracellular medium and in the cell lysate, as previously described (Riganti et al., 2005), to check the cytotoxicity of doxorubicin. Absorbance at 340 nm was measured for 10 min with a Lambda 3 spectrophotometer (Perkin Elmer). Both intracellular and extracellular enzyme activity was expressed as µmol NADH oxidized min−1, then extracellular LDH activity was calculated as percentage of the total LDH activity in the dish.
Trypan blue staining
After a 6 h incubation under different experimental conditions, cell monolayers were washed and allowed to grow for another 24 h in fresh medium, then detached with trypsin/EDTA and resuspended in 1 mL of PBS. 10 µL of 20% (w/v) Trypan blue were added to each sample. After a 1 min incubation at room temperature, 10 µL of each cellular suspension were analysed under a light microscope and the Trypan blue-positive cells were counted as percentage of dead cells on a total number of 200 cells.
Annexin V/propidium iodide (PI) assay
Cells were incubated for 6 h in the experimental conditions described under the Results section, then they were washed and cultured for other 24 h in fresh medium. After this incubation time, cells were rinsed twice with fresh PBS, detached with the Cell Dissociation Solution (Sigma Chemical Co.) and incubated for 10 min at room temperature in 1 mL of binding buffer (100 mmol·L−1 HEPES/NaOH, 140 mmol·L−1 NaCl, 25 mmol·L−1 CaCl2, pH 7.5) containing 10 µmol·L−1 annexin V-fluorescein isothiocyanate conjugate (FITC) and 5 µmol·L−1 PI. The cell suspensions were washed three times with fresh PBS and rinsed with 1 mL of binding buffer. The fluorescence of each sample was recorded using a FACSCalibur system (Becton Dickinson). For each analysis 10 000 events were collected; the green fluorescence (for annexin V-FITC) was selected using a 530 nm band pass filter, while the red fluorescence (for PI) was obtained with a 640 nm longpass filter. The percentage of cells positive for annexin V, PI or both was calculated by the Cell Quest software (Becton Dickinson).
Cell cycle analysis
Cells, incubated for 6 h in the experimental conditions described under the Results section and cultured for the subsequent 24 h in fresh medium, were washed twice with fresh PBS, detached with the Cell Dissociation Solution (Sigma Chemical Co.) and resuspended in 100 µL of ice-cold PBS. Samples were incubated for 1 h at 4°C in presence of 500 µL of 70% ethanol, then centrifuged at 1200×g for 5 min and rinsed with 300 µL of citrate buffer (50 mmol·L−1 Na2HPO4, 25 mmol·L−1 sodium citrate, 0.1% Triton X-100), containing 10 µg·mL−1 PI and 1 mg·mL−1 RNAse (from bovine pancreas). After a 15 min incubation in the dark, the intracellular fluorescence was detected by a FACSCalibur system (Becton Dickinson). For each analysis, 10 000 events were collected and a gate was drawn on the forward scatter/side scatter dot plot to exclude dead cells and debris. The results of the cell cycle analysis were elaborated by the Cell Quest software (Becton Dickinson).
Electrophoretic mobility shift assay (EMSA)
Cells were plated in 60 mm diameter dishes at confluence and 10 µg of nuclear proteins were used to detect NF-kB translocation as described (Aldieri et al., 2003). The probe containing the HIF-1α oligonucleotide consensus sequence was labelled with [γ-32P]ATP (Amersham Bioscience, Piscataway, NJ) (3000 Ci mmol−1, 250 µCi), using T4 polynucleotide kinase (Roche, Basel, Switzerland). The sequence of oligonucleotide was: 5′-TCTGTACGTGACCACACTCACCTC-3′; 3′-AGACATGCACTGGTGTGAGTGGAG-5′ (Santa Cruz Biotechnology). 10 µg of extracts were incubated for 20 min with 20 000 cpm of 32P-labelled double-stranded oligonucleotide at 4°C. The DNA-protein complex was separated on a not-denaturating 4% polyacrylamide gel in TBE buffer (0.4 mol·L−1 Tris, 0.45 mol·L−1 boric acid, 0.5 mol·L−1 EDTA, pH 8.0). After electrophoresis, the gel was dried and autoradiographed by exposure to X-ray film for 48 h.
Statistical analysis
All data in text and figures are provided as means ± SE. The results were analysed by a one-way analysis of variance (anova) and Tukey's test. P < 0.05 was considered significant.
Materials
Foetal bovine serum, RPMI 1640, HAM's F12 and DMEM medium were supplied by BioWhittaker (Verviers, Belgium); plasticware for cell culture was from Falcon (Becton Dickinson, Bedford, MA, USA). KN93 was purchased from Calbiochem (La Jolla, CA, USA). Electrophoresis reagents were obtained from Biorad (Hercules, CA, USA). When not otherwise specified, the other reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Results
Artemisinin inhibits SERCA activity and increases [Ca++]i levels in HT29 cells
Artemisinin and the structurally related sesquiterpene lactone parthenolide, reduced SERCA activity in HT29 cells, dose-dependently (Figure 1). Artemisinin and parthenolide were less effective than thapsigargin and cyclopiazonic acid, two well-known SERCA inhibitors (Seidler et al., 1989): however, at 10 µmol·L−1 they significantly reduced the activity of SERCA (Figure 1). The expression of SERCA, detected by Western blotting in the immunoprecipitated samples, did not change under any of our experimental conditions (data not shown). We then checked the [Ca++]i levels in HT29 cells by using the fluorescent probe FURA-AM. Artemisinin and parthenolide elicited a significant transient increase of [Ca++]i which reached the maximum value between 3 and 5 min after drug addition (Figure 2A). Under each experimental condition, the [Ca++]i levels returned to the baseline within 30 min (Figure 2A) and was stable during a further 30 min period (not shown). When HT29 cells were pre-loaded with the Ca++ chelator BAPTA, none of these drugs was able to increase [Ca++]i (Figure 2B).
Figure 1.
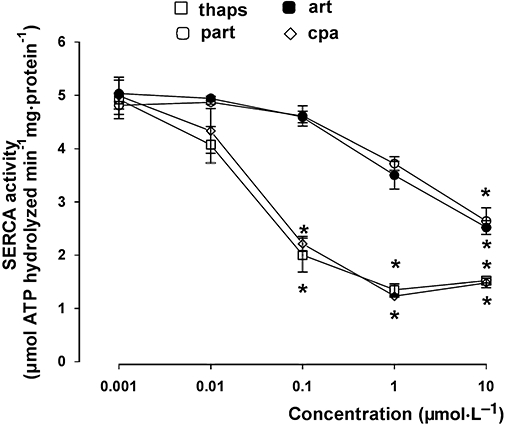
Effects of thapsigargin, parthenolide, artemisinin and cyclopiazonic acid on SERCA activity in HT29 cells. 50 µg of purified SERCA protein (see Methods section) were incubated in the absence or presence of thapsigargin (thaps), parthenolide (part), artemisinin (art), cyclopiazonic acid (cpa), at different concentrations. SERCA activity was measured as described under the Methods section. Measurements were performed in duplicate and data are presented as means ± SE (n = 4). SERCA activity in control cells was 4.82 ± 0.13 µmol ATP hydrolyzed min−1mg·protein−1 (n = 4; not shown in the figure). Significance versus CTRL: *P < 0.001. SERCA, sarcoplasmic/endoplasmic reticulum Ca++-ATPase.
Figure 2.
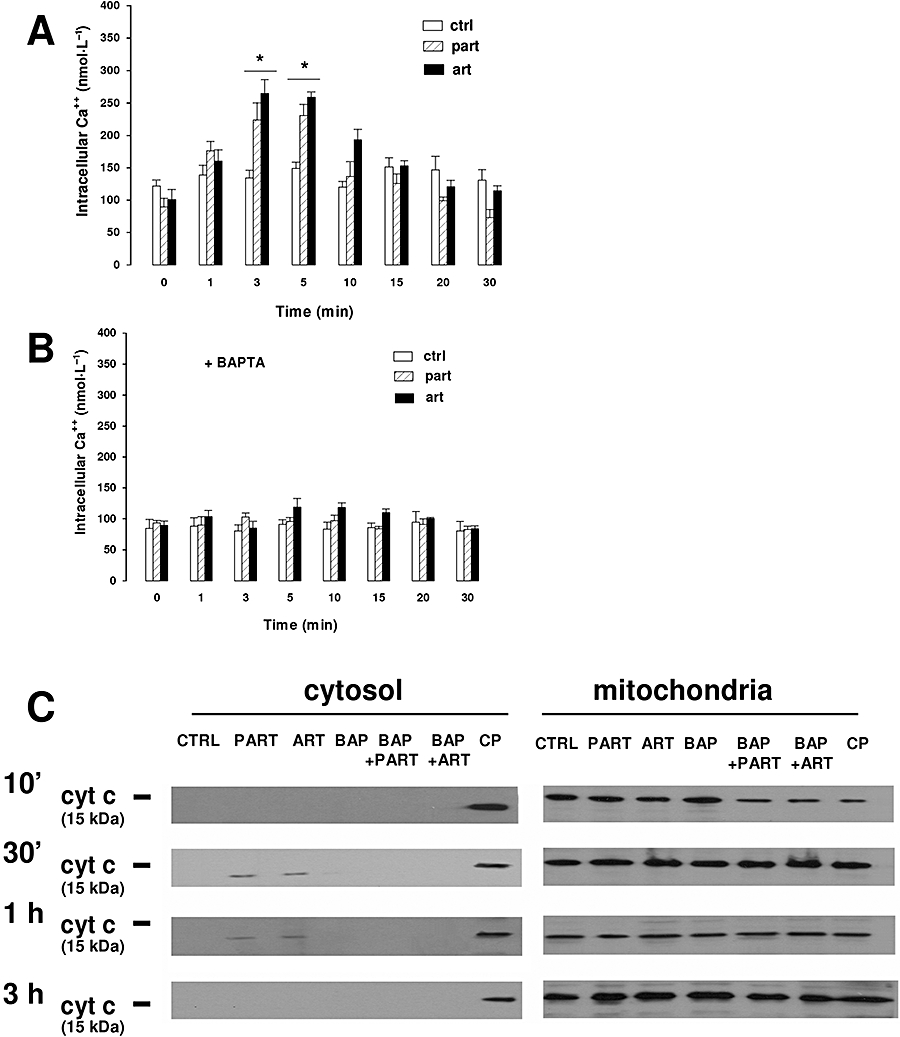
Effects of parthenolide and artemisinin on [Ca++]i. (A) Cells were grown on sterile glass coverslips for 24 h, washed with PBS and incubated for 10 min in HEPES-Ca buffer containing 10 µmol·L−1 FURA-AM, in the absence (ctrl) or presence of either parthenolide (part, 10 µmol·L−1) or artemisinin (art, 10 µmol·L−1). Fluorescence was detected throughout the first 30 min, as described under Methods section. Measurements were performed in duplicate and data are presented as means ± SE (n = 6). Significance of each drug versus ctrl: *P < 0.005. (B) Cells were grown on sterile glass coverslips as described previously, incubated for 1 h with 10 µmol·L−1 BAPTA-AM, then washed with PBS, and subjected to the same experimental procedure described in panel A. Measurements were performed in duplicate and data are presented as means ± SE (n = 4). (C) Cells were incubated for different times in the absence (CTRL) or in the presence of either parthenolide (PART, 10 µmol·L−1) or artemisinin (ART, 10 µmol·L−1). When indicated, cells were pre-loaded with the Ca++ chelator BAPTA (BAP) (see above). As a positive control, several samples were previously incubated for 24 h with the pro-apoptotic drug camptothecin (CP, 10 nmol·L−1). Cytosolic and mitochondrial fractions were separated and subjected to Western blotting analysis for cytochrome c, as described under Methods section. The Figure is representative of three experiments with similar results. BAPTA-AM, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethylester; PBS, phosphate buffer saline.
To assess whether the increase of [Ca++]i elicited by parthenolide and artemisinin was associated with the activation of an intrinsic pathway of apoptosis, we measured the release of cytochrome c from mitochondria into the cytosol in HT29 cells incubated with artemisinin and parthenolide for different times (Figure 2C). In untreated cells, as well as after a 10 min incubation with the sesquiterpene lactones, cytochrome c was not released from mitochondria. Only after 30 min, artemisinin and parthenolide induced a weak increase of cytosolic cytochrome c, which was still present after 1 h, but was no longer detectable after 3 h. When cells were pre-loaded with BAPTA, the release of cytochrome c was completely prevented (Figure 2C).
None of the drugs exerted a cytotoxic effect, as the release of LDH in the supernatant was not modified throughout the assay time in all the experimental conditions analysed (data not shown).
Artemisinin and parthenolide increase Pgp levels and reduce doxorubicin accumulation and toxicity in HT29 cells
An increase of [Ca++]i has been observed to potentiate the ouabain-induced expression of the mdr1/Pgp gene in a human lung cancer cell line (Baudouin-Legros et al., 2003). In HT29 cells, artemisinin and parthenolide significantly increased the mRNA for Pgp as a function of time. Significantly higher mRNA levels were detectable after a 3 h incubation with each drug and increased further after a 6 h incubation (Figure 3A). A 3 h incubation with either artemisinin or parthenolide induced a slight increase of the amount of Pgp protein; a stronger induction was detected after a 6 h incubation (Figure 3B and Figure S1). Pre-incubation with BAPTA-AM prevented the increase of both Pgp mRNA and protein elicited by the drugs, without changing the basal expression of Pgp (Figure 3 and Figure S1). In parallel, a 6 h incubation with the drugs caused a significant decrease of intracellular doxorubicin accumulation and of doxorubicin toxicity (assessed as release of LDH) (Figure 4A): a 1 h pre-incubation with BAPTA-AM completely prevented both the effects elicited by the drugs. In the absence of doxorubicin, none of these agents exerted a significant increase of LDH activity in the culture medium (data not shown). In the presence of the drugs, the number of Trypan blue-positive or annexin V- and PI-positive cells was strongly reduced in comparison with the HT29 cells treated with doxorubicin alone (Figure 4B and Figure S2); however, pre-incubation with BAPTA-AM prevented the effects of SERCA inhibitors on cell viability and apoptosis (Figure 4B and Figure S2).
Figure 3.
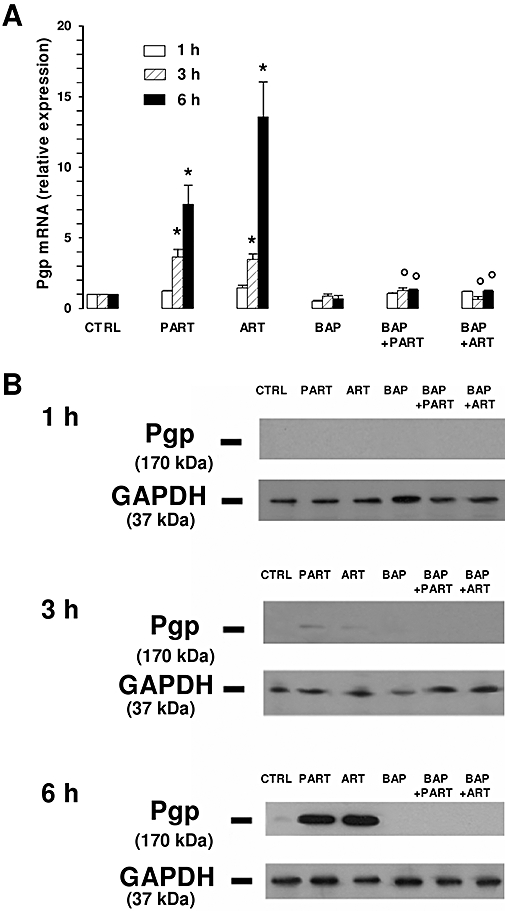
Effects of parthenolide and artemisinin on Pgp expression. HT29 cells were incubated for 1, 3 and 6 h in the absence (CTRL) or presence of either parthenolide (PART, 10 µmol·L−1) or artemisinin (ART, 10 µmol·L−1). When indicated, cells were pre-incubated for 1 h with BAPTA-AM (BAP, 10 µmol·L−1), then washed in PBS and incubated in the absence or presence of one of the drugs as described above. (A) Total RNA was extracted and retro-transcribed by RT-PCR, performed at 1 h, 3 h and 6 h as indicated in the Methods section. Measurements were performed in triplicate and data are presented as means ± SE (n = 3). Versus CTRL: *P < 0.05. Versus PART or ART respectively: °P < 0.05. (B) To detect the Pgp protein, cells were lysed and the whole cellular lysate was immunoprecipitated with an anti-Pgp polyclonal antibody. The immunoprecipitated proteins were subjected to Western blotting, using the same antibody (see Methods section). The expression of GAPDH, the product of an housekeeping gene, was used as a control of equal protein loading. The Figure is representative of three experiments with similar results. BAPTA-AM, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethylester; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PBS, phosphate buffer saline; Pgp, P-glycoprotein; RT-PCR, real-time polymerase chain reaction.
Figure 4.
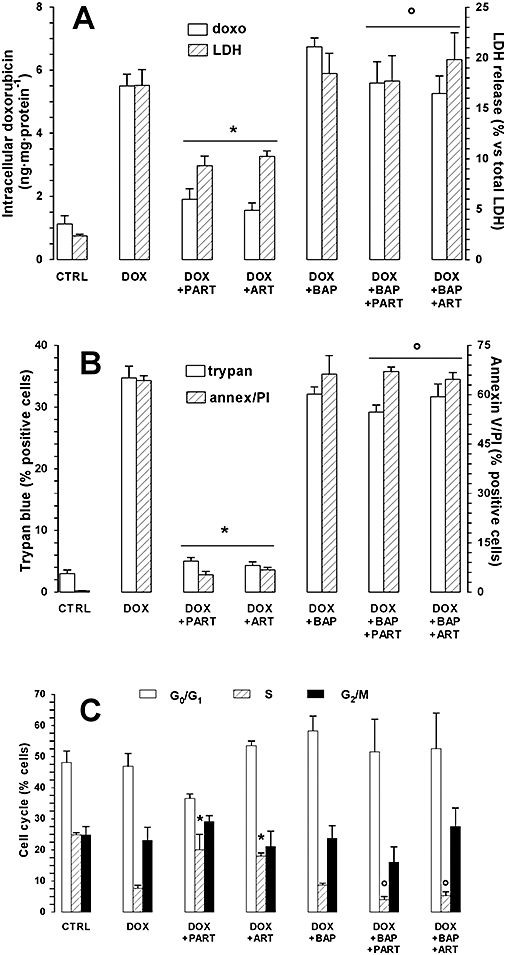
Effects of parthenolide and artemisinin on doxorubicin accumulation and cytotoxicity (assessed as: LDH release, Trypan blue staining, percentage of apoptotic cells, percentage of cells in cycle). HT29 cells were cultured in the absence (CTRL) or in the presence of doxorubicin (5 µmol·L−1, DOX) for 6 h, together with parthenolide (PART, 10 µmol·L−1) or artemisinin (ART, 10 µmol·L−1); when indicated, cells were pre-incubated for 1 h with BAPTA-AM (BAP, 10 µmol·L−1), then washed in PBS before the incubation with the other drugs. (A) Doxorubicin intracellular accumulation (doxo) and release of LDH activity in the culture supernatant (LDH) were measured as described in Methods section. Measurements were performed in duplicate and data are presented as means ± SE (n = 4). The significance of DOX versus CTRL was P < 0.001 (not reported in the figure). Versus DOX: *P < 0.001. Versus DOX + PART or DOX + ART respectively: °P < 0.02. (B) Trypan blue staining (trypan) and FACS analysis of annexin V-FITC and PI-positive HT29 cells (annex/PI) were performed as reported in the Methods section. Measurements were performed in duplicate and data are presented as means ± SE (n = 3). The significance of DOX versus CTRL was P < 0.001 (not reported in the figure). Versus DOX: *P < 0.001. Versus DOX + PART or DOX + ART respectively: °P < 0.001. (C) Cells were incubated for 6 h as indicated above, then washed and grown for a further 24 h in fresh medium; after this time, cells were permeabilized and treated with PI, as described under Methods section. Cell cycle analysis of G0/G1 phase, S phase and G2/M phase was obtained by the FACSCalibur system using the Cell Quest software (see Methods section for details). The measurements were performed in duplicate and data are presented as means ± SE (n = 3). Concerning the percentage of cells in S phase, the significance of DOX versus CTRL was P < 0.05 (not reported in the figure). Versus DOX: *P < 0.05. Versus DOX + PART or DOX + ART respectively: °P < 0.05. FITC, fluorescein isothiocyanate conjugate; LDH, lactate dehydrogenase; PBS, phosphate buffer saline; PI, propidium iodide.
We then investigated whether artemisinin or parthenolide might affect the cell-cycle arrest induced by doxorubicin. HT29 cells were incubated for 6 h in the presence of doxorubicin, with or without one of the sesquiterpene drugs, then washed and grown for a further 24 h in fresh medium. After this incubation time, cells were permeabilized and assessed for the cell cycle phases by FACS analysis. Doxorubicin lowered the percentage of cells entering the S phase, relative to the untreated cells, as shown in Figure 4C and in Figure S2. When doxorubicin was co-incubated with either artemisinin or parthenolide, the percentage of cells in S phase remained significantly higher than that found in cells treated with doxorubicin alone (Figure 4C). Again, cell loading with BAPTA completely prevented the effect of the drugs on cell cycle. The number of cells in G0/G1 and G2/M phase did not change under any experimental conditions (Figure 4C and Figure S2).
Inhibition of calmodulin kinase II reversed the effect of artemisinin and parthenolide on Pgp expression and doxorubicin accumulation and toxicity
In order to investigate the mechanism which may connect increased [Ca++]i to Pgp induction, we incubated HT29 cells with KN93, which is known to inhibit the calmodulin-dependent kinase II (CaMKII) (Kuhn et al., 1980); (Zhu et al., 2003). KN93 prevented the increase of Pgp gene transcription (Figure 5A) and of Pgp protein expression (Figure 5B and Figure S1) elicited by artemisinin and parthenolide at each time point. In parallel, the reduction of doxorubicin accumulation, cytotoxicity and pro-apoptotic effect exerted by the different drugs was completely prevented in the presence of KN93 (Figure 6 and Figure S2). Similarly, the CaMKII inhibitor abolished the effects of the sesquiterpene drugs on the cell cycle progression, measured in the cells incubated for 6 h with doxorubicin or parthenolide/artemisinin, and then cultured for further 24 h in fresh medium (Figure 6C and Figure S2). When used alone, KN93 did not significantly affect Pgp mRNA and protein expression (Figure 5 and Figure S1), intracellular doxorubicin accumulation and doxorubicin-induced extracellular release of LDH. Moreover, it changed neither the number of HT29 cells positive for Trypan blue and annexin V/PI nor the percentage of cells entering the S phase (Figure 6, Figure S2).
Figure 5.
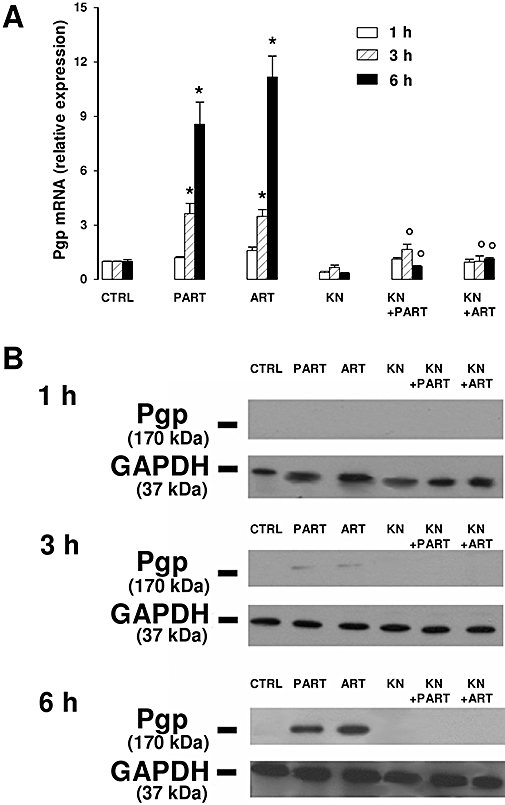
Effect of the CaMKII inhibitor KN93 on Pgp mRNA and protein expression. HT29 cells were incubated for 1 h, 3 h or 6 h in the absence (CTRL) or presence of either parthenolide (PART, 10 µmol·L−1) or artemisinin (ART, 10 µmol·L−1), alone or together with KN93 (KN, 10 µmol·L−1). At the end of the incubation time, cells were subjected to the following investigations. (A) Total RNA was extracted and RT-PCR was performed at 1 h, 3 h and 6 h as indicated in the Methods section. Measurements were performed in triplicate and data are presented as means ± SE (n = 3). Versus CTRL: *P < 0.05. Versus PART or ART respectively: °P < 0.05. (B) Pgp was immunoprecipitated from the whole cellular lysate using an anti-Pgp antibody, then subjected to Western blotting, as described in Methods section. The expression of GAPDH was used as a control of equal protein loading. The Figure is representative of three experiments with similar results. CaMKII, calmodulin-dependent kinase II; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Pgp, P-glycoprotein; RT-PCR, real-time polymerase chain reaction.
Figure 6.
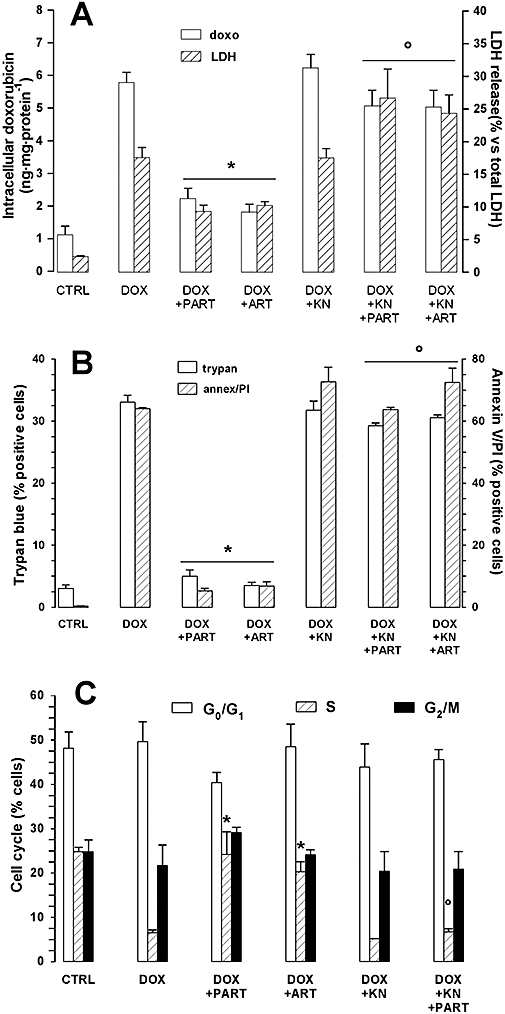
Effect of the CaMKII inhibitor KN93 on doxorubicin accumulation and cytotoxicity (assessed as: LDH release, Trypan blue staining, percentage of apoptotic cells, percentage of cells in cycle). HT29 cells were in the absence (CTRL) or in the presence of doxorubicin (5 µmol·L−1, DOX) for 6 h, together with either parthenolide (PART, 10 µmol·L−1) or artemisinin (ART, 10 µmol·L−1), alone or together with KN93 (KN, 10 µmol·L−1). (A) The cells were tested for the intracellular doxorubicin accumulation (doxo), while the extracellular culture medium was checked for LDH activity (LDH) as previously indicated (see Methods section). Measurements were performed in duplicate and data are presented as means ± SE (n = 3). The significance of DOX versus CTRL was P < 0.001 (not reported in the figure). Versus DOX: *P < 0.05. Versus DOX + PART or DOX + ART respectively: °P < 0.05. (B) Cells were stained with Trypan blue (trypan) and assessed for the positive reaction for annexin V and PI by FACS analysis (annex/PI), as reported in the Methods section. The measurements were performed in duplicate and data are presented as means ± SE (n = 3). The significance of DOX versus CTRL was P < 0.001 (not reported in the figure). Versus DOX: *P < 0.001. Versus DOX + PART or DOX + ART respectively: °P < 0.001. (C) Cells were incubated for 6 h as indicated above, then washed and let grow for further 24 h in fresh medium; after this time, cells were permeabilized and stained with PI, as described under Methods section. The percentage of HT29 cells in G0/G1 phase, S phase and G2/M phase was measured by the FACSCalibur system using the Cell Quest software (see Methods section for details). The measurements were performed in duplicate and data are presented as means ± SE (n = 3). As far as the percentage of cells in S phase is concerned, the significance of DOX versus CTRL was P < 0.05 (not reported in the figure). Versus DOX: *P < 0.05. Versus DOX + PART or DOX + ART respectively: °P < 0.05. CaMKII, calmodulin-dependent kinase II; LDH, lactate dehydrogenase; PI, propidium iodide.
The transcription factor HIF-1α is activated by artemisinin and parthenolide via CaMKII in HT29 cells
It has been reported that CaMKII promotes the phosphorylation and the nuclear translocation of HIF-1α (Yuan et al., 2005). In HT29 cells, incubation with either artemisinin or parthenolide induced phosphorylation of HIF-1α, which was absent in untreated cells and inhibited in the presence of KN93 (Figure 7A). By EMSA assay, in the same experimental conditions, we observed a very slight binding of HIF-1α to DNA in non-stimulated cells (Figure 7B), whereas, after incubation with either artemisinin or parthenolide, the nuclear translocation of HIF-1α was significantly enhanced (Figure 7B). A similar increase was obtained after a 3 h incubation in hypoxic conditions, taken as a positive control. In the presence of KN93, no increase of HIF-1α translocation was elicited by artemisinin and parthenolide. Our results suggest that these drugs promote the phosphorylation and the nuclear translocation of HIF-1α via CaMKII in HT29 cells (Figure 7A and B).
Figure 7.
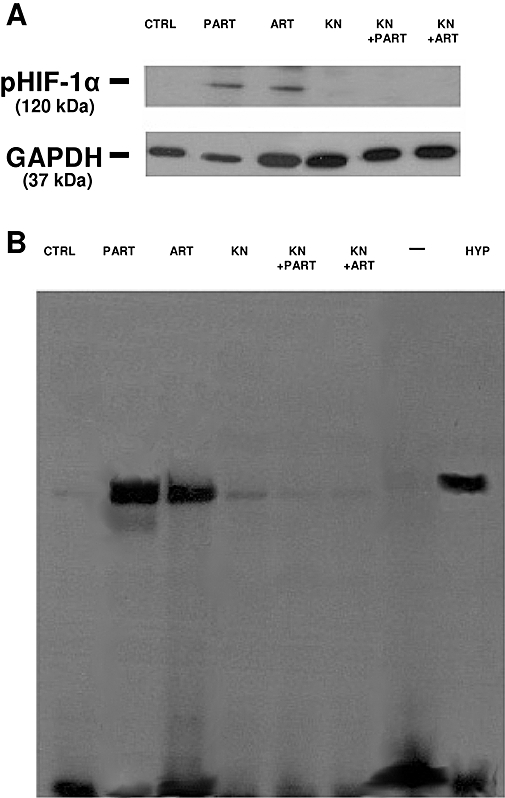
Effect of parthenolide, artemisinin and KN93 on HIF-1α phosphorylation and nuclear translocation. HT29 cells were incubated for 3 h in the absence (CTRL) or in the presence of either parthenolide (PART, 10 µmol·L−1) or artemisinin (ART, 10 µmol·L−1), alone or together with KN93 (KN, 10 µmol·L−1), then the following investigations were performed. (A) Western blotting detection of phospho(Ser)-HIF-1α (pHIF-1α). Whole cell lysates were incubated with an anti-HIF-1α antibody. Subsequently the immunoprecipitated proteins were separated by SDS-PAGE and probed with an anti-phospho(Ser) antibody as described in the Methods section. The expression of GAPDH was used as a control of equal protein loading. The Figure is representative of three experiments with similar results. (B) EMSA detection of HIF-1α nuclear translocation. EMSA was performed on nuclear extracts as detailed in Methods section. In each experiment, one lane was loaded with double-distilled water (–) instead of cellular extracts. The lane marked with HYP was loaded with nuclear extracts obtained from HT29 cells incubated for 3 h in a humidified hypoxic atmosphere (3% O2, 5% CO2, 37°C), to achieve a maximal HIF-1α activation. The Figure is representative of three experiments with similar results. EMSA, electrophoretic mobility shift assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HIF-1α, hypoxia-inducible factor-1α.
Discussion and conclusions
Since the first isolation from Artemisia annua in 1972, artemisinin, also known as qinghaosu, has attained a worldwide use as an antimalarial drug (Golenser et al., 2006). Different hypotheses have been made as to its mechanism of action: the drug has been proposed to alter the redox balance of P. falciparum, to interfere with parasite transport proteins, to disrupt the parasite mitochondrial function and to modulate the host immune function (Golenser et al., 2006). Artemisinin has been shown to bind and to inhibit the PfATP6 protein (the SERCA orthologue of P. falciparum), as does thapsigargin, a sesquiterpene lactone already known to inhibit protozoan and mammalian SERCA (Eckstein-Ludwig et al., 2003); (Uhleman et al., 2005).
In our work, we first investigated whether artemisinin inhibited SERCA activity and increasesd [Ca++]i also in a mammalian cell line, the human colon cancer HT29 cells, in comparison with thapsigargin and cyclopiazonic acid, two well-known SERCA inhibitors (Seidler et al., 1989). We analysed also the effect of parthenolide, a sesquiterpene lactone structurally similar to artemisinin, which exhibits pro-apoptotic (Kim et al., 2005), anti-inflammatory and antiseptic (Aldieri et al., 2003); (Li et al., 2006) properties. In our experiments, both artemisinin and parthenolide reduced the activity of purified SERCA, although less potently than the known SERCA inhibitors thapsigargin and cyclopiazonic acid. The small differences in the chemical structure of these drugs may account for this difference. At the concentration of artemisinin used in the present work (10 µmol·L−1), the inhibition of SERCA is expected to be specific: indeed when PfATP6 is expressed in Xenopus laevis oocytes, no other transporters are inhibited even at 50 µmol·L−1 artemisinin (Eckstein-Ludwig et al., 2003).
As a consequence of SERCA inhibition, artemisinin is believed to elicit an increase of [Ca++]i in HT29 cells. Artemisinin increased the transient rise in [Ca++]i induced by 60 mmol·L−1 KCl in guinea pig ventricular myocytes (Ai et al., 2001). In our experimental conditions artemisinin, as well as parthenolide, induced a significant increase of basal [Ca++]i with superimposable kinetics. To our knowledge, this is the first work reporting that artemisinin may inhibit SERCA pumps and increase [Ca++]i levels in human cells. This increase was followed by a weak release of cytochrome c from mithocondria into cytosol, an index of the activation of an intrinsic apoptotic pathway in cells. The peak of [Ca++]i increase preceded the peak of the cytochrome c release, as expected, and both events declined rapidly after having reached the maximum. We may hypothesize that, as the [Ca++]i declined towards the baseline 5 min after the exposure to the drug, cells were exposed to a pro-apoptotic signal for a very short time. Cytochrome c is usually degraded by proteasomes (Ferraro et al., 2008), allowing the activation of the anti-apoptotic programs and the cell survival. Indeed we did not detect any significant increase of LDH activity in the extracellular medium in the presence of either parthenolide or artemisinin alone: this result may suggest that the increase of [Ca++]i levels elicited by the sesquiterpene drugs triggers a weak activation of the intrinsic pro-apoptotic pathway, which does not lead to a significant cell death of HT29 cells.
Increased [Ca++]i has been correlated with mdr1/Pgp gene expression: in human lung cancer cells, thapsigargin enhances the ouabain-dependent Pgp expression and this effect was blunted by the calcium chelator BAPTA (Baudouin-Legros et al., 2003). Untreated HT29 cells express very low amounts of Pgp mRNA and protein: artemisinin and parthenolide increased the level of cytosolic [Ca++]i and enhanced the Pgp gene transcription and protein expression. The effects on both mRNA and protein were time-dependent for both drugs. These sesquiterpene lactones also reduced the intracellular accumulation of doxorubicin and the cytotoxic effects of doxorubicin in HT29 cells, as far as LDH release, decrease of cell viability, induction of apoptosis and cell cycle derangement were concerned.
Recently, interest has grown in the possibility of using natural terpenes in the treatment of MDR. For instance, it has been shown that some natural terpenes reduce rhodamine efflux in mouse lymphoma cells and in multidrug resistant human breast cancer cells (Molnár et al., 2006). Different sesquiterpenes modulate the activity of Leishmania tropica Pgp, which shows 37% homology with mammalian Pgp (Cortés-Selva et al., 2005). However, no data are available as to a direct effect of sesquiterpenes on Pgp mRNA levels and protein expression.
We hypothesized that Ca++ mobilization may be responsible for the increase of Pgp gene transcription. Indeed, in the presence of BAPTA, artemisinin and parthenolide did not induce a significant change of [Ca++]i in HT29 cells and did not increase the Pgp expression. The [Ca++]i levels seem to play a key role in regulating doxorubicin efficacy in HT29 colon cancer cells, as no inhibition of the intracellular doxorubicin accumulation and of the doxorubicin cytotoxicity was observed in the presence of BAPTA.
A rapid increase of [Ca++]i has pleiotropic effects on human cells (Chen et al., 2003). For instance, calcium may enhance the transcription of several genes through the activation of CaMK proteins, the Ras/Raf/MEK/extracellular signal regulated kinases and the cyclic adenosine monophosphate (AMP) response element-binding protein (Chen et al., 2003). In HT29 cells, the sesquiterpene lactone-induced [Ca++]i increase was transient and returned to the baseline after a few minutes. This rapid increase of [Ca++]i may activate calmodulin and CaMKII, as inferred from the inhibitory effect of KN93. CaMKII is a pleiotropic mediator of calcium signalling and its activity is a fine sensor of the [Ca++]i increase (Dupont et al., 2003). In HT29 cells, the addition of the CaMKII inhibitor KN93 abolished the up-regulation of Pgp elicited by artemisinin and parthenolide, and made the drug-stimulated cells as sensitive to doxorubicin's effects as resting cells. Taken together, our results suggest that CaMKII could affect the activity of a transcription factor acting on the mdr1 gene promoter.
Different transcription factor-binding sites are located on the mdr1 gene (Takara et al., 2006). Among them, a hypoxia responsive element (HRE), specific for HIF-1α, has been identified (Comerford et al., 2002). Hypoxic areas of tumors are more resistant to chemotherapeutic drugs, due to their enhanced expression of Pgp (O'Donnell et al., 2006). Moreover, increased synthesis of cytokines and hormones and the activation of different tyrosine kinase receptors may elevate HIF-1α activity even in normoxic cancer cells (Zhou and Brüne, 2006). Changes of the intracellular calcium levels have been variously related to HIF-1α activation (Berchner-Pfannschmidt et al., 2004); (Yuan et al., 2005). For instance, HIF-1α expression and activity was enhanced by the calcium-induced activation of PKC-α (Hui et al., 2006). HIF-1α may be phosphorylated on serine by different kinases (O'Donnell et al., 2006), which enhance HIF-1α activity or stability, up-regulating the target genes controlled by HRE (Sodhi et al., 2001); (Suzuki et al., 2001). Interestingly, in rat pheochromocytoma cells the activation of CaMKII significantly induced the transcription of HIF-1α-dependent genes under normoxic conditions (Yuan et al., 2005). Our results show that also in human HT29 colon cancer cells, expressing very low basal levels of HIF-1α, the two lactones elicited a transient increase of [Ca++]i inducing a clear nuclear translocation, phosphorylation and DNA-binding activity of HIF-1α. As the CaMKII inhibitor KN93 completely prevented such effects, we hypothesize that CaMKII may control both the activation of HIF-1α and the transcription of the mdr1 gene in HT29 cells.
In summary, our results showed that artemisinin and parthenolide were able to inhibit SERCA activity and to increase the [Ca++]i levels in HT29 cells. The transient increase of [Ca++]i may activate CaMKII, which in turn phosphorylates and activates the transcription factor HIF-1α. As a consequence of HIF-1α nuclear translocation, Pgp is overexpressed, intracellular accumulation of doxorubicin is reduced and cytotoxic effects of doxorubicin are prevented. Therefore, HT29 cells, when treated with artemisinin and parthenolide, become more resistant to doxorubicin. Our results were not cell type-specific: artemisinin and parthenolide decreased with similar mechanisms the efficacy of doxorubicin in human colon cancer LoVo cells, in human liver cancer HepG2 cells and in human breast cancer MCF-7 cells (Figures S3–S8). Furthermore, the same effects elicited by artemisinin and parthenolide were also exerted by the SERCA inhibitors thapsigargin and cyclopiazonic acid (data not shown). Therefore, SERCA activity and the regulation of [Ca++]i are likely to play a crucial role in Pgp expression and doxorubicin resistance in human cancer cells. In addition to their anti-malarial properties, artemisinin and its derivatives are cytotoxic for cancer cells and the pleiotropic nature of their antitumor effect has been recently discussed (Efferth, 2006); (Nakase et al., 2008). It has been observed that the artemisinin derivative artesunate is similarly active towards drug-sensitive and multidrug resistant cell lines which overexpress Pgp (Efferth, 2006). Due to its anti-proliferative and anti-angiogenic properties, parthenolide has been proposed as an adjuvant drug in chemotherapy (Sweeney et al., 2005); a phase I clinical trial has shown the safety of orally administered parthenolide in cancer patients (Curry et al., 2004).
Our results suggest that artemisinin and parthenolide could interfere with the action of antineoplastic drugs, when administered together, making cancer cells more resistant to chemotherapy. At present, parthenolide is only employed in preclinical and clinical trials, whereas the number of patients taking artemisinin for malaria therapy or prophylaxis is high (Mohanty et al., 2006). Due to their broad therapeutic activity, anthracyclines are widely used in clinical protocols and often represent the first-line therapy against solid and haematological malignancies (Cortes-Funes and Coronado, 2007). Clinicians should take into account that the efficacy of doxorubicin might be reduced in patients subjected to a concomitant artemisinin therapy.
Acknowledgments
This work has been supported with grants from Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS), Compagnia di San Paolo, Regione Piemonte (Ricerca Sanitaria Finalizzata CIPE A201 2004/2005 e 2006) and Ministero dell'Università e della Ricerca.
Sophie Doublier is recipient of a Research Fellowship funded by FIRMS, Torino, Italy.
Glossary
Abbreviations
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethylester
- CaMKII
calmodulin-dependent kinase II
- EMSA
electrophoretic mobility shift assay
- FITC
fluorescein isothiocyanate conjugate
- FBS
foetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HIF-1α
hypoxia-inducible factor-1α
- HRE
hypoxia responsive element
- LDH
lactate dehydrogenase
- MDR
multidrug resistance
- NF-kB
nuclear factor-kappa B
- Pgp
P-glycoprotein
- PI
propidium iodide
- SERCA
sarcoplasmic/endoplasmic reticulum Ca++-ATPase
Conflict of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Densitometric analysis of Western blotting experiments. Panel A concerns the experimental conditions reported in Figure 3B, and panel B is referred to the experimental conditions reported in Figure 5B of the manuscript.The band intensity, calculated by ImageJ Software (National Institutes of Health, Research Services Branch, Bethesda, MD, USA; available from http://rsb.info.nih.gov/ij/), and expressed as arbitrary units, is presented as mean ± SE (n = 3). Versus CTRL: *P < 0.05; versus PART or ART respectively: °P < 0.05.
Figure S2 Effect of artemisinin, BAPTA-AM and KN93 on the apoptosis (A) and on the cell cycle arrest (B) induced by doxorubicin. HT29 cells were incubated for 6 h in the absence (CTRL-DOXO) or presence of 5 mmol·L-1 doxorubicin (CTRL) plus artemisinin (ART, 10mmol·L-1); when indicated, cells were pre-incubated for 1 h with BAPTA-AM (BAP, 10 mmol·L-1) or co-incubated with KN93 (KN, 10 mmol·L-1), alone or in different combinations. After these incubation times, cells were washed and let grow in fresh medium for 24 h, then stained with annexin V-FITC (FL1-H) and PI (FL2-H) as reported in the Methods section. To evaluate the cell cycle, cells were permeabilized with ethanol and incubated with PI as indicated under the Methods section. The dot plots and the histograms, obtained by the FACS analysis of cellular fluorescence, are representative of three experiments with similar results, obtained in HT29 cells incubated with artemisinin. The incubation with parthenolide produced superimposable results (see Figures 4B,C and 6B,C). BAPTA-AM, 1,2-bis(2- aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid-acetoxymethylester; FITC, fluorescein isothiocyanate conjugate; PI, propidium iodide.
Figure S3 Effects of thapsigargin, parthenolide, artemisinin and cyclopiazonic acid on SERCA activity in LoVo, HepG2 and MCF-7 cells. 50 mg of purified SERCA protein were incubated in the absence or presence of thapsigargin (thaps, open square), parthenolide (part, open circle), artemisinin (art, solid circle), cyclopiazonic acid (cpa, open diamond), at different concentrations. SERCA activity was measured as described under the Methods section. Measurements were performed in duplicate and data are presented as means ± SE (n = 3). SERCA activity in control cells was 5.71 ± 0.28 mmol ATP hydrolyzed min-1 mg·protein-1 for LoVo cells, 4.52 ± 0.19 mmol ATP hydrolyzed min-1 mg·protein-1 for HepG2 cells, 4.89 ± 0.07 mmol ATP hydrolyzed min-1 mg·protein-1 for MCF-7 cells (not shown in the figure). Significance versus CTRL: *P < 0.001 (LoVo); *P < 0.005 (HepG2); *P < 0.001 (MCF-7). SERCA, sarcoplasmic/endoplasmic reticulum Ca++-ATPase.
Figure S4 Effects of parthenolide and artemisinin on [Ca++]i in LoVo (A and B), HepG2 (C and D), MCF-7 (E and F) cells. Cells were grown on sterile glass coverslips for 24 h, washed with PBS and incubated for 10 min in Hepes-Ca buffer containing 10 mmol·L-1 FURA-AM, in the absence (CTRL, open bars) or presence of parthenolide (PART, 10 mmol·L-1, hatched bars) and artemisinin (ART, 10 mmol·L-1, solid bars). When indicated, cells were pre-incubated for 1 h with 10 mmol·L-1 BAPTA-AM, then treated as reported above. Fluorescence was detected throughout the first 30 min, as described under Methods section. Measurements were performed in duplicate and data are presented as means ± SE (n = 4). Significance of each drug versus CTRL: *P < 0.005 (LoVo); *P < 0.005 (HepG2); *P < 0.05 (MCF-7). BAPTA-AM, 1,2-bis(2- aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid-acetoxymethylester; PBS, phosphate buffer saline.
Figure S5 Effects of parthenolide and artemisinin on Pgp mRNA (A) and protein (B and C) in LoVo, HepG2 and MCF-7 cells. Cells were incubated for 3 h (A) or 6 h (B) in the absence (CTRL) or presence of parthenolide (PART, 10 mmol·L-1) and artemisinin (ART, 10 mmol·L-1). When indicated, cells were pre-incubated for 1 h with BAPTA-AM (BAP, 10 mmol·L-1) or co-incubated with KN93 (KN, 10 mmol·L-1). (A) Total RNA was extracted, retro-transcribed and subjected to RT-PCR, performed as indicated under the Methods section. Measurements were performed in triplicate and data are presented as means ± SE (n = 3). Significance for LoVo cells: versus CTRL: *P < 0.02; versus PART or ART respectively: °P < 0.05. Signifi- cance for HepG2 cells: versus CTRL: *P < 0.005; versus PART or ART respectively: °P < 0.05. Significance for MCF-7 cells: versus CTRL: *P < 0.05; versus PART or ART respectively: °P < 0.05. (B and C) To detect the Pgp protein, cells were lysed and the whole cellular lysate was immunoprecipitated with an anti-Pgp polyclonal antibody. The immunoprecipitated proteins were subjected to Western blotting, using the same antibody (see Methods section). The expression of GAPDH, the product of an housekeeping gene, was used as a control of equal protein loading. The figure is representative of three experiments with similar results. GAPDH, glyceraldehyde-3- phosphate dehydrogenase; Pgp, P-glycoprotein; RT-PCR, realtime polymerase chain reaction.
Figure S6 Effects of parthenolide and artemisinin on doxorubicin accumulation, cytotoxicity and viability in LoVo (A and B), HepG2 (C and D) and MCF-7 (E and F) cells. Cells were grown for 6 h in the absence (CTRL) or in the presence of doxorubicin (5 mmol·L-1, DOX), together with either parthenolide (PART, 10mmol·L-1) or artemisinin (ART, 10 mmol·L-1); when indicated, cells were pre-incubated for 1 h with BAPTA-AM (BAP, 10mmol·L-1) or co-incubated with KN93 (KN, 10 mmol·L-1), alone or in different combinations. Doxorubicin intracellular accumulation, release of LDH activity in the culture supernatant, positivity of Trypan blue and FACS analysis for annexin V and PI-positive cells were measured as described under Methods section. Measurements were performed in duplicate and data are presented as means ± SE (n = 3). The significance of DOX versus CTRL was P < 0.05 for all the cell lines (not reported in the figure). Significance for LoVo cells: versus DOX: *P < 0.002; versus DOX + PART or DOX + ART respectively: °P < 0.002. Signifi- cance for HepG2 cells: versus DOX: *P < 0.05; versus DOX + PART or DOX + ART respectively: °P < 0.02. Signifi- cance for MCF-7 cells: versus DOX: *P < 0.005; versus DOX + PART or DOX + ART respectively: °P < 0.005. BAPTAAM, 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid-acetoxymethylester; PI, propidium iodide.
Figure S7Effects of parthenolide and artemisinin on the cell cycle arrest induced by doxorubicin in LoVo, HepG2 and MCF-7 cells. Cells were grown for 6 h in the absence (CTRL) or presence of doxorubicin (5 mmol·L-1, DOX), together with either parthenolide (PART, 10 mmol·L-1) or artemisinin (ART, 10 mmol·L-1); when indicated, cells were pre-incubated for 1 h with BAPTA-AM (BAP, 10mmol·L-1) or co-incubated with KN93 (KN, 10 mmol·L-1), alone or in combination. After this incubation time, cells were washed and let grow for 24 h in fresh medium, then they were permeabilized in ethanol and incubated with PI as indicated in the Methods section. Cell cycle analysis of G0/G1 phase (open bars), S phase (hatched bars) and G2/M phase (solid bars) was obtained by a FACSCalibur system using the Cell Quest software. Measurements were performed in duplicate and data are presented as means ± SE (n = 2). The significance of DOX versus CTRL was P < 0.05 for all the cell lines (not reported in the figure). Significance for LoVo cells: versus DOX: *P < 0.05; versus DOX + PART or DOX + ART respectively: °P < 0.05. Significance for HepG2 cells: versus DOX: *P < 0.05; versus DOX + PART or DOX + ART respectively: °P < 0.05. Significance for MCF-7 cells: versus DOX: *P < 0.05; versus DOX + PART or DOX + ART respectively: °P < 0.05. BAPTA-AM, 1,2-bis(2- aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid-acetoxymethylester.
Figure S8 Effect of parthenolide, artemisinin and KN93 on HIF-1α phosphorylation (pHIF-1α) in LoVo cells, HepG2 cells and MCF-7 cells. Cells were incubated for 3 h in the absence (CTRL) or presence of either parthenolide (PART, 10 mmol·L-1) or artemisinin (ART, 10mmol·L-1), alone or together with KN93 (KN, 10 mmol·L-1). Cell lysates were incubated with an anti-HIF-1α antibody, subsequently the immunoprecipitated proteins were separated by SDS-PAGE and probed with an anti-phosphoserine antibody as described under the Methods section. The expression of GAPDH was used as a control of equal protein loading. The figure is representative of three experiments with similar results. GAPDH, glyceraldehyde-3- phosphate dehydrogenase; HIF-1α, hypoxia-inducible factor-1α. Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ai J, Gao HH, He SZ, Wang L, Luo DL, Yang BF. Effect of matrine, artemisinin and tetrandrine on cytosolic [Ca2+]i in guinea pig ventricular myocytes. Acta Pharmacol Sin. 2001;22:512–516. [PubMed] [Google Scholar]
- Aldieri E, Atragene D, Bergandi L, Riganti C, Costamagna C, Bosia A, et al. Artemisinin inhibits inducible nitric oxide synthase and nuclear factor NF-kB activation. FEBS Lett. 2003;552:141–144. doi: 10.1016/s0014-5793(03)00905-0. [DOI] [PubMed] [Google Scholar]
- Baudouin-Legros M, Brouillard F, Tondelier D, Hinzpeter A, Edelman A. Effect of ouabain on CFTR gene expression in human Calu-3 cells. Am J Physiol Cell Physiol. 2003;284:C620–C626. doi: 10.1152/ajpcell.00457.2002. [DOI] [PubMed] [Google Scholar]
- Berchner-Pfannschmidt U, Petrat F, Doege K, Trinidad B, Freitag P, Metzen E, et al. Chelation of cellular calcium modulates hypoxia-inducible gene expression through activation of hypoxia-inducible factor-1 α. J Biol Chem. 2004;279:44976–44986. doi: 10.1074/jbc.M313995200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhuang S, Cassenaer S, Casteel DE, Gudi T, Boss GR, et al. Synergism between calcium and cyclic GMP in cyclic AMP response element-dependent transcriptional regulation requires cooperation between CREB and C/EBP-β. Mol Cell Biol. 2003;23:4066–4082. doi: 10.1128/MCB.23.12.4066-4082.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible Factor-1-dependent Regulation of the Multidrug Resistance (MDR1) Gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- Cortes-Funes H, Coronado C. Role of anthracyclines in the era of targeted therapy. Cardiovasc Toxicol. 2007;7:56–60. doi: 10.1007/s12012-007-0015-3. [DOI] [PubMed] [Google Scholar]
- Cortés-Selva F, Jiménez IA, Muñoz-Martínez F, Campillo M, Bazzocchi IL, Pardo L, et al. Dihidro-b-agarofuran sesquiterpenes: a new class of reversal agents of the multidrug resistance phenotype mediated by P-Glycoprotein in the protozoan parasite leishmania. Cur Pharm Des. 2005;11:3125–3139. doi: 10.2174/1381612054864920. [DOI] [PubMed] [Google Scholar]
- Curry EA, Murry DJ, Yoder C, Fife K, Armstrong V, Nakshatri H, et al. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest New Drugs. 2004;22:299–305. doi: 10.1023/B:DRUG.0000026256.38560.be. [DOI] [PubMed] [Google Scholar]
- Dupont G, Houart G, De Koninc P. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium. 2003;34:485–497. doi: 10.1016/s0143-4160(03)00152-0. [DOI] [PubMed] [Google Scholar]
- Eckstein-Ludwig U, Webb RJ, van Goethem IDA, East JM, Lee AG, Kimura M, et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- Efferth T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr Drug Targets. 2006;7:407–421. doi: 10.2174/138945006776359412. [DOI] [PubMed] [Google Scholar]
- Ferraro E, Pulicati A, Cencioni MT, Cozzolino M, Navoni F, di Martino S, et al. Apoptosome-deficient cells lose cytochrome c through proteasomal degradation but survive by autophagy-dependent glycolysis. Mol Biol Cell. 2008;19:3576–3588. doi: 10.1091/mbc.E07-09-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006;36:1427–1441. doi: 10.1016/j.ijpara.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Hallam TJ, Sanchez A, Rink TJ. Stimulus-response coupling in human platelets. Changes evoked by platelet-activating factor in cytoplasmic free calcium monitored with the fluorescent calcium indicator quin2. Biochem J. 1984;218:819–827. doi: 10.1042/bj2180819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien TT, White NJ. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- Hui AS, Bauer AL, Striet JB, Schnell PO, Czyzyk-Krzeska MF. Calcium signaling stimulates translation of HIF-1a during hypoxia. FASEB J. 2006;20:466–475. doi: 10.1096/fj.05-5086com. [DOI] [PubMed] [Google Scholar]
- Kim JH, Liu L, Lee S-O, Kim Y-T, You K-R, Kim D-G. Susceptibility of Cholangiocarcinoma Cells to Parthenolide-Induced Apoptosis. Cancer Res. 2005;65:6312–6320. doi: 10.1158/0008-5472.CAN-04-4193. [DOI] [PubMed] [Google Scholar]
- Krishna S, Woodrow C, Webb R, Penny J, Takeyasu K, Kimura M, et al. Expression and Functional Characterization of a Plasmodium falciparum Ca21-ATPase (PfATP4) Belonging to a Subclass Unique to Apicomplexan Organisms. J Biol Chem. 2001;276:10782–10787. doi: 10.1074/jbc.M010554200. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, O’Callaghan JP, Juskevich J, Lovenberg W. Activation of brain tryptophan hydroxylase by ATP-Mg2+: dependence on calmodulin. Proc Natl Acad Sci USA. 1980;77:4688–4691. doi: 10.1073/pnas.77.8.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dong Y, Tu Y, Lin Z. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signalling pathway NF-kappa B translocation. Int Immunopharmacol. 2006;6:1243–1250. doi: 10.1016/j.intimp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Mohanty S, Patel DK, Pati SS, Mishra SK. Adjuvant therapy in cerebral malaria. Indian J Med Res. 2006;124:245–260. [PubMed] [Google Scholar]
- Molnár J, Gyémánt N, Tanaka M, Hohmann J, Bergmann-Leitner E, Molnár P, et al. Inhibition of multidrug resistance of cancer cells by natural diterpenes, triterpenes and carotenoids. Curr Pharm Des. 2006;12:287–311. doi: 10.2174/138161206775201893. [DOI] [PubMed] [Google Scholar]
- Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferring conjugation. Int J Pharmaceut. 2008;354:28–33. doi: 10.1016/j.ijpharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- O’Donnell JL, Joyce MR, Shannon AM, Harmey J, Geraghty J, Bouchier-Hayes D. Oncological implications of Hypoxia Inducible Factor-1a (HIF-1a) expression. Cancer Treat Rev. 2006;32:407–416. doi: 10.1016/j.ctrv.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Riganti C, Miraglia E, Viarisio D, Costamagna C, Pescarmona GP, Ghigo D, et al. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005;65:516–525. [PubMed] [Google Scholar]
- Seidler NW, Jonaz I, Veghg M, Martonosill A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Miyazaki H, Gutkind JS. MAPK and akt act cooperatively but independently on Hypoxia Inducible Factor-1a in rasV12 upregulation of VEGF. Biochem Biophys Res Commun. 2001;287:292–300. doi: 10.1006/bbrc.2001.5532. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Tomida A, Tsuruo T. Dephosphorylated Hypoxia Inducible Factor 1a as a mediator of p53-dependent apoptosis during hypoxia. Oncogene. 2001;20:5779–5788. doi: 10.1038/sj.onc.1204742. [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, et al. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther. 2005;4:1004–1012. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]
- Takara K, Sakaeda T, Okumura K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des. 2006;12:273–286. doi: 10.2174/138161206775201965. [DOI] [PubMed] [Google Scholar]
- Uhleman AC, Cameron A, Eckstein-Ludwig U, Fischbarg J, Iserovich P, Zuniga FA, et al. A single aminoacid residue can determine the sensitivity of SERCA to artemisinins. Nat Struct Mol Biol. 2005;12:628–629. doi: 10.1038/nsmb947. [DOI] [PubMed] [Google Scholar]
- Wibom R, Hagenfeldt L, von Dobeln U. Measurement of ATP production and respiratory chain enzyme activities in mitochondria isolated from small muscle biopsy samples. Anal Biochem. 2002;311:139–151. doi: 10.1016/s0003-2697(02)00424-4. [DOI] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Raman Bhasker C, Semenza GL, Prabhakar NR. Ca2++/Calmodulin kinase-dependent activation of Hypoxia Inducible Factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–4328. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- Zhou J, Brüne B. Cytokines and hormones in the regulation of Hypoxia Inducible Factor-1a (HIF-1 a) Cardiovasc Hematol Agents Med Chem. 2006;4:189–197. doi: 10.2174/187152506777698344. [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, et al. Linkage of a1-adrenergic stimulation to apoptotic heart cell death through protein kinase A–independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


