Abstract
Background and purpose
The expression and function of P2X7 receptors in osteoclasts is well established, but less is known about their role in osteoblast-like cells. A study in P2X7 receptor knockout mice suggested the involvement of these receptors in bone formation. We have investigated the expression and pharmacology of several P2X receptors in two human osteosarcoma cell lines to see if they could be involved in bone turnover in man.
Experimental approach
Reverse transcriptase-polymerase chain reaction and Western blotting were used to study P2X2, P2X4 and P2X7 receptor expression at mRNA and protein levels, respectively, in human osteoblast-like cells. P2X7 receptor pharmacology was studied by measuring pore formation in the presence of different agonists and antagonists using the YO-PRO 1 uptake method.
Key results
P2X4 and P2X7 receptor mRNA and protein were found to be expressed by these cell lines. No evidence was found for P2X4/P2X7 receptor heteropolymerization. 2′-3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (DBzATP) was equipotent to ATP and the antagonists used were either ineffective or weakly blocked pore formation.
Conclusions and implications
This study demonstrates that P2X4 and P2X7 receptors are expressed by human osteoblast-like cells. The affinities of the different agonists suggest that the P2X7 receptor is mainly responsible for pore formation although P2X4 receptors may also be involved. The low affinity of DBzATP and the weak action of the antagonists support the previously described atypical pharmacology of the P2X7 receptor in osteoblasts. Targeting the P2X7 receptor in osteoblasts could represent a promising new treatment for bone diseases such as osteoporosis and rheumatoid arthritis.
Keywords: P2X7 receptor, human osteoblast-like cells, pore formation, YO-PRO 1, ATP, DBzATP, MG63 cells, SaOS2 cells
Introduction
ATP can activate two receptor types, ligand-gated ion channel P2X receptors and G protein-coupled P2Y receptors (Suprenant et al., 1996; North and Barnard, 1997); nomenclature follows (Alexander et al., 2008). The P2X7 receptor is the largest (595 amino acids) member of the P2X receptor family (Suprenant et al., 1996; North and Barnard, 1997). It also has the longest C-terminus (242 amino acids) of the P2X family of receptors (Khakh, 2001a).
A distinguishing characteristic of the P2X7 receptor is the formation, upon prolonged stimulation, of a non-selective pore in the cell membrane permeable to molecules as large as 900 Daltons, such as ethidium bromide and quinolinium 4-[(3-methyl-2(3H)-benzoxazolylidene)methyl]-1-[3-(trimethylammonio) propyl]-diiodide (YO-PRO 1) (Steinberg et al. 1987; Chessell et al. 1997; Virginio et al. 1999; Hibell et al., 2000). It is believed that pore formation involves the long C-terminus of the P2X7 receptor (Suprenant et al., 1996). However, P2X2 and P2X4 receptors, which have shorter C-termini, can still form pores (Khakh, 2001a; Vial et al., 2004). North, (2002) suggested two mechanisms for the progressive permeability of the P2X7 receptor to cations and larger molecules on activation, the dilation hypothesis and the extra protein hypothesis. An additional characteristic of the P2X7 receptor is its very low affinity for ATP compared with other P2X receptors (EC50 value 300 µM; North and Barnard, 1997). Furthermore, 2′-3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (DBzATP) was claimed to be the most potent agonist by some studies (Chessell et al., 1998; Michel et al., 1999; Lee et al., 2006a), although most of these studies were performed in transfected cells.
P2X7 receptors were initially found in haemopoietic cells, but have since also been identified in other cells such as blood, microglia, brain, nerves, epithelial, lung and spleen cells (Suprenant et al., 1996; Collo et al., 1997; Deuchars et al., 2001; Di Virgilio et al., 2001; North, 2002; Kobayashi et al., 2005). The receptor is also expressed in many pathological conditions such as leukaemia (Thunberg et al., 2002; Wiley et al., 2002; Zhang et al., 2004), intestinal epithelial carcinoma (Coutinho-Silva et al., 2005), cervical cancer (Wang et al., 2004a; 2005) and systemic lupus erythematosus (Elliott et al., 2005). It is thought to be involved in intercellular communication (Chiozzi et al., 1997) and has also been described as a death receptor, because cell death by either necrosis or apoptosis resulting from its stimulation has been reported in many cells (Di Virgilio et al., 1998; Sluyter and Wiley, 2002; Wang et al., 2004b; Elliott et al., 2005). In addition, it was found to be important in immune and inflammatory responses such as cytokine release (Ferrari et al., 1997; Di Virgilio et al., 2001; Solle et al., 2001; Labasi et al., 2002; Bulanova et al., 2005). Additionally, P2X7 receptors were found to be involved in the release of the anti-inflammatory protein, intracellular interleukin-1 receptor antagonist type-1, which can potentially change receptor expression (Wilson et al., 2004).
In bone, many studies have confirmed the expression of P2X7 receptors by osteoclasts and their function in osteoclasts has been clearly described (Hoebertz et al., 2000; 2003; Naemsch et al., 2001). The involvement of P2X7 receptors in the process of calcium signalling between osteoblasts and osteoclasts has also been documented (Jorgensen et al., 2002). (Gartland et al., 2003) reported that blockade of the receptor prevented the formation of multinucleated osteoclasts from human peripheral blood, increased osteoclast apoptosis and eventually inhibited osteoclastic resorption. Ke et al. (2003) found that deletion of the P2X7 receptor in mice caused increased resorption proving the significance of the receptor in osteoclast function. However, they also found that multinucleated osteoclasts can be produced even in the absence of the P2X7 receptor.
Less is known about P2X7 receptor expression and function in osteoblasts. Nakamura et al. (2000) showed expression of P2X7 receptors by human osteoblast-like MG63 cells at the mRNA level. Gartland et al. (2001) demonstrated the expression of P2X7 receptors by two human osteosarcoma cell lines (SaOS2 and Te85) and primary human bone-derived cells using reverse transcriptase-polymerase chain reaction (RT-PCR). They also showed high levels of the receptor protein were found in the SaOS2, but not in the Te85 cells, and varying levels in the primary human cells. P2X7 receptor pore formation was shown in SaOS2 cells, but not in Te85 cells. In SaOS2 and human primary cells, receptor activation caused pore formation, and changes in cell morphology which eventually led to apoptosis. As pore formation in osteoblasts needed 60 min incubation with agonist, while only 5 min is usually needed for haemopoietic cells, the authors suggested an atypical pharmacology of the receptor in these cells (Gartland et al., 2001). The importance of P2X7 receptors in bone formation was established in the study by Ke et al. (2003). They compared P2X7 receptor expression and function in osteoblasts from knockout and wild-type mice using RT-PCR and pore formation studies, and found that the knockout mice had reduced bone formation. As P2X7 receptors appear to be involved in bone formation, more information is needed about their expression and pharmacology in osteoblasts. Our study therefore aimed to characterize these receptors more fully in two human osteoblast-like cell lines, MG63 and SaOS2, which are at different stages of development of the osteoblast phenotype (Arnett and Henderson, 1998). SaOS2 cells are believed to be the closest to the mature osteoblast (Hughes and Aubin, 1998). Our results showed that functional P2X7 receptors are expressed by both MG63 and SaOS2 cells. The receptors had an atypical pharmacology as DBzATP was equipotent to ATP. We also showed expression of P2X4 receptors in both cell lines, but our data suggest that it is the P2X7 receptor that is responsible for pore formation.
Methods
Cell culture
MG63 and SaOS2 osteosarcoma cell lines (The European Collection of Cell Cultures, Salisbury, Wiltshire, UK) were grown in 25 cm2 flasks in Dulbecco's Modified Eagle's Medium (Cambrex, Berks, UK) containing 2 mmol·L−1 glutamine and 4500 mg·L−1 glucose and supplemented with 5% foetal calf serum (Invitrogen, Paisley, UK). The cells were grown at 37°C in a humidified atmosphere with 5% CO2 in air, and the medium was changed every 3 or 4 days. Cells were passaged using trypsin-EDTA (0.025% trypsin Worthington Biochemical Corporation, Reading, UK; 0.025% EDTA (ethylenediamine tetraacetic acid)).
RT-PCR
The human P2X2 receptor gene primers (5′–3′) used were: sense – CCCAAATTCCACTTCTCCAA and antisense – GTC CAGGTCACAGTCCCAGT. The human P2X4 receptor gene primers (5′–3′) used were: sense – ACCGTGCTGTGTGACAT CAT and antisense – TGAGTGCTTGTGGAGTGGAG. The human P2X7 receptor gene primers (5′–3′) used were: sense – GTTCCTCTGACCGAGGTT and antisense – CAGGTCTTCTG GTTCCCT. All primers were obtained from Invitrogen and were checked against GenBank for selectivity.
RNA was extracted from cells using the MIDAS RNA extraction kit (Biogene, Cambridge, UK) according to the manufacturer's instructions, and treated with DNase (Ambion, Warrington, UK). RT reactions were carried out to prepare cDNA from 1 µg RNA using avian myeoloblastosis virus (AMV) RT according to the manufacturer's instructions (Promega, Southampton, UK). Reactions were performed in parallel without the RT enzyme. cDNA samples were stored at −20°C until needed. PCR reactions (25 µL) were performed using 1 µL of the RT reaction or water and 10 pmol of each primer. All PCR reactions were run in a Perkin Elmer 480 Thermal Cycler as follows: 94°C for 3 min, 60°C for 1 min, 72°C for 1 min and then 35 cycles at: 94°C for 30 s, 60°C for 1 min, 72°C for 1 min. PCR samples and marker (0.3 µg·lane−1, New England Biolabs, Herts, UK) were then separated in 1.5% agarose gels containing 1.27 µmol·L−1 ethidium bromide using TBE buffer (90 mmol·L−1 Tris base, 90 mmol·L−1 boric acid, 2.5 mmol·L−1 EDTA). The bands were visualized with a UV-transilluminator (Fotodyne Incorporated, New Berlin, WI, USA), and photographed using a Polaroid camera. The bands obtained were excised from the gels, incubated in 50 µL sterile water, kept at 4°C for 48 h and the cDNA eluted into the water and sequenced using the Big Dye® Terminator V 3.1 Cycle Sequencing kit (Applied Biosystems, Warrington, UK).
Receptor deglycosylation
Deglycosylation of the P2X7 receptor in MG63 and SaOS2 cell membranes obtained as described previously (Yates et al., 2006) was studied using a Glycoprotein Deglycosylation Kit (Calbiochem, Nottingham, UK). According to the manufacturer's instructions, samples were centrifuged at 15 306 g at 4°C for 15 min, and the pellet resuspended in 30 µL deionized water. Half the sample was incubated with a total of 2.5 U of the enzymes (N-glycosidase F, α2-3,6,8,9-Neuroaminidase and Endo-α-N-acetylgalactosminidase), while the other half was incubated with distilled water. Both samples were incubated for 3 h at 37°C and the reaction was then stopped with 3X Laemmli sample buffer (0.19 mol·L−1 Tris base, 12% SDS, 30% glycerol, 15% β-mercaptoethanol, 1 mg·mL−1 bromophenol blue) and they were then stored at −20°C until use.
Western blotting
Cell lysis was performed as described previously (Thomas et al., 2006). Cell nuclei were isolated using lysis buffer (10 mmol·L−1 Tris base, 10 mmol·L−1 NaCl, 5 mmol·L−1 MgCl2, 0.5% Nonidet P 40, pH 7.4), the lysed cells were centrifuged at 400 g at 4°C for 5 min and the pellet was resuspended in 4 mL lysis buffer, vortexed and centrifuged as above. Protein concentrations were determined using the bicinchonic acid method (Perbio Science UK Ltd., Cramlington, UK)
Western blotting was performed using standard methods (Thomas et al., 2006). Membrane samples of human embryonic kidney cells (HEK393) transfected with the P2X7 receptor (HEK7) were used as a positive control (Michel et al., 1999). The samples and molecular weight marker (Precision Plus Protein Standards marker, Bio-Rad Laboratories, Hertfordshire, UK) were loaded onto 10% polyacrylamide gels and separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis in running buffer (25 mmol·L−1 Tris base, 190 mmol·L−1 glycine, 0.05% SDS, pH 8.3). The separated proteins were then blotted on to 0.2 µm nitrocellulose membranes (GE, Little Chalfont, UK), washed in Tris-buffered saline with Tween 20 (TBST, 2 mmol·L−1 Tris, 15 mmol·L−1 NaCl, 0.1% Tween-20, pH 7.5) and blocked for 1 h, at room temperature, in TBST supplemented with 5% w·v−1 fat-free dried milk (BLOTTO; Marvel, Premier International Ltd., Spalding, UK). The membranes were then incubated with the relevant primary antibody in 1% BLOTTO at 4°C overnight. For re-adsorption of the anti-P2X7 receptor antibody with its corresponding peptide, 1.2 µg of peptide was incubated at 4°C overnight with the rP2X7 antibody in 1% w·v−1 BLOTTO before use. Re-adsorption of the anti-P2X2 and anti-P2X4 receptor antibodies with 4 and 3.2 µg of their corresponding peptides, respectively, was also performed. Membranes were washed five times in TBST and incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:20 000, Vector Laboratories, Peterborough, UK) for 1 h at RT. The membranes were washed as above in TBST, the bands visualized using enhanced chemiluminiscent detection (Super Signal®, West Dura, Perbio Science), and exposed to high performance chemiluminescent X-ray film (GE).
Immunocytochemistry
Immunocytochemistry was performed as described previously (Thomas et al., 2006) with several modifications as detailed below. Both cell lines were grown on 13 mm coverslips treated with 2% 3-aminopropyltriethoxy silane in acetone in 24 well cluster plates in the appropriate growth media. Cells were fixed in 2% formaldehyde in 0.1 mol·L−1 phosphate-buffered saline (PBS, 145 mmol·L−1 NaCl, 96.4 mmol·L−1 NaHPO4, 21.5 mmol·L−1 NaH2PO4), pH 7.4, for 15 min at room temperature and subsequently washed three times in PBS (each 5 min). Coverslips were blocked for 30 min in 3% goat serum, 1% bovine serum albumin and 0.1% Triton X-100 in PBS. The coverslips were incubated with rP2X7 receptor primary antibody (1:1000) in blocking buffer for 24–72 h at 4°C. To confirm the labelling seen, the rP2X7 receptor antibody was pre-adsorbed for 48 h in blocking solution with 0.8 µg of its corresponding peptide. After washing as above, coverslips were then incubated with an anti-rabbit secondary antibody conjugated to Cy3 (Millipore, Watford, UK), 1:800, for 1 h at room temperature in the dark. The coverslips were washed as above and finally dipped in distilled water to remove any residual buffer salts before being left to dry in the dark. Coverslips were mounted on ethanol-rinsed slides using an anti-fade agent (fluorescent mounting medium, DAKO UK Ltd., Ely, UK) and stored at 4°C. Slides were visualized using a Leica DMRA2 microscope (Leica, Wetzlar, Germany), images were recorded using a digital camera (DC 500, Leica) and the Leica FW 4000 software and images were processed using Adobe Photoshop.
YO-PRO 1 Uptake
P2X7 receptor activation was studied using a modified version of the YO-PRO 1 uptake method (Michel et al., 1999). Cells were grown in 25 cm2 flasks until confluent, and then collected using trypsin/EDTA. The cells were resuspended in 1 mL of growth medium and centrifuged at 179×g at room temperature for 5 min. The cell pellet was then resuspended in 10 mL Dulbecco's PBS (Invitrogen), counted and centrifuged again at 200×g for 5 min at room temperature. The cell pellet was resuspended in 10 mL ice-cold assay buffer pH 7.4 (5 mmol·L−1 KCl, 0.5 mmol·L−1 CaCl2, 10 mmol·L−1 glucose, 10 mmol·L−1 HEPES, 10 mmol·L−1 N-methyl-D-glucamine and 280 mmol·L−1 sucrose) and centrifuged at 200×g for 5 min at room temperature and resuspended again in assay buffer at 37°C. Approximately 100 000 cells per well were added to 96-well black plates (Greiner Bio-One Ltd., Stonehouse, UK) containing buffer or an agonist and 1 µmol·L−1 YO-PRO 1 in assay buffer and the plate was incubated at 37°C for various times. YO-PRO 1 fluorescence was monitored in a 96-well plate reader (Fluostar Optima, BMG Labtechnologies Ltd., Aylesbury, UK) using an excitation wavelength of 485 nm and an emission wavelength of 520 nm. To determine the optimum time needed for the pore formation, MG63 and SaOS2 cells were incubated with 0.8 mmol·L−1 ATP for different periods (5–30 min) and the uptake was measured. To find the best assay buffer for YO-PRO 1 uptake in MG63 and SaOS2 cells, five different buffers were studied. In addition to the assay buffer above (A), 0.8 mmol·L−1 ATP was used to induce YO-PRO 1 uptake in the following buffers: (B) assay buffer containing 0.1 mmol·L−1 CaCl2, (C) the same buffer containing 0.1 mmol·L−1 EDTA in place of CaCl2 (Michel et al., 1999), (D) PBS and (E) a buffer containing 125 mmol·L−1 KCl, 1 mmol·L−1 EDTA, 5 mmol·L−1 glucose and 20 mmol·L−1 HEPES, pH 7.4 (Gartland et al., 2001). Experiments were also performed to investigate receptor desensitization. Here, flasks of cells were incubated at 37°C for 20 min with or without 3 mmol·L−1 ATP and the YO-PRO 1 uptake was then determined.
Different P2X receptor antagonists were studied to investigate whether they could block pore formation induced by ATP and DBzATP. For this purpose, cells were incubated with different concentrations of the antagonists for 1 h in the presence of 1 µmol·L−1 YO-PRO 1 in assay buffer at 37°C, then ATP or DBzATP was added and the assay performed.
An experiment was performed to investigate the potential involvement of P2X4 receptors in the YO-PRO 1 uptake. Cells were pre-incubated with 10 µmol·L−1 ivermectin for 10 min and then YO-PRO 1 uptake induced by 1 and 0.5 mmol·L−1 ATP or DBzATP was measured.
Data expression and statistical analysis
For the YO-PRO 1 uptake studies, data were expressed as % of maximum uptake of each agonist and EC50 values were calculated using Prism 4.0 (GraphPad Software, La Jolla, CA, USA).
Results were analysed using one-way anova. Multiple comparisons were then made using Bonferroni or Dunnett's post hoc tests in Prism 4.0. P values less than 0.05 were considered to be statistically significant. The results were expressed as the mean ± SD.
Drugs, chemicals and antibodies
All chemicals were of Analar grade and were obtained from Sigma (Poole, Dorset, UK) or Fisher (Leicester, UK), unless otherwise stated.
ATP, DBzATP, adenosine-5′-(3-thiotriphosphate) (ATPγS), brilliant blue G (BBG), adenosine 5′-triphosphate, periodate oxidized, sodium salt (oATP) and pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) tetrasodium salt (PPADS) were obtained from Sigma and 2-(methylthio)adenosine 5′-triphosphate tetrasodium salt (2-Me-S-ATP) and 4-[(2S)-2-[(5-isoquinolinylsulphonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulphonic acid ester (KN-62) were obtained from Tocris (Bristol, UK).
YO-PRO 1 1 mmol·L−1 solution in DMSO was obtained from Invitrogen. Polyclonal rabbit anti-C-terminal P2X2, anti-P2X4, and anti-P2X7 receptor antibodies and their corresponding peptides were obtained from Alomone Labs, Jerusalem, Israel and are referred to as rP2X2, rP2X4 and rP2X7 antibodies respectively.
Results
RT-PCR
Reverse transcriptase-polymerase chain reaction for P2X4 (Figure 1B) and P2X7 receptor mRNA (Figure 1C), using MG63 and SAOS2 cell RNA samples gave bands of the expected sizes [200 base pairs (bp) for the P2X4 and 100 bp for the P2X7 receptor]. No bands were seen in control samples (water or no AMV in the RT reaction) (Figure 1B,C). To confirm these results, sequencing was carried out on the bands excised from the gel and the expected sequence of nucleotides was obtained for each receptor (data not shown). No bands were seen using the P2X2 receptor primers in either cell line (Figure 1A). A human pancreatic cell line, BON-1, was used as a positive control (Lynch et al., 1999) to demonstrate that the primers did amplify the expected P2X2 receptor product of 200 bp (Figure 1A).
Figure 1.
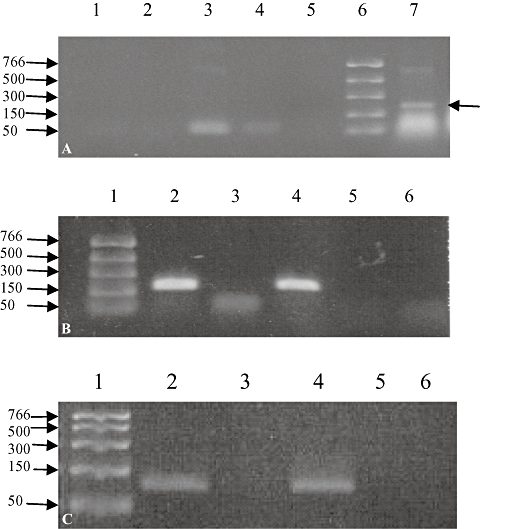
Representative example of RT-PCR for P2X2, P2X4 and P2X7 receptor mRNAs in MG63 and SaOS2 cell samples. RT-PCR was performed on RNA extracted from MG63 and SaOS2 cells using primers for the human P2X2 (A), P2X4 (B) and P2X7 (C) receptors. In (A), no bands were seen for MG63 (lanes 1 and 2) or SaOS2 (lanes 3 and 4) cells in the presence (lanes 1 and 3) or absence (lanes 2 and 4) of the RT enzyme. Human BON-1 pancreatic cell cDNA was used as a positive control (lane 7) and gave the expected band of 200 bp (arrow). The marker ladder is in lane 6. In (B) and (C) bands were seen in lanes 2 and 4 for MG63 and SaOS2 samples, respectively, corresponding to P2X4 (200 bp, B) or P2X7 (100 bp, C) receptors. No bands were seen when MG63 (lane 3) and SaOS2 (lane 5) samples were incubated without the RT enzyme or when sterile water (lane 6) was used in the PCR reaction instead of the RT sample (lane 6). The marker ladder is shown in lane 1. n = 2–5. RT-PCR, reverse transcriptase-polymerase chain reaction.
Western blotting
Membranes of HEK7 cells were used as a positive control. Using the rP2X7 receptor antibody, bands were seen in the positive control, SaOS2 and MG63 cell membranes, lysates, and nuclei at 68 ± 5 kDa, and at 89 ± 3 kDa (Figure 2A,C). Bands larger than 100 kDa were also seen in the SaOS2 and MG63 lysate and nuclei samples (data not shown). Following pre-incubation of the rP2X7 receptor antibody with its corresponding peptide, the bands in the HEK7 positive control, SaOS2 and MG63 membranes, lysates and nuclei either disappeared or were greatly reduced in intensity (Figure 2B,D).
Figure 2.
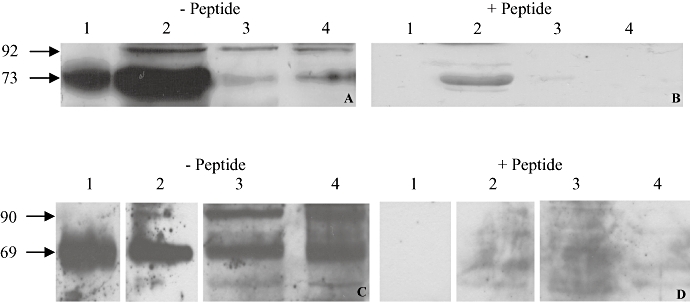
Representative example of Western blotting for P2X7 receptors in SaOS2 and MG63 cells. Positive control (10 µg of HEK7 cell membrane, lane 1), and 25 µg of SaOS2 (A & B) or MG63 (C & D) cell membranes (lane 2), lysate (lane 3) and nuclei (lane 4) were separated on 10% gels and bands detected after prior incubation of the rP2X7 receptor antibody (1:1000) with (B & D) or without (A & C) its corresponding peptide (1.2 µg). Bands were seen at 73 and 92 kDa in SaOS2 cells (A) and at 69 and 90 kDa in MG63 cells (C). All these bands disappeared or were greatly reduced in intensity after preincubation with the peptide (B & D). n = 10.
Additionally, Western blotting was performed to investigate the expression of the other pore-forming P2X receptors, P2X2 and P2X4, in SaOS2 and MG63 cells. Rat brain membranes were used as a positive control. Using the rP2X4 receptor antibody, bands were seen in the positive control and MG63 and SaOS2 cell lysate samples at 64 ± 1.8 kDa for the P2X4 receptor (Figure 3A). Following pre-incubation of the rP2X4 receptor antibody with its corresponding peptide, all these bands disappeared (Figure 3B). Using the anti-P2X2 receptor antibody, a band at 60 ± 1.8 kDa was seen in all samples but, following pre-incubation of the rP2X2 receptor antibody with its corresponding peptide, only the positive control band disappeared (data not shown).
Figure 3.
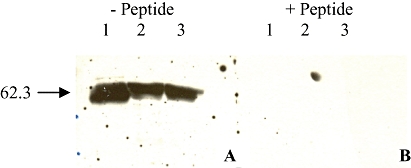
Representative example of Western blotting for P2X4 receptors in SaOS2 and MG63 cells. Positive control (60 µg of rat brain membranes, lane 1), and 12.5 µg of MG63 (lane 2 A and B) and SaOS2 (lane 3 A and B) cell lysates were separated on 10% gels and bands detected after prior incubation of the rP2X4 receptor antibody (1:1000) with (B) or without (A) its corresponding peptide (3.2 µg). A band was seen in all the samples at 62.3 kDa (A), which disappeared after preincubation with the peptide (B). n = 5.
P2X7 receptor deglycosylation
Deglycosylation of MG63 and SaOS2 cell membranes reduced the apparent molecular mass of the P2X7 receptor from 68 ± 1.7 kDa to 60 ± 25 kDa and from 70 ± 1.9 kDa to 61 ± 2 kDa in MG63 and SaOS2 cells, respectively (Figure 4). The higher molecular mass band (∼89 kDa) was not detected in any of these experiments.
Figure 4.
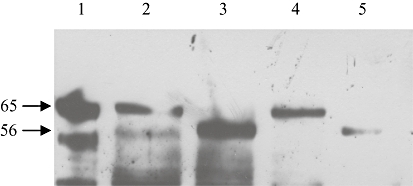
Representative example of Western blotting of the effect of deglycosylation of P2X7 receptors in MG63 and SaOS2 cell membranes. Positive control (5 µg of HEK7 cell membranes, lane 1), 100 µg of glycosylated MG63 (lane 2) and SaOS2 (lane 4) cell membranes, and 100 µg of deglycosylated MG63 (lane 3) and SaOS2 (lane 5) cell membranes were separated on 10% gels and bands detected using the rP2X7 antibody (1:2000). Bands were seen in the positive control at 63.7 kDa, glycosylated MG63 cell membranes at 65.2 kDa, deglycosylated MG63 cell membranes at 56.1 kDa, glycosylated SaOS2 cell membranes at 66.7 kDa and deglycosylated SaOS2 cell membranes at 58.4 kDa. No higher bands were seen in any of the samples. n = 3.
P2X7/P2X4 receptor co-immunoprecipitation
P2X4 and P2X7 receptors were immunoprecipitated from MG63 and SaOS2 cell lysates using the rP2X4 and rP2X7 receptor antibodies, respectively. After separation and blotting as described above, membranes were probed with the same antibodies. Immunoprecipitation of both receptors was seen with their corresponding antibodies but no bands were obtained for the P2X7 receptor co-immuno-precipitated with the rP2X4 receptor antibody or vice versa (data not shown).
Immunocytochemistry
Much more intense labelling of the nuclei compared with the cytoplasm was observed when cells were incubated with the rP2X7 antibody. A representative example of MG63 cells is shown in Figure 5A. No labelling was seen in control cells incubated with the blocking solution in the absence of the antibody (data not shown). To confirm the labelling seen, the rP2X7 receptor antibody was pre-adsorbed with its corresponding peptide and the labelling disappeared or was much weaker (Figure 5B). Similar results were seen with SaOS2 cells (data not shown).
Figure 5.
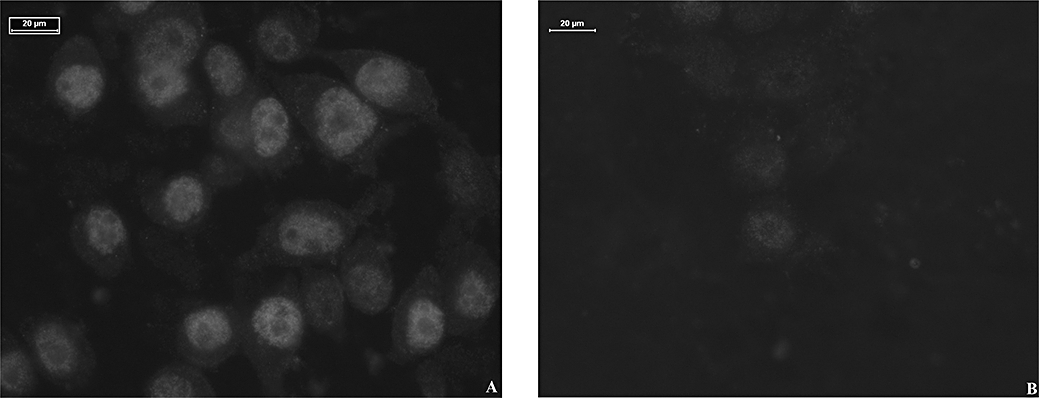
Representative example of immunocytochemistry in MG63 cells. MG63 cells were incubated with the rP2X7 receptor antibody (1:1000) after prior incubation with (B) or without (A) its corresponding peptide (0.8 µg) and detected with an anti-rabbit secondary antibody conjugated to Cy3 (1:800). n = 3.
YO-PRO 1 uptake
Initial experiments investigated the optimum assay buffer and incubation period for the agonists in both cell lines. The highest uptake was obtained with assay buffer (A) described by Michel et al. (1999) and uptake was also obtained with buffer (B) (data not shown). No uptake above baseline was obtained with buffers (C), (D) or (E) (data not shown). An incubation period of 5 min gave the highest uptake in both cell lines and a significant decrease in uptake was seen after 20 and 30 min incubation in SaOS2 cells (data not shown). YO-PRO 1 uptake was lower than control levels when cells were pre-incubated for 60 min with ATP (data not shown). All subsequent experiments were therefore carried out using assay buffer (A) and 5 min incubation.
Effect of agonists on YO-PRO 1 uptake
All the agonists used, ATP, DBzATP, ATPγS and 2Me-S-ATP, were found to induce YO-PRO 1 uptake in SaOS2 cells with similar potency (Figure 6, Table 1). ATP and DBzATP also induced uptake in MG63 cells with similar potency (Table 1). YO-PRO 1 uptake induced by ATP and 2Me-S-ATP at concentrations higher than 1 mmol·L−1 was less than that induced by 1 mmol·L−1. Table 1 compares the EC50 values of the agonists used in this study with those reported in the literature for the influx of intracellular calcium in cells transfected with human P2X2, P2X4 and P2X7 receptors. With the exception of the data for DBzATP, EC50 values in this study were closer to those for P2X7 receptors than for P2X2 or P2X4 receptors.
Figure 6.
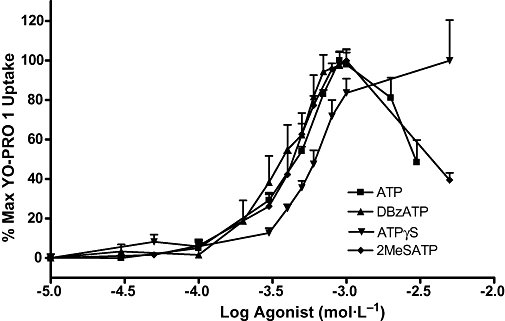
Concentration-effect curves for ATP, DBzATP, ATPγS and 2MeSATP in SaOS2 cells. SaOS2 cells (approximately 100 000 cells per well) were incubated with each agonist and 1 µmol·L−1 YO-PRO 1 in 0.5 mmol·L−1 CaCl2-containing assay buffer at 37°C for 5 min. YO-PRO 1 fluorescence was monitored in a 96-well plate reader using an excitation wavelength of 485 nm, and an emission wavelength of 520 nm. Data are expressed as % maximum YO-PRO 1 uptake for each agonist. n = 3–19. 2MeSATP, 2-(methylthio)adenosine 5′-triphosphate tetrasodium salt; ATPγS, adenosine-5′-(3-thiotriphosphate); DBzATP, 2′-3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; YO-PRO 1, quinolinium 4-[(3-methyl-2(3H)-benzoxazolylidene)methyl]-1-[3-(trimethylammonio) propyl]-diiodide.
Table 1.
Comparison of EC50 values (µmol·L−1 ± SD) for ATP, DBzATP, ATPγS and 2MeSATP obtained here with literature values (µmol·L−1) for human P2X2, P2X4 and P2X7 receptors
| Agonist | MG63a | SaOS2a | hP2X2b | hP2X4c | hP2X7c |
|---|---|---|---|---|---|
| ATP | 360 ± 100 | 430 ± 200 | 1 | 0.48 | 95 |
| DBzATP | 500 ± 100 | 360 ± 200 | 0.42 | 0.49 | 4.7 |
| ATPγS | ND | 600 ± 100 | 1.35 | 11 | >100 |
| 2MeSATP | ND | 420 ± 100 | 0.81 | 2.2 | >100 |
EC50 values (µmol·L−1 ± SD) obtained in this study for MG63 and SaOS2 cells.
EC50 values (µmol·L−1) for the influx of intracellular calcium measured in human astrocytoma cells transfected with human P2X2 (hP2X2) receptors (Lynch et al., 1999).
EC50 values (µmol·L−1) for the influx of intracellular calcium measured in human astrocytoma cells transfected with human P2X4 (hP2X4) or human P2X7 (hP2X7) receptors (Bianchi et al., 1999).
2MeSATP, 2-(methylthio)adenosine 5′-triphosphate tetrasodium salt; ATPγS, adenosine-5′-(3-thiotriphosphate); DBzATP, 2′-3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; ND, not determined.
Receptor desensitization with ATP in MG63 and SaOS2 cells did not alter the concentration-effect curves for ATP compared with control cells. The EC50 values for ATP in the control and treated MG63 cells were 0.44 ± 0.02 mmol·L−1 and 0.43 ± 0.07 mmol·L−1, respectively, while in SaOS2 cells, the EC50 value for ATP was 0.58 ± 0.05 mmol·L−1 in both control and treated cells.
Effect of antagonists on YO-PRO 1 uptake
Figures 7–10 show the concentration-effect curves for ATP in the presence of different concentrations of BBG, KN-62, oATP and PPADS in MG63 (A) and SaOS2 (B) cells. EC50 values for ATP and DBzATP were not significantly altered by any of the antagonists. The EC50 values for the highest antagonist concentrations used are shown in Table 2. In MG63 cells, 0.2 µmol·L−1 BBG significantly increased the uptake induced by 30 and 300 µmol·L−1 ATP, but 5 µmol·L−1 BBG significantly reduced the maximum YO-PRO 1 uptake induced by ATP by approximately 20% (Figure 7A). BBG had no effect on YO-PRO 1 uptake induced by ATP in SaOS2 cells (Figure 7B). KN-62 did not significantly alter the maximum YO-PRO 1 uptake induced by ATP in MG63 cells (Figure 8A). In SaOS2 cells, 10 µmol·L−1 KN-62 significantly reduced the maximum YO-PRO 1 uptake induced by ATP by approximately 11% (Figure 8B). In MG63 cells, 30 and 300 nmol·L−1 PPADS significantly decreased the maximum uptake induced by ATP by approximately 11 and 17% respectively (Figure 9A). PPADS did not significantly alter YO-PRO 1 uptake induced by ATP in SaOS2 cells (Figure 9B). oATP had no significant effect on ATP-induced YO-PRO 1 uptake in MG63 (Figure 10A) or SaOS2 (Figure 10B) cells.
Figure 7.
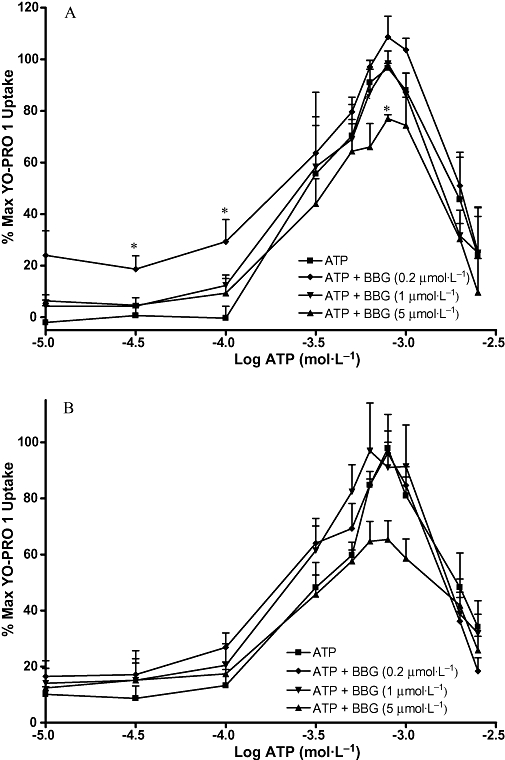
ATP concentration-effect curves in MG63 and SaOS2 cells in the presence of BBG. MG63 (A) or SaOS2 (B) cells (approximately 100 000 cells per well) were incubated with 1 µmol·L−1 YO-PRO 1 in assay buffer in the presence of water (vehicle for BBG), or 0.2 µmol·L−1, 1 µmol·L−1, or 5 µmol·L−1 BBG at 37°C for 1 h followed by ATP for 5 min. YO-PRO 1 fluorescence was monitored in a 96-well plate reader using an excitation wavelength of 485 nm, and an emission wavelength of 520 nm. Data are expressed as % of maximum uptake of ATP in the presence of the vehicle. Statistical analyses were performed using one-way anova with Bonferroni post hoc test. *P < 0.05 versus control. n = 3–4. BBG, brilliant blue G; YO-PRO 1, quinolinium 4-[(3-methyl-2(3H)-benzoxazolylidene)methyl]-1-[3-(trimethylammonio) propyl]-diiodide.
Figure 10.
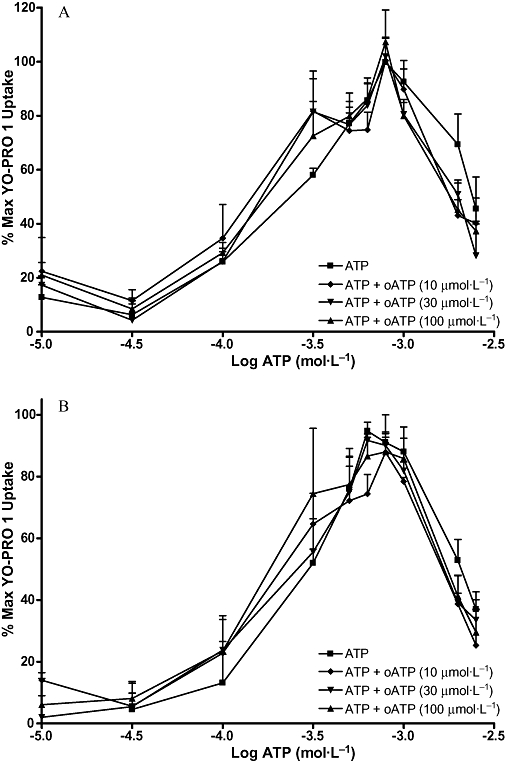
ATP concentration-effect curves in MG63 and SaOS2 cells in the presence of oATP. MG63 (A) or SaOS2 (B) cells (approximately 100 000 cells per well) were incubated with 1 µmol·L−1 YO-PRO 1 in assay buffer in the presence of water (vehicle for oATP), 10 µmol·L−1, 30 µmol·L−1, or 100 µmol·L−1 oATP at 37°C for 1 h followed by ATP for 5 min. YO-PRO 1 fluorescence was monitored in a 96-well plate reader using an excitation wavelength of 485 nm, and an emission wavelength of 520 nm. Data are expressed as % of maximum uptake of ATP in the presence of the vehicle. Statistical analyses were performed using one-way anova with Bonferroni post hoc test. *P < 0.05 versus control. n = 3. oATP, adenosine 5′-triphosphate, periodate oxidized, sodium salt; YO-PRO 1, quinolinium 4-[(3-methyl-2(3H)-benzoxazolylidene)methyl]-1-[3-(trimethylammonio) propyl]-diiodide.
Table 2.
EC50 values (µmol·L−1 ± SD) for ATP and DBzATP in the absence or presence of the highest concentration of the antagonists in MG63 and SaOS2 cells
| MG63a | SaOS2a | |
|---|---|---|
| ATP | 280 ± 100 | 290 ± 40 |
| ATP + BBG 5 µmol·L−1 | 280 ± 100 | 290 ± 40 |
| ATP | 170 ± 50 | 190 ± 10 |
| ATP + KN-62 10 µmol·L−1 | 130 ± 10 | 160 ± 90 |
| ATP | 180 ± 10 | 290 ± 120 |
| ATP + oATP 100 µmol·L−1 | 120 ± 500 | 110 ± 90 |
| ATP | 260 ± 10 | 170 ± 80 |
| ATP + PPADS 0.3 µmol·L−1 | 160 ± 40 | 150 ± 10 |
| DBzATP | ND | 340 ± 150 |
| DBzATP + BBG 5 µmol·L−1 | ND | 440 ± 150 |
| DBzATP | ND | 260 ± 30 |
| DBzATP + KN-62 10 µmol·L−1 | ND | 230 ± 30 |
MG63 or SaOS2 cells (approximately 100 000 cells per well) were incubated with 1 µmol·L−1 YO-PRO 1 in assay buffer in the presence of water or DMSO (vehicle), or BBG, KN-62, oATP or PPADS at 37°C for 1 h followed by ATP or DBzATP for 5 min. YO-PRO 1 fluorescence was monitored in a 96-well plate reader using an excitation wavelength of 485 nm, and an emission wavelength of 520 nm. EC50 values (µmol·L−1 ± SD) were calculated using Prism. The data for the highest antagonist concentrations used are shown here. No differences were seen with any of the lower concentrations. n = 3–4.
BBG, brilliant blue G; DBzATP, 2′-3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; KN-62, 4-[(2S)-2-[(5-isoquinolinylsulphonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulphonic acid ester; ND, not determined; oATP, adenosine 5′-triphosphate, periodate oxidized, sodium salt; PPADS, pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) tetrasodium salt; YO-PRO 1, quinolinium 4-[(3-methyl-2(3H)-benzoxazolylidene)methyl]-1-[3-(trimethylammonio) propyl]-diiodide.
Figure 8.
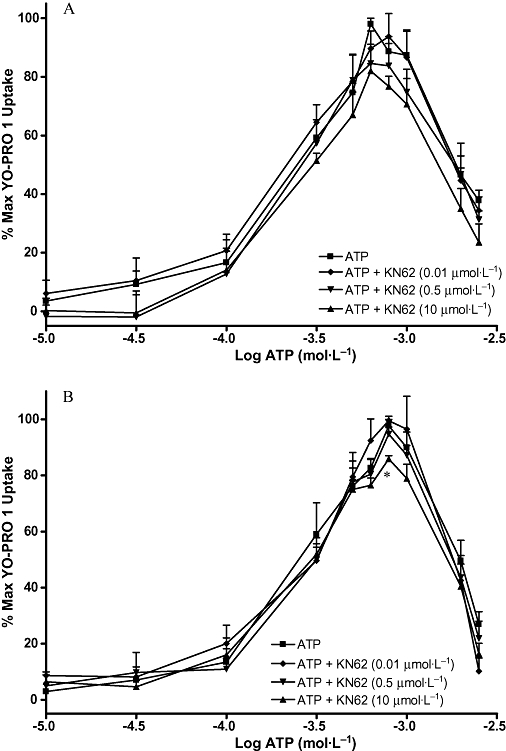
ATP concentration-effect curves in MG63 and SaOS2 cells in the presence of KN-62. MG63 (A) or SaOS2 (B) cells (approximately 100 000 cells per well) were incubated with 1 µmol·L−1 YO-PRO 1 in assay buffer in the presence of DMSO (vehicle for KN-62), 0.01 µmol·L−1, 0.5 µmol·L−1, or 10 µmol·L−1 KN-62 at 37°C for 1 h followed by ATP for 5 min. YO-PRO 1 fluorescence was monitored in a 96-well plate reader using an excitation wavelength of 485 nm, and an emission wavelength of 520 nm. Data are expressed as % of maximum uptake of ATP in the presence of the vehicle. Statistical analyses were performed using one-way anova with Bonferroni post hoc test. *P < 0.05 versus control. n = 3. KN-62, 4-[(2S)-2-[(5-isoquinolinylsulphonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulphonic acid ester; YO-PRO 1, quinolinium 4-[(3-methyl-2(3H)-benzoxazolylidene)methyl]-1-[3-(trimethylammonio) propyl]-diiodide.
Figure 9.
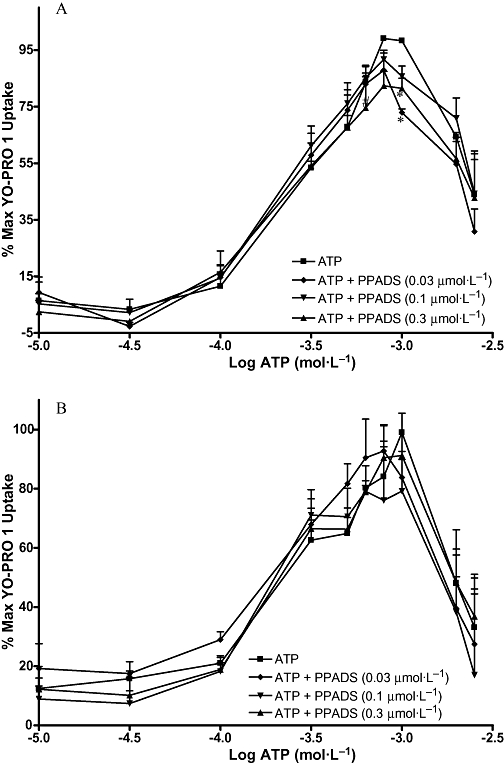
ATP concentration-effect curves in MG63 and SaOS2 cells in the presence of PPADS. MG63 (A) or SaOS2 (B) cells (approximately 100 000 cells per well) were incubated with 1 µmol·L−1 YO-PRO 1 in assay buffer in the presence of water (vehicle for PPADS), 0.03 µmol·L−1, 0.1 µmol·L−1, or 0.3 µmol·L−1 PPADS at 37°C for 1 h followed by ATP for 5 min. YO-PRO 1 fluorescence was monitored in a 96-well plate reader using an excitation wavelength of 485 nm, and an emission wavelength of 520 nm. Data are expressed as % of maximum uptake of ATP in the presence of the vehicle. Statistical analyses were performed using one-way anova with Bonferroni post hoc test. *P < 0.05 and #P < 0.001 versus control. n = 3. PPADS, pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) tetrasodium salt; YO-PRO 1, quinolinium 4-[(3-methyl-2(3H)-benzoxazolylidene)methyl]-1-[3-(trimethylammonio) propyl]-diiodide.
YO-PRO 1 uptake induced by DBzATP in SaOS2 cells was not significantly altered by BBG or KN-62 (Table 2).
Effect of ivermectin on YO-PRO 1 uptake
Ivermectin had no significant effect on the uptake of YO-PRO 1 induced by ATP or DBzATP in MG63 or SaOS2 cells. In MG63 cells 1 mmol·L−1 ATP increased YO-PRO 1 uptake by 82 ± 16% compared with control, while in the presence of ivermectin the increase was 73 ± 14%. 1 mmol·L−1 DBzATP increased YO-PRO 1 uptake by 99 ± 14% compared with control, while in the presence of ivermectin the increase was 98 ± 13%. In SaOS2 cells, 1mM ATP increased YO-PRO 1 uptake by 65 ± 19% compared with control, while in the presence of ivermectin the increase was 67 ± 14%. 1 mmol·L−1 DBzATP increased YO-PRO 1 uptake by 91 ± 14% compared with control, while in the presence of ivermectin the increase was 89 ± 13%.
Discussion
Reverse transcriptase-polymerase chain reaction was utilized to investigate the expression of P2X2, P2X4 and P2X7 receptors at the mRNA level in both cell lines. Both P2X4 and P2X7 receptor mRNAs were detected in MG63 and SaOS2 cells and the identity of the DNA bands was confirmed by sequencing for both receptors. P2X4 receptor mRNA has been found previously in MG63 cells (Nakamura et al., 2000) but not in SaOS2 cells. Our data for P2X7 receptor mRNA confirm previous findings for both cell lines (Nakamura et al., 2000; Gartland et al., 2001). P2X2 receptor mRNA was not detected in MG63 or SaOS2 cells suggesting that the receptor is not expressed or present in very low amounts in these cells. The positive control for P2X2 receptor mRNA demonstrated that P2X2 receptor cDNA could be detected with these PCR primers.
Western blotting detected both P2X4 and P2X7 receptors in MG63 and SaOS2 cells confirming the RT-PCR results. The specificity of the labelling for both antibodies was confirmed by the marked reduction in intensity or disappearance of the receptor bands in all samples following pre-adsorption of the primary antibodies with their corresponding peptides. We are not aware of any previous reports describing the expression of the P2X4 receptor protein in osteoblast-like cells so our results are the first demonstration of this receptor in these cells and suggest that P2X4 receptors may have a role in osteoblast functions.
Western blotting detected two bands of approximately 68 and 89 kDa for the P2X7 receptor in both cell lines. The 89 kDa band has been reported in other cells (Wang et al., 2005). They claimed that it was the mature and functional form of the receptor, and that the 68 kDa form was the deglycosylated receptor. However, after deglycosylation, the mass of the 68 kDa band was reduced by approximately 8 kDa and the 89 kDa band was not seen in these membrane samples suggesting that it is not found in large quantities at the cell surface. Our results therefore suggest that the 68 kDa band represents the functional form of the receptor in human osteoblasts (as it is the glycosylated form) and that the functional form may differ from one cell type to another. Other studies have also suggested this smaller form of the receptor to be the full-length protein (Lee et al., 2006b). The 89 kDa band might represent the extra protein needed for pore formation according to a hypothesis suggested earlier (North, 2002; Faria et al., 2005; Liang and Schwiebert, 2005). An alternative explanation for this 89 kDa band might be that it is a P2X4/P2X7 receptor heteromer. The possibility of such heteromeric receptors was suggested in a recent study (Guo et al., 2007). We therefore investigated whether P2X4/P2X7 heteromeric receptors could be detected in MG63 or SaOS2 cells. However, using co-immunoprecipitation techniques, we were unable to find any evidence for the existence of P2X4/P2X7 heteromeric receptors. Our results suggest that these heteromers are likely to be cell-type-dependent. The most likely explanation therefore for the 89 kDa band seen in this study is that it is an intracellular form of the P2X7 receptor.
We found that the P2X7 receptor was expressed in all the samples used (membranes, nuclei and cell lysates) from both the MG63 and SaOS2 cell lines. Expression levels of P2X7 receptors in both cell lines were similar (data not shown). Results from the immunocytochemistry also showed strong expression in nuclei in both cell lines, mirroring results in THP1 cells (Kidd, unpublished data) and guinea pig smooth muscle cells (Menzies et al., 2003). The specificity of the labelling in the immunocytochemistry was demonstrated by the abolition of staining in cells following pre-adsorption of the primary antibody with its corresponding peptide. The reason for the strong expression of the P2X7 receptor in the nuclei is not clear. It could be related to calcium transport into the nucleus, especially as the intracellular ATP concentration is sufficient to activate the receptor.
We could not detect P2X2 receptor protein in either MG63 or SaOS2 cells in agreement with the lack of detectable mRNA. The P2X2 receptor antibody successfully detected a band in the positive control which was abolished by pre-adsorption with the corresponding peptide thus confirming the validity of our results.
Once we had shown that MG63 and SaOS2 cells expressed two pore-forming receptors, YO-PRO 1 uptake was studied in both cell lines to determine which receptor was responsible for pore formation.
Different agonists were used to compare their potency at the P2X4 and P2X7 receptors. The results showed that all the agonists were equipotent in inducing YO-PRO 1 uptake with high EC50 values in the millimolar range. DBzATP was not more potent than ATP, in contrast to results obtained with transfected receptors (Chessell et al., 1998; Michel et al., 1999; Lee et al., 2006a). However, other studies in cells expressing native P2X7 receptors have either found DBzATP to be equipotent to ATP (Hickman et al., 1994; Coutinho-Silva et al., 1999; Gartland et al., 2001) or to be inactive when used at concentrations up to 100 µmol·L−1 (Cario-Toumaniantz et al., 1998). Therefore, it is possible that ATP and DBzATP are equipotent in cells expressing endogenous P2X7 receptors and/or that the high receptor number in transfected cells affects the potency of DBzATP.
In a previous study on osteoblasts, Gartland et al. (2001) found that a 1 h incubation with agonists was needed for pore formation. In contrast, in our study, a 5 min incubation period was found to give maximal pore formation. This discrepancy might be due to methodological differences in the buffer and dye used (ethidium bromide used by Gartland et al., 2001). Based on the 1 h incubation period needed for pore formation, Gartland et al. (2001) suggested that P2X7 receptors have an atypical pharmacology in osteoblasts. We also believe that P2X7 receptors have an atypical pharmacology in osteoblast-like cells but our data are based on the low potency of DBzATP compared with ATP.
Comparison of agonist potencies for our data with those for recombinant P2X2, P2X4 and P2X7 receptors suggested that pore formation in this study is mediated by P2X7 receptors. This is consistent with the results from the RT-PCR and Western blotting for the P2X2 receptor. Although P2X4 receptors are expressed in these cells, these results suggest that they do not contribute significantly to pore formation. The absence of demonstrable receptor desensitization provides further confirmation for the lack of involvement of P2X4 receptors in pore formation in osteoblast-like cells. P2X2 and P2X4 receptors, unlike P2X7 receptors, desensitize upon long exposure to agonists (North and Barnard, 1997; North, 2002; Fountain and North, 2006; Sperlagh et al., 2006).
Further evidence for the involvement of P2X7 rather than P2X4 receptors in pore formation was provided with ivermectin. It is a positive allosteric modulator of P2X4 receptors and used to distinguish current carried by P2X4 receptors from that induced by P2X2, P2X3 or P2X7 receptor stimulation (North, 2002; Gever et al., 2006). The lack of effect of ivermectin on YO-PRO 1 uptake induced by ATP and DBzATP suggests that this uptake is mainly due to P2X7 receptor activation. However, it should be noted that previous studies with ivermectin have only been performed on the P2X4 receptor channel and not the pore (Lalo et al., 2007; Zhang et al., 2007); Emmett et al., 2008), so these results cannot be taken as definitive proof for lack of involvement of the P2X4 receptor.
Interestingly, ATP and 2-Me-S-ATP, at concentrations higher than 1 mmol·L−1, were found to reduce YO-PRO 1 uptake, an effect also observed on inducing ethidium uptake by ATP in HEK7 cells (Humphreys et al., 1998; Lee et al., 2006a). This may be due to these agonists binding to the calcium in the buffer (Dweck et al., 2005; Saris and Carafoli, 2005) thus reducing the effective concentration. Another, more likely explanation, given the low concentration of calcium used in the current study, is that concentrations higher than 1 mmol·L−1 lead to dephosphorylation of the P2X7 receptor on tyrosine-343. This was seen after repeated application of 100 µmol·L−1 DBzATP on rat P2X7 receptors where a decline in current recorded was observed (Kim et al., 2001). This dephosphorylation might cause either a change in channel conformation, or disrupt the protein-protein interactions needed for pore formation. Receptor desensitization can be ruled out as an explanation as no evidence was obtained for it here and it has not been reported by other studies.
We tested a number of P2X receptor antagonists to see whether they could block ATP- and DBzATP-induced YO-PRO 1 uptake. Concentration-effect curves for the agonists in the presence of different concentrations of the antagonists were studied. The concentration-effect curves showed that the main action of the antagonists, BBG, KN-62 and PPADS, was to produce a small reduction in the maximum YO-PRO 1 uptake induced by ATP. There was no shift in the EC50 values for either agonist with any of the antagonists. oATP had no effect on ATP-induced YO-PRO 1 uptake. We observed that the effect of the antagonists differed between the MG63 and SaOS2 cell lines. The most likely explanation for this finding is the different level of differentiation of these two cell lines (Arnett and Henderson, 1998).
Initial experiments were performed to determine the best pre-incubation period for the antagonists to inhibit YO-PRO 1 uptake and 1 h was found to be the best pre-incubation time (data not shown). The pre-incubation period of P2X receptor antagonists is quite variable between studies. A 30 min pre-incubation period with PPADS, oATP or KN-62 was found to be sufficient to study the antagonist effect of these compounds on DBzATP-induced YO-PRO 1 uptake in HEK7 cells (Michel et al., 2000). This study also reported that 60 min pre-incubation did not increase the antagonist effect of these compounds (Michel et al., 2000). Another study used a 20 min pre-incubation period for KN-62 against DBzATP-induced YO-PRO 1 uptake (White et al., 2005). In human osteoblasts a pre-incubation period of 60 min with PPADS was used to block DBzATP-induced blebbing (Gartland et al., 2001).
The non-competitive inhibition seen with BBG, KN-62 and PPADS in MG63 and SaOS2 cells is similar to data reported in the literature for other cell lines (Michel et al., 2000). BBG has been reported to be more potent at P2X7 receptors compared with the other receptors with an IC50 value in the nanomolar range (Hibell et al., 2000; Jiang et al., 2000; Khakh et al., 2001b; Sperlagh et al., 2006). Thus, the inhibition seen here supports the hypothesis that the P2X7 receptor is responsible for pore formation. KN-62 is reported to be the most potent P2X7 receptor antagonist with a nanomolar IC50 value (Sperlagh et al., 2006). However, it was not the most potent antagonist in this study. The reason for this difference is not clear, but may be related to the atypical pharmacology of the P2X7 receptor in human osteoblast-like cells. This is supported by the lack of effect of oATP, another P2X7 receptor antagonist (Michel et al., 2000; Gever et al., 2006). Another explanation for these results may be the involvement of the P2X4 receptor in pore formation, but this is unlikely as discussed above for a variety of reasons. Furthermore, PPADS, which also had a small inhibitory effect on YO-PRO 1 uptake, is reported to be approximately 10-fold more potent at P2X4 receptors compared with P2X7 receptors (Khakh et al., 2001b).
The results presented in this study confirm the expression of the P2X7 receptor in the human osteoblast-like cell lines, MG63 and SaOS2, at the protein and mRNA level. Furthermore, the expression of P2X4 receptor mRNA has been confirmed and the first evidence for the expression of the receptor protein in these cell lines has been obtained. However, heteropolymerization of the P2X4 and P2X7 receptors appears very unlikely in these cell lines. Taken together our results suggest that it is the P2X7 receptor which is responsible for YO-PRO 1 uptake in these two osteoblast-like cell lines. The weak potency of the agonists and the lack of effect of desensitization or ivermectin all militate against an involvement of the P2X4 receptor. The unexpectedly low affinity of DBzATP and the weak action of the antagonists provide further evidence for the atypical pharmacology of the P2X7 receptor in osteoblast cells. Expression of P2X7 receptors in osteoblasts has interesting pathophysiological implications as they are involved in cytokine release and cell death in many cell types. Therefore, they could be involved in conditions such as osteoporosis and rheumatoid arthritis.
Acknowledgments
SM Alqallaf was funded by the Kingdom of Bahrain. We gratefully acknowledge Mrs Carole Elford for technical assistance and Dr Jack Ham for the gift of BON-1 cells.
Glossary
Abbreviations
- AMV
avian myeoloblastosis virus
- ATPγS
adenosine-5′-(3-thiotriphosphate)
- BBG
brilliant blue G; bp, base pairs
- DBzATP
2′-3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate
- EDTA
ethylenediamine tetraacetic acid
- KN-62
4-[(2S)-2-[(5-isoquinolinylsulphonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulphonic acid ester
- oATP
adenosine 5′-triphosphate, periodate oxidized, sodium salt; PBS, phosphate-buffered saline
- PPADS
pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) tetrasodium salt; YO-PRO 1, quinolinium 4-[(3-methyl-2(3H)-benzoxazolylidene)methyl]-1-[3-(trimethylammonio) propyl]-diiodide
Conflicts of interest
None.
References
- Arnett TR, Henderson B, editors. Methods in Bone Biology. London: Chapman and Hall; 1998. [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn.) 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Bulanova E, Budagian V, Orinska Z, Hein M, Petersen F, Thon L, et al. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol. 2005;174:3880–3890. doi: 10.4049/jimmunol.174.7.3880. [DOI] [PubMed] [Google Scholar]
- Cario-Toumaniantz C, Loirand G, Ferrier L, Pacaud P. Non-genomic inhibition of human P2X7 purinoceptor by 17β-oestradiol. J Physiol. 1998;508:659–666. doi: 10.1111/j.1469-7793.1998.659bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Michel AD, Humphrey PPA. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br J Pharmacol. 1997;121:1429–1437. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Michel AD, Humphrey PPA. Effects of antagonists at the human recombinant P2X7 receptor. Br J Pharmacol. 1998;124:1314–1320. doi: 10.1038/sj.bjp.0701958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiozzi P, Sanz JM, Ferrari D, Falzoni S, Aleotti A, Buell GN, et al. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol. 1997;138:697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacol. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Persechini PM, Bisaggio RDC, Perfettini J-L, Neto AC, Kanellopoulos JM, et al. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol. 1999;276:C1139–C1147. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Stahl L, Cheung K-K, de Campos NE, de Oliveira Souza C, Ojcius DM, et al. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1024–1035. doi: 10.1152/ajpgi.00211.2004. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TFC, et al. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, et al. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- Dweck D, Reyes-Alfonso JA, Potter JD. Expanding the range of free calcium regulation in biological solutions. Anal Biochem. 2005;347:303–315. doi: 10.1016/j.ab.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Elliott J, McVey J, Higgins C. The P2X7 receptor is a candidate product of murine and human lupus susceptibility loci: a hypothesis and comparison of murine allelic products. Arthritis Res Ther. 2005;7:R468–R475. doi: 10.1186/ar1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett DS, Feranchak A, Kilic G, Puljak L, Miller B, Dolovcak S, et al. Characterization of ionotrophic purinergic receptors in hepatocytes. Hepatology. 2008;47:698–705. doi: 10.1002/hep.22035. [DOI] [PubMed] [Google Scholar]
- Faria RX, DeFarias FP, Alves LA. Are second messengers crucial for opening the pore associated with P2X7 receptor? Am J Physiol Cell Physiol. 2005;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281:15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- Gartland A, Hipskind RA, Gallagher JA, Bowler WB. Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J Bone Min Res. 2001;16:846–856. doi: 10.1359/jbmr.2001.16.5.846. [DOI] [PubMed] [Google Scholar]
- Gartland A, Buckley KA, Bowler WB, Gallagher JA. Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calc Tissue Int. 2003;73:361–369. doi: 10.1007/s00223-002-2098-y. [DOI] [PubMed] [Google Scholar]
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflügers Arch Eur J Physiol. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72:1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- Hibell AD, Kidd EJ, Chessell IP, Humphrey PPA, Michel AD. Apparent species differences in the kinetic properties of P2X7 receptors. Br J Pharmacol. 2000;130:167–173. doi: 10.1038/sj.bjp.0703302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, el Khoury J, Greenberg S, Schieren I, Silverstein SC. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84:2452–2456. [PubMed] [Google Scholar]
- Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR. Expression of P2 receptors in bone and cultured bone cells. Bone. 2000;27:503–510. doi: 10.1016/s8756-3282(00)00351-3. [DOI] [PubMed] [Google Scholar]
- Hoebertz A, Arnett TR, Burnstock G. Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol Sci. 2003;24:290–297. doi: 10.1016/S0165-6147(03)00123-8. [DOI] [PubMed] [Google Scholar]
- Hughes FJ, Aubin JE. Culture of cells of the osteoblast lineage. In: Arnett TR, Henderson B, editors. Methods in Bone Biology. London: Chapman and Hall; 1998. pp. 1–49. [Google Scholar]
- Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Mackenzie AB, North RA, Suprenant A. Brilliant Blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- Jorgensen NR, Henriksen Z, Sorensen OH, Eriksen EF, Civitelli R, Steinberg TH. Intercellular calcium signalling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J Biol Chem. 2002;277:7574–7580. doi: 10.1074/jbc.M104608200. [DOI] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nature RevNeurosci. 2001a;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, et al. International Union of Pharmacology.XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001b;53:107–118. [PubMed] [Google Scholar]
- Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, et al. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol. 2005;481:377–390. doi: 10.1002/cne.20393. [DOI] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donova C, McCurdy S, Lira P, Payette MM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Lalo U, Verkhratsky A, Pankratov Y. Ivermectin potentiates ATP-induced ion currents in cortical neurones: Evidence for functional expression of P2X4 receptors? Neurosci Lett. 2007;421:158–162. doi: 10.1016/j.neulet.2007.03.078. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jo S, Lee GE, Jeong LS, Kim YC, Park CS. Establishment of an assay for P2X7 receptor-mediated cell death. Mol Cells. 2006a;22:198–202. [PubMed] [Google Scholar]
- Lee DH, Park KS, Kong ID, Kim JW, Han BG. Expression of P2 receptors in human B cells and Epstein-Barr virus-transformed lymphoblastoid cell lines. BMC Immunol. 2006b;7:22. doi: 10.1186/1471-2172-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Schwiebert EM. Large pore formation uniquely associated with P2X7 purinergic receptor channels. Focus on. Are second messengers crucial for opening the pore associated with P2X7 receptor? Am J Physiol Cell Physiol. 2005;288:C240–C242. doi: 10.1152/ajpcell.00532.2004. [DOI] [PubMed] [Google Scholar]
- Lynch KJ, Touma E, Niforatos W, Kage KL, Burgard EC, Van Biesen T, et al. Molecular and functional characterization of human P2X2 receptors. Mol Pharmacol. 1999;56:1171–1181. doi: 10.1124/mol.56.6.1171. [DOI] [PubMed] [Google Scholar]
- Menzies J, Paul A, Kennedy C. P2X7 subunit-like immunoreactivity in the nucleus of visceral smooth muscle cells of the guinea pig. Auton Neurosci. 2003;106:103–109. doi: 10.1016/S1566-0702(03)00078-X. [DOI] [PubMed] [Google Scholar]
- Michel AD, Chessell IP, Humphrey PPA. Ionic effects on human recombinant P2X7 receptor function. Arch Pharmacol. 1999;359:102–109. doi: 10.1007/pl00005328. Naunyn-Schmied. [DOI] [PubMed] [Google Scholar]
- Michel AD, Kaur R, Chessell IP, Humphrey PPA. Antagonist effects on human P2X7 receptor-mediated cellular accumulation of YO-PRO-1. Br J Pharmacol. 2000;130:513–520. doi: 10.1038/sj.bjp.0703368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naemsch LN, Dixon SJ, Sims SM. Activity-dependent development of P2X7 current and Ca2+ entry in rabbit osteoclasts. J Biol Chem. 2001;276:39107–39114. doi: 10.1074/jbc.M105881200. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Uezono Y, Narusawa K, Shibuya I, Oishi Y, Tanaka M, et al. ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am J Physiol Cell Physiol. 2000;279:C510–C519. doi: 10.1152/ajpcell.2000.279.2.C510. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard E. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Saris N-EL, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochem. 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- Sluyter R, Wiley JS. Extracellular adenosine 5′-triphosphate induces a loss of CD23 from human dendritic cells via activation of P2X7 receptors. Int Immunol. 2002;14:1415–1421. doi: 10.1093/intimm/dxf111. [DOI] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Steinberg T, Newman A, Swanson J, Silverstein S. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- Suprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytoloytic P2z receptor for extracellular ATP identified as a P2x receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Liddell JE, Murphy LS, Pache DM, Kidd EJ. An antibody to the β-secretase cleavage site on amyloid-β-protein precursor inhibits amyloid-β production. J Alzheimers Dis. 2006;10:379–390. doi: 10.3233/jad-2006-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunberg U, Tobin G, Johnson A, Soderberg O, Padyukov L, Hultdin M, et al. Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. Lancet. 2002;360:1935–1939. doi: 10.1016/S0140-6736(02)11917-9. [DOI] [PubMed] [Google Scholar]
- Vial C, Roberts JA, Evans RJ. Molecular properties of ATP-gated P2X receptor ion channels. Trends Pharmacol Sci. 2004;25:487–493. doi: 10.1016/j.tips.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nature Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li X, Wang L, Feng Y-H, Zeng R, Gorodeski G. Antiapoptotic effects of estrogen in normal and cancer human cervical epithelial cells. Endocrinology. 2004a;145:5568–5579. doi: 10.1210/en.2004-0807. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang L, Feng Y-H, Li X, Zeng R, Gorodeski GI. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol. 2004b;287:C1349–C1358. doi: 10.1152/ajpcell.00256.2004. [DOI] [PubMed] [Google Scholar]
- Wang L, Feng Y-H, Gorodeski GI. Epidermal growth factor facilitates epinephrine inhibition of P2X7-receptor-mediated pore formation and apoptosis: a novel signaling network. Endocrinol. 2005;146:164–174. doi: 10.1210/en.2004-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N, Butler PEM, Burnstock G. Human melanomas express functional P2X7 receptors. Cell Tissue Res. 2005;321:411–418. doi: 10.1007/s00441-005-1149-x. [DOI] [PubMed] [Google Scholar]
- Wiley JS, Dao-Ung LP, Gu BJ, Sluyter R, Shemon AN, Li C, et al. A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: a molecular study. Lancet. 2002;359:1114–1119. doi: 10.1016/S0140-6736(02)08156-4. [DOI] [PubMed] [Google Scholar]
- Wilson HL, Francis SE, Dower SK, Crossman DC. Secretion of intracellular IL-1 receptor antagonist (Type 1) is dependent on P2X7 receptor activation. J Immunol. 2004;173:1202–1208. doi: 10.4049/jimmunol.173.2.1202. [DOI] [PubMed] [Google Scholar]
- Yates L, Clark JH, Martin TJ, James S, Broadley KJ, Kidd EJ. Radioligand binding and functional responses of ligands for human recombinant adenosine A3 receptors. Auton Autacoid Pharmacol. 2006;26:191–200. doi: 10.1111/j.1474-8673.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zheng G, Ma X, Yan Y, Li G, Rao Q, et al. Expression of P2X7 in human hematopoietic cell lines and leukemia patients. Leuk Res. 2004;28:1313–1322. doi: 10.1016/j.leukres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sanchez D, Gorelik J, Klenerman D, Lab M, Edwards C, et al. Basolateral P2X4-like receptors regulate the extracellular ATP-stimulated epithelial Na+ channel activity in renal epithelia. Am J Physiol Renal Physiol. 2007;292:F1734–1740. doi: 10.1152/ajprenal.00382.2006. [DOI] [PubMed] [Google Scholar]


