Abstract
Background and purpose:
The present study was designed to determine how ginsenoside Rg1, an active ingredient in ginseng root, exerts its oestrogenic effects. We hypothesize that Rg1 may exert oestrogen-like actions in MCF-7 cells by activating the mitogen-activated protein kinase (MAPK) pathway in a ligand-independent manner.
Experimental approach:
MCF-7 cells were co-incubated with the MAPK inhibitor PD98059 to determine whether the stimulant effects of Rg1 on cell proliferation, the induction of IGF-IR and pS2, the functional transactivation of oestrogen receptor-α (ERα), as well as ERα phosphorylation are dependent on MAPK. The time-dependent responses of mitogen-activated protein kinase kinase (MEK) and extracellular signal-regulated protein kinase (ERK) to Rg1 in MCF-7 cells were studied. The responses of MEK phosphorylation to Rg1 in oestrogen receptor (ER)-negative HEK293 cells were also determined. The effects of Rg1 on cell proliferation and IGF-IR protein expression were studied in the presence of tyrosine kinase inhibitor genistein to elucidate the involvement of tyrosine kinase in mediating these effects.
Key results:
The oestrogenic effects of Rg1 in MCF-7 cells were abolished in the presence of PD98059. Rg1 could induce MEK protein expression and the phosphorylation level of MEK and ERK significantly in a time- and dose-dependent manner. Rg1 activated MEK phosphorylation in ER-negative HEK293 cells in a time- and dose-dependent manner. Rg1 induction of cell proliferation and IGF-IR protein expression was abolished by co-treatment with genistein.
Conclusions and implications:
Taken together, these results show that the MAPK pathway is involved in mediating the oestrogen-like actions of Rg1 in MCF-7 cells and suggest that Rg1 may activate ERα via MEK/ERK in a ligand-independent manner.
Keywords: MAPK, ERα, phytoestrogens, ginsenoside Rg1
Introduction
The ginseng root, a precious herb used in Traditional Chinese Medicine, is widely studied in the West. It has been safely used in China for more than 2000 years as a tonic to combat stress. In recent years, ginseng has become one of the most commonly used alternative herbal medicines in the West. Huang (1999) has reported that ginsenosides are the major ingredient of ginseng to account for its pharmacological actions. Among the 30 ginsenosides, ginsenoside Rg1 is one of the most active and abundant compounds found in ginseng. The pharmacological effects of Rg1 in the skeletal system, central nervous system and cardiovascular system as well as its involvement in the pathogenesis of endometrial, breast and ovarian cancers have been studied (Shibata, 2001). Recently, Rg1 has been identified as a potent phytoestrogen (Chan et al., 2002). With the demonstration of oestrogenic effects at picomolar range, Rg1 appears to be the most potent phytoestrogen investigated as other naturally occurring compounds such as flavonoids, coumestan derivatives and lignans demonstrate little oestrogenic activity in the micromolar range (Murkies et al., 1998; Tham et al., 1998; Cassidy, 1999). Owing to the side effects of hormone replacement therapy, Rg1 might serve as an alternative agent for treatment of postmenopausal symptoms. However, the molecular action of ginsenoside Rg1 remains unclear.
We have shown previously that ginsenoside Rg1 can stimulate the growth of human breast cancer MCF-7 cells, as well as activate the oestrogen response element (ERE)-dependent luciferase activities in HeLa cells without directly binding to the oestrogen receptor-α (ERα) (Chan et al., 2002; Chen et al., 2006). Furthermore, we have demonstrated that Rg1 can activate the insulin-like growth factor-I receptor (IGF-IR)-mediated pathway (Chen et al., 2006). Recently, we have demonstrated that Rg1 preferentially activates ERα and rapidly induces the phosphorylation of ERα at the AF-1 domain (Lau et al., 2008). These results suggest that the action of Rg1 is ER-dependent but it exerts its oestrogen-like effects via a distinct molecular pathway.
In the classical pathway, oestrogen binds and induces conformational changes of ERs. The hormone-receptor complex then binds to a promoter containing the ERE to modulate the transcription of oestrogen-regulated genes. ER activity can also be stimulated in the absence of ligand binding by modulating different signal transduction pathways, including the mitogen-activated protein kinase (MAPK) and protein kinase A pathways. Ligand-independent ER activators that function via the MAPK pathway include insulin-like growth factor-I (IGF-I) (Lee et al., 1997), epidermal growth factor (EGF) (Kato et al., 1995), serum (Karas et al., 1998) and leptin (Catalano et al., 2004). For ligand-independent activation of ER, the MAPK pathway is normally activated by growth factors through the membrane-associated receptor tyrosine kinases (e.g. IGF-IR and EGFR), which in turn activate Ras, followed by the activation of the protein kinase Raf. The activated Raf then mediates signal transduction to mitogen-activated protein kinase kinase (MEK/MAPKK) and downstream extracellular signal-regulated protein kinase (ERK) (Pearson et al., 2001). Finally, ERK induces the phosphorylation of ERα at Ser118 residue to modulate the activity of AF-1 (Kato et al., 1995; Bunone et al., 1996; Lu et al., 2002). The activation of unliganded ERα via the MAPK cascade has been demonstrated in many tumour-derived cell lines (Kato et al., 1995; Bunone et al., 1996). In MCF-7 cells, a large body of evidence suggests that IGF-I and EGF activate the A/B domain of ERα via the MEK/ERK pathway (Bunone et al., 1996; Levin, 2003). In COS-1 cells, growth factors induce the activation of ERα via MAPK-mediated phosphorylation at serine 118 in the A/B domain of ERα (Kato et al., 1995). Given that unliganded ERα can be activated by MAPK signal and that ginsenoside Rg1 is able to activate the IGF-IR-mediated pathway, we hypothesize that ginsenoside Rg1 mimics growth factors to activate ERα via the MEK/ERK pathway to exert oestrogen-like activities in MCF-7 cells.
Methods
Culture and treatment of human breast cancer (MCF-7) and human embryonic kidney (HEK293) cell lines
Ginsenoside Rg1 was purified as described previously (Chan et al., 2002). The purity of Rg1 was determined by HPLC and found to be more than 99% pure. MCF-7 cells and HEK293 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 5% foetal bovine serum (FBS) (for MCF-7) or 10% FBS (for HEK293) and penicillin 100 u·mL−1 and streptomycin 100 µg·mL−1 at 37°C in a humidified atmosphere of 95% air and 5% CO2. When the cells reached 50–60% confluence, the medium was changed to 1% charcoal-stripped serum in phenol red-free DMEM and cultured for 3–5 days before treatment.
Cell proliferation assays
MCF-7 cells were plated in 96-well plates at a density of 5 × 103 cells per well; 24 h after being plated, the cells were cultured in phenol red-free DMEM medium containing 1% charcoal-stripped serum for 48 h and subsequently treated with 1 pmol·L−1 Rg1, 10 nmol·L−1 oestradiol or 50 ng·mL−1 IGF-I in the presence or absence of 50 µmol·L−1 PD98059 or genistein for 48 h. After being incubated, the growth of the cells was quantified using the MTS proliferation assay according to the manufacturer's instructions. The 96-well plate was incubated at 37°C for 2.5 h and then read on a microplate reader at a wavelength of 490 nm.
Immunoblotting
For Western blotting, cells were lysed with Nonidet P-40 buffer (20 mmol·L−1 Tris-HCl, pH 7.5, 150 mmol·L−1 NaCl, 1 mmol·L−1 CaCl2, 1 mmol·L−1 MgCl2, 10% glycerol, 1% Nonidet P-40) containing protease inhibitors (aprotinin 2 µg·mL−1, leupeptin 2 µg·mL−1, 1 mmol·L−1 PMSF) and phosphatase inhibitors (1 mmol·L−1 sodium orthovanadate, 10 mmol·L−1 NaF). Lysates were centrifuged at 10 000× g for 30 min at 4°C and protein concentrations were analysed by the method of Bradford. Equal amounts of proteins (5 µg) were separated by SDS-PAGE on 10% reducing gels at a constant voltage (150 V) for 1 h as previously described (Chen and Wong, 2004), and transblotted onto PVDF membranes. Immuno-detection was performed after blocking non-specific binding sites on the membrane with 5% skimmed milk. The blots were probed with polyclonal rabbit anti-human IGF-IRβ, MEK1/2, phospho-MEK (Ser 218/Ser 222), ERK1/2, phospho-ERK (Tyr 204) (1:2000), monoclonal rabbit anti-human phospho-ERα (specific to Ser118, hyper-phosphorylation might result in the appearance of two bands) (1:2000; Upstate), ERα (1:2800) or monoclonal mouse anti-human β-actin as the primary antibody (1:5000). This was followed by incubation with the goat anti-rabbit or anti-mouse antibody conjugated with horseradish peroxidase (1:2000) as the secondary antibody for 1 h. The antigen-antibody complexes were then detected with enhanced chemiluminescence (ECL) reagent and visualized by the Lumi-Imager using Lumi Analyst version 3.10 software.
Real-time RT-PCR
MCF-7 cells were treated with 10 nmol·L−1 oestradiol, 50 ng·mL−1 IGF-I or 1 pmol·L−1 Rg1 in the presence or absence of 50 µmol·L−1 PD98059 for 48 h. The medium and test compounds were replenished at 24 h. After 2 days of treatment, total RNA was isolated from cells by using Trizol reagent according to the standard protocol. Total RNA was reverse-transcribed in 20 µL of a reaction mixture that contained reverse transcription buffer, deoxynucleotide triphosphate mixture, random primers and MultiScribe reverse transcriptase, using a high-capacity cDNA reverse transcription kit, at 25°C for 10 min, 37°C for 2 h and 85°C for 5 s. The sequences of the PCR primers for pS2 and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH)were 5′-ATGGCCACCATGGAGAACAAGG-3′ (pS2 forward) and 5′-CATAAATTCACACTCCTCTTCTGG-3′ (pS2 reverse), and 5′-ACCACAGTCCATGCCTACAC-3′ (GAPDH forward) and 5′-TTCACCACCCTGTTGCTGTA-3′ (GAPDH reverse). PCR was carried out in 20 µL reaction mixture containing 10 µL iQ SYBR green supermix and 0.5 µL of cDNA template. The PCR was performed in an ABI 7900HT Fast Real-Time PCR system using the following cycle parameters: 1 cycle of 95°C for 1 min, and 40 cycles of 95°C for 20 s, 52°C for 20 s and 72°C 18 s. Standard curves were generated using serially diluted solutions of cDNA derived from untreated MCF-7 cells. The target gene transcripts in each sample were normalized on the basis of its GAPDH.
Transient transfection of MCF-7 cells and luciferase assay
MCF-7 cells were seeded into 24-well plates (37 500 cells per well) and cultured in phenol red-free DMEM containing 1% charcoal-stripped serum. The cells were transfected by Lipofectamine 2000 reagent. The ERE-containing luciferase reporter plasmid vERETkluc, 0.7 µg, together with 0.1 µg of an inactive control plasmid pRL-TK, a Renilla luciferase control vector, were cotransfected into the cells in triplicate. Six hours after transfection, indicated amounts of oestradiol, IGF-I and Rg1 were added in the presence or absence of 50 µmol·L−1 PD98059. After 24 h incubation, the cells were lysed, the luciferase activity was measured by a dual luciferase reporter assay system and the signal detected by a TD-20/20 Luminometer. Oestrogen promoter activity was expressed as firefly luciferase values normalized by pRL-TK Renilla luciferase values. Experiments were performed in triplicate and the results of a representative experiment are presented.
Materials
MCF-7 cells (No. HTB-22, a human breast cancer cell line) and HEK293 cells (No. CRL-1573, a human embryonic kidney cell line) were obtained from ATCC (Rockville, MD, USA); penicillin, streptomycin and Lipofectamine 2000 reagent, Invitrogen (Carlsbad, CA, USA); MTS proliferation assay (CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay), Promega Corp; Bradford method, Bio-Rad, (Hercules, CA, USA); PVDF membranes (Immobilin-P), Millipore Corp. (MA, USA); polyclonal rabbit anti-human IGF-IRβ, MEK1/2, phospho-MEK (Ser218/Ser222), ERK1/2, phospho-ERK (Tyr204) and goat anti-rabbit or anti-mouse antibody conjugated with horseradish peroxidase, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); ERα, oestradiol, PD98059 (2-amino-3-methoxyflavone), Sigma (St. Louis, MO, USA); monoclonal mouse anti-human β-actin, Abcam (Cambridge, UK). The Lumi Analyst version 3.10 software was from Roche (Mannheim, Germany) and the high-capacity cDNA reverse transcription kit and ABI 7900HT fast real-time PCR system, Applied Biosystems (Foster City, CA, USA); iQ SYBR Green Supermix, Bio Rad Laboratories (Hercules, CA, USA). The ERE-containing luciferase reporter plasmid vERETkluc was kindly provided by Dr Vincent Giguere (McGill University, Montreal, Quebec, Canada). The dual luciferase reporter assay system was from Promega (Madison, WI, USA); the TD-20/20 Luminometer, Turner Design (Sunnyvale, CA, USA).
Statistical analysis
Results are expressed as means ± SEM. Statistical analysis was performed using Student's t-test. A P-value <0.05 was considered statistically significant.
Results
MEK inhibitor PD98059 abolished the oestrogen-like activities induced by Rg1 in human breast cancer (MCF-7) cells
Our previous study demonstrated that the effective concentration of Rg1 for the stimulation of MCF-7 cell proliferation was 1 pmol·L−1 (Chan et al., 2002) and this concentration was used throughout the present study. As shown in Figure 1A, treatment of MCF-7 cells with 10 nmol·L−1 E2, 50 ng·mL−1 IGF-I or 1 pmol·L−1 Rg1 for 48 h resulted in an approximately 1.68-, 1.52- and 1.35-fold increase in cell number respectively. MAPK cascade is an important signalling pathway that mediates growth factor-dependent cell proliferation in many cell types (Ballif et al., 2001). In order to determine if the effect of Rg1 on cell proliferation was MAPK-dependent, MCF-7 cells were co-incubated with 50 µmol·L−1 PD98059, a specific MAPK inhibitor, for 48 h. At this concentration, PD98059 has previously been shown to prevent the full activation of MEK1/2 without competing with ATP or substrate (Alessi et al., 1995). Our results indicate that co-treatment of MCF-7 cells with PD98059 completely abolishes the induction of cell proliferation by 1 pmol·L−1 Rg1 (Figure 1A).
Figure 1.
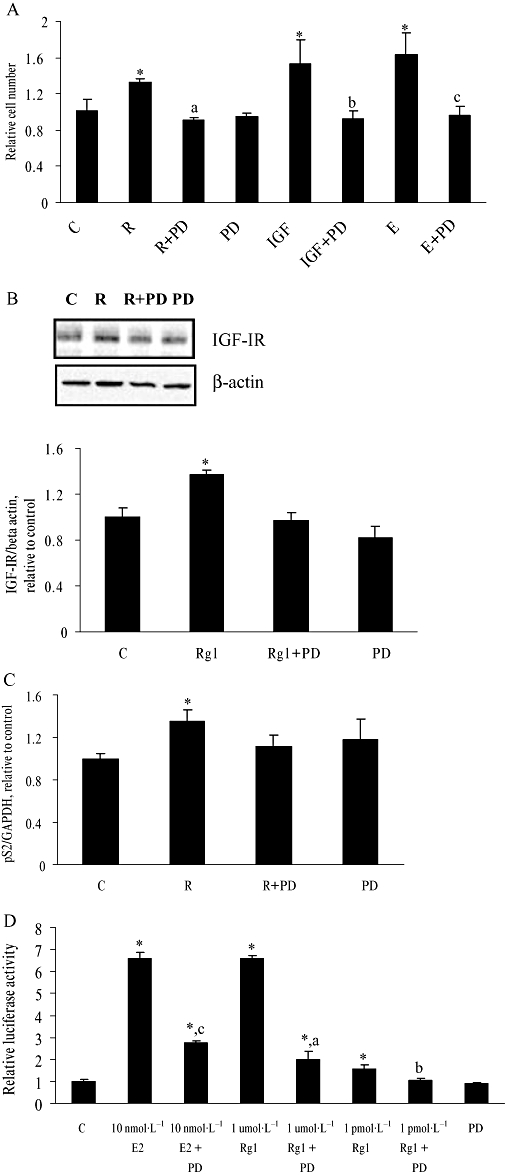
Effect of PD98059 on Rg1-induced cell proliferation, IGF-IR and pS2 expression, and ERE-containing promoter activities in human breast cancer (MCF-7) cells. (A) MCF-7 cells were treated with vehicle (C), 10 nmol·L−1 17(-oestradiol (E), 50 ng·mL−1 IGF-I (IGF) or 1 pmol·L−1 Rg1 (R) in the presence or absence of 50 (mol·L−1 PD98059 (PD) for 48 h. Cell number was then determined by MTS assay. The results are representative of three independent experiments and are expressed as means ± SEM. *P < 0.05 versus control, aP < 0.05 versus R, bP < 0.05 versus IGF, cP < 0.05 versus E, n = 3. (b,c) MCF-7 cells were treated with vehicle (C), 1 pmol·L−1 Rg1 (R) in the presence or absence of 50 (mol·L−1 PD98059 (PD) for 48 h. For (B), proteins were isolated and fractionated using 10% SDS-PAGE and subjected to Western blotting by using specific antibodies. The signals were detected and quantified by the Lumi-Imager. The lower panel shows the graphic representation of the IGF-IR protein expression level, which is expressed as a ratio to the expression of (-actin. For (C), total RNA was isolated and subjected to real-time RT-PCR analysis for pS2 and GAPDH. (D) MCF-7 cells were cotransfected with 0.7 µg of this reporter construct, together with 0.1 µg of an inactive control plasmid pRL-TK, a Renilla luciferase control vector using the Lipofectamine 2000 reagent according to the manufacture's instructions. At 6 h after transfection, the transfected MCF-7 cells were treated with vehicle (C), 1 pmol·L−1 Rg1, 1 (mol·L−1 Rg1 or 10 nmol·L−1 E2 in the presence or absence of 50 (mol·L−1 PD98059 (PD) for another 24 h. Activities of luciferase encoded by experimental and internal control plasmid were measured sequentially with the DLR assay reagents. The ERE firefly luciferase activities were normalized for pRL-TK Renilla luciferase values. One hundred per cent represents the ERE luciferase activity of the control. In (B), (C) and (D) the results were obtained from three independent experiments and expressed as mean ± SEM. *P < 0.05 versus control, aP < 0.05 versus 1 (mol·L−1 Rg1, bP < 0.05 versus 1 pmol·L−1 Rg1, cP < 0.05 versus 10 nmol·L−1 E2, n = 3.
The fact that Rg1 exerts its oestrogen-like activity without direct interaction with ERα suggests other signalling pathways might be involved in mediating its actions. Crosstalk between ERα and the IGF-IR signalling pathways has been observed in human breast cancer cells (Dupont and Le Roith, 2001). Our previous study demonstrated that the activation of the IGF-IR-mediated pathway is involved in the action of Rg1 and 1 pmol·L−1 Rg1 could induce the protein expression of IGF-IR- and ER-dependent pS2 gene transcription significantly in MCF-7 cells (Chen et al., 2006). To determine whether MAPK is involved in the induction of IGF-IR protein and pS2 mRNA expression by Rg1, MCF-7 cells were co-treated with 50 µmol·L−1 PD98059 for 48 h. The induction effects of Rg1 on the IGF-IR protein and pS2 mRNA expression in MCF-7 cells were completely abolished in the presence of PD98059 (Figure 1B and C). These results indicate that MAPK is involved in the induction of IGF-IR protein and pS2 mRNA expression by Rg1.
The MAPK signal is generally involved in enhancing functional activation of the ER in a ligand-independent manner. Thus, it might have a role in mediating the stimulant effects of Rg1 on the ERE-containing promoter. As shown in Figure 1D, Rg1 induced ERE-dependent luciferase activity significantly at 1 pmol·L−1 and 1 µmol·L−1, indicating that Rg1 at either the pico- or micromolar range stimulates the transcription of target genes via the promoter-containing ERE. To confirm that the transcriptional activity of ER by Rg1 is mediated by MAPK, MCF-7 cells were co-incubated with 50 µmol·L−1 PD98059 for 24 h. Figure 1D shows that PD98059 reduced the responses of ERE-containing promoter activities to 1 pmol·L−1 and 1 µmol·L−1 Rg1 by 100% and 70% respectively. These results indicate that the induction of ERE-containing promoter activities by Rg1 is dependent on MAPK.
Ginsenoside Rg1 induced MEK, but not ERK expression in MCF-7 cells
The activities of MAPKs are involved in numerous cellular responses including cell growth and differentiation; and MEK and ERK are the two major signalling molecules in the MAPK-mediated pathway. To determine whether the actions of Rg1 are mediated by an alteration in the protein expressions of MEK and ERK, MCF-7 cells were treated with 1 pmol·L−1 Rg1 for 24 and 48 h. As shown in Figure 2A, 1 pmol·L−1 Rg1 stimulated MEK, but not ERK, protein expression in MCF-7 cells. To determine the optimal time for the increased expression of MEK protein induced by Rg1, MCF-7 cells were treated with 1 pmol·L−1 Rg1, 10 nmol·L−1 E2 or its vehicle for 0–72 h (Figure 2B). The results show that 1 pmol·L−1 Rg1 and 10 nmol·L−1 E2 significantly increase MEK protein expression in a time-dependent manner and the induction reaches a maximum of 1.29-fold and 1.34-fold by 48 h respectively. These results indicate that Rg1 can induce MEK expression in MCF-7 cells.
Figure 2.
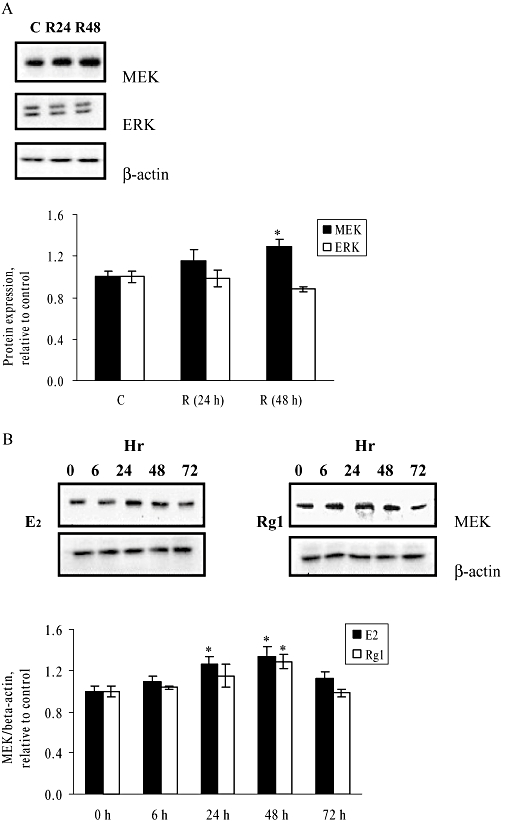
Effect of 1 pmol·L−1 Rg1 on protein expressions of MEK and ERK in MCF-7 cells. (A) MCF-7 cells were stimulated with vehicle (C) and 1 pmol·L−1 Rg1 for 24 or 48 h. After SDS-PAGE, blots were immunoblotted with specific antibodies. The signals were detected and quantified by the Lumi-Imager. The lower panel shows the graphic representation of the MEK and ERK protein expression levels, which are expressed as a ratio to the expression of (-actin. (B) MCF-7 cells were cultured and treated with 10 nmol·L−1 17(-oestradiol (E2) or 1 pmol·L−1 Rg1 (Rg1) for 0, 6, 24, 48 and 72 h. After SDS-PAGE, blots were immunoblotted with specific antibodies. The signals were detected and quantified by the Lumi-Imager. The lower panel shows the graphic representation of the MEK/(-actin protein expression level, which is expressed as a ratio to the basal reading where time 0 (untreated as basal) equals to 1. In (A) and (B) the results are representative of three independent experiments and are expressed as means ± SEM. *P < 0.05 versus control, n = 3.
Ginsenoside Rg1 stimulates MEK and ERK phosphorylation in MCF-7 cells
Activation of the MAPK pathway is mediated by the phosphorylation of its signalling molecules which include MEK and ERK. Phosphorylation of MEK on serines 218 and 222 is both necessary and sufficient for MEK to be activated and able to phosphorylate ERK at tyrosine 204 (Cobb and Goldsmith, 1995). To determine whether Rg1 activates the MAPK cascade in MCF-7 cells, we measured the degree of phosphorylation of MEK and ERK in MCF-7 cells in response to treatment with 1 pmol·L−1 Rg1 for 24 and 48 h. The response of MEK in MCF-7 cells to treatment with IGF-I 50 ng·mL−1 of for 5 min was used as a positive control. The degree of phosphorylation is calculated as the ratio of phospho-MEK to MEK or phospho-ERK to ERK protein expression correspondingly. As shown in Figure 3A, 1 pmol·L−1 Rg1 increased MEK phosphorylation in MCF-7 cells by 56.5% and 92% at 24 and 48 h respectively. The level of MEK phosphorylation induced by Rg1 at 24 h was similar to that induced by treatment with 50 ng·mL−1 IGF-I for 5 min. In the case of ERK phosphorylation (Figure 3B), 1 pmol·L−1 Rg1 increased ERK phosphorylation in MCF-7 cells by 48.7% and 58% at 24 and 48 h respectively. The levels of ERK phosphorylation induced by Rg1 in MCF-7 cells at both time points were smaller than that induced by 5 min of IGF-I (50 ng·mL−1) treatment. These results indicate that the pathway mediated by MAPK is activated at the same time as when the oestrogenic effects of Rg1 are observed.
Figure 3.
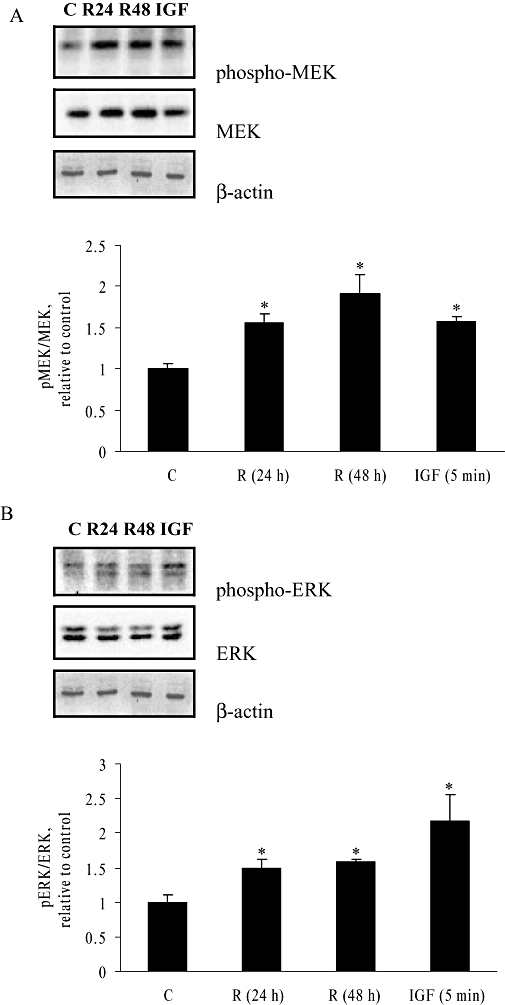
Effect of 1 pmol·L−1 Rg1 on MEK and ERK phosphorylation in MCF-7 cells. MCF-7 cells were stimulated with vehicle (C) and 1 pmol·L−1 Rg1 for 24 or 48 h. Stimulation by 50 ng·mL−1 IGF-I (IGF) for 5 min was used as a positive control. After SDS-PAGE, blots were immunoblotted with specific antibodies. The signals were detected and quantified by the Lumi-Imager. (A) Upper panel shows the immunoblot against phosphor-MEK, MEK and β-actin. Lower panel shows the graphical representation of the degree of phosphorylation, which is expressed as a ratio of pMEK to MEK. (B) Upper panel shows the immunoblot against phosphor-ERK, ERK and β-actin. Lower panel shows the graphical representation of the degree of phosphorylation which is expressed as a ratio of pERK to ERK. The results are representative of three independent experiments expressed as means ± SEM. *P < 0.05 versus control, n = 3.
Time course and dose-dependent activation of MEK and ERK by Rg1 in MCF-7 cells
In human breast cancer cells, ligand-independent ER activators, such as EGF and IGF-I, induce phosphorylation of MEK/ERK within 15 min (Bartucci et al., 2001; Song et al., 2007). To determine whether Rg1 mimics growth factors in activating the MAPK pathway in MCF-7 cells, the levels of MEK and ERK phosphorylation in response to short-term (minute-hour) Rg1 treatment were studied (Figure 4). For MEK, Rg1 induced a rapid and maximal response (1.83-fold) within 5 min in MCF-7 cells and the strong activation was sustained for 60 min. In the case for ERK, Rg1 initiated its activation in the first 5 min and produced a maximal activation (2.11-fold) by 10 min in MCF-7 cells. To determine the concentration-dependent responses of MEK and ERK to Rg1, MCF-7 cells were treated with various concentrations of Rg1 for 10 min. As shown in Figure 5, Rg1 significantly induced MEK and ERK phosphorylation in MCF-7 cells in a dose-dependent manner at 10−14 to 10−10 mol·L−1 with a maximal induction at 10 nmol·L−1 and 1 µmol·L−1 Rg1 respectively. The rapid responses of MEK and ERK to Rg1 indicate that Rg1 is a potent activator of the MAPK pathway and suggest that Rg1 might act like growth factors in exerting oestrogenic effects via MAPK in a ligand-independent manner.
Figure 4.
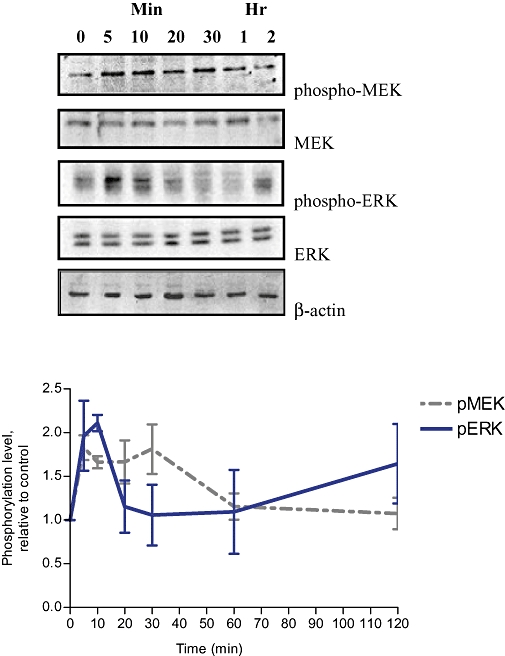
Time course of MEK and ERK activation by Rg1 in MCF-7 cells. MCF-7 cells were stimulated with 1 pmol·L−1 Rg1 for various lengths of time (0, 5 min, 10 min, 20 min, 30 min, 1 h and 2 h). After SDS-PAGE, blots were immunoblotted with specific antibodies. The signals were detected and quantified by the Lumi-Imager. Upper panel shows the immunoblot against phospho-MEK, MEK, phospho-ERK, ERK and β-actin. Lower panel shows the degree of phosphorylation which is calculated first as a ratio of pMEK to MEK or a ratio of pERK to ERK and finally expressed as a ratio to the basal reading where time 0 (untreated as basal) equals 1. The results shown were obtained from three independent experiments and expressed as mean ± SEM; n = 3.
Figure 5.
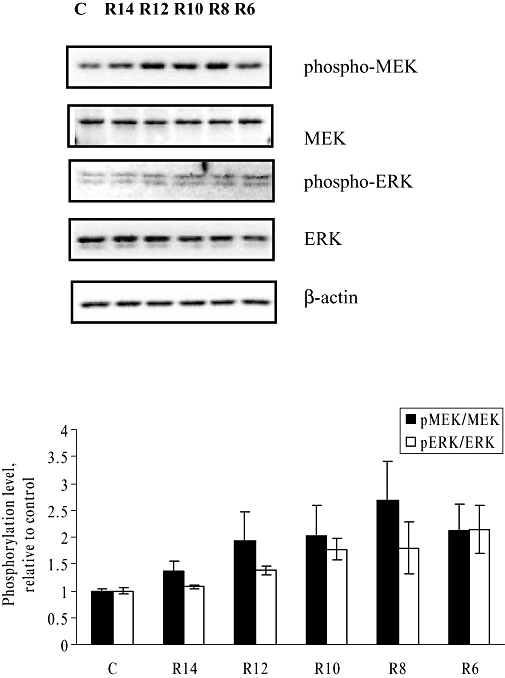
Dose-dependent effect of Rg1 on MEK and ERK activation in MCF-7 cells. MCF-7 cells were treated with 10−14 (R14), 10−12 (R12), 10−10 (R10), 10−8 (R8), and 10−6 mol·L−1 (R6) of Rg1 for 10 min. After SDS-PAGE, blots were immunoblotted with specific antibodies. The signals were detected and quantified by the Lumi-Imager. Upper panel shows the immunoblot against phospho-MEK, MEK, phospho-ERK, ERK and β-actin. Lower panel shows the degree of phosphorylation which is calculated first as a ratio of pMEK to MEK or a ratio of pERK to ERK and finally expressed as a ratio to the basal reading where time 0 (untreated as basal) equals 1. The results shown were obtained from three independent experiments and expressed as mean ± SEM; n = 3.
Ginsenoside Rg1 induced serine phosphorylation of ERα via MAPK in MCF-7 cells
Serine 118 is the major phosphorylation site in ERα for ligand-independent activation by the MAPK-mediated pathway. Results from our recent study showed that Rg1 mimics growth factors and activates ERα by phosphorylation at its serine 118 residue (S118) position in MCF-7 cells in a time-dependent manner (Lau et al., 2008). To determine whether the effect of Rg1 on ERα phosphorylation is dependent on MAPK, MCF-7 cells were pretreated with 50 µmol·L−1 PD98059 for 2 h before the addition of 1 pmol·L−1 Rg1 for 24 h. As shown in Figure 6, treatment of MCF-7 cells with 10 nmol·L−1 E2 for 30 min significantly increased ERα phosphorylation. Similarly, ERα phosphorylation was induced by treatment with 50 ng·mL−1 of IGF-I for 30 min in MCF-7 cells and the increase was abolished in the presence of PD98059. Most importantly, treatment of cells with 1 pmol·L−1 Rg1 for 24 h increased ERα phosphorylation significantly by 34% without altering its protein expression. The induction of ERα phosphorylation by Rg1 was also abolished in the presence of PD98059. The result indicated that phosphorylation of ERα by Rg1 was MAPK-dependent and suggested that Rg1 might act like IGF-I to induce ERα phosphorylation in a ligand-independent manner.
Figure 6.
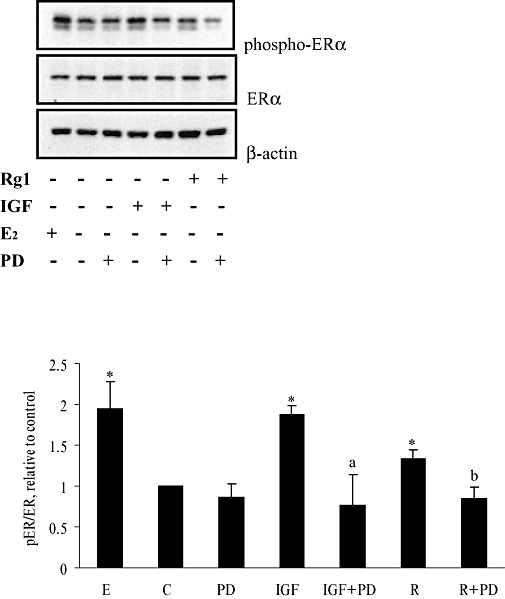
Effect of inhibition of the MAPK pathway on the serine phosphorylation of ERα induced by Rg1 in MCF-7 cells. MCF-7 cells were pretreated with vehicle (0.1% DMSO) or 50 (mol·L−1 PD98059 (PD) for 2 h before the addition of 1 pmol·L−1 Rg1 (R) or 50 ng·mL−1 IGF-I (IGF) for 30 min. The response to stimulation with 10 nmol·L−1 E2 for 30 min was used as a positive control. After SDS-PAGE, blots were immunoblotted with specific antibodies. The signals were detected and quantified by the Lumi-Imager. Strong activation might result in hyper-phosphorylation (appearance of two bands) of ERα. Quantification of phosphorylation of ERα was calculated by combining the band intensities of both bands. Upper panel shows the immunoblot against phosphor-ERα, ERα and β-actin. Lower panel shows the degree of the phosphorylation, which is expressed as a ratio of pERα to ERα. The result is representative of three independent experiments expressed as mean ± SEM. *P < 0.05 versus control, aP < 0.05 versus IGF, bP < 0.05 versus R, n = 3.
Rg1 activated MEK phosphorylation in human embryonic kidney (HEK293) cells
To determine whether the activation of MEK-mediated pathways by Rg1 requires the presence of ER, the responses of MEK phosphorylation in ER-negative HEK293 cells to Rg1 were determined. HEK293 cells were treated with 1 pmol·L−1 of Rg1 or 50 ng·mL−1 IGF-1 for different periods of time. As shown in Figure 7A, IGF-I and Rg1 significantly induced MEK phosphorylation in HEK293 cells within 1 and 3 min, respectively, without altering MEK protein expression. Activation of MEK by both IGF-I and Rg1 was sustained for 30 min, but the magnitude of the increase by IGF-I was larger than that of Rg1. Maximal MEK phosphorylation induced by Rg1 in HEK293 cells occurred at 3 min after application of the stimulus.
Figure 7.
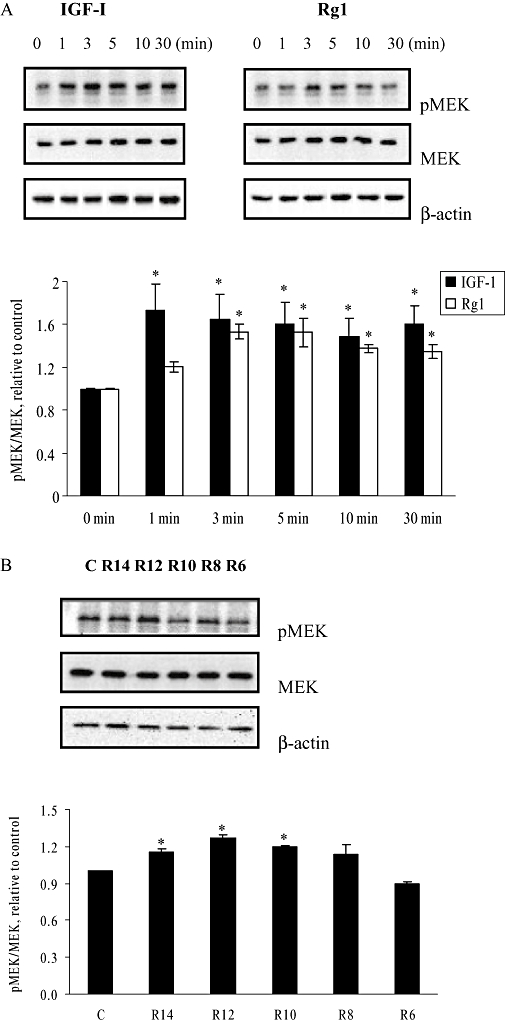
Effects of Rg1 on serine-phosphorylation of MEK in human embryonic kidney (HEK293) cells. (A) HEK293 cells were treated with 50 ng·mL−1 IGF-1 and 1 pmol·L−1 Rg1 for varying lengths of time (0, 1, 3, 5, 10 and 30 min). (B) HEK293 cells were treated with 10−14 (R14), 10−12 (R12), 10−10 (R10), 10−8 (R8), and 10−6 mol·L−1 (R6) of Rg1 for 3 min. Proteins were isolated and fractionated using 10% SDS-PAGE and immunoblotted with pMEK and MEK antibody. The signals were detected and quantified by the Lumi-Imager. Results were obtained from three independent experiments and expressed as mean ± SEM. *P < 0.05 versus control, n = 3.
To determine whether Rg1 activated MEK in a dose-dependent manner, HEK293 cells were treated with various concentrations of Rg1 for 3 min. As shown in Figure 7B, Rg1 significantly induced MEK phosphorylation in HEK293 cells in a dose-dependent manner at concentrations 10−14 to 10−10 mol·L−1 with a maximal induction at 1 pmol·L−1 Rg1. These results indicate that the activation of MEK phosphorylation by Rg1 is not dependent on ER.
Tyrosine kinase inhibitor, genistein, abolishes the stimulant effects of Rg1 on cell proliferation and IGF-IR protein expression in MCF-7 cells
To determine whether the effect of Rg1 on cell proliferation and IGF-IR protein expression in human breast cancer cells is dependent on tyrosine kinase, MCF-7 cells were co-incubated with 50 µmol·L−1 genistein, a specific tyrosine kinase inhibitor, for 48 h. At this concentration, genistein was previously shown to inhibit the intrinsic tyrosine kinase activities of growth factor receptors (Pagliacci et al., 1994; Shao et al., 1998). As shown in Figure 8A, treatment of MCF-7 cells with 1 pmol·L−1 Rg1 for 48 h resulted in an approximately 1.33-fold increase in cell number (P < 0.05). Co-treatment of MCF-7 cells with 50 µmol·L−1 genistein completely abolished the induction of cell proliferation by 1 pmol·L−1 Rg1. As shown in Figure 8B, 1 pmol·L−1 Rg1 increased IGF-IR protein expression in MCF-7 cells by 1.38-fold (P < 0.05). Co-treatment of MCF-7 cells with genistein abolished the induction effects of Rg1 on IGF-IR protein expression. These results indicate that tyrosine kinase is involved in the up-regulation of cell proliferation and IGF-IR expression in MCF-7 cells by ginsenoside Rg1.
Figure 8.
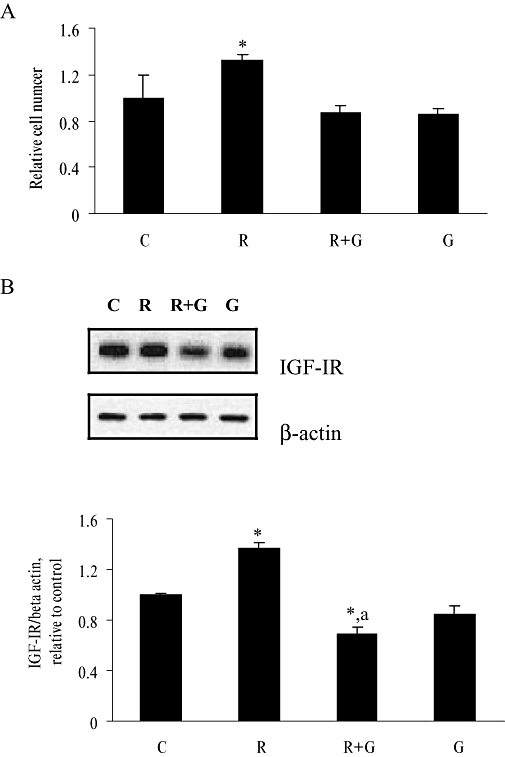
Effect of tyrosine kinase inhibitor, genistein, on Rg1-induced cell proliferation, IGF-IR protein expression in MCF-7 cells. MCF-7 cells were treated with 1 pmol·L−1 Rg1 (R) in the presence and absence of 50 µmol·L−1 genistein (G) for 48 h. (A) Cell number was then determined by MTS assay. (B) Proteins were isolated and fractionated using 10% SDS-PAGE and immunoblotted with IGF-IR and β-actin antibodies. The signals were detected and quantified by the Lumi-Imager. The results are representative of three independent experiments and are expressed as means ± SEM. *P < 0.05 versus control, aP < 0.05 versus R, n = 3.
Discussion
Ginsenoside Rg1, a newly identified phytoestrogen, possesses a wide range of pharmacological activities in the skeletal system, central nervous system and cardiovascular system (Shibata, 2001). Unlike other well-characterized phytoestrogens such as genistein (Chen and Wong, 2004), the high potency of Rg1 in inducing ERE-luciferase activity (EC50 0.05 pmol·L−1) without binding ERα (Chan et al., 2002; Chen et al. 2006) suggests that it might exert oestrogen-like activities via unique signalling pathways. The present study clearly demonstrated that the MAPK pathway is involved in mediating the oestrogenic action of Rg1 in MCF-7 cells, including cell proliferation, induction of IGF-IR and oestrogen-responsive pS2 expression as well as functional transactivation of ERα. The fact that Rg1 could activate pS2 expression and that the induction could be abolished by PD98059 suggest that Rg1 induces active oestrogen signalling via MAPK pathways. Moreover, Rg1 was shown to induce ERα phosphorylation at the AF-1 domain in MCF-7 cells via the activated MAPK in a ligand-independent manner. Furthermore, our results show that Rg1 can induce MEK phosphorylation in an ER-independent manner and that its stimulant effects in MCF-7 cells are dependent on tyrosine kinase.
Activation of the MAPK pathway is known to be required for growth factor-induced cellular proliferation. As cell survival has been shown to be dependent on MEK signalling (Ballif and Blenis, 2001) and ER activation found to be induced by constitutively expressed active MAPK kinase kinase (MEK1) in Ishikawa cells (Lee et al., 2000), it is highly possible that Rg1 exerts oestrogenic effects via the up-regulation of MEK expression and activity in MCF-7 cells. The results of the present study clearly confirm our speculation that Rg1 induce cell proliferation via activation of the MEK/ERK pathway. Our results show that 1 pmol·L−1 Rg1 mimics the stimulant effect of 10−8 mol·L−1 E2 on MEK expression, and that the induction of MEK and ERK phosphorylation by Rg1 in MCF-7 cells occurs rapidly and reaches a maximum within 10 min. The rapid activation of MEK and ERK in MCF-7 cells induced by Rg1 was dose-dependent. As ginsenosides have been shown previously to have a direct interaction with specific membrane proteins at the cell surface (Kimura et al., 1994; Brann et al., 1995), the rapid responses of the MEK/ERK pathway to Rg1 observed in the present study suggest that Rg1 initiate its actions in MCF-7 cells at the cell surface. A similar action has been demonstrated for other xenoestrogens in which membrane-initiated signalling pathways were involved in inducing ERK activities (Bulayeva and Watson, 2004).
Mitogen-activated protein kinase signal plays an important role in the functional activation of unliganded ERα (Kato et al., 1995; Bunone et al., 1996) and ligand-independent activation of the ER by MAPK-activating agents appears to be dependent only on the ER AF-1 domain (Bunone et al., 1996; EI-Tanani and Green, 1997). Numerous studies have demonstrated that phosphorylation of serine 118 is required for the full activity of the ERα AF-1 domain and that the human ERα is phosphorylated by ERK at this site (Kato et al., 1995; Bunone et al., 1996; Lu et al., 2002). Our recent study showed that Rg1 could induce serine 118 phosphorylation of ERα in MCF-7 cells in a time-dependent manner without altering the expression of ERα protein (Lau et al., 2008). The present study further showed that the induction of ERα phosphorylation at S118 in MCF-7 cells could be abolished by co-incubation with an MAPK inhibitor, suggesting that ligand-independent activation of ERα by Rg1 requires the functional activity of MAPK-mediated pathway.
In a recent study we found that the phosphorylation of ERα at S118 by Rg1 occurred rapidly in MCF-7 cells within 5 min of treatment (Lau et al., 2008). The results in the present study indicate that Rg1 also activates MEK and ERK phosphorylation in MCF-7 cells within 5 min of treatment (Figure 4). Thus, it is not clear whether the activation of MEK and ERK phosphorylation by Rg1 occurs upstream of the S118 phosphorylation of ERα in MCF-7 cells. The results obtained with the ER-negative HEK293 cells indicate that Rg1 can activate MEK phosphorylation in the absence of ER. Also the effect of Rg1 on MEK phosphorylation in HEK293 cells was shown to be rapid (within 3 min of incubation) and highly potent, with an optimal effect at 1 pmol·L−1, in agreement with our previous findings that the effective concentration of Rg1 at stimulating the growth of MCF-7 cells and ERE-dependent luciferase activity is in the pmol·L−1 range (Chan et al., 2002). Thus, the results of the present study provide evidence to support the fact that activation of MEK by Rg1 occurs prior to the activation of ER in MCF-7 cells.
Our results also showed that the stimulant effects of Rg1 on cell proliferation and IGF-IR protein expression in MCF-7 cells could be abolished in the presence of tyrosine kinase inhibitor. As MAPK cascade might be activated by membrane-associated receptor tyrosine kinases (e.g. IGF-IR and EGFR), our results suggest that Rg1 exerts its oestrogen-like effects via the activation of receptor tyrosine kinases such as IGF-IR. In addition, our previous study demonstrated that Rg1 activates not only IGF-IR expression but also the phosphorylation of its downstream signalling molecules in MCF-7 cells (Chen et al., 2006). Therefore, the present study further strengthens our hypothesis that Rg1 exerts its oestrogen-like activities via crosstalk between receptor tyrosine kinases and ER signal transduction pathways.
The discovery that the MAPK pathway is involved in the oestrogen-like action of ginsenoside provides new insights into the understanding of the pharmacological actions of ginseng. In vivo and in vitro experiments have shown that ginsenoside Rg1 has a stimulant effect on the CNS, including neurotropic and neuroprotective effects (Chen et al., 2003; Radad et al., 2004). There is much evidence indicating that 17β-oestradiol has the ability to improve spatial learning and memory impairment, and provide neuroprotection, via an effect on the MAPK pathway (Gardona-Gomez et al., 2002; Mize et al., 2003; Chen et al., 2001). Thus, it is highly likely that the effect of Rg1 in brain tissue is mediated through the activation of the MAPK pathway. Moreover, in recent studies, phytoestrogens such as genistein and coumestrol were shown to promote bone metabolism and differentiation via the up-regulation of both the expression and activities of MAPK signalling molecules (Kanno et al., 2004; Pie et al., 2006). Thus, it would be of interest to determine whether Rg1 can mimic E2 to improve brain function and bone formation via the activation of the MEK/ERK pathway. Further study is needed to determine the role of the MAPK pathway in mediating the molecular actions of Rg1 in other tissues, such as brain and bone.
In summary, the present study provides new evidence to support our hypothesis that Rg1 exerts oestrogen-like activities via the ligand-independent activation of the ERα pathway in MCF-7 cells. Our results show that Rg1 exerts its oestrogenic actions in the absence of direct interaction with ER, first, by activation of a tyrosine kinase- and MEK/ERK-mediated pathway; second, by MAPK-dependent phosphorylation of ERα at S118 site; subsequently, these effects lead to transactivation of ERα that mediate the transcription of oestrogen-responsive genes. To conclude, ginsenoside Rg1 is a new class of phytoestrogen that exerts its oestrogen-like activities via the activation of the MAPK-mediated signalling pathway.
Acknowledgments
This work was supported by the Areas of Excellence Scheme Established under the University Grants Committee of the Hong Kong Special Administrative Region, China (AOE/P-10/01), the RGC General Research Fund (POLYU 5636/07M) and the Research Studentship from the Central Allocation Grant from the Research Committee of The Hong Kong Polytechnic University. We also thank Wei Zhou and Dr Dennis Labahn for their generous provision of the vectors.
Glossary
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- E2
17β-estradiol
- ER
oestrogen receptor
- ERE
oestrogen response element
- ERK
extracellular signal-regulated protein kinase
- FBS
foetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IGF-I
insulin-like growth factor-I
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- RT-PCR
reverse transcriptase-polymerase chain reaction
- S118
serine 118 residue
Conflict of interest
None.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001;12:397–408. [PubMed] [Google Scholar]
- Bartucci M, Morelli C, Mauro L, Ando S, Surmacz E. Differential insulin-like growth factor I receptor signaling and function in estrogen receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Cancer Res. 2001;61:6747–6754. [PubMed] [Google Scholar]
- Brann DW, Hendry LB, Mahesh VB. Emerging diversities in the mechanism of action of steroid hormones. J Steroid Biochem Mol Biol. 1995;52:113–133. doi: 10.1016/0960-0760(94)00160-n. [DOI] [PubMed] [Google Scholar]
- Bulayeva NN, Watson CS. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect. 2004;112:1481–1487. doi: 10.1289/ehp.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Cassidy A. Potential tissue selectivity of dietary phytoestrogens and estrogens. Curr Opin Lipidol. 1999;10:47–52. doi: 10.1097/00041433-199902000-00009. [DOI] [PubMed] [Google Scholar]
- Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279:19908–19915. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- Chan RY, Chen WF, Dong A, Guo D, Wong MS. Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng. J Clin Endocrinol Metab. 2002;87:3691–3695. doi: 10.1210/jcem.87.8.8717. [DOI] [PubMed] [Google Scholar]
- Chen J, Gong Y-S, Zhang J-T. Effects of 17β-estradiol and total ginsenoside on the spatial learning and memory impairment of ovariectomized rats. Chin Pharm J. 2001;36:522–526. [Google Scholar]
- Chen WF, Wong MS. Genistein enhances insulin-like growth factor signaling pathway in human breast cancer (MCF-7) cells. J Clin Endocrinol Metab. 2004;89:2351–2359. doi: 10.1210/jc.2003-032065. [DOI] [PubMed] [Google Scholar]
- Chen WF, Lau WS, Cheung PY, Guo DA, Wong MS. Activation of insulin-like growth factor I receptor-mediated pathway by ginsenoside Rg1. Br J Pharmacol. 2006;147:542–551. doi: 10.1038/sj.bjp.0706640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XC, Fang F, Zhu YG, Chen LM, Zhou YC, Chen Y. Protective effect of ginsenoside Rg1 on MPP+-induced apotosis in SHSY5Y cells. J Neural Transm. 2003;110:835–845. doi: 10.1007/s00702-003-0005-y. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. How MAP Kinase are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Dupont J, Le Roith D. Insulin-like growth factor I and oestradiol promote cell proliferation of MCF-7 breast cancer cells: new insights into their synergistic effects. J Clin Pathol. 2001;54:149–154. doi: 10.1136/mp.54.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EI-Tanani MKK, Green CD. Two separate mechanisms for ligand-independent activation of the estrogen receptor. Mol Endocrinol. 1997;11:928–937. doi: 10.1210/mend.11.7.9939. [DOI] [PubMed] [Google Scholar]
- Gardona-Gomez GP, Mendez P, Doncarlos LL, Azcoitia I, Garcia-Segura LM. Interaction of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Biol. 2002;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Huang KC. The Pharmacology of Chinese Herbs. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- Kanno S, Hirano S, Kayama F. Effects of the phytoestrogen coumestrol on RANL-ligand-induced differentiation of osteoclasts. Toxicology. 2004;203:211–220. doi: 10.1016/j.tox.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Karas RH, Gauer EA, Bieber HE, Baur WE, Mendelsohn ME. Growth factor activation of the estrogen receptor in vascular cells occurs via a mitogen-activated protein kinase-independent pathway. J Clin Invest. 1998;101:2851–2861. doi: 10.1172/JCI1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kimura T, Saunders PA, Kim HS, Rheu HM, Oh KW, Ho IK. Interactions of ginsenosides with ligand-bindings of GABA(A) and GABA(B) receptors. Gen Pharmacol. 1994;25:193–199. doi: 10.1016/0306-3623(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Lau WS, Chan RYK, Guo DA, Wong MS. Ginsenoside Rg1 exerts estrogen-like activities via ligand-independent activation of ERα pathway. J Steroid Biochem Mol Biol. 2008;108:64–71. doi: 10.1016/j.jsbmb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Lee H, Jiang F, Wang Q, Nicosia SV, Yang J, Su B, et al. MEKK1 activation of human estrogen receptor and stimulation of the agonistic activity of 4-hydroxytamoxifen in endometrial and ovarian cancer cells. Mol Endocrinol. 2000;14:1882–1896. doi: 10.1210/mend.14.11.0554. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135–140. doi: 10.1016/s0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17:309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- Lu Q, Ebling H, Mittler J, Baur WE, Karas RH. MAP kinase mediates growth factor-induced nuclear translocation of estrogen receptor α. FEBS Lett. 2002;516:1–8. doi: 10.1016/s0014-5793(02)02432-8. [DOI] [PubMed] [Google Scholar]
- Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- Murkies AL, Wilcox G, Davis SR. Phytoestrogens. J Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- Pagliacci MC, Smacchia M, Migliorati G, Grignani F, Riccardi C, Nicoletti I. Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur J Cancer. 1994;30A:1675–1682. doi: 10.1016/0959-8049(94)00262-4. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson TB, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions derived from Panax notoginseng. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Pie JE, Park JH, Park YH, Ryu YM, Kim KN, Suh SW, et al. Effect of genistein on the expression of bone metabolism genes in OVX mice using a cDNA microarray. J Nutr Biochem. 2006;17:157–164. doi: 10.1016/j.jnutbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenoside Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:42–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–4857. [PubMed] [Google Scholar]
- Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16:S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving IGF-IR, matrix metalloproteinases and EGFR to activate MAPK in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–4101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham DM, Gardner CD, Haskell WL. Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab. 1998;83:2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]


