Abstract
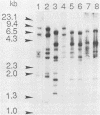
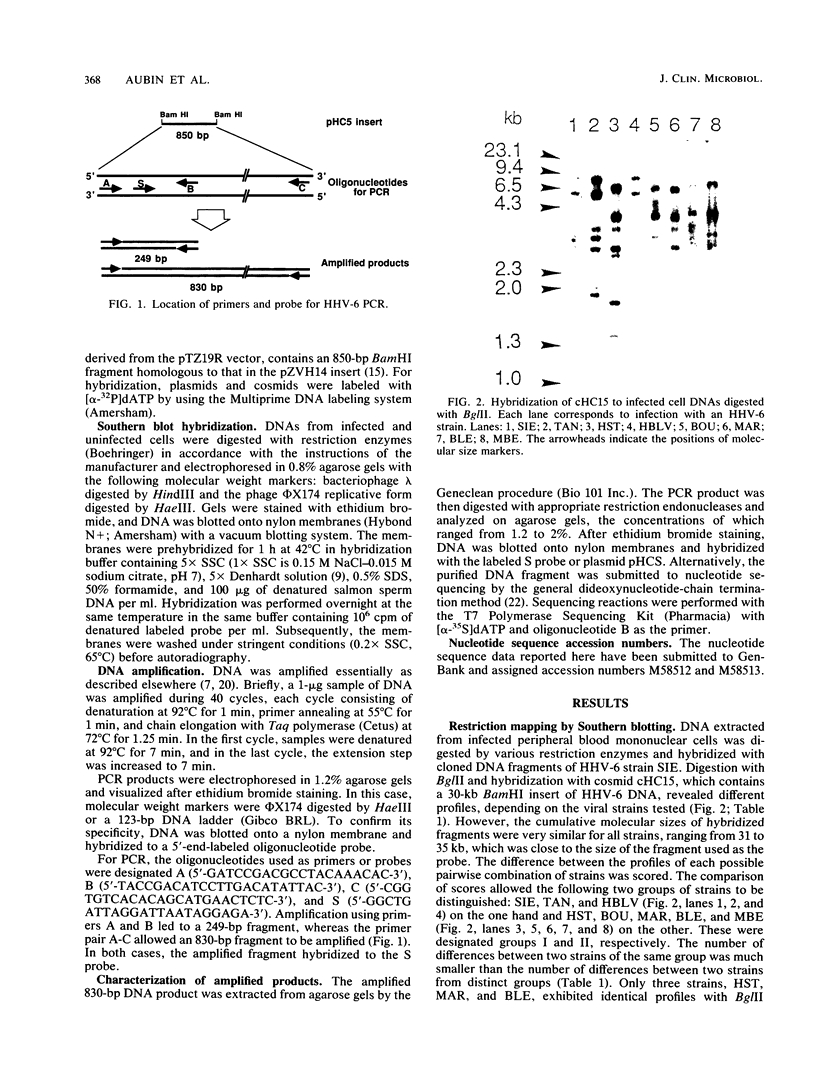
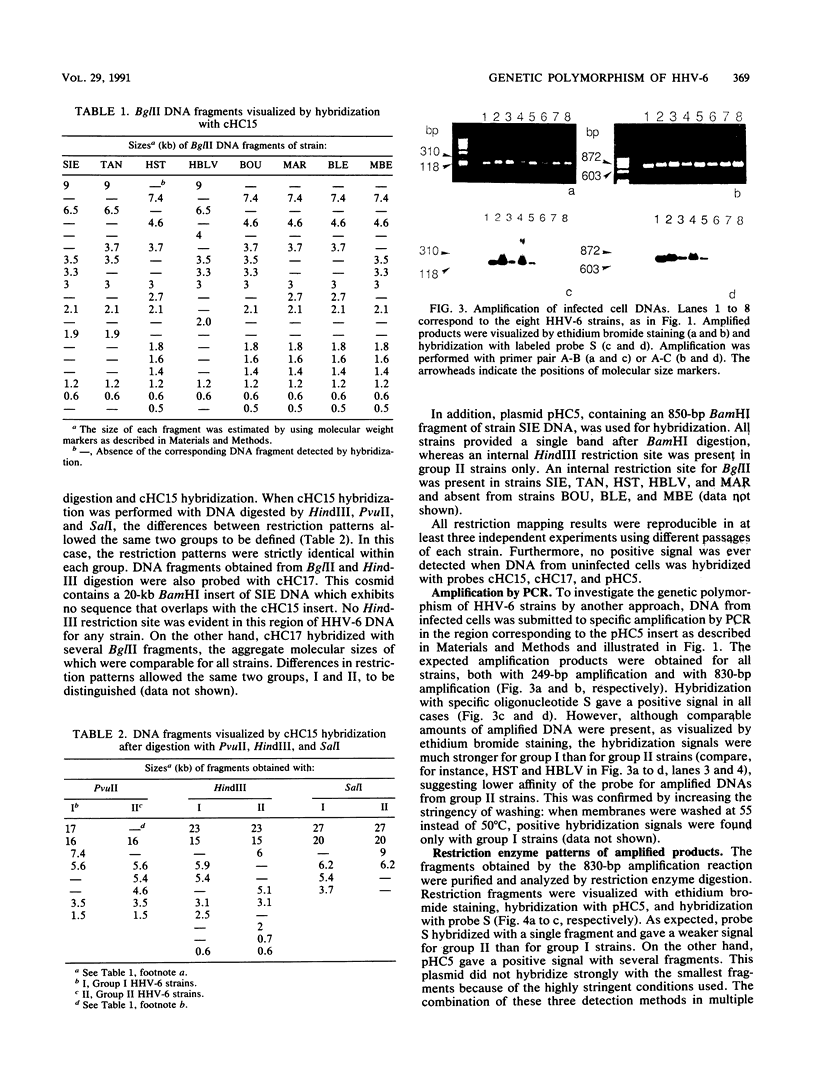
Eight human herpesvirus 6 (HHV-6) strains were studied by Southern blot and polymerase chain reaction. DNA from infected cells was digested by a panel of restriction enzymes and hybridized with cloned BamHI fragments corresponding to about 30% of the HHV-6 strain SIE genome. In parallel, this DNA was amplified by polymerase chain reaction using pairs of primers derived from the strain SIE nucleotide sequence. Subsequently, amplification products were analyzed by hybridization, digestion with restriction endonucleases, and partial nucleotide sequencing. Overall results indicated that all strains were closely related to one another. However, concordant differences in restriction patterns allowed at least two groups to be distinguished, typified by strains SIE and HST, respectively. Differences between the two groups were found to reflect a limited number of punctual changes in nucleotide sequences. These results strengthen the idea of a unique HHV-6 species with genetic polymorphism. In addition, this study provides useful markers for the diagnosis and molecular epidemiology of HHV-6 infections.
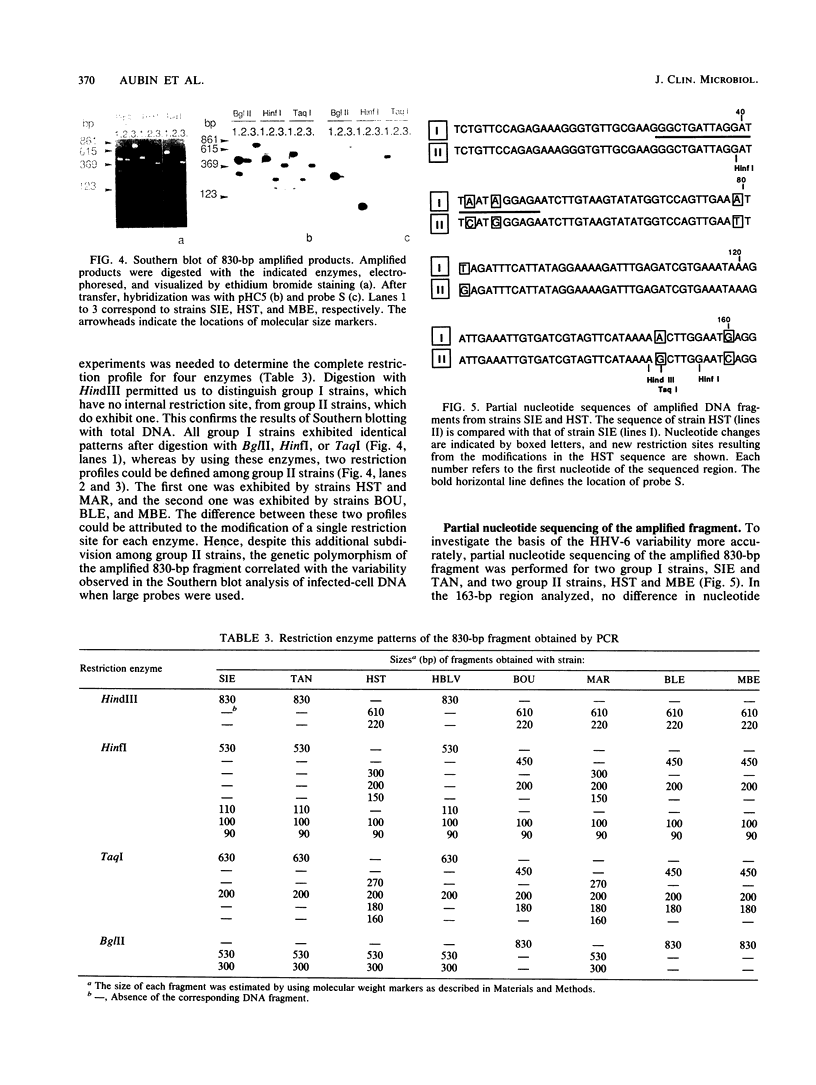
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P. The molecular epidemiology of cytomegalovirus transmission among children attending a day care center. J Infect Dis. 1985 Oct;152(4):760–768. doi: 10.1093/infdis/152.4.760. [DOI] [PubMed] [Google Scholar]
- Agut H., Guetard D., Collandre H., Dauguet C., Montagnier L., Miclea J. M., Baurmann H., Gessain A. Concomitant infection by human herpesvirus 6, HTLV-I, and HIV-2. Lancet. 1988 Mar 26;1(8587):712–712. doi: 10.1016/s0140-6736(88)91520-6. [DOI] [PubMed] [Google Scholar]
- Agut H., Kérouédan D., M'Pelé P., Collandre H., Samba-Lefèvre C., Rosenheim M., Ingrand D., Itoua-Ngaporo A., Gentilini M., Montagnier L. Co-cultivation of human herpesvirus 6 and HIV1 from blood lymphocytes. Res Virol. 1989 Jan-Feb;140(1):23–26. doi: 10.1016/s0923-2516(89)80080-9. [DOI] [PubMed] [Google Scholar]
- Becker W. B., Engelbrecht S., Becker M. L., Piek C., Robson B. A., Wood L., Jacobs P. New T-lymphotropic human herpesviruses. Lancet. 1989 Jan 7;1(8628):41–41. doi: 10.1016/s0140-6736(89)91691-7. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Downing R. G., Sewankambo N., Serwadda D., Honess R., Crawford D., Jarrett R., Griffin B. E. Isolation of human lymphotropic herpesviruses from Uganda. Lancet. 1987 Aug 15;2(8555):390–390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Gompels U. A., Craxton M. A., Honess R. W., Ward K. DNA homology between a novel human herpesvirus (HHV-6) and human cytomegalovirus. Lancet. 1988 Jan 2;1(8575-6):63–64. doi: 10.1016/s0140-6736(88)91049-5. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Schirmer E. C., Wyatt L. S., Katsafanas G., Roffman E., Danovich R. M., June C. H. Isolation of a new herpesvirus from human CD4+ T cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):748–752. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett R. F., Gallagher A., Gledhill S., Jones M. D., Teo I., Griffin B. E. Variation in restriction map of MHV-6 genome. Lancet. 1989 Feb 25;1(8635):448–449. doi: 10.1016/s0140-6736(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Ablashi D. V., Salahuddin S. Z., Kramarsky B., Franza B. R., Jr, Pellett P., Buchbinder A., Memon S., Wong-Staal F., Gallo R. C. Molecular studies of HHV-6. J Virol Methods. 1988 Sep;21(1-4):179–190. doi: 10.1016/0166-0934(88)90064-x. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Salahuddin S. Z., Ablashi D. V., Schachter F., Wong-Staal F., Gallo R. C. Genomic analysis of the human B-lymphotropic virus (HBLV). Science. 1986 Oct 31;234(4776):601–603. doi: 10.1126/science.3020691. [DOI] [PubMed] [Google Scholar]
- Kikuta H., Lu H., Matsumoto S., Josephs S. F., Gallo R. C. Polymorphism of human herpesvirus 6 DNA from five Japanese patients with exanthem subitum. J Infect Dis. 1989 Sep;160(3):550–551. doi: 10.1093/infdis/160.3.550. [DOI] [PubMed] [Google Scholar]
- Lawrence G. L., Chee M., Craxton M. A., Gompels U. A., Honess R. W., Barrell B. G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990 Jan;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., Pellett P., Stewart J., Goldsmith C., Sanderlin K., Black J., Warfield D., Feorino P. Characteristics of human herpesvirus-6. J Infect Dis. 1988 Jun;157(6):1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sonoda S., Kawakami K., Miyata K., Oki T., Nagata T., Okuno T., Kamanishi K. Human herpesvirus 6 and exanthem subitum. Lancet. 1988 Jun 25;1(8600):1463–1463. doi: 10.1016/s0140-6736(88)92275-1. [DOI] [PubMed] [Google Scholar]
- Tedder R. S., Briggs M., Cameron C. H., Honess R., Robertson D., Whittle H. A novel lymphotropic herpesvirus. Lancet. 1987 Aug 15;2(8555):390–392. doi: 10.1016/s0140-6736(87)92404-4. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]