Abstract
Background and purpose
Changes in smooth muscle tone of the prostate gland are involved in aetiology of symptomatic prostatic hyperplasia, however the control mechanisms of prostatic smooth muscle are not well understood. Here, we have examined the role of internal Ca2+ compartments in regulating slow wave activity in the guinea pig prostate.
Experimental approach
Standard intracellular membrane potential recording techniques were used.
Key results
The majority (89%) of impaled cells displayed ‘slow wave’ activity. Cyclopiazonic acid (10 µmol·L−1) transiently depolarized (3–9 mV) the membrane potential of the prostatic stroma and transiently increased slow wave frequency. Thereafter, slow wave frequency slowly decreased over 20–30 min. Ryanodine transiently increased slow wave frequency, although after 30 min exposure slow wave frequency and time course returned to near control values. Caffeine (1 mmol·L−1) reduced slow wave frequency, accompanied by membrane depolarization of about 8 mV. Blockade of inositol trisphosphate receptor (IP3R)-mediated Ca2+ release with 2-aminoethoxy-diphenylborate (60 µmol·L−1) or Xestospongin C (3 µmol·L−1) or inhibiting phospholipase C and IP3 formation using U73122 (5 µmol·L−1) or neomycin (1 and 4 mmol·L−1) reduced slow wave frequency, amplitude and duration. The mitochondrial uncouplers, p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (1–10 µmol·L−1), carbonyl cyanide m-chlorophenylhydrazone (1–3 µmol·L−1) or rotenone (10 µmol·L−1), depolarized the membrane (8–10 mV) before abolishing electrical activity.
Conclusion and implications
These results suggest that slow wave activity was dependent on the cyclical release of Ca2+ from IP3-controlled internal stores and mitochondria. This implies that intracellular compartments were essential in the initiation and/or maintenance of the regenerative contractile activity in the guinea pig prostate gland.
Keywords: cyclopiazonic acid, IP3-dependent Ca2+ stores, pacemaker activity, prostate, slow waves, smooth muscle
Introduction
We have previously reported the presence of distinct electrical activities and cell types in the smooth muscle stroma of the guinea pig prostate and speculated as to their functional roles (Exintaris et al., 2002; Lang et al., 2004; Exintaris et al., 2006; Lang et al., 2006). Overall, it is likely that a specialized group of c-Kit immunoreactive interstitial cells (Van der Aa et al., 2003; Shafik et al., 2005) that lie between the glandular epithelium and smooth muscle stroma have a similar role to intestinal interstitial cells of Cajal (ICC), generating the pacemaker signal that manifests as slow wave activity and ensuing contractility in the smooth muscle cells of the prostate (Exintaris et al., 2002). As changes in smooth muscle tone are involved in the aetiology of age-dependent, prostate-specific conditions such as benign prostatic hyperplasia (BPH), knowledge of the electrical properties of these cell types and their interactions with nerves and the effects of the hormonal environment is of considerable medical interest. Selective modulation of slow wave currents may well provide a different, and perhaps more selective avenue for modulating stromal excitability and smooth muscle tone.
The time course of the prostatic slow wave is complex, consisting of distinct alternating depolarizing and repolarizing phases with several superimposed spike potentials (Exintaris et al., 2002). Investigations into the membrane channel currents in freshly isolated smooth muscle cells of the guinea pig prostate have revealed that the slow waves are likely to arise from the opening and closing of L-type Ca2+ channels and the opposing influence of a 4AP-sensitive K+ current respectively. Tetraethyl ammonium (TEA)-sensitive large conductance Ca2+-activated K+ channels (BK) and TEA-insensitive delayed rectifier K+ channels are likely to regulate the number and duration of these superimposed spikes (Kurokawa et al., 1998; Oh et al., 2003; Lang et al., 2004). It is also likely that intracellular Ca2+ stores are involved in the regulation of the spike potentials as 10 µmol·L−1 cyclopiazonic acid (CPA) attenuated all the transient outward K+ currents in isolated stromal myocytes (Lang et al., 2004). Thus, as with other hollow smooth muscle organs, intracellular calcium stores are likely to play a significant role in facilitating electrical rhythmicity in the prostate gland. This is also supported by the observation that the depolarizing transient of the slow wave is nifedipine-insensitive but is abolished within several minutes of Ca2+ being removed from the bathing solution (Exintaris et al., 2002), implying the contribution of another Ca2+ source, possibly through receptor-activated mechanisms or the cycling of Ca2+ through various intracellular compartments. Accordingly, in this report we have investigated the specific contribution of various intracellular compartments in determining the frequency or time course of slow waves in the guinea pig prostate. Specifically, we have considered the roles of mitochondrial Ca2+ release, ryanodine receptor (RyR)-dependent Ca2+ release and inositol trisphosphate receptor (IP3R)-dependent release of Ca2+ from intracellular stores.
Methods
All animal care and experimental procedures were approved by the Victorian College of Pharmacy Animal Ethics Committee at Monash University. Immature guinea pigs (250–400 g; provided by Monash University Animal Services) were killed by stunning and exsanguination and the dorsal prostate glands removed through an abdominal incision. In the electrophysiological experiments, individual glands (5 mm × 5 mm) of the dorsal lobe were pinned firmly to the bottom of an organ bath (volume 1 mL) mounted on the stage of an inverted microscope and superfused at 3–4 mL min−1 with physiological saline solution (composition (in mmol·L−1): NaCl 120, KCl 5, CaCl2 2.5, MgCl2 1, NaH2PO4 1, NaHCO3 25 and glucose 11, bubbled with a 95% O2: 5% CO2 gas mixture to establish a pH of 7.3–7.4) at 37°C. Recordings of membrane potential were made from the prostate stroma using a standard unity-gain pre-amplifier and microelectrodes with resistances of 60–80 MΩ when filled with 2 mol·L−1 KCl. Changes in the membrane potential were digitized and stored using a TL1 DMA analogue-to-digital interface (Axon Instruments), Axotape 6 software (Axon Instruments) and a personal computer.
In tension experiments, each gland was divided longitudinally in half to provide four preparations in total. The preparations were placed into 2.5 mL organ baths filled with physiological saline solution. Each tissue was tied between a Perspex tissue holder and a transducer and placed under 1 g tension. Tissues were immersed in Krebs-Henseleit solution (composed of (mmol·L−1): NaCl 118, KCl 4.7, MgSO4.7H20 1.1, KH2PO4 1.18, NaHCO3 25.0, glucose 11.66 and CaCl2.2H20 2.5) at 37°C and bubbled with 95% O2, 5% CO2. During the 60 min equilibration period, tissues were electrically field stimulated using trains of 20 pulses at 10 Hz every 50 s and 0.5 ms pulse duration at 60 V. Responses were recorded isometrically with Grass FT.03 tension transducers using a Powerlab system (AD Instruments, Castle Hill, NSW, Australia).
Experimental protocol
After equilibration, phenylephrine (100 µmol·L−1) was applied to the bath, serving as an internal standard. After 2 min, the tissue was then washed with Krebs-Henseleit solution for 2 min. This solution was then replaced with Ca2+-free Krebs, composed of (mmol·L−1): NaCl 120, KCl 4.7, MgSO4.7H20 1.1, KH2PO4 1.18, NaHCO3 25.0, glucose 11.66 and EDTA 1. The tissue was left to rest for 30 min before phenylephrine was again added to the bath for 2 min. After 2 min of washing, Krebs-Henseleit solution was again replaced with Ca2+-free Krebs and the desired drug was applied for 30 min. A final dose of phenylephrine was applied for 2 min and then washed.
Analysis of area under the curve (AUC)
Each response to phenylephrine was measured as an AUC over a 2 min period beginning from the addition of phenylephrine. The AUC for all responses was then calculated as a percentage of the control response, that is, the first response to phenylephrine without any treatment or inhibitors. Statistical differences in the control and ‘test’ responses were determined by a two-tailed Student's t-tests for paired data and differences were considered significant at P < 0.05.
Drugs used
The following drugs were used: 2-aminoethoxy-diphenylborate (2-APB) and neomycin (Calbiochem, San Diego, CA, USA), caffeine (BDH Biochemicals), CPA, rotenone, ryanodine, U73122, Xestospongin C, p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP), carbonyl cyanide m-chlorophenylhydrazone (CCCP), (Sigma, St Louis, MO, USA). The concentration of all stock solutions ranged between 0.1 mmol·L−1 and 10 mmol·L−1. Most drugs were dissolved in filtered distilled water and diluted with physiological saline to their final concentrations as indicated. CPA and U73122 were dissolved in dimethyl sulphoxide (DMSO) to provide stock solutions. Caffeine was dissolved directly into the physiological saline solution. Stock solutions were generally added 1:1000 dilution. During the intracellular microelectrode and tension recording experiments, solutions were vigorously bubbled with the gas mixture to restore any changes of pH. 0.1% ethanol or DMSO had no effect on the spontaneous activity of the prostate.
Data analysis
Various parameters of the spontaneous slow waves were measured: the membrane potential 1000 ms before the onset of each slow wave, the frequency of slow wave discharge, the overall amplitude consisting of the amplitude of the depolarization and the first spike of the slow wave, the amplitude and half amplitude duration of the depolarization and the peak after-hyperpolarization. The parameters of three or four responses were averaged and compared with those measured after 30 s–1 min, 10–20 min or >30 min of exposure to a ‘test’ drug. A number of similar experiments were then averaged as indicated. In most experiments, a paired Student's t-test was used for tests of significance unless otherwise indicated; P < 0.05 was considered to be statistically significant.
The receptors and ion channel nomenclature used in this manuscript conform to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Results
The spontaneous electrical events recorded in the stromal wall of the prostate gland consisted of slow waves, pacemaker potentials, spontaneous transient depolarizations and spike potentials (Exintaris et al., 2006). In this study, we have characterized the slow wave activity as this is the most likely to contribute to the resting tone of the prostate. The majority (89%) of impaled cells (n= 62 cells) displayed spontaneous slow wave activity.
Role of intracellular Ca2+ stores
Exposure of prostatic preparations to CPA (10 µmol·L−1, for 30–60 min), which depletes internal Ca2+ stores by blocking the Ca-ATPase (SERCA) pump on the sarcoplasmic endoplasmic reticulum, led to a transient depolarization of the membrane potential from −57.4 ± 2.8 mV to −53.9 ± 3.5 mV (P < 0.05, n= 11) (Figure 1). This transient depolarization occurred within the first few minutes of exposure to CPA and was not accompanied by a significant change in slow wave frequency, initial spike amplitude or half amplitude duration [control 6.04 ± 0.56 min−1, 35.2 ± 1.5 mV and 1103 ± 153 s respectively; in CPA (after 1 min) 6.21± 0.66 min−1, 29.3 ± 6.5 mV and 1076 ± 63 ms respectively; all P > 0.05, n= 11]. In six preparations, impalements were maintained for at least 20 min in the presence of 10 µmol·L−1 CPA. In these preparations the membrane potential slowly repolarized to −56.8 ± 2.4 mV (control −58.1 ± 4.5 mV; n= 6), while the frequency of slow wave activity was reduced to 2.3 ± 0.4 min−1 (control frequency 6.2 ± 0.7 min−1; P < 0.05, n= 6). In contrast, the amplitude of the initial spike and the number of spikes superimposed on the depolarization both increased after this 20 min exposure to CPA (in control 35.8 ± 2.8 mV and 3.3 ± 0.8 spikes respectively; in CPA 49.8 ± 2.1 mV and 8.8 ± 1.8 spikes respectively; P < 0.05 n= 6).
Figure 1.
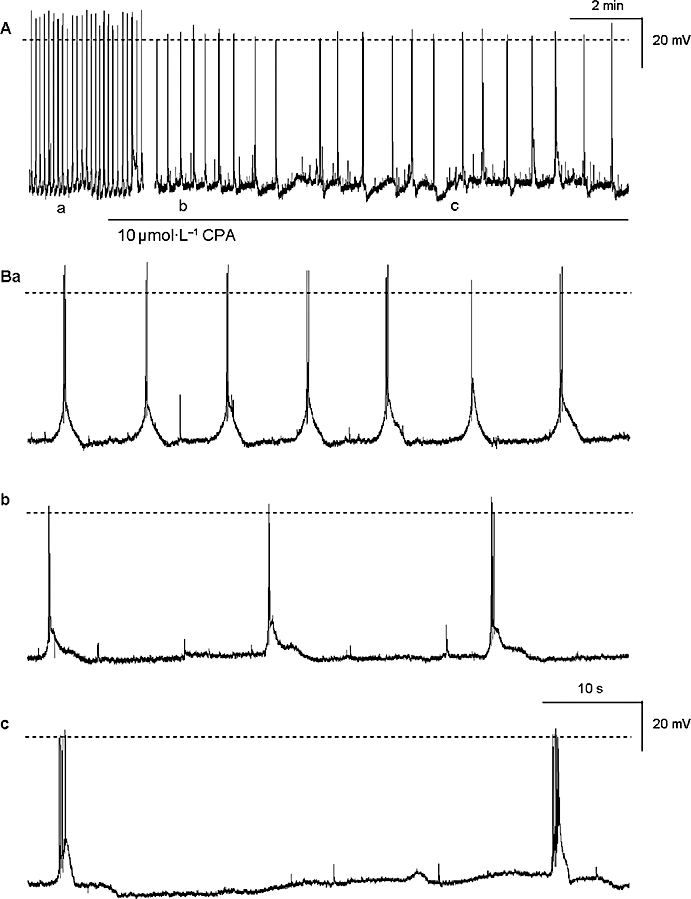
The effects of CPA (10 µmol·L−1) on the slow wave activity recorded in the guinea pig prostate gland (A) are shown on an expanded time scale in (B). CPA significantly decreased the frequency of the slow waves (Bb-c) and after 20 min exposure, also increased the number of superimposed spike potentials on the depolarizing transients (Bc). The broken horizontal line indicates 0 mV. CPA, cyclopiazonic acid.
Effects of caffeine & ryanodine
The role of RyR-mediated release of internal Ca2+ in the generation of prostatic slow waves was examined using two activators of RyR, caffeine and ryanodine. Exposure to ryanodine (30 µmol·L−1) resulted in a transient increase in the frequency of the spontaneous electrical activity, which peaked at 155 ± 19% of control after 1 min [control 5.0 ± 1.0 min−1; in ryanodine (1 min) 7.33 ± 0.99 min−1; P < 0.05, n= 4] and slowly decayed over the washout period (10–30 min) (Figure 2). After 30 min, the membrane potential and slow wave frequency returned to near control values [in control −58.1 ± 0.7 mV and 6.3 ± 0.4 min−1 respectively; in ryanodine (30 min) −56.3 ± 0.5 mV and 6.9 ± 0.7 min−1 respectively; P > 0.05, n= 4]. In contrast, the peak after-hyperpolarization after a 30 min exposure to ryanodine was 3 mV positive of control (control −60.9 ± 0.8 mV; in ryanodine −57.4 ± 0.8 mV; P < 0.05, n= 4). The half-amplitude duration and overall amplitude of the slow wave were not significantly affected by ryanodine (877 ± 145 ms and 59.2 ± 3.6 mV respectively; in ryanodine 973 ± 77 ms and 58.3 ± 5.9 mV respectively; all P > 0.05, n= 4).
Figure 2.
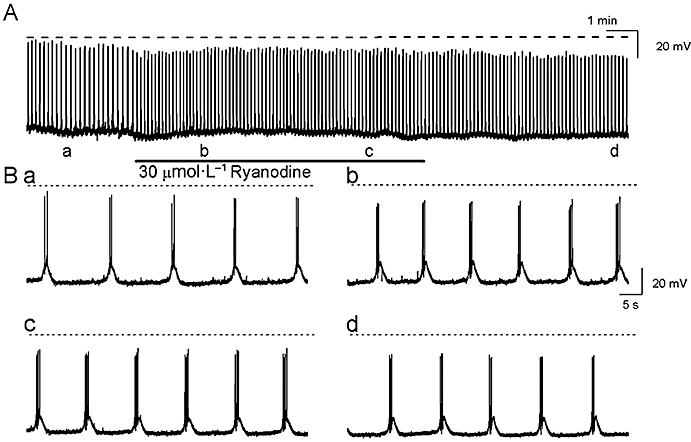
The effects of ryanodine (30 µmol·L−1) on the slow waves (A) are shown on an expanded time scale in (B). Ryanodine caused a transient increase in the slow wave frequency (Bb) before returning to near control values during the washout period. The broken horizontal line indicates 0 mV.
In seven prostatic preparations, exposure to caffeine (1 mmol·L−1 for 30 min) resulted in a significant depolarization of both the membrane potential (control −63.1 ± 1.4 mV; in caffeine −55.4 ± 1.2 mV; P < 0.05, n= 7) and the peak negative value of the after-hyperpolarization following each slow wave (control −66.0 ± 1.2 mV; in caffeine −57.5 ± 1.1 mV; P < 0.05, n= 7). This was accompanied by a significant decrease in the frequency of the spontaneous electrical events to 84 ± 8% of control (control frequency 6.6 ± 0.5 min−1; in caffeine 5.56 ± 0.7 min−1; P < 0.05, n= 7). After 30 minutes exposure to caffeine (1 mmol·L−1), the half-amplitude duration and amplitude of the initial spike of the slow wave were not significantly different from control (in control 1019 ± 86 ms and 58.3 ± 5.9 mV respectively; in caffeine 1110 ± 118 ms and 58.3 ± 5.9 mV respectively; both P > 0.05, n= 4).
Effects of IP3-induced Ca2+ release
2-aminoethoxy-diphenylborate has been previously used to inhibit inositol 1,4,5-trisphosphate receptor (IP3R)-mediated release from internal stores (Wu et al., 2000). In five prostatic preparations, application of 2-APB (60 µmol·L−1 for 10 min) to the prostate resulted in a time-dependent decrease in slow wave frequency. In two of these five preparations, all electrical activity was abolished within 10 min exposure to 2-APB (60 µmol·L−1). In the remaining three preparations, the frequency of slow wave discharge was significantly reduced [in control 4.5 ± 0.3 min−1 (n= 5); in 2-APB 2.4 ± 0.2 min−1 (n= 3); unpaired t test P < 0.05]. This effect was not accompanied by a significant change in the initial spike amplitude and the half-amplitude duration of the recorded slow waves in 2-APB (in control 59.5 ± 1.2 mV and 813 ± 73 ms respectively; in 2-APB 57.2 ± 1.7 mV and 725 ± 57 ms respectively; unpaired t test P > 0.05). In five experiments, Xestospongin C (1–3 µmol·L−1) for >5 min reduced the frequency of slow wave discharge to 90 ± 4% of control (Figure 3). Other parameters were little affected. The number of spikes and duration were 4.4 ± 0.3 spikes and 1134 ± 126 ms in control and 3.5 ± 0.5 spikes and 1144 ± 97 ms in the presence of Xestospongin C respectively (n= 5, P > 0.05).
Figure 3.
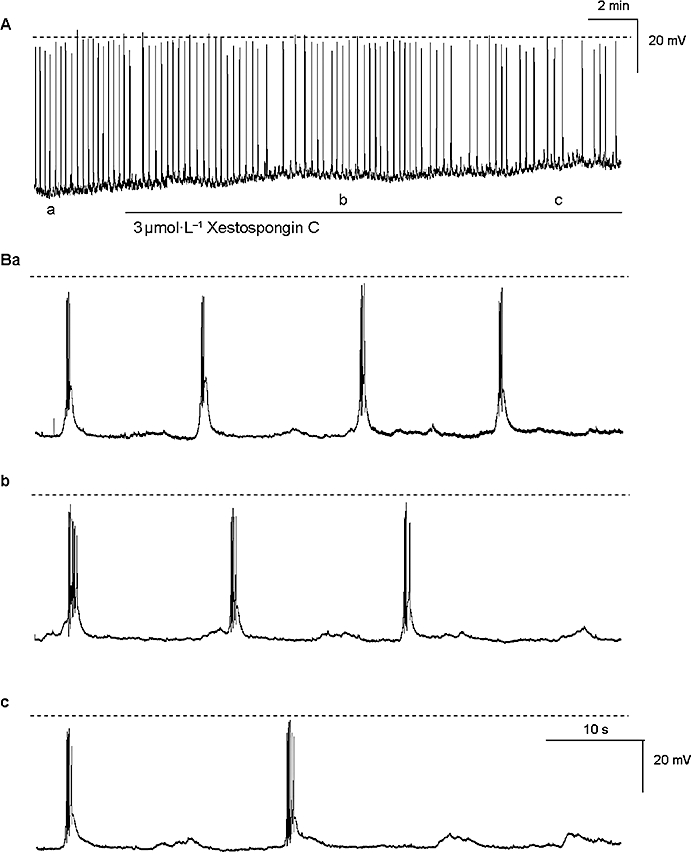
The time scale of (A) was expanded in (B) and shows that after more than 5 min exposure to Xestospongin C (3 µmol·L−1), the frequency of the slow wave activity was significantly reduced (Bb-c) when compared with the control (Ba). The broken horizontal line indicates 0 mV.
Effects of neomycin and U73122
We have further examined the involvement of IP3-dependent Ca2+ release in the generation of the prostatic slow waves using U73122 and neomycin, inhibitors of phosphatidylinositol-specific phospholipase C that reduce the formation of IP3. U73122 (5 µmol·L−1 for >40 min) reduced the frequency of slow wave discharge to 78 ± 5.8% (P < 0.05, n= 4) (in control 8.66 ± 1.02 min−1 in U73122 6.68 ± 0.59 min−1) without significantly affecting the membrane potential, spike amplitude, half amplitude duration or after-hyperpolarization (in control −59.8 ± 3.9 mV, 49.4 ± 0.95 mV, 843 ± 151 ms and −61.9 ± 3.0 mV respectively; in U73122 −58.6 ± 4.5 mV, 50.1 ± 2.9 mV, 990 ± 71 ms and −60.8 ± 4.7 mV respectively; all P > 0.05, n= 5). Neomycin (1 mmol·L−1 for 20 min) rapidly (within 1 min) evoked a membrane depolarization of 4.5 ± 2 mV that was maintained throughout the exposure period (in control −61.5 ± 1.2 mV; in neomycin −56.8 ± 2.9 mV (1 min) and −52.3 ± 4.8 mV (20 min) respectively) (P < 0.05, n= 4) (Figure 4). The half amplitude duration of the slow waves in the presence of neomycin (1 mmol·L−1) was also reduced to 84.3 ± 6.2% of control (in control −1246 ± 148 ms; in neomycin 1058 ± 85 ms; P < 0.05, n= 4). The frequency, spike amplitude and after-hyperpolarization of the recorded slow waves were not significantly affected [in control 6.71 ± 2.45 min−1, 47.9 ± 7.9 mV and −63.3 ± 1.2 mV; in neomycin (20 min) 4.03 ± 1.07 min−1, 48 ± 7.4 mV and −54.7 ± 5.3 mV respectively; all P > 0.05] (Figure 9Cb). However, when preparations were exposed to neomycin (4 mmol·L−1), all slow wave activity rapidly ceased and the membrane depolarized to −43 mV (Figure 4).
Figure 4.
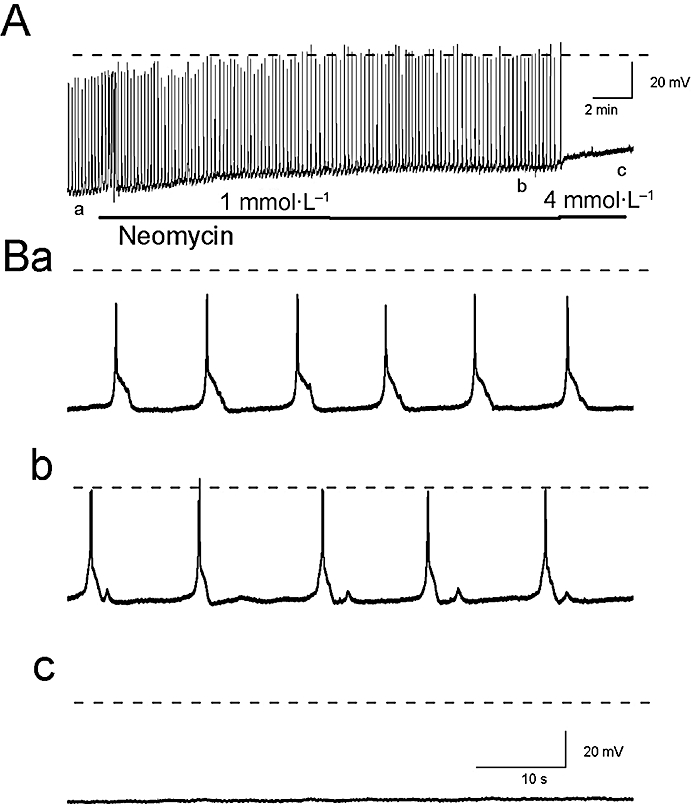
The effects of neomycin (1–4 mmol·L−1) on the slow wave activity of the guinea pig prostate (A) are depicted in an expanded time scale (B). Neomycin (1 mmol·L−1) depolarized the resting membrane potential and reduced the half-amplitude duration of the transient depolarization (Bb). At a higher concentration (4 mmol·L−1), neomycin lead to a further depolarization of the membrane potential and abolished slow wave activity (Bc). The broken horizontal line indicates 0 mV.
Mitochondrial Ca2+ release
We have previously reported that mitochondria may also play a role in intracellular calcium handling resulting in the modulation of the amplitude and/or frequency of pacemaker activity in nifedipine-arrested prostatic preparations (Lang et al., 2006). In this study, the mitochondrial uncouplers, FCCP and CCCP, and the respiratory inhibitor rotenone were used to assess the role that mitochondria play in modulating the spontaneous slow wave activity recorded in the guinea pig prostatic stroma. Application of FCCP (1–3 µmol·L−1 for >15 min) (n= 3), CCCP (1 µmol·L−1 for >15 min) (n= 5) or rotenone (10 µmol·L−1 > 5 min) (n= 3) resulted in membrane depolarization (8–10 mV) before the cessation of all electrical activity (Figure 5). This was reversible upon 30–60 min washout in Krebs solution.
Figure 5.
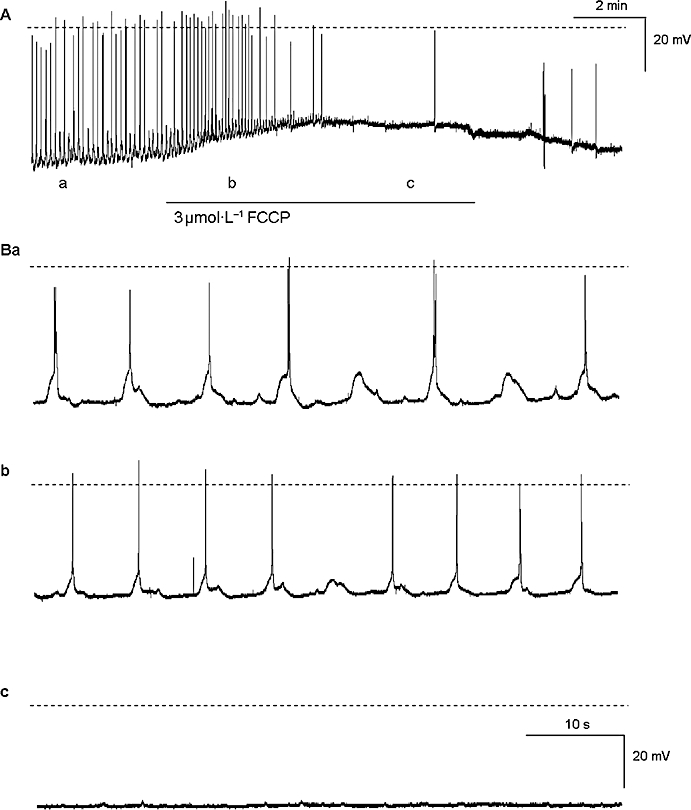
The effects of FCCP (3 µmol·L−1) on the slow wave activity in the guinea prostate (A) are shown on an expanded timescale in (B). FCCP caused a depolarization in resting membrane potential (Ab, Bb) and after 15 min, FCCP abolished slow wave activity (Ac, Bc). The broken horizontal line indicates 0 mV. FCCP, p-trifluoromethoxy carbonyl cyanide phenyl hydrazone.
Contractile studies
We have previously reported that approximately 50% of preparations of guinea pig prostate can generate spontaneous contractions irregular in both their amplitude (between 0.1 and 3 mN in magnitude) and frequency (2 to 10 min−1). Phenylephrine (1–10 µmol·L−1), carbachol (1–10 µmol·L−1) and a raised K+ (20–66 mmol·L−1) physiological saline solution can all induce contractions in both quiescent and spontaneously contracting preparations. In the present experiments, contractions to phenylephrine (100 µmol·L−1 for 2 min) were used to establish the degree of inhibition of either IP3 formation or action induced by 2-APB (60 µmol·L−1) or neomycin (1 µmol·L−1). CPA (10 µmol·L−1), caffeine (1 mmol·L−1) and ryanodine (1 µmol·L−1) were also used in this set of experiments. The AUC of the phenylephrine contractions recorded after a prolonged (for 30 min) exposure to these modulating agents (in the presence of Ca2+-free physiological saline solution) were then expressed as a percent of the AUC of the control contractions recorded in the absence of these agents. Contractions to phenylephrine consisted of a number of transient occasionally fused contractions, often accompanied by an increase in the baseline tension. In the presence of CPA (10 µmol·L−1), caffeine (1 mmol·L−1), ryanodine (1 µmol·L−1), 2-APB (60 µmol·L−1) or neomycin (1 mmol·L−1), the AUC values for phenylephrine (100 µmol·L−1 for 2 min)-evoked contractions were reduced (all P < 0.05) (Figure 6). Moreover, the inhibitory effects of these agents were mostly irreversible during the washout period (for 30 min), with the exception of caffeine which was readily reversed upon washout.
Figure 6.
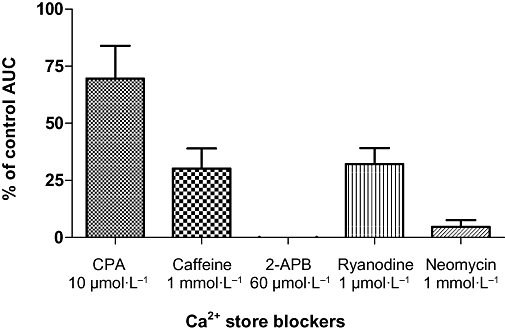
Effects of 10 µmol·L−1 CPA, 1 mmol·L−1 caffeine, 60 µmol·L−1 2-APB, 1 mmol·L−1 neomycin, 1 µmol·L−1 ryanodine on the phenylephrine-evoked contractions of isolated strips of the guinea pig prostate. The strips were exposed to these modulating agents, in the presence of Ca2+-free Krebs solution for 30 min. The AUC of the phenylephrine contractions (100 µmol·L−1 for 2 min) was then recorded and expressed as a percent of the AUC of the control contractions, recorded in the absence of these agents. The AUC values of phenylephrine-evoked contractions were reduced significantly (P < 0.05; n= 6–7) by 10 µmol·L−1 CPA, 1 mmol·L−1 caffeine, 60 µmol·L−1 2-APB, 1 mmol·L−1 neomycin or 1 µmol·L−1 ryanodine. 2-APB, 2-aminoethoxy-diphenylborate; AUC, area under the curve; CPA, cyclopiazonic acid.
Discussion
In this study, we have extended our previous investigations in the prostate gland in order to ascertain the specific contribution of various intracellular compartments to the configuration of the slow wave. Slow wave activity is of particular interest as it is the most likely to contribute to contractility, thereby dictating the resting tone of the prostate; an increase in smooth muscle tone contributes to many of the urinary voiding symptoms associated with BPH (Exintaris et al., 2006).
RyR and IP3-dependent mediated Ca2+ release
In human prostatic stromal smooth muscle cells, both spontaneous and phenylephrine-induced Ca2+ transients were suppressed following application of 20 mmol·L−1 caffeine suggesting that intracellular Ca2+ stores play an important role in facilitating the regenerative and agonist-induced activity in these cells (Wu et al., 2005). At the high concentrations of caffeine used in this study (20 mmol·L−1), it is conceivable that both RyR-mediated and IP3R-dependent Ca2+ release were affected (MacMillan et al., 2005). In our present study, the Ca-ATPase inhibitor CPA (10 µmol·L−1) reduced slow wave frequency over 30 min confirming that intracellular calcium stores are involved in maintaining autorhythmicity in the guinea pig prostate gland (Exintaris et al., 2006; Lang et al., 2006). However, spontaneous slow wave discharge did not appear to be dependent on the cycling of intracellular Ca2+ through ryanodine-sensitive Ca2+ stores as ryanodine only initially transiently increased slow wave discharge before the frequency settled to near control values (Figure 2) and low concentrations of caffeine only reduced the frequency of slow wave discharge by approximately 10%. In contrast, we have previously shown that 100 µmol·L−1 CPA and 30 µmol·L−1 ryanodine significantly reduced the TEA-sensitive BK Ca2+-activated K+ currents in freshly dispersed stromal myocytes. This implies that Ca2+ release from ryanodine-sensitive internal stores is involved in the generation of the whole cell K+ outward currents, thereby contributing to the regenerative spikes superimposed on the slow waves. In this study, ryanodine did not significantly attenuate any of the characteristics of the prostatic slow wave; however, the superimposed spikes were not studied in detail.
In the current study, blockade of IP3R-mediated Ca2+ release with 60 µmol·L−1 2-APB and 3 µmol·L−1 Xestospongin C significantly reduced slow wave discharge. Similarly, inhibiting phospholipase C and IP3 formation using U73122 (5 µmol·L−1) or neomycin (1 and 4 mmol·L−1) significantly reduced slow wave frequency, amplitude and duration (Figure 5) or abolished activity altogether indicating that IP3-dependent Ca2+ stores have a significant role in the generation or maintenance of slow wave activity in the guinea pig prostate gland. It remains to be seen whether the blocking effects of the agents that interfere with IP3-dependent Ca2+ release are predominantly on the prostatic interstitial cells (PIC), smooth muscle cells or both cell types. The cycling of Ca2+ through IP3-dependent mechanisms does appear to be involved in generating electrical activity in the agonist-evoked responses in the smooth muscle cells of the prostate gland. For example, phenylephrine-induced contractions of freshly dispersed human stromal cells have been reported to be partly dependent on the cycling of Ca2+ through IP3-dependent mechanisms (Eckert et al., 1995). Similarly, phenylephrine-evoked contractions were reduced in the presence of 2-APB or neomycin in the current study (Figure 6). However, both U73122 and neomycin have also been used to block of contractions elicited by a raised K+ saline, suggesting that their inhibitory effect may be partly due to their blockade of voltage-dependent Ca2+ channels (Lang et al., 2002).
In the literature, there is ample support to suggest that IP3R-mediated Ca2+ release is involved in generating pacemaker activity in ICC. For example, calcium oscillations in rabbit urethral interstitial cells are initiated by calcium release from ryanodine-sensitive intracellular stores and that conversion of the primary oscillation to a propagated calcium wave depends upon IP3-induced calcium release (McHale et al., 2006; Sergeant et al., 2006a,b). In the gastrointestinal system, the cycling of Ca2+ through IP3-dependent Ca2+ stores also appears to regulate pacemaker discharge. It has been postulated that during the onset of the intestinal pacemaker potential, the release of Ca2+ from IP3 stores leads to the activation of mitochondrial Ca2+ uptake and the lowering of the localized Ca2+ concentration near the plasma membrane. This fall in Ca2+ then activates the cationic-selective channels to generate the pacemaker potential (Ward et al., 2000; Hirst et al., 2003). In contrast, potentials recorded in the ICC of the myenteric plexus (ICC-MY) and those in the smooth muscle (ICC-IM) of guinea pig stomach generate spontaneous depolarizations which are blocked by not only inhibitors of IP3-dependent Ca2+ release and disruption of mitochondrial handling but also Cl– channel blockers (Hirst et al., 2002). Further experiments will unequivocally demonstrate the role of IP3-induced Ca2+ release on pacemaker activity in the guinea pig prostate gland.
Mitochondria
Recent studies indicate that mitochondria may also play a role in intracellular calcium handling resulting in the modulation of the amplitude and/or frequency of slow wave activity in various smooth muscles, for example, small intestine (Ward et al., 2000). Mitochondria are more likely to buffer cytosolic Ca2+ rather than contribute to the concentration of intracellular Ca2+ directly. In this study, the mitochondrial uncouplers, FCCP (1 µmol·L−1) and CCCP (3 µmol·L−1), and the respiratory chain inhibitor, rotenone (10 µmol·L−1), significantly reduced slow wave activity recorded in the smooth muscle cells of the guinea pig prostatic stroma (Figure 5). Such agents that disrupt mitochondrial Ca2+ handling have been previously shown to affect both smooth muscle and/or interstitial cells. For example, in guinea pig detrusor smooth muscle cells, it has been postulated that CCCP may inactivate L-type Ca2+ channels by interrupting the capacity of the mitochondria to buffer Ca2+ near the plasma membrane. Alternatively, CCCP and other mitochondrial inhibitors may affect Ca2+ handling by interacting with ICC cells directly (Kubota et al., 2003). In the guinea pig prostate, the propagated pacemaker potentials were significantly reduced when Ca2+ mobilization between the intracellular stores and mitochondria was disrupted using 1 µmol·L−1 CCCP (Lang et al., 2006). In the current study, the underlying pacemaker potentials did not appear to be significantly affected by rotenone, although slow wave activity was significantly reduced. Mitochondrial inhibitors can therefore reduce or abolish slow wave activity by affecting pacemaker activity which affect the slow wave activity arising from the smooth muscle cells in the guinea pig prostate gland. Alternatively, these agents affect both cell types.
In conclusion, this study has demonstrated that regenerative electrical activity arising from the smooth muscle cells of the guinea pig prostate is dependent on the cycling of Ca2+ from IP3-dependent Ca2+ stores and the buffering of Ca2+ from mitochondria. Selective modulation of slow wave activity may well provide a different, novel and perhaps more specific avenue for modulating stromal excitability and ensuing smooth muscle tone in the prostate. It remains to be seen whether changes in populations of the smooth muscle cells or PIC or the mechanisms underlying rhythmicity in these cells change with age; information which could provide a better understanding of the aetiology of prostate-specific conditions such as BPH.
Acknowledgments
This work has been supported by the National Health and Medical Research Council of Australia.
Glossary
Abbreviations
- BK
large conductance Ca2+-activated K+ channel
- BPH
benign prostatic hyperplasia
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- FCCP
p-trifluoromethoxy carbonyl cyanide phenyl hydrazone
- ICC
interstitial cells of Cajal
- IP3R
inositol trisphosphate receptor
- PIC
prostatic interstitial cells
- RyR
ryanodine receptor
- TEA
tetraethyl ammonium
Conflict of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC). 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RE, Schreier U, Drescher P, Madsen PO, Derouet H, Becht E, et al. Regulation of prostatic smooth muscle contractility by intracellular second messengers: implications for the conservative treatment of benign prostatic hyperplasia. Urol Int. 1995;54:6–21. doi: 10.1159/000282685. [DOI] [PubMed] [Google Scholar]
- Exintaris B, Klemm MF, Lang RJ. Spontaneous slow wave and contractile activity of the guinea pig prostate. J Urol. 2002;168:315–322. [PubMed] [Google Scholar]
- Exintaris B, Nguyen DT, Dey A, Lang RJ. Spontaneous electrical activity in the prostate gland. Auton Neurosci. 2006;126–127:371–379. doi: 10.1016/j.autneu.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Beckett EA, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002;540:1003–1012. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Hashitani H, Fukuta H, Kubota H, Kohri K, Suzuki H. Role of mitochondria in the generation of spontaneous activity in detrusor smooth muscles of the Guinea pig bladder. J Urol. 2003;170:628–633. doi: 10.1097/01.ju.0000069428.46133.d5. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y, Kojima K, Kanayama H, Kagawa S, Minami K, Nakaya Y. Activation of the Ca2+-activated K+ channel via protein kinase A-dependent phosphorylation in human prostatic smooth muscle cells. Int J Urol. 1998;5:482–486. doi: 10.1111/j.1442-2042.1998.tb00394.x. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Hashitani H, Keller S, Takano H, Mulholland EL, Fukuta H, et al. Modulators of internal Ca2+ stores and the spontaneous electrical and contractile activity of the guinea-pig renal pelvis. Br J Pharmacol. 2002;135:1363–1374. doi: 10.1038/sj.bjp.0704609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Mulholland E, Exintaris B. Characterization of the ion channel currents in single myocytes of the guinea pig prostate. J Urol. 2004;172:1179–1187. doi: 10.1097/01.ju.0000135456.65892.ed. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Nguyen DT, Matsuyama H, Takewaki T, Exintaris B. Characterization of spontaneous depolarizations in smooth muscle cells of the Guinea pig prostate. J Urol. 2006;175:370–380. doi: 10.1016/S0022-5347(05)00003-0. [DOI] [PubMed] [Google Scholar]
- MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol. 2005;569:533–544. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale N, Hollywood M, Sergeant G, Thornbury K. Origin of spontaneous rhythmicity in smooth muscle. J Physiol. 2006;570:23–28. doi: 10.1113/jphysiol.2005.098376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Kim KM, Chung YS, Hong EK, Shin SY, Kim SJ. Ion-channel currents of smooth muscle cells isolated from the prostate of guinea-pig. BJU Int. 2003;92:1022–1030. doi: 10.1111/j.1464-410x.2003.04510.x. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Ca2+ signalling in urethral interstitial cells of Cajal. J Physiol. 2006a;576:715–720. doi: 10.1113/jphysiol.2006.115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Interstitial cells of Cajal in the urethra. J Cell Mol Med. 2006b;10:280–291. doi: 10.1111/j.1582-4934.2006.tb00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafik A, Shafik I, el-Sibai O. Identification of c-kit-positive cells in the human prostate: the interstitial cells of Cajal. Arch Androl. 2005;51:345–351. doi: 10.1080/014850190944456. [DOI] [PubMed] [Google Scholar]
- Van der Aa F, Roskams T, Blyweert W, De Ridder D. Interstitial cells in the human prostate: a new therapeutic target? Prostate. 2003;56:250–255. doi: 10.1002/pros.10264. [DOI] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525(Pt 2):355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Fry PM, Sui G, Fry CH. Intracellular Ca2+ regulation in a human prostate stromal cell culture. Neurourol Urodyn. 2005;24:81–88. doi: 10.1002/nau.20088. [DOI] [PubMed] [Google Scholar]
- Wu J, Kamimura N, Takeo T, Suga S, Wakui M, Maruyama T, et al. 2-Aminoethoxydiphenyl borate modulates kinetics of intracellular Ca(2+) signals mediated by inositol 1,4,5-trisphosphate-sensitive Ca(2+) stores in single pancreatic acinar cells of mouse. Mol Pharmacol. 2000;58:1368–1374. doi: 10.1124/mol.58.6.1368. [DOI] [PubMed] [Google Scholar]


