Abstract
Background and purpose:
Obesity is a severe health problem in the modernized world and understanding the central nervous mechanisms underlying food-seeking behaviour and reward are at the forefront of medical research. Cannabinoid receptors have proven an efficient target to suppress hunger and weight gain by their pharmacological inactivation.
Experimental approach:
A standard fasted protocol and a novel long-term home-cage observation system with free-feeding animals were used to assess the feeding behaviour of mice treated with the CB1 antagonist AM251. Similarly, the effects of the phytocannabinoid Δ9-tetrahydrocannabivarin (Δ9-THCV), which behaves like a CB1 antagonist, were also determined in free-feeding animals.
Key results:
AM251 suppressed food intake and weight gain in fasted and non-fasted animals. The suppression of food intake by AM251 (10 mg·kg−1) endured for a period of 6–8 h when administered acutely, and was continuous when injected for four consecutive days. Pure Δ9-THCV also induced hypophagia and weight reduction at doses as low as 3 mg·kg−1. No rebound was observed on the following day with all drug groups returning to normal activity and feeding regimes. However, a Δ9-THCV-rich cannabis-extract failed to suppress food intake and weight gain, possibly due to residual Δ9-tetrahydrocannabinol (Δ9-THC) in the extract. This Δ9-THC effect was overcome by the co-administration of cannabidiol.
Conclusions and implications:
The data strongly suggest (i) the long-term home-cage observation system is a sensitive and obesity-relevant tool, and (ii) the phytocannabinoid Δ9-THCV is a novel compound with hypophagic properties and a potential treatment for obesity.
Keywords: obesity, hypophagia, phytocannabinoids, Δ9-THCV, AM251, home cage, mice, CB1 receptor
Introduction
Increased consumption of high levels of sugar and saturated fats, combined with reduced physical activity, have raised obesity rates 3-fold or more since 1980 in modern western countries. Obesity and overweight pose a major risk for serious diet-related chronic diseases, and childhood obesity has already attained epidemic levels in some regions in UK. Effective weight management requires understanding of its basic physiology and pharmacology and towards this end the endocannabinoid system of the hypothalamus and cortico-limbic system plays an integral part in the regulation of caloric intake in hunger and satiety (for a recent review, see Pagotto et al., 2006).
The endocannabinoid system interacts with the appetite suppressing hormone leptin, which is released by adipocytes and stimulates the release of peptides within the hypothalamus to decrease appetite. Administration of leptin reduces levels of two endocannabinoids, 2-arachidonoyl glycerol (2-AG) and anandamide in obese mice (ob/ob) and normal rats (Di Marzo et al., 2001) confirming that the endocannabinoid system appears to be negatively regulated by leptin.
Centrally, the regulation of appetite and feeding behaviour is mediated by CB1 receptors. The hyperphagic effects observed after exogenous administration of cannabinoid receptor agonists such as Δ9-tetrahydrocannabinol (Δ9-THC), 2-AG or anandamide can be reversed by CB1 receptor antagonists, but not by the CB2 receptor antagonist SR144528 (Williams and Kirkham, 2002a). Moreover, CB1 knockout mice display a number of characteristics including increased leanness, reduced food intake, resistance to diet-induced obesity and a significant loss in visceral fat levels compared with wild-type controls (Cota et al. 2003; Ravinet-Trillou et al., 2003), which are concordant with CB1-receptor contribution to obesity.
Endogenous cannabinoids and CB1 receptors are located in the hypothalamus and other brain areas associated with feeding and reward (Wittman et al. 2007). Infusion of anandamide or 2-AG into the nucleus accumbens (Kirkham et al. 2002; Soria-Gomez et al. 2007) or hypothalamus (Jamshidi and Taylor, 2001) and intra-paraventricular injections of Δ9-THC (Verty et al. 2005) all induce hyperphagia together with neural activation of posterior and ventromedial hypothalamus.
The appetite enhancing properties of exogenously administered cannabinoids (smoking marijuana or administration of Δ9-THC in the synthetic form of Dronabinol) are part of the therapy for weight loss in patients suffering from HIV and cancer (Timpone et al, 1997; Haney et al. 2005; Walsh et al, 2005). By contrast, antagonists for the neural CB1 receptor are hypophagic (Gadde and Allison, 2006; Pagotto et al, 2006). SR141716A (rimonabant) is the first and prototypic antagonist synthesized by Rinaldi-Carmona et al. in 1994 and numerous other derivatives have been developed since. Rimonabant was marketed in the UK and other European countries as Acomplia and was indicated for use in conjunction with diet and physical exercise for obese patients with associated risk factors.
In animal studies, acute or chronic administration of rimonabant suppresses food intake (Colombo et al, 1998; Vickers et al, 2003), reduces the consumption of palatable food (Simiand et al, 1998; Gallate and McGregor, 1999) and prevents the hyperphagia induced by Δ9THC (Jarrett et al., 2005; Jarrett et al.,2007). Other CB1 receptor antagonists such as AM251 or AM281 have similar effects (McLaughlin et al., 2003; Shearman et al., 2003; Zhou and Shearman, 2004), most likely owing to a reduction of the perceived palatability of the food administered (Jarrett et al., 2007) and thus explaining why CB1 antagonists/inverse agonists reduce food intake of both highly palatable (reviewed in Cota et al., 2006) and familiar laboratory chow (Hildebrandt et al., 2003; Verty et al., 2004) in normal ad libitum or obesity models (Thiebot et al., 2006 for a review).
A more detailed characterization of the ingredients of cannabis has revealed the existence of the phytocannabinoid (-)-Δ9-tetrahydrocannabivarin (Δ9-THCV) (Gill et al. 1970; Merkus 1971), which expresses the pharmacological profile of a CB1 antagonist in vitro and in vivo (Thomas et al., 2005; Pertwee et al., 2007). We therefore assessed whether this phytocannabinoid also reduces food intake and weight in an established test situation, using non-fasted mice in a home-cage observation system.
Typically, food intake is assessed in either fasted or satiated conditions. Fasting includes a 24 h food deprivation schedule prior to drug treatment followed by a period of 1 h in which food intake of regular chow or highly palatable diets is measured in an open-field-type environment. In contrast, non-fasted animals are tested in their home cages with free access to regular rodent chow. As effects of drugs are recorded, an independent yet related issue concerns drug effects on motor activity which if increased may lead to hyperactivity and this may support body weight reduction independent of any effect on food consumption. Therefore, a more complete assessment of drug-related hypophagic properties also requires the determination of activity levels in an open arena. Here, we combined the two approaches by using a novel automated home-cage video observation method (PhenoTyper) that enabled continuous monitoring of cage floor locomotion prior to and after administration of drug. In addition, we also determined visits to both the food hopper containing regular mouse chow, and the water bottle to confirm that weight reduction correlates with reduced food/water consumption. Moreover, we explored whether the activity profile may be useful as an overall index of altered food intake pattern caused by the anorectic agent. To validate this method, we used the CB1 antagonist AM251, and continued to establish whether the novel phytocannabinoid antagonist Δ9-THCV has similar properties.
Methods
Subjects
Male C57 BL6 mice (25–32 g) (Harlan, UK and Harlan, Italy) were used in these experiments. The mice were group housed (10 per cage) prior to testing and kept in a holding room with a 12 h light/dark cycle (lights off 19 h 00 min), temperature was maintained at 23 ± 2°C and 40–60% relative humidity. Prior to testing animals were allowed free access to food (standard rodent chow) and water.
Drug treatments and groups
Drugs were prepared using one of two methods: (i) AM251 and Tween 80 were dissolved in ethanol and prepared fresh on each experimental day in a solution of two parts of Tween 80 by weight (Experiment 1, PhenoTyper); ethanol was evaporated under vacuum and the residue re-suspended in saline; or (ii) in all other cases, drugs were evaporated and then dissolved in the solution of ethanol (98%), cremaphor and distilled water. Prior to testing animals were matched for body weight and assigned to drug groups. Drugs were injected i.p. at a volume of 0.1 mL·10 g−1 body weight. The following doses and groups were tested: AM251 (10 mg·kg−1), Δ9-THCV-pure (3 mg·kg−1, 10 mg·kg−1 or 30 mg·kg−1), Δ9-THCV-rich botanical drug substance (BDS) (containing 0.48 mg·kg−1, 0.96 mg·kg−1, 1.44 mg·kg−1, 3 mg·kg−1, 10 mg·kg−1 or 30 mg·kg−1Δ9-THCV), cannabidiol (CBD) (10 mg·kg−1), Δ9-THCV-rich BDS (3 mg·kg−1)+CBD (10 mg·kg−1) and vehicles (Tween 80 or cremaphor solution), for group sizes, see the individual figure legends.
Apparatus and testing procedure
Two different behavioural tasks were applied:
24 h food deprivation task
Experiments were performed following the protocol of Wiley et al. (2005) with minor modifications; 24 h before the beginning of the feeding trial (at about 12 h 00 min), all food was removed from the home cages. The next day mice were weighed and then injected with drug, and a feeding session was performed 30 min post drug treatment during which mice were individually placed in a clear plastic cage lined with thick paper and allowed free access to a pre-determined amount of laboratory chow for 1 h. At the end of the session, mice were removed from the observation cage and weighed before being returned to their home cages. The food left by animals in the test cage, including crumbs, was measured, and the amount consumed was calculated.
Automated home-cage observation in non-deprived mice
The PhenoTyper is a video-based observation system that allows long-term continuous monitoring of behavioural activity in mice. Boxes consisted of clear Perspex walls and floors (30 × 30 × 35 cm) and contained a white nesting house (10 × 10 × 5 cm), food hopper and water bottle allowing free access to food and water. Vision between cages was obscured by grey Perspex boards. PhenoTypers were filled with saw-dust and the top unit of each cage contained a built-in digital infrared sensitive video camera and infrared lighting sources for video tracking. The infrared sources provided a constant and even illumination of the ground floor of the cage and enabled continuous behavioural recordings in both dark and light periods. Animals were individually placed in the PhenoTyper cages and subjected to conditions identical to those of the standard holding rooms. Up to 32 PhenoTyper cages in one laboratory were used simultaneously, and video tracks were recorded by two computers (16 PhenoTypers per PC) using the computer-based tracking software Ethovision 3.1 pro.
From the video tracks, the following parameters were extracted: (i) total locomotion in the arena summarized in hourly bins during experimental days; (ii) time spent in the area in front of the feeder (Figure 1C) and, similarly, time spent in the area in front of the water bottle. The area definitions were kept close to the feeder/water bottle with dimensions for the food and water zones of 15 × 5 cm and 7 × 5 cm respectively in order to minimize artifacts. In addition to these parameters, the body weight of each mouse, weight of food hopper (food consumption) and the weight of water bottle (water intake) were also determined on a daily basis. Hoarding was observed only occasionally and unrelated to drug treatment regimes. As a clear definition of food consumption was impractical in these animals, hoarding subjects were omitted from the analysis.
Figure 1.
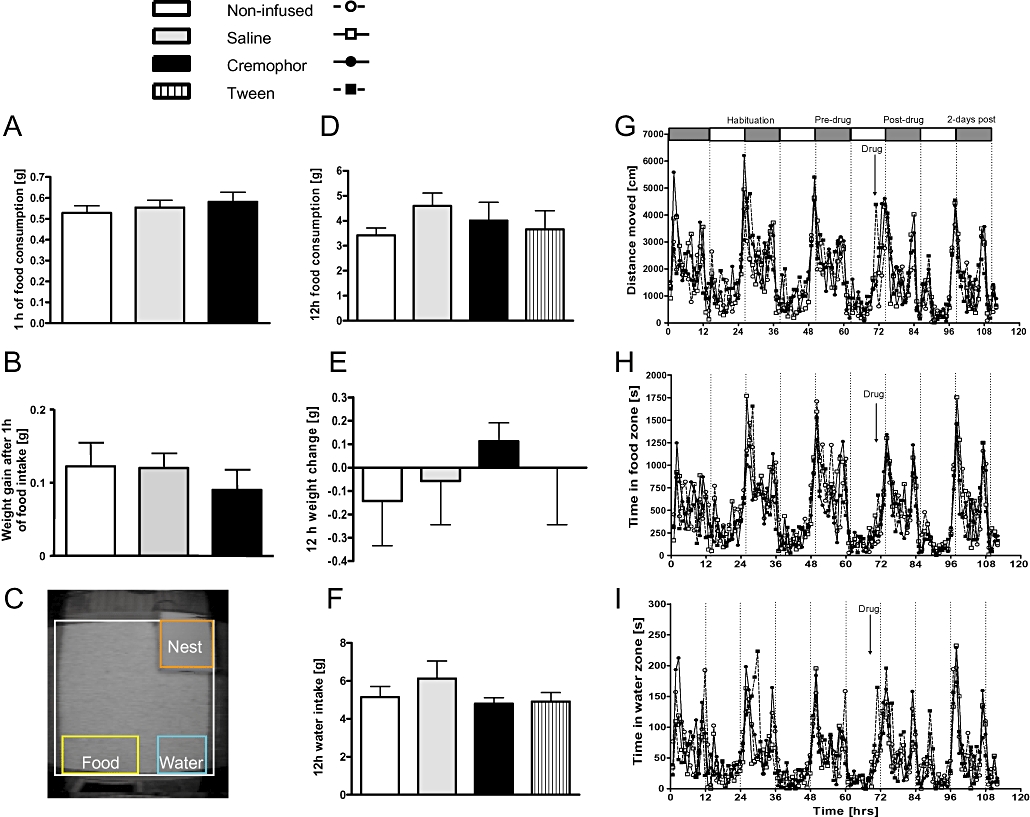
Systemic infusion of various control solvents had no effect on feeding behaviour of fasted and non-fasted animals. No difference in food intake (A) or body weight (B) was observed in fasted animals following administration of control solvents. (C) Home-cage activity observation system (‘PhenoTyper’) indicating the location of different zones of interest: food, water and the nest. Non-fasted animals presented with no difference in food intake (D), body weight (E) or water intake (F) when injected with solvents relative to non-infused controls. PhenoTyper analysis of overall activity (G), time spent in food zone (H) and water zone (I), respectively, indicated normal circadian rhythmicity in C57Bl/6 mice with higher locomotor activity during the night cycle. Animals sleep more during the light phase and increase their eating and drinking during the night (shaded bars). Also indicated is the period of habituation as well as pre-drug, post-drug and recovery days. Data shown are means ± s.e.mean (n= 7 per group).
After habituation of 3–4 days, drug administration was performed acutely via an i.p. injection of a single drug dose at 17 h 00 min during the light phase of the circadian cycle. In Experiment 1C, we also employed a repeated dosing regime and administered the drug daily for four consecutive days. Recording of the animals’ behaviour, body weights and weights of the food hopper as well as the water bottle continued for up to 2 days post injection with all measurements taken between 10 h 00 min and 11 h 00 min during the light cycle. In the repeated dosing procedure in Experiment 1C the body weights, food hopper and water bottle weights were recorded twice daily (between 16 h 00 min and 17 h 00 min) followed by the administration of AM251 (10 mg·kg−1) or Tween, and again each morning post drug treatment (between 10 h 00 min and 11 h 00 min).
Drugs and apparatus
AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) was obtained from Tocris Cookson (UK), CBD, Δ9-THCV-pure and Δ9-THCV-rich BDS were supplied by GW Pharmaceuticals (Porton Down, Wiltshire, UK). Tween 80, the mixture of ethanol (98%) and cremaphor EL were from Sigma (UK) and these together with distilled water (ratio 1 : 1 : 18) were used as vehicles in these experiments. Δ9-THCV-rich BDS contained 57.1% Δ9-THCV and 11.9% Δ9-THC and numerous other cannabinoid and terpenoids in minute amounts.
The PhenoTyper and Ethovision 3.1 pro were obtained from Noldus (Wageningen, Netherlands).
Statistical analysis
All data were analysed using the PC-based statistics package Prism 4.01 (Graphpad, San Diego, CA, USA). Individual animals that were outliers (values exceeding two standard deviations from the average) were excluded from the data analysis (n= 3; no more than 1 in each experimental group). Two-way repeated measures analysis of variance (anova) was employed with drug-treatment as between subject factor and time as within-subject factor; one-way anova and t-tests using Bonferroni adjustment were conducted for group comparisons of body weight, food intake and water consumption. To confirm the relationship between food intake, water intake and the time spent in the respective zones, correlation coefficients were calculated and reliability was determined using Pearson coefficient analysis. For all tests, the null hypothesis was rejected for probability values exceeding 5% (P > 0.05).
Results
Experiment 1: validation of the PhenoTyper method as a test for anorectic properties of drugs
Experiment 1 consisted of three parts. We first (Experiment 1A) compared the effects of various solvents on feeding behaviour in the traditional fasting paradigm and in a free-feeding protocol with a long-term assessment in ‘PhenoTyper’ home cages. Then, we established the cannabinoid CB1 antagonist AM251, widely known for its anorectic properties, as a positive control (Experiment 1B), before confirming the effectiveness of AM251 in a repeated administration regime (Experiment 1C).
Experiment 1A: the effect of different solvents on feeding behaviour in deprived and non-deprived mice
Cannabinoids were dissolved in Tween 80 and saline or in Cremaphor and ethanol; these solvents, especially when given in high doses, are likely to affect behaviour in mice. Particularly sensitive is food intake and in the first experiment we compared some widely used solvents for their effect on food intake in both fasted and non-fasted animals.
Figure 1A shows a summary of the results for food consumption after 24 h of fasting. During the 1 h testing phase, untreated mice ate marginally less than saline or cremaphor-injected mice, but this difference was not significant (F < 1 for factor treatment). Similarly, weight gain during 1 h of eating did not differ between groups (F < 1) (Figure 1B). The same outcome was attained in non-deprived animals during home-cage observation in PhenoTyper boxes. After 3–4 days of habituation, weights were taken, animals injected and weighed again on the next day (Figure 1E). There was no weight difference between the control groups (F < 1; note that Tween 80 was also tested in this protocol). In line with this result, food consumption (Figure 1D) and water intake (Figure 1F) also did not differ between the four control groups (all F < 1). These results establish that neither of the solvents affected food intake in the short or long term.
Recording free-feeding activity in the PhenoTyper enabled determination of activity levels in predetermined zones (see Figure 1C). Locomotor activity of animals during the recording periods are depicted in Figure 1 for overall distance moved per hour (G), time spent in the zone fronting the feeder (H) and time in the water zone (I). For all areas, we observed a clear difference in activity for day and night. In agreement with previous analyses, C57BL/6 mice were more active during the dark phase (De Visser et al., 2005; De Visser et al.,2007) and presented with two activity peaks, one at the beginning and one at the end of each dark phase. This was similarly observed in all zones and was not sensitive to vehicle injection (Figure 1G–I). Consequently, overall locomotor activity and time spent in food or water zones did not differ between treatment groups (all F < 1.3 for the dark phase post-injection – 72–84 h in Figure 1G–I).
Experiment 1B: the effect of the cannabinoid receptor antagonist AM251 on weight after acute treatment in fasted and non-fasted mice: selective reduction of feeding behaviour
We next examined the well-known property of AM251 to reduce food consumption in animals starved for 24 h. Although food consumption was drastically and reliably reduced by administration of AM251 (Figure 2A; t= 4.5; df = 18, P= 0.0003), there was no reliable difference in weight gain induced by the drug over 1 h (Figure 2B; t < 1). It appeared that the 1 h recording period is too short, and long-term assessment in PhenoTypers confirmed not only a suppression of food intake in the AM251 group over 12 h post treatment (Figure 2C; t= 3.2; df = 13, P= 0.0072) but also a weight reduction in the AM251 group relative to a small weight gain in controls (Figure 2D; t= 6.3; df = 13, P < 0.0001) and reduced water intake (Figure 2E; t= 5.5; df = 13, P= 0.0001).
Figure 2.
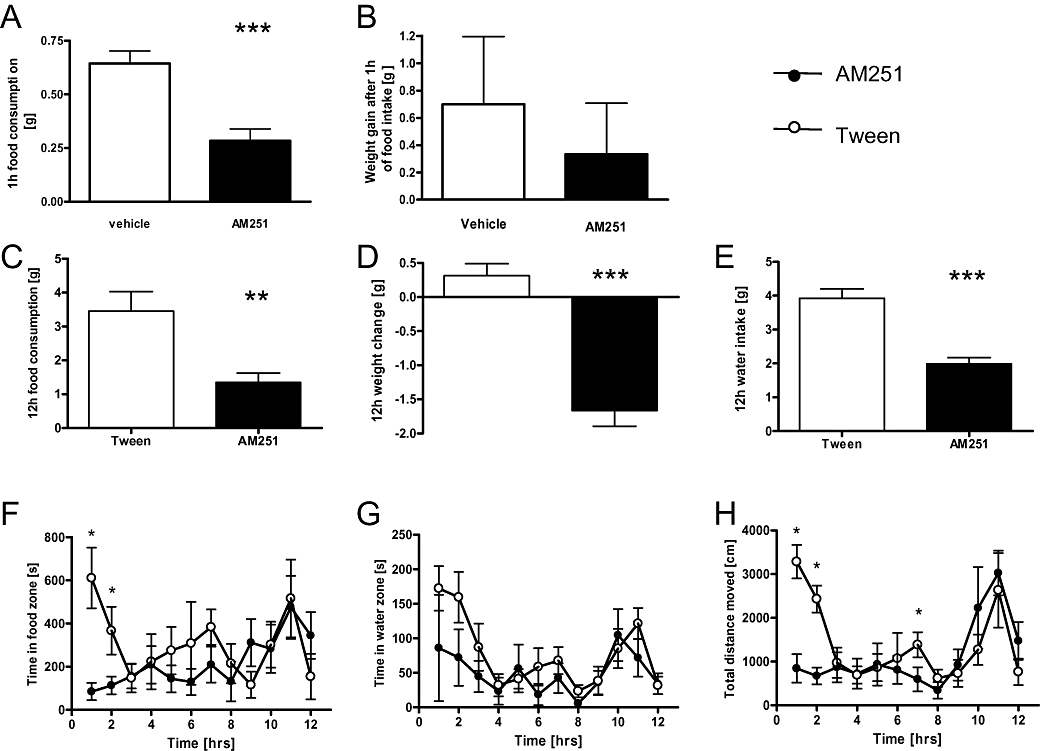
Treatment with AM251 produced a reduction in food intake in both fasted and non-fasted animals. (A) AM251 decreased the food consumption of fasted mice but no difference in body weight was observed (B). Non-fasted animals treated with AM251 displayed a decrease in food intake (C), body weight (D) and water intake (E) compared with Tween 80-treated controls in the 24 h following drug exposure. They also spent less time in the food zone compared with controls (F) and were less active (H). No difference between groups was evident for time spent in the water zone (G). Data shown are means ± s.e.mean (n= 8 per group). Asterisks indicate significance: *P < 0.05; **P < 0.01; ***P < 0.001 (t-test).
Figure 2F depicts the time spent in the zone adjacent to the feeder summarized in hourly bins during the dark phase post treatment. Clearly, during the early hours of darkness, AM251-treated mice spent less time in the food zone compared with vehicle controls; thus, statistical analysis yielded a significant effect of drug (F(1,168) = 3.92; P < 0.05). Reduced activity in the water zone was also observed in the AM251 group (F(1,168) = 5.19; P= 0.02) (Figure 2G). Finally, overall locomotor activity was lower in the AM251 group, especially during the first few hours of the night (Figure 2H), which may be related to less feeding and less hunger. Because activity between treatment groups did not differ towards the final hours of the night, we only observed a significant interaction between treatment and hour (F(11,168) = 2.69; P= 0.003).
Experiment 1C: the effect of repeated dosing of AM251 on weight in non-deprived mice
Given that AM251 at a dose of 10 mg·kg−1 was effective in reducing weights when administered acutely, we reasoned that at the same dose it could also exert anorectic properties under a repeated dosing regime. If there were a continuous and sustained weight loss, this should be detectable in PhenoTypers as activity in the food zone should be lower on all days. Relative to Tween 80 treated controls, AM251 administration caused a sustained weight reduction in animals (Figure 3A). The weight reduction was most prominent on day 1 and progressively waned until on day 4 no further weight loss was established. At this point in time, mice had already lost 11.5% of their pre-drug body weight (Figure 3B) with AM251-treated animals displaying a significant suppression of weight gain throughout testing (F(1,84) = 75.96; P < 0.0001). During the same period, controls gained little weight and were reliably different from AM251 mice (F(1,36) = 36.81; P < 0.0001) on days 1 and 2 (see asterisks in Figure 3A). At the same time, AM251 mice ate less each day (Figure 3C; F(1,36) = 43.93; P= 0.0011) and drank less (Figure 3D; F(1,36) = 32.41; P < 0.0001) than controls (see asterisks for individual differences).
Figure 3.
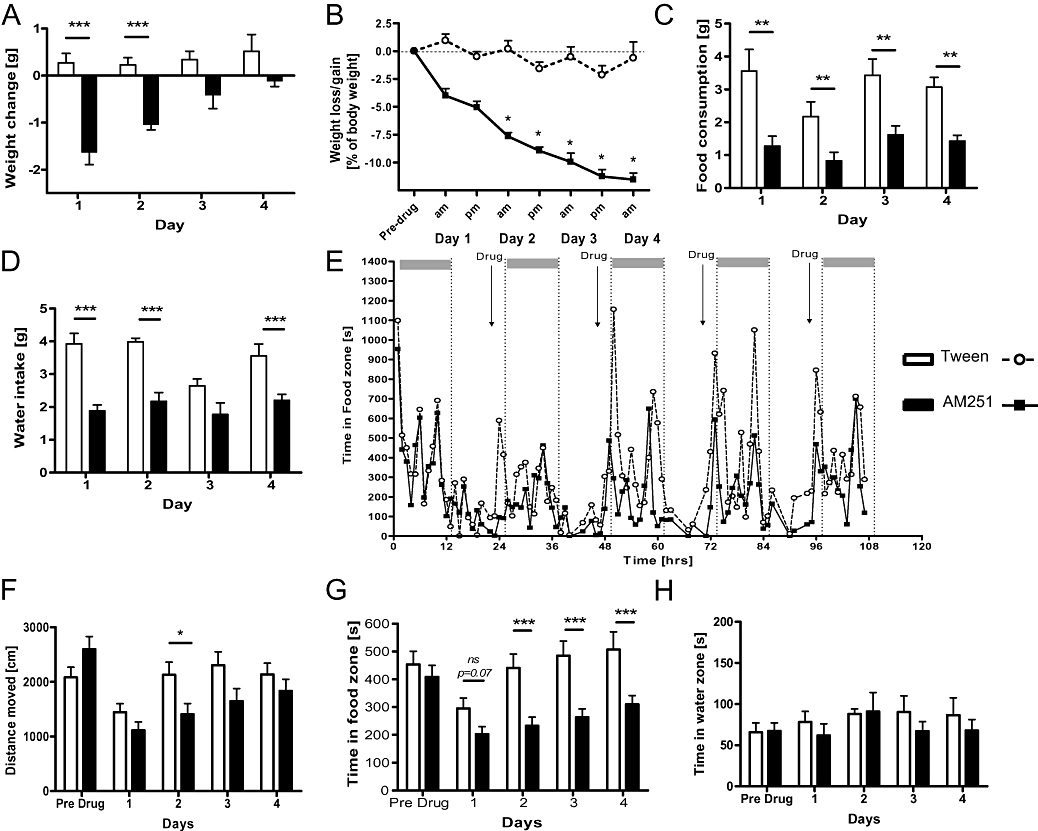
Repeated infusion of AM251 for 4 days suppressed body weight and food intake of non-food deprived animals. (A) AM251-treated mice lost weight compared with controls during the first 2 days and maintained a reduced weight level throughout treatment (B). Note that all animals lost small amounts of weight during the day. Reduced food consumption (C) and water intake (D) was evident on all treatment days in the AM251 group. (E and G) Long-term home-cage observation chart of time spent in the food zone during the treatment regime confirmed a reduction during all nights following drug treatment. Time in water zone (H) and overall activity (F) remained relatively unaffected by AM251. Data shown are means ± s.e.mean (n= 7 per group). Asterisks indicate significance *P < 0.05; **P < 0.01; ***P < 0.001 (t-test).
Locomotor activity between the two groups was monitored for five consecutive days and particular focus was given to the time spent in the food zone (Figure 3E). Overall activity seemed to decline after initial drug infusion (Figure 3F; interaction of drug × day F(4,48) = 2.63; P < 0.05), AM251-injected mice spent less time in the food zone on all nights following drug treatment (Figure 3E, G; F(1,48) = 36.19; P < 0.0001) supporting the notion that less time was spent exploring food and consuming it. In contrast, time spent in the water zone (Figure 3H) was not altered by AM251 (all F < 1). Analysis confirmed a significant correlation between the amount of food consumed and time spent in the food zone (r= 0.37; P= 0.0026, see Figure S1A).This establishes that weight loss and the suppression of feeding induced by AM251 is paralleled by a reduction in the time spent around the feeder.
Experiment 2: effect of Δ9_THCV on weight in non-deprived mice
The cannabis plant constituent, Δ9-THCV, also behaves like a CB1 receptor antagonist (Pertwee et al., 2007) and thus may exert some anorectic properties. Pure Δ9-THCV was administered acutely and we established a time course of efficacy by testing whether animals that eat less under Δ9-THCV exposure continue to do so or whether they rebound on the next day and eat more to compensate for the loss.
Measurement of weight gain/loss both on the day post treatment and 2 days later revealed a significant difference between drug doses (Figure 4A: F(3,28) = 12.16; P < 0.0001 for drug × day interaction). While weight loss for the first day was apparent for all drug doses relative to controls (see asterisks), weight gain was similar for all groups on day 2 when no further drug (F < 1) was administered. The weight reduction was paralleled by reduced food intake (Figure 4B) at all doses of Δ9-THCV (F(3,29) = 9.75; P= 0.0002), and less water intake was observed for 3 mg·kg−1 and 10 mg·kg−1 doses (Figure 4C; F(3,29) = 4.78; P= 0.0089). Overt locomotor activity as determined by PhenoTyper analysis also was reduced after Δ9-THCV administration (Figure 4D), but not on the following day. This effect was independent of Δ9-THCV dose (F(3,286) = 16.24; P < 0.0001) over the dark phase post-drug; post-hoc planned comparison to controls (all F > 4.7; P < 0.05), but the 10 mg·kg−1Δ9-THCV group also differed from the lower (3 mg·kg−1) and higher (30 mg·kg−1) doses (all F > 7.6; P≤ 0.01). This effect of Δ9-THCV was paralleled by a reduction of time spent in the food zone (Figure 4E; F(3,286) = 11.4; P < 0.0001) and water zone (Figure 4F; F(3,286) = 12.10; P= 0.0001). Furthermore, it became clear that Δ9-THCV reduced activity in the food zone only in the dark phase following drug administration (Figure 4G), and animals returned to their normal feeding rhythm on the following day. All doses had similar effects on time spent in the food zone, yet they differed in activity in the water zone. Only 3 mg·kg−1 and 10 mg·kg−1 groups spent reliably less time in the water zone compared with controls and the 30 mg·kg−1 group (Figure 4H). Again, they returned to normal in the following dark phase. Despite this overt reduction in locomotor activity in the food or water zone following Δ9-THCV administration, all mice presented with two activity boosts, one at the beginning and one at the end of the dark phase, in agreement with the typical activity pattern found in C57BL/6 mice (see Figure 1). Furthermore, the behavioural comparison of time spent in the food zone (Figure 4G) correlated well with the amount of food consumed following drug treatment (Figure 4B; r= 0.82, P < 0.0001 see Figure S1B) and during the dark phase of the following day when no drug was administered (r= 0.38; P < 0.05). Similarly, a correlation of water intake and time in water zone was observed following drug treatment (Figure 4C and H; r= 0.5, P= 0.007, see Figure S1D) thus lending further support to the notion that time in food zone and water zone are genuine measures of the amount of food and water consumed and reflect the anorectic properties of Δ9-THCV.
Figure 4.
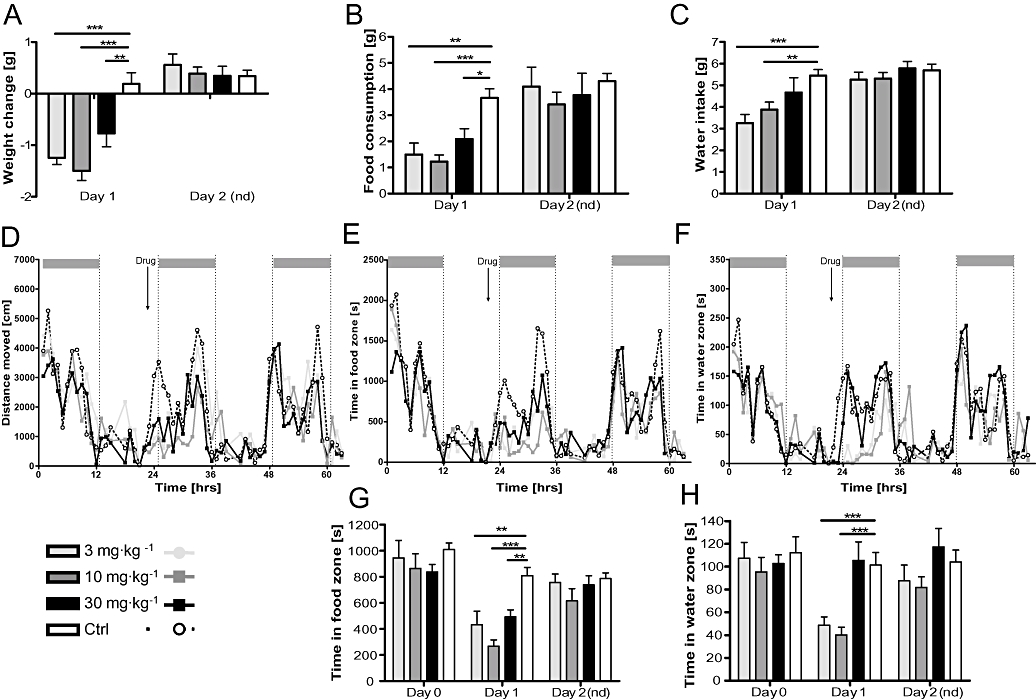
Acute infusion of pure Δ9-THCV suppressed feeding and body weight of mice. (A) During the 12 h following drug treatment, Δ9-THCV (3–30 mg·kg−1) was anorectic relative to vehicle, but animals showed normal weight gain on the recovery day. This was coincident with hypophagia (B) and reduced water intake (C) on the day following drug treatment. Overall activity (D), time spent in the food (E) and water zone (F) were reduced during the night following drug exposure, but fully recovered in the following night. (G and H) Pooled results for time spent in the food and water zone, respectively, during the night (i.e. activity phase of the mice comparing pretreatment baseline, post-treatment and recovery. Δ9THCV reduced visits to the food zone in all anorectic doses. Data shown are means ± s.e.mean (n= 7 per group). Asterisks indicate significance, *P < 0.05; **P < 0.01; ***P < 0.001 (t-test). Δ9-THCV, Δ9-tetrahydrocannabivarin.
Experiment 3: the effect of Δ9-THCV extracts on feeding in non-deprived mice
The cannabis plant extract Sativex which contains a 1:1 combination of Δ9-THC to CBD plus other phytocannabinoids and terpenoids is effective in the treatment of pain and sleep disorders (see Russo et al., 2007 for review), therefore, it was determined that other cannabis extracts may also have therapeutic benefits and a plant extract rich in Δ9-THCV (Δ9-THCV BDS) was tested in this protocol (Δ9-THCV = 57.1%, Δ9-THC = 11.9%). Δ9-THC itself can increase food consumption (see Introduction for details) and we reasoned that the presence of Δ9-THC potentially may counteract the anorectic properties of Δ9-THCV, and vice versa. Administration doses were calculated on the basis of Experiment 2 to give the same final doses of Δ9-THCV (3, 10 and 30 mg·kg−1). As expected, acute administration of Δ9-THCV BDS did not affect weights (Figure 5A; F < 1.1), food consumption (Figure 5B; F < 1.2) or water intake (F < 1.7, P > 0.15, Figure S2A). It is obvious from the long-term locomotor activity measurement that Δ9-THCV BDS did not affect overall activity (Figure 5C), visits to the food zone (Figure 5D) or water zone (Figure S2B) in the dark cycle following drug administration (F < 1 for main effects of drug and interaction).
Figure 5.
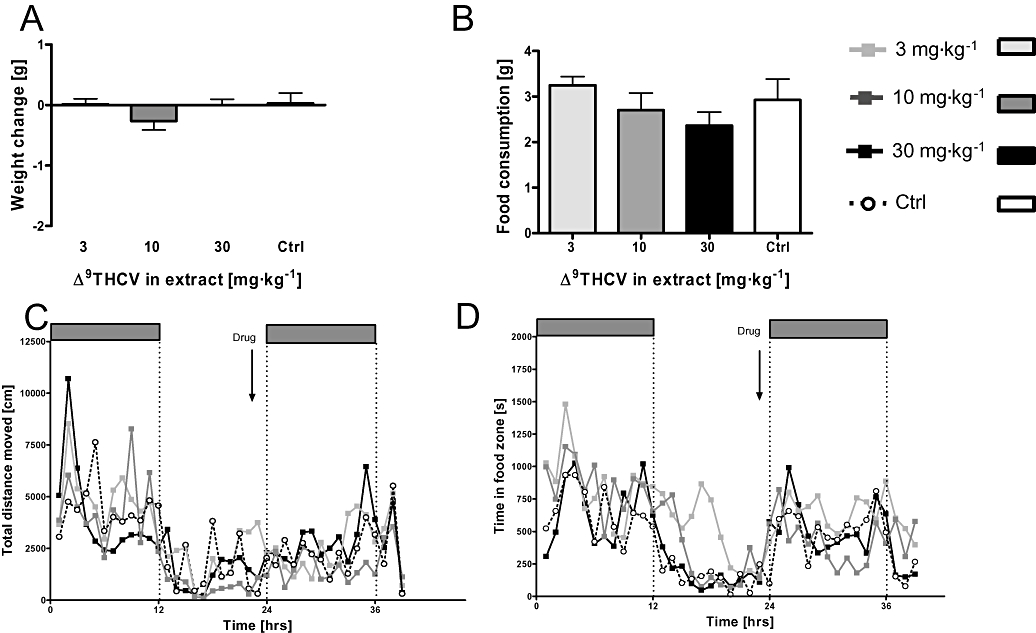
The same doses that were effective in Figure 4 tested as Δ9THCV extract. There was no change in weight gain (A) and food consumption (B) in the 12 h following treatment. Long-term activity measurements also revealed no difference in locomotor activity (C) or time in food zone (D). Data shown are means ± s.e.mean (n= 8 per group). Δ9-THCV, Δ9-tetrahydrocannabivarin.
Such a lack of effect with the Δ9-THCV BDS may be due to Δ9-THC residual within the BDS as it contains a ratio of 1 : 4.8 of Δ9-THC: Δ9-THCV. Lowering the amount of Δ9-THCV would in turn reduce the amount of Δ9-THC administered to the animals and may bring about the anorectic properties of the Δ9-THCV BDS. We therefore re-examined the hypophagic effects using three small doses of Δ9-THCV BDS (0.48, 0.96 and 1.44 mg·kg−1Δ9-THCV and 0.1, 0.2 and 0.3 mg·kg−1Δ9-THC respectively) (Figure 6).
Figure 6.
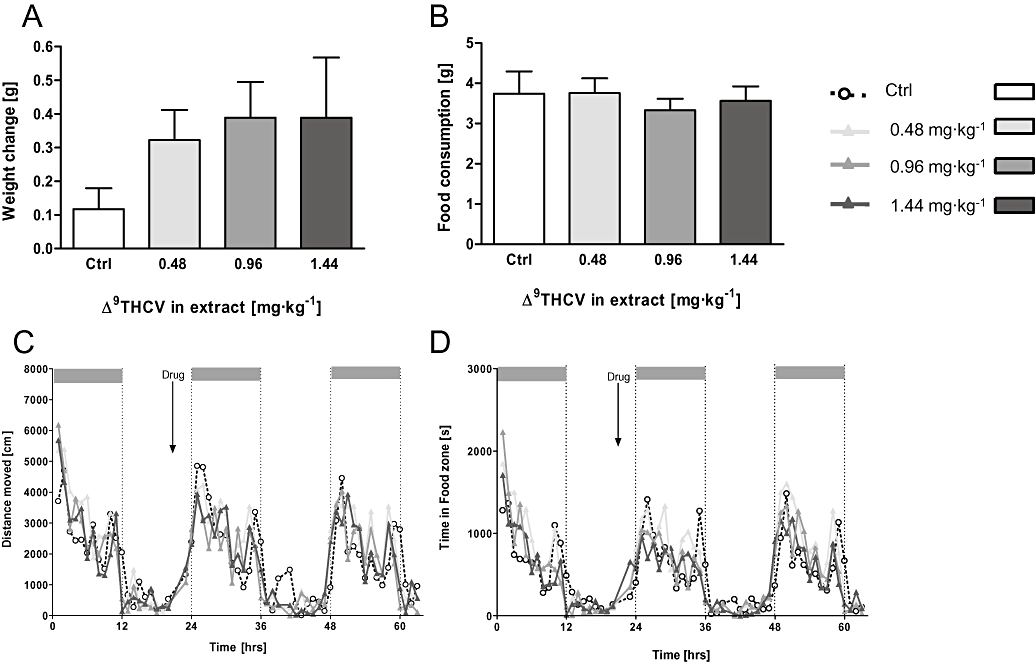
Lower doses of Δ9-THCV extract (0.48–1.44 mg·kg−1) also failed to affect feeding behaviour of non-deprived mice. (A) Body weight of all Δ9-THCV groups increased during the 12 h recording period, but this was not significant. (B) Similarly, there was no difference observed for food intake and the activity chart also did not reveal a drug-related change in locomotor activity (C) or time spent in the food zone (D). Data shown are means ± s.e.mean (n= 9 per group). Δ9-THCV, Δ9-tetrahydrocannabivarin.
As with the higher doses, no differences in mouse weight (F < 1.6; Figure 6A), food consumption (Figure 6B) or water intake (all F < 1; Figure S2C) were observed for the lower doses of Δ9-THCV BDS and this was confirmed in all activity-related measures taken in the PhenoTyper (Figure 6C and D; Figure S2D; all F < 1.2).
Experiment 4: the effect of CBD on the effects of Δ9-THC within Δ9-THCV BDS on food intake
The absence of hypophagic properties of Δ9-THCV BDS in Experiment 3, even at very low doses, would appear to be due to the presence of Δ9-THC in the extract. Nevertheless, Δ9-THCV within the extract seems to antagonize the hyperphagic effects of the Δ9-THC. We therefore postulated that administration of CBD at a dose of 10 mg·kg−1, which is known to antagonize the in vivo effects of Δ9-THC (Fadda et al., 2004), might facilitate the hypophagic properties of Δ9-THCV in the extract through a non-CB1 receptor-mediated mechanism even at low doses. Consequently, Δ9-THCV BDS was administered in conjunction with CBD and food intake recorded.
All groups displayed a small but not reliable increase in body weight (all F < 1; Figure 7A), but combined administration of Δ9-THCV BDS+CBD yielded a strong trend towards a reduction in food intake compared with controls and just failed to attain significance (Figure 7B; df = 14; t= 1.91; P= 0.07); it was, however, reliable for water intake (Figure 7C; t= 2.71; P= 0.01). Treatment with CBD alone did not significantly reduce food consumption (t= 1.64; P= 0.12) or water intake (t= 1.83; P= 0.08). Although overall locomotor activity (Figure 7D) during the night following drug infusion was similar in all drug groups (all F < 1.4), and there was also no difference in time spent in the water zone (Figure 2E; F < 1), the time spent in the food zone yielded a reliable difference (Figure 7E and F); co-administration of Δ9-THCV BDS+CBD reduced time spent in the food zone relative to the pretreatment period (pre v post drug treatment: df = 14; t= 4.69; P= 0.0003), and relative to controls [F(1,154) = 8.21; P= 0.01] thereby explaining the overall trend towards reduced food intake that was observed. Further analysis confirmed a significant correlation between time spent in the food zone and food consumed for all treatments in the dark phase following drug infusion (r= 0.50; P= 0.01, Figure S1C) and also on the following night when no drug was administered (r= 0.64; P= 0.0004), again supporting the notion that time spent in the food zone is directly related to the amount of food consumed. No significant differences were observed for CBD treatment alone and time spent in the food zone (all F < 2.4; P > 0.05). Food consumption and visits to the food zone were back to normal on the following day; there was no rebound.
Figure 7.
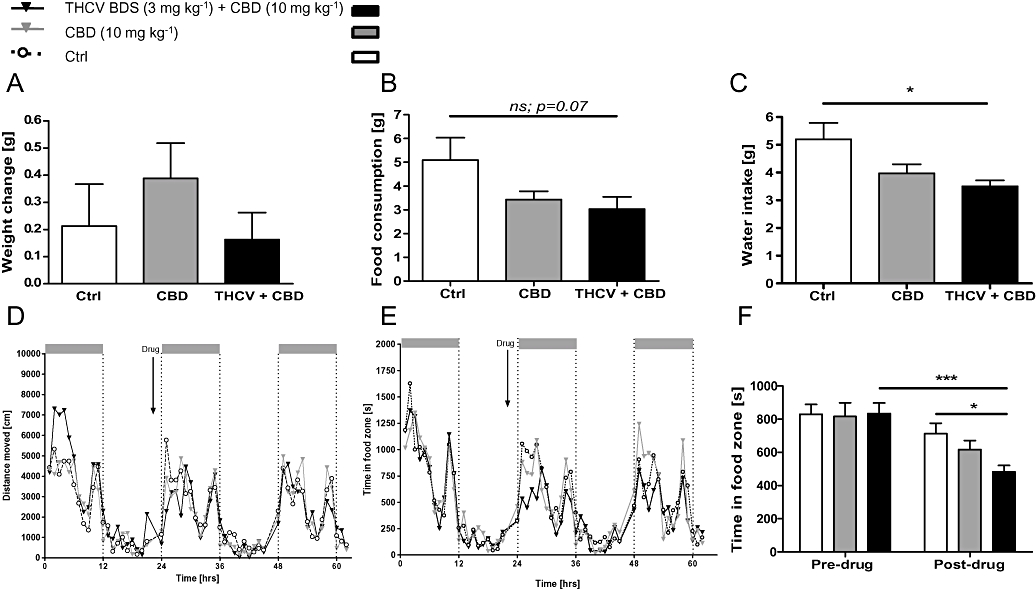
Co-administration of CBD with Δ9THCV extract suppressed feeding behaviour in non-deprived mice. (A) Body weights were unaffected by injection of CBD (10 mg·kg−1) alone or in conjunction with Δ9THCV extract (3 mg·kg−1Δ9THCV) during 12 h post-treatment. (B) Δ9THCV+CBD and CBD alone only presented with a trend towards a reduction in food intake compared with controls, while Δ9THCV+CBD drank less water than controls (C). Analysis of behaviour in home cages revealed that combined drug administration induced no effect on overall locomotor activity (D), but a significant reduction in time spent in the food zone following drug treatment and compared with controls (E and F). This was most obvious during the first 5–7 h post-treatment. Data shown are means ± s.e.mean (n= 8 per group). Asterisks indicate significance, *P < 0.05; ***P < 0.001 (t-test). CBD, cannabidiol; Δ9-THCV, Δ9-tetrahydrocannabivarin.
Discussion
Here we confirmed the hypophagic properties of the CB1 receptor antagonist AM251 both in a standard fasted protocol, but also in normal animals exposed to their standard diet in home cages. This was extended by the observation that visits to the food zone were directly correlated with food intake and weight gain/loss, and that the drug was effective in reducing food intake for several hours of the activity period of the mice without rebound. This observation was extended to the phytocannabinoid Δ9_THCV, which also behaves like a CB1 receptor antagonist, but was not repeated with the biological drug substance rich in Δ9_THCV, possibly due to residual Δ9_THC in the extract. However, co-administration of CBD together with Δ9_THCV BDS revealed a trend towards rescuing the hypophagic activity of the extract.
AM251 and Δ9_THCV are hypophagic: validation in long-term home-cage observation system
Our initial experiments confirm numerous published results (e.g. Wiley et al., 2005) of cannabinoid receptor antagonist-induced anorectic properties. As these were widely observed in fasted animals over a period of only 1 h or so, we reasoned that a more long-term and non-fasted procedure that simultaneously establishes overall activity, circadian rhythmicity and evidence of the time course of drug action would provide a richer understanding of the behavioural changes and possible confounds inherent to CB1 receptor antagonists (for some examples, see McLaughlin et al., 2005; Thornton-Jones et al., 2005). Towards this end, the ‘PhenoTyper’ proved a reliable and sensitive test system with the minor disadvantage that hoarding of food into the nest could not be prevented and some crumbs not consumed remained in front of the food hopper. Hoarding was indeed observed in a small number of cases unrelated to drug treatment and these animals were excluded from the study.
The most prevalent advantage of home-cage observations, however, is the establishment of drug actions in a combined cross-sectional and longitudinal fashion such that animals exposed to different drugs/doses are assessed pretreatment (baseline), post-treatment and at recovery. This would readily reveal any drug-related rebound effect on food intake, but we failed to observe such effects with CB1 receptor antagonists, even when administered repeatedly. Interestingly, we observed a strong correlation between overall food intake, weight gain and visits to the food zone suggesting that these measures interact and either one is sufficient to establish hypophagic drug properties. We prefer the time in the food zone as this provides a longitudinal and handling-free record of the animals food-seeking behaviour. It was used only in normal mice in this study, but could be used in obese animal models including Zucker rats and diet-induced obese (DIO) mice.
AM251 reduced body weight, food intake and exploratory behaviour
The CB1 receptor antagonist AM251 resulted in a similar decrease in body weight and food intake in fasted and non-fasted animals. These results corroborate previous studies which have observed hypophagia and weight reduction in both rats and mice treated with AM251 or SR141716A (Rowland et al., 2001; McLaughlin et al., 2003; McLaughlin et al.2005; Shearman et al., 2003; Chambers et al., 2004; Verty et al., 2004; Zhou and Shearman, 2004; Wiley et al., 2005; Chen et al., 2006; Gardner and Mallet, 2006) and lend further support to the home-cage observation system as an effective and convenient experimental method to study the anorectic properties of various drugs. When repeated dosing over long periods is envisaged, which most closely reflects the therapeutic treatment regime, home-cage observation combines continuous monitoring of exploratory behaviour with food and water intake whereas fasted models cannot be used. AM251 given on four consecutive days as single injections reduced both body weight and food intake and maintained the drug group on an overall lower weight with no effect on exploratory behaviour. This is in agreement with previous studies, which have observed no effects of either AM251 (Shearman et al., 2003) or SR141716A on locomotor activity (Verty et al., 2004; Wiley et al., 2005; Gardner and Mallet, 2006). The absence of a progressive weight loss must be acknowledged in light of an 8–10% reduction in weight relative to pre-drug body weights. As a consequence, it may be difficult to induce further suppression of body weight when animals are maintained on a free-feeding regime. This sensitization agrees with previous studies demonstrating that the anorectic efficacy of AM251 (Hildebrandt et al., 2003; Chen et al., 2006) and SR141716A (Colombo et al., 1998; Ravinet-Trillou et al., 2003; Vickers et al., 2003) waned following repeated administration, with no further decrease in body weight observed after 4–5 days of treatment although the suppression in weight gain could be sustained for up to 28 days (Vickers et al., 2003).
Different to our approach, previous studies have assessed the effects of CB1 receptor antagonists including SR141716A and AM251 in obese Zucker rats or DIO mice (Hildebrandt et al., 2003; Ravinet-Trillou et al., 2003; Vickers et al., 2003; Chambers et al., 2004; Chen et al., 2004). Both these studies and those in normal animals (Colombo et al., 1998; Vickers et al., 2003; Wiley et al., 2005; Chen et al., 2006) reported reductions in food intake and body weight at similar amounts as observed in our experiments. Although we have not assessed cellular mechanisms of action of AM251, the similarities with published work on AM251 and SR141716A suggest anorectic effects may indeed be mediated by CB1 receptors within the hypothalamus and other brain areas associated with the regulation of appetite (Jamshidi and Taylor, 2001; Kirkham et al., 2002; Verty et al., 2005; Soria-Gomez et al., 2007; Wittman et al., 2007).
Δ9-THCV as a possible treatment for obesity
Δ9-tetrahydrocannabivarin purified from cannabis extracts or synthetic Δ8 or Δ9−THCV appears to act as a competitive antagonist on CB1 receptors (Thomas et al., 2005; Pertwee et al., 2007). In line with this action is our finding that Δ9-THCV exerts hypophagic properties and significantly reduces body weight at a low dose of 3 mg·kg−1. At similar doses, synthetic Δ9-THCV (O-4394) attenuated Δ9-THC-induced hypothermia and antinociception confirming the potency as a CB1 receptor antagonist (Pertwee et al., 2007). It is therefore surprising that the Δ9-THCV BDS containing identical amounts of Δ9-THCV was without anorectic properties. We attribute this fact to the presence of Δ9-THC and other chemicals still residual within the extract. Opposite to CB1 antagonists, Δ9_THC is a known hyperphagic agent in both humans and rodents (Williams et al., 1998; Koch, 2001; Hart et al., 2002; Williams and Kirkham, 2002b; Wiley et al., 2005) at doses above 0.5 mg·kg−1 (Williams et al., 1998; Higgs et al., 2003) and this might explain the lack of effect at high doses of Δ9-THCV BDS containing up to 6 mg kg−1Δ9-THC. When the dose of Δ9-THCV was lowered, the amount of Δ9-THCV may simply have been too low to be effective (<1.5 mg·kg−1Δ9-THCV) and a more long-term treatment regime may be necessary to result in an accumulation of Δ9-THCV to effective suprathreshold levels as has been suggested before with other CB1 antagonists (Colombo et al., 1998; Hildebrandt et al., 2003; Ravinet-Trillou et al., 2003; Vickers et al., 2003; Chen et al., 2006). Alternatively, we cannot exclude possible hyperphagic effects of other constituents remaining as part of the Δ9-THCV extract, which may act alone or in conjunction with Δ9-THC. Overall, it thus appears that the current ratio of 5:1 for Δ9-THCV: Δ9-THC in the extract is not in favour of Δ9-THCV to reveal its anorectic potential; experiments need to be conducted in which the ratio is titrated until the Δ9-THCV effect becomes detectable.
A similar result was obtained by us with a CBD-rich BDS. Despite a more favourable ratio of 8:1 (CBD:Δ9-THC) (Fadda et al., 2004) our cognitive set-up did not reveal a memory enhancing effect of the CBD extract.
We therefore reasoned that co-administration of pure CBD, which does not act via CB1 receptors, may support the Δ9-THCV effect in the extract and help to overcome the Δ9-THC-induced hyperphagia. CBD could act as a cytochrome P450 (CYP) isozyme inhibitor and prevent the metabolism of Δ9-THC to its more potent 11 hydroxy metabolite (see Pertwee, 2004). Previous studies have found that CBD is able to antagonize Δ9-THC-induced effects on catalepsy, anxiety and antinociception (see Pertwee, 2004 for review) and that higher doses of CBD alone (30–50 mg kg−1) than tested here can be anorectic (Sofia and Knobloch, 1976; Silveira Filho and Tufik, 1981), but the exact conditions for this effect remain unclear (see Wiley et al., 2005 for negative results). CBD (10 mg kg−1) alone induced a small although non-significant reduction in food intake and weight gain. By contrast, co-administration with Δ9-THCV BDS induced a significant suppression of time spent in the food zone of the home cage during the night following treatment. Normalization of food intake occurred on the following day confirming the time course of the effect and suggesting a possible synergistic effect of CBD on Δ9-THCV in the extract, which warrants further mechanistic and more detailed pharmacological and dose-profiling studies. Such a synergism is novel for cannabinoid drugs with differing pharmacological properties and extends previous studies with similar effects between opioid and CB1 receptor antagonists (Kirkham and Williams 2001; Chen et al., 2004).
In summary, our results demonstrate hypophagic effects of AM251 in a standard fasted protocol or a free-feeding home-cage observation system. The phytocannabinoid Δ9-THCV which also behaves like a CB1 antagonist induced hypophagia and a reduction in body weight at low doses. The absence of any suppression in food intake and weight gain with the Δ9-THCV rich extract could be a result of residual Δ9-THC within the extract.
Acknowledgments
This work was supported in part by grants from GW Pharmaceuticals to GR, PF and RGP and NIDA to GR.
Glossary
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- BDS
botanical drug substance
- CBD
cannabidiol
- DIO
diet-induced obese
- Δ9-THC
Δ9-tetrahydrocannabinol
- Δ9-THCV
Δ9-tetrahydrocannabivarin
Conflict of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Supporting figure for Experiments 1C, 2 and 4. Correlational analysis of food and water intake and time spent in the respective zones. Reliable correlations between food consumption and time spent in food zone were observed for all three experiments [Experiment 1C, repeated infusions of AM251 (A); Experiment 2, Δ9THCV pure (B) and Experiment 4, Δ9THCV extract+CBD (C)]. Treatment with Δ9THCV pure also resulted in a significant correlation between water intake and time in water zone (D).
Figure S2 Supporting figure for Experiments 3 and 4. The effect of D9THCV extract alone and co-administration with CBD on water intake and time spent in the water zone. (A and C) No effect on water intake was observed with Δ9THCV extract at all doses (0.48–30 mg kg-1). (B and D) Similarly, there were no drug-related changes for time spent in the water zone. (E) Co-administration of Δ9THCV extract with CBD or CBD alone also induced no effect on time in the water zone. Data shown are means ± s.e.mean.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist, AM251, causes a sustained reduction of daily food intake in the rat. Physiol Behav. 2004;82:863–869. doi: 10.1016/j.physbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Huang RRC, Shen CP, MacNeil DJ, Fong TM. Synergistic effects of cannabinoid inverse agonist AM251 and opioid antagonist nalmefene on food intake in mice. Brain Res. 2004;999:227–230. doi: 10.1016/j.brainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Frassetto A, Fong TM. Effects of the CB1 cannabinoid receptor inverse agonist AM251 on food intake and body weight in mice lacking μ-opioid receptors. Brain Res. 2006;1108:176–178. doi: 10.1016/j.brainres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- De Visser L, van den Bos R, Kuurman WW, Kas MJH, Spruijt BM. Novel approach to the behavioural characterization of inbred mice: automated home cage observations. Genes, Brain and Behav. 2005. pp. 1–9. [DOI] [PubMed]
- De Visser L, van den Bos R, Stoker AK, Kas MJH, Spruijt BM. Effects of genetic background and environmental novelty on wheel running as a rewarding behaviour in mice. Behav Brain Res. 2007;177:290–297. doi: 10.1016/j.bbr.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Fadda P, Robinson L, Fratta W, Pertwee RG, Riedel G. Differential effects of THC or CBD-rich cannabis extracts on working memory in rats. Neuropharmacol. 2004;47:1170–1179. doi: 10.1016/j.neuropharm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Allison DB. Cannabinoid-1 receptor antagonist, rimonabant, for management of obesity and related risks. Circulation. 2006;114:974–984. doi: 10.1161/CIRCULATIONAHA.105.596130. [DOI] [PubMed] [Google Scholar]
- Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology. 1999;142:302–308. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Gill EW, Paton WDM, Pertwee RG. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature. 1970;228:134–136. doi: 10.1038/228134a0. [DOI] [PubMed] [Google Scholar]
- Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV + marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology. 2005;181:170–178. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Δ9-tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Higgs S, Williams CM, Kirkham TC. Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after Δ9-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology. 2003;165:370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Kelly-Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur J Pharmacol. 2003;462:125–132. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett MM, Limebeer CL, Parker LA. Effect of Delta9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav. 2005;86:475–479. doi: 10.1016/j.physbeh.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Scantlebury J, Parker LA. Effect of Delta(9)-tetrahydrocannabinol on quinine palatability and AM251 on sucrose and quinine palatability using the taste reactivity test. Physiol Behav. 2007;90:425–430. doi: 10.1016/j.physbeh.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM. Synergistic effects of opioid and cannabinoid antagonists on food intake. Psychopharmacology. 2001;153:267–270. doi: 10.1007/s002130000596. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JE. Δ9-THC stimulates food intake in Lewis rats effects on chow, high-fat and sweet high-fat diets. Pharmacol Biochem Behav. 2001;68:539–543. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR141716A and AM251 suppress food intake and food-reinforced behaviour in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology. 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- Merkus FWHM. Cannabivarin and tetrahydrocannabivarin, two new constituents of hashish. Nature. 1971;232:579–580. doi: 10.1038/232579a0. [DOI] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sciences. 2004;76 doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, et al. The psychoactive plant cannabinoid, Δ9-tetrahydrocannabinol, is antagonized by Δ8- and Δ9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinet-Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology. 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- Russo EB, Guy GW, Robson PJ. Cannabis, Pain and Sleep: Lessons from therapeutic clinical trials of Sativex®, a cannabis-based medicine. Chemistry and Biodiversity. 2007;4:1729–1743. doi: 10.1002/cbdv.200790150. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, et al. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrie P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Sofia RD, Knobloch LC. Comparative effects of various naturally occurring cannabinoids on food, sucrose and water consumption by rats. Pharmacol Biochem Behav. 1976;4:591–599. doi: 10.1016/0091-3057(76)90202-1. [DOI] [PubMed] [Google Scholar]
- Silveira Filho NG, Tufik S. Comparative effects between cannabidiol and diazepam on neophobia, food intake and conflict behaviour. Res Comm Psychol Psychiat Behav. 1981;6(3):251–266. [Google Scholar]
- Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, et al. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebot MH, Chaperon F, Fride E, Onaivi EM. Behavioural effects of endocannabinoids. In: Onaivi ES, Suiura T, Di Marzo V, editors. Endocannabinoids. Boca Raton: Taylor & Francis; 2006. pp. 303–347. [Google Scholar]
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, et al. Evidence that the plant cannabinoid Delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Jones ZG, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology. 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, et al. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. The DATRI 004 Study Group. Division of AIDS Treatment Research Initiative. AIDS Res. Hum. Retroviruses. 1997;13:305–315. doi: 10.1089/aid.1997.13.305. [DOI] [PubMed] [Google Scholar]
- Verty ANA, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett. 2004;354:217–220. doi: 10.1016/j.neulet.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Verty AN, McGregor IS, Mallet PE. Paraventricular hypothalamic CB(1) cannabinoid receptors are involved in the feeding stimulatory effects of Delta(9)-tetrahydrocannabinol. Neuropharmacol. 2005;49:1101–1109. doi: 10.1016/j.neuropharm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology. 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Walsh D, Kirkova J, Davis MP. The efficacy and tolerability of long-term use of dronabinol in cancer-related anorexia: a case series. J Pain Symptom Manage. 2005;30:493–495. doi: 10.1016/j.jpainsymman.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, et al. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral Δ9-THC. Physiol Behav. 1998;65(2):343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Reversal of Δ9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav. 2002a;71:341–348. doi: 10.1016/s0091-3057(01)00694-3. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol Behav. 2002b;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Wittman G, Deli L, Kallo I, Hrabovszky E, Watanabe M, Liposits Z, et al. Distribution of type 1 cannabinoid receptor (CB1)-immunoreactive axons in the mouse hypothalamus. J Comp Neurol. 2007;503:270–279. doi: 10.1002/cne.21383. [DOI] [PubMed] [Google Scholar]
- Zhou D, Shearman LP. Voluntary exercise augments acute effects of CB1-receptor inverse agonist on body weight loss in obese and lean mice. Pharmacol Biochem Behav. 2004;77:117–125. doi: 10.1016/j.pbb.2003.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


