Abstract
Background and purpose:
The aims of the present work were to study the mechanism of the reverse rate dependency of different interventions prolonging cardiac action potential duration (APD).
Experimental approach:
The reverse rate-dependent lengthening effect of APD-prolonging interventions and the possible involvement of IKr (rapid component of the delayed rectifier potassium current) and IK1 (inward rectifier potassium current) were studied by using the standard microelectrode and the whole-cell patch-clamp techniques in dog multicellular ventricular preparations and in myocytes isolated from undiseased human and dog hearts.
Key results:
All applied drugs – dofetilide (1 µmol·L−1), BaCl2 (10 µmol·L−1), BAY-K-8644 (1 µmol·L−1), veratrine (1 µg·mL−1) – lengthened APD in a reverse rate-dependent manner regardless of their mode of action, suggesting that reverse rate dependency may not represent a specific mechanism of APD prolongation. The E-4031-sensitive current (IKr) and the Ba2+-sensitive current (IK1) were recorded during repolarizing voltage ramps having various steepness and also during action potential waveforms with progressively prolonged APD. Gradually delaying repolarization results in smaller magnitude of IKr and IK1 currents at an isochronal phase of the pulses. This represents a positive feedback mechanism, which appears to contribute to the reverse rate-dependent prolongation of action potentials.
Conclusions and implications:
Action potential configuration may influence the reverse rate-dependent APD prolongation due to the intrinsic properties of IKr and IK1 currents. Drugs lengthening repolarization by decreasing repolarizing outward, or increasing depolarizing inward, currents are expected to cause reverse rate-dependent APD lengthening with high probability, regardless of which current they modify.
Keywords: antiarrhythmic drugs, cardiac electrophysiology, IKr, reverse rate dependency, IK1, action potential duration
Introduction
In cardiac muscle, the course of repolarization is set by the interplay between different voltage- and time-dependent currents. The contribution of each current strictly depends on the relation between its kinetic properties and the voltage profile during the electrical cycle, which varies with heart rate. Accordingly, repolarization changes caused by blockade (or enhancement) of individual currents are rate-dependent. In spite of the widely different properties of currents, repolarization response to their modulation is consistently larger at slow heart rates (long cycle lengths), a phenomenon named ‘reverse rate dependency’ (RRD) (Hondeghem and Snyders, 1990; Jurkiewicz and Sanguinetti, 1993). Blockade of outward potassium currents or increase of inward currents is associated with RRD and instability of repolarization (induction of early after-depolarizations) at slow rates (Hohnloser and Woosley, 1994; Varro et al., 2004). In most cases RRD is not explained by the features of drug–channel interaction (Carmeliet, 1992); it might be viewed as a general property, the mechanism of which has not been fully elucidated. RRD is undesirable because it minimizes drug effects on repolarization during tachyarrhythmias and enhances their proarrhythmic (torsadogenic) potential at normal or slow rates. The rapid component of the delayed rectifier potassium current (IKr) is considered to have an important role in repolarization. Results of previous work in guinea-pig myocytes indicate that, due to its peculiar gating properties (rectification), slower repolarization causes IKr to decrease, which in turn, slows repolarization (Rocchetti et al., 2001). Albeit, through a different molecular mechanism, inward rectification is also a property of the inward rectifier potassium current (IK1), which might thus behave similarly to IKr. Due to their positive feedback interaction with repolarization rate IKr (and IK1) may represent autoregenerative mechanisms in repolarization. This view might provide clues to the mechanism of RRD and help to interpret the detrimental effect of IKr blockade on repolarization stability. Nonetheless, significant differences exist between guinea-pigs and larger mammals in gating kinetics and rectification properties of IKr and of the slow component of the delayed rectifier potassium current (IKs). Thus, whether the interpretations suggested from previous studies can be generalized remains unknown. Moreover, while most studies were performed in isolated myocytes, ion changes in the restricted extracellular space may contribute to RRD occurring in the intact tissue.
The aims of the present study were: (i) to study RRD of different action potential duration (APD)-prolonging interventions in multicellular preparations of cardiac muscle from dogs; and (ii) to test whether the peculiar dependency of IKr on repolarization rate holds true for dog and human cardiomyocytes and whether such a feature is shared by IK1.
Methods
Human
Cells were prepared from undiseased donor hearts. The experimental protocol complied with the Declaration of World Medical Association proclaimed in Helsinki (Cardiovascular Research 1997; 35:2–4) and was approved by the Ethical Review Board of the Albert Szent-Györgyi Medical University (No. 51-57/1997 OEj). Proper consent was obtained for use of each individual's tissue for experimentation. The hearts were obtained from general organ donors; their valves were utilized for pulmonary and aortic valve transplantation surgery. Before explantation of the hearts, the patients did not receive any medication except for dobutamine, furosemide and plasma expanders.
Animals
All experiments were carried out in compliance with the Guide for the Care and Use of Laboratory Animals (USA NIH publication NO 85-23, revised 1985). The protocols were approved by the Review Board of the Committee on Animal Research of the Albert Szent-Györgyi Medical University (54/1999 Oej).
Conventional microelectrode technique
Adult mongrel dogs (8–14 kg) of either sex were used. Following anaesthesia (sodium pentobarbital, 30 mg·kg−1 administered intravenously), the hearts were rapidly removed and immediately rinsed in modified Locke's solution containing (in mmol·L−1): NaCl 120, KCl 4, CaCl2 1.0, MgCl2 1, NaHCO3 22 and glucose 11. The pH of this solution was 7.35–7.40 when saturated with the mixture of 95% O2 and 5% CO2 at 37°C. Tips of the papillary muscles obtained from the right ventricle were individually mounted in a tissue chamber having a volume of 50 mL. Each preparation was initially stimulated (HSE stimulator type 215/II, Hugo Sachs Elektronik, March-Hugstetten, Germany) at a basic cycle length of 1000 ms, by using rectangular constant current pulses of 2 ms in duration. At least 60 min was allowed for each preparation to equilibrate before the measurements were initiated. Temperature of the superfusate was kept constant at 37°C. Transmembrane potentials were recorded by using conventional microelectrode technique. Microelectrodes filled with 3 mol·L−1 KCl and having tip resistances of 5–20 MΩ were connected to the input of a high-impedance electrometer (Experimetria Ltd., Budapest, Hungary). Outputs from the amplifier were displayed on a dual-beam oscilloscope (Tektronix 2230 100 MHz digital storage oscilloscope, Beaverton, OR, USA).
The maximum diastolic potential, action potential amplitude and action potential duration at 50 and 90% of depolarization (APD50 and APD90) were automatically measured by using a software developed in our laboratory (HSE-APES) running on a 386-microprocessor based, IBM compatible computer containing an ADA 3300 analogue-to-digital data acquisition board (Real Time Devices Inc., State College, PA, USA) with a maximum sampling frequency of 40 kHz. In each experiment, baseline action potential characteristics were first determined during continuous pacing at 1 Hz, and then while pacing cycle length was sequentially varied between 300 and 5000 ms. Twenty-five action potentials were evoked at each cycle length, and the cycle length was then changed so that ‘quasi’ steady-state frequency response relations could rapidly be generated. After control measurements the preparations were superfused for 40 min with saline containing the compound under study, and then the electrophysiological measurements were resumed. Efforts were made to maintain the same impalement throughout each experiment. If, however, an impalement became dislodged, adjustment was attempted, and if the action potential characteristics (action potential amplitude and APD90) of the re-established impalement deviated by less than 5% from the previous measurement, the experiment continued.
Whole-cell configuration of the patch-clamp technique
Left ventricular myocytes were enzymatically dissociated from hearts of mongrel dogs following anaesthesia (sodium pentobarbital, 30 mg·kg−1, intravenously) as described previously in detail (Varro et al., 2000). Human left ventricular myocytes were isolated by an enzymatic dissociation procedure as performed previously (Iost et al., 1998).
One drop of cell suspension was placed within a transparent recording chamber mounted on the stage of an inverted microscope (TMS, Nikon, Tokyo, Japan), and the myocytes were allowed to settle and adhere to the bottom for at least 5 min before superfusion was initiated. Only rod-shaped cells with clear cross striations were used. HEPES-buffered Tyrode's solution served as the normal superfusate. This solution contained (in mmol·L−1): NaCl 144, NaH2PO4 0.33, KCl 4.0, CaCl2 1.8, MgCl2 0.53, glucose 5.5 and HEPES 5.0 at pH of 7.4.
Patch-clamp micropipettes were fabricated from borosilicate glass capillaries (Clark, Reading, UK) by using a P-97 Flaming/Brown micropipette puller (Sutter Co, Novato, CA, USA). These electrodes had resistances between 1.5 and 2.5 MΩ when filled with pipette solution containing (in mmol·L−1): K-aspartate 100, KCl 25, ATP 5, MgCl2 1, EGTA 10 and HEPES 5. The pH of this solution was adjusted to 7.2 by KOH. When measuring IK1 and IKr currents, 1 µmol·L−1 nisoldipine (gift from Bayer AG, Leverkusen, Germany) was added to the external solution to eliminate L-type Ca2+ current (ICa). The IKr was separated from IKs by using the selective IKs blocker L-735 821 (100 nmol·L−1, a gift from Merck-Sharpe & Dohme, West-Point, PA, USA). Membrane currents were recorded with Axopatch-1D and 200B patch-clamp amplifiers (Axon Instruments, Union City, CA, USA) using the whole-cell configuration of the patch-clamp technique. After establishing a high (1–10 GΩ)-resistance seal by gentle suction, the cell membrane beneath the tip of the electrode was disrupted by suction or by application of short electrical pulses. The series resistance was typically 4–8 MΩ before compensation (50–80%, depending on the voltage protocol). The holding potential was −80 mV. Experiments where the series resistance was high, or substantially increased during measurement, were discarded. Membrane currents were digitized by using a 333 kHz analog-to-digital converter (Digidata 1200, Axon Instruments) under software control (pClamp 8, Axon Instruments). Analyses were performed by using pClamp 8 software after low-pass filtering at 1 kHz. All data were collected at 37°C.
Results
Rate dependency of APD prolongation by compounds with different mode of action
The following interventions were tested in dog papillary muscle preparations: 1 µmol·L−1 dofetilide (completely blocks IKr at this concentration), 10 µmol·L−1 BaCl2 (IK1 blocker), 1 µmol·L−1 Bay K-8644 (ICa enhancer), 1 µg·mL−1 veratrine (Na+ current activator). Some 10 µmol·L−1 BaCl2 was used for the blockade of IK1 because we found earlier that at this concentration BaCl2 selectively blocks about 60% of IK1 at −60 mV. Some 1 µmol·L−1 Bay K-8644 significantly enhanced ICa (from 369.4 ± 50.2 pA to 1473.8 ± 233.6 pA at 0 mV, n = 4). The amplitude of slowly inactivating TTX-sensitive Na+ current was found to be 176.1 ± 39.3 pA (n = 4) in the absence and 984.2 ± 291.0 pA (n = 4) in the presence of 1 µg·mL−1 veratrine at −20 mV.
Rate-dependent modulation of APD by the test interventions is illustrated in Figure 1. APD prolongation was maximal at long cycle lengths with all agents, suggesting that RRD may not be characteristic of a specific mechanism of APD prolongation. At short cycle lengths, the extent of APD prolongation was similar for dofetilide and veratrine, both larger than observed with BaCl2 and Bay K-8644. However, the largest RRD was caused by enhancement of sustained inward currents by veratrine or Bay K-8644. The extent of RRD obtained with IKr and IK1 blockade was substantially similar in size. These results indicate that both IKr and IK1 may contribute substantially to RRD, as in absence of IKr or IK1, the RRD was relatively small.
Figure 1.
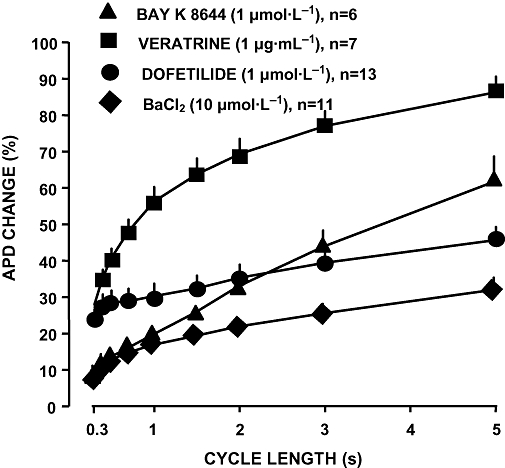
Reverse rate-dependent APD prolongation by IKr block (1 µmol·L−1 dofetilide), IK1 block (10 µmol·L−1 BaCl2), ICa activation (1 µmol·L−1 BAY-K 8644) and by INa activation (1 µg·mL−1 veratrine) in dog right ventricular papillary muscle. Note that the most prominent reverse rate-dependent APD lengthening was elicited by enhancement of ICa and INa. Data are expressed as mean ± SEM. APD, action potential duration; ICa, L-type Ca2+ current; IK1, inward rectifier potassium current; IKr, rapid component of the delayed rectifier potassium current; n, number of measurements.
Dependency of IKr and IK1 on repolarization rate
These experiments were carried out by the patch-clamp technique in single dog ventricular myocytes. From a holding potential of −80 mV, currents were activated/rectified by a depolarizing step to 20 mV (150 ms), followed by a repolarizing ramp of variable rate (Figures 2 and 3). IKr and IK1 were defined as difference currents, resulting from subtraction of the traces recorded in the presence of 1 µmol·L−1 E-4031 and 10 µmol·L−1 BaCl2 respectively from control ones. Peak IKr amplitudes evoked with different repolarization rates did not differ consistently, but IKr increase was faster with steeper ramps. Thus, current amplitude measured at the same time during the ramp (isochronal amplitude) was smaller as repolarization rate was decreased (Figure 2). Similar results were obtained when IK1 was measured (Figure 3).
Figure 2.
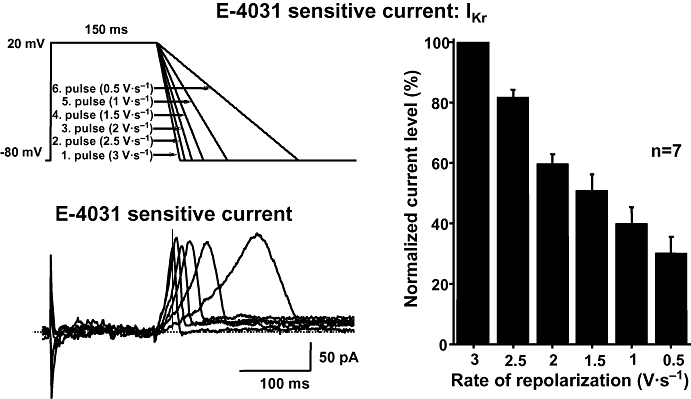
Profile of the E-4031-sensitive current (IKr) recorded in a dog ventricular myocyte. The current was elicited by applying voltage ramps having different rates of repolarization (top left) and was dissected as an E-4031-sensitive difference current (bottom left). The vertical line represents the isochronal phase at the time when the current levels were measured. The magnitude of IKr during voltage ramps with gradually slower repolarization at an isochronal point of the pulses (168 ms) is shown in the right. The magnitude of the current is normalized to the current level measured during the steepest ramp. Data are expressed as mean ± SEM. IKr, rapid component of the delayed rectifier potassium current; n, number of measurements.
Figure 3.
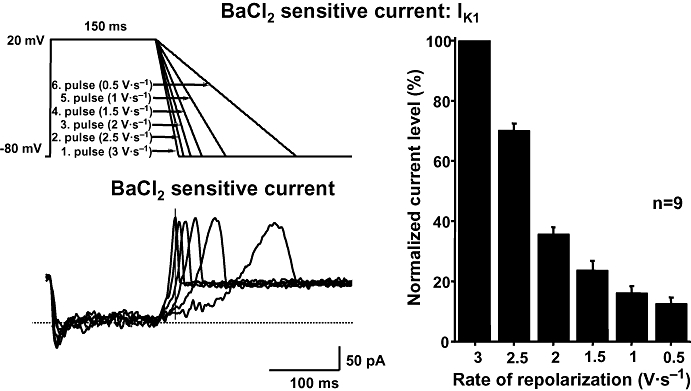
The Ba2+-sensitive current (IK1) obtained at different rates of repolarization in a dog ventricular myocyte. Transmembrane currents were elicited by applying voltage ramps with different repolarization rate (top left). IK1 was assessed as a Ba2+-sensitive difference current (bottom left). The vertical line represents the isochronal phase at the time when the current levels were measured. The magnitude of IK1 during voltage ramps with gradually slower repolarization at an isochronal point of the pulses (174 ms) is shown in the right. The magnitude of the current is normalized to the current level measured during the steepest ramp. Data are expressed as mean ± SEM. IK1, inward rectifier potassium current; n, number of measurements.
Figures 2 and 3 (right) summarizes the results of isochronal IKr and IK1 measurements (at 168 and 174 ms after ramp start respectively), as a function of repolarization rate at values encompassing the physiological range. IKr dependency on repolarization rate was almost linear over the range tested; IK1 dependency was steeper at high repolarization rates, to become shallower at lower ones. These results confirm that in dog myocytes IKr expression at a given time during repolarization is proportional to repolarization rate. Although with different shape, the same proportionality was observed also with IK1.
Dependence of IKr and IK1 on action potential morphology
Because the currents mediated by IKr and IK1 are known to contribute significantly to ventricular repolarization, their dependency on the rate of repolarization was studied. In these experiments, action potential waveforms were used as the command signal to drive membrane potential of dog ventricular myocytes under voltage-clamp conditions. The action potential waveforms were previously recorded from dog papillary muscle by conventional microelectrodes at stimulation cycle lengths of 400, 700, 1000, 2000, 3000 and 5000 ms, thus representing a wide range of repolarization rates and APDs. IKr and IK1 evoked by the action potential waveforms were defined as difference currents, resulting from subtraction of the traces recorded in the presence of 1 µmol·L−1 E-4031 or 10 µmol·L−1 BaCl2, respectively, from the control traces. Peak IKr amplitudes evoked with different action potential waveforms did not differ consistently, but IKr increase was faster with shorter action potentials. Thus, current amplitude measured at the same time during the repolarization phase (isochronal amplitude) was smaller as repolarization rate was decreased (Figure 4). Similar results were obtained when IK1 was analysed (Figure 5). Thus, isochronal IKr and IK1 magnitudes (measured at 160 and 171 ms respectively) decreased as the action potential was prolonged (Figures 4 and 5).
Figure 4.
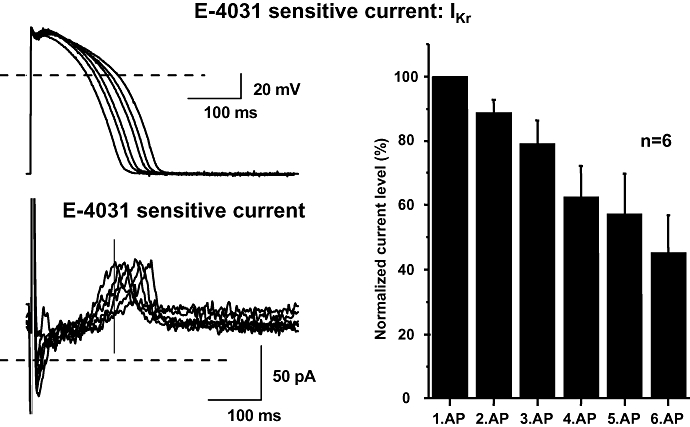
E-4031-sensitive (IKr) current recorded during action potentials with gradually longer repolarization phase in dog ventricular myocytes. Transmembrane currents were elicited by applying action potentials as command pulses (top left) recorded in previous experiments using conventional microelectrodes at pacing cycle lengths of 400, 700, 1000, 2000, 3000 and 5000 ms. The holding potential was −80 mV. The E-4031-sensitive current was recorded as difference current (bottom left). The vertical line represents the isochronal phase at the time when the current levels were measured. The magnitude of IKr during action potentials with gradually longer repolarization at an isochronal point of the pulses (160 ms) is shown in the right. The magnitude of the current is normalized to the current level measured during the shortest action potential. Data are expressed as mean ± SEM. IKr, rapid component of the delayed rectifier potassium current; n, number of measurements.
Figure 5.
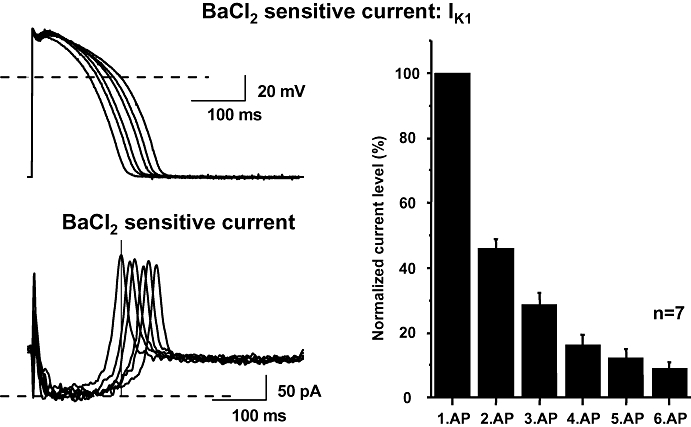
Ba2+-sensitive (IK1) current recorded during action potentials with gradually longer repolarization phase in dog ventricular myocytes. Transmembrane currents were elicited by applying action potentials as command pulses (top left) recorded in previous experiments using conventional microelectrodes at pacing cycle lengths of 400, 700, 1000, 2000, 3000 and 5000 ms. The Ba2+-sensitive current was recorded as difference current (bottom left). The vertical line represents the isochronal phase at the time when the current levels were measured. The magnitude of IK1 during action potentials with gradually longer repolarization at an isochronal point of the pulses (171 ms) is shown in the right. The magnitude of the current is normalized to the current level measured during the shortest action potential. Data are expressed as mean ± SEM. IK1, inward rectifier potassium current; n, number of measurements.
Dependence of IKr and IK1 on repolarization rate and action potential configuration in human ventricular myocytes
The measurements described above were repeated in ventricular myocytes isolated from three undiseased human donor hearts. As shown by the representative records presented in Figure 6, IKr and IK1 difference currents obtained with the action potential pulses in the human myocytes were similar to those recorded previously in dog ventricular myocytes. This finding suggests that the dependence of IKr and IK1 currents on the rate of repolarization in dog and human ventricular cells is largely similar.
Figure 6.
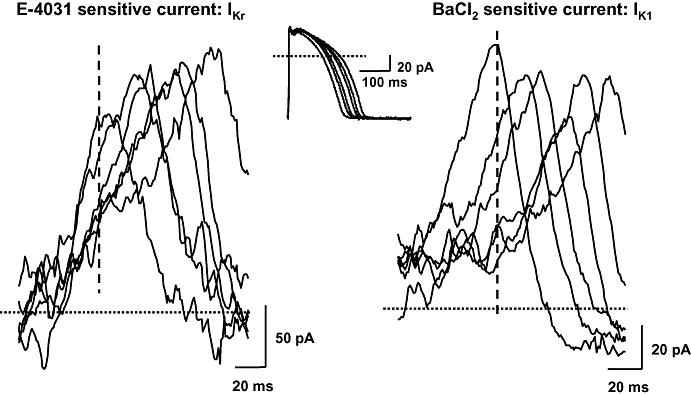
The E-4031-sensitive current (IKr) and the Ba2+-sensitive current (IK1) during action potentials with gradually longer repolarization phase, as recorded in an undiseased human left ventricular myocyte. Transmembrane currents were elicited by applying action potentials as command pulses (top) recorded in previous experiments using conventional microelectrodes at pacing cycle lengths of 400, 700, 1000, 2000, 3000 and 5000 ms. The E-4031-sensitive (IKr, left) and the Ba2+-sensitive (IK1 right) currents were recorded as difference currents. IK1, inward rectifier potassium current; IKr, rapid component of the delayed rectifier potassium current.
Discussion
In most species, including dog and human, the APD of the ventricular muscle is dependent on the heart rate. Slower frequency results in longer repolarization, and faster frequency results in shorter repolarization. The mechanism of the rate-dependent repolarization changes, which represent normal behaviour of the cardiac muscle seems to be complex (Boyett and Jewell, 1978; Hua and Gilmour, 2004) and is not fully understood. The results of this study indicate that the increased duration and thereby the decreased rate of repolarization decreases the contribution of IKr and IK1 currents to the repolarization process during the time course of the action potential at slow rates, which in turn produces a further slowing of repolarization after augmenting plateau inward current. It means that there is positive feedback between IKr or IK1 and the repolarization rate of action potentials. Generally, as a negative feedback mechanism tends to stabilize, the positive feedback mechanisms make any kind of system more unstable. Accordingly, the negative feedback mechanism found between the IKs and the action potential repolarization (Varro et al., 2000) stabilizes the repolarization process, and the positive feedback between IKr or IK1 and the repolarization rate makes the repolarization more unstable, especially when the repolarization force is weaker due to other reasons, for instance during slow heart rates. Consequently, diminishing of repolarization force results in greater APD lengthening at slow than at faster heart rate. Therefore, this positive feedback mechanism, decrease of IKr and IK1 seems to play an important role in the reverse rate-dependent nature of the drug-induced prolongation of action potential, as the reverse rate-dependent nature of repolarization lengthening appears to be further amplified by the intrinsic property of cardiac membranes including the rectifying gating kinetics of IKr and IK1.
To explain the mechanism of the reverse rate-dependent lengthening of action potential, several hypotheses were proposed (Figure 7). Jurkiewicz and Sanguinetti (1993), based on experimental results obtained in guinea-pig ventricular myocytes, suggested that during fast rates the deactivation of IKs is incomplete and significant accumulation of IKs occurs, which would greatly attenuate the APD-lengthening influence of drugs blocking other outward or activating inward currents. However, the deactivation kinetics of IKs in dog (Gintant, 1996) and human heart (Virag et al., 2001) is much faster than that of the guinea-pig and, as such, it is largely over 200 ms after the beginning of diastole. Therefore, the accumulation of IKs does not explain the reverse rate-dependent APD prolongation in species other than the guinea-pig. Indeed, it was recently reported that in dog ventricular myocytes, during fast rates of pulsing, significant IKs accumulation was not observed (Stengl et al., 2003) arguing that the IKs hypothesis does not explain well the RRD in the dog heart.
Figure 7.
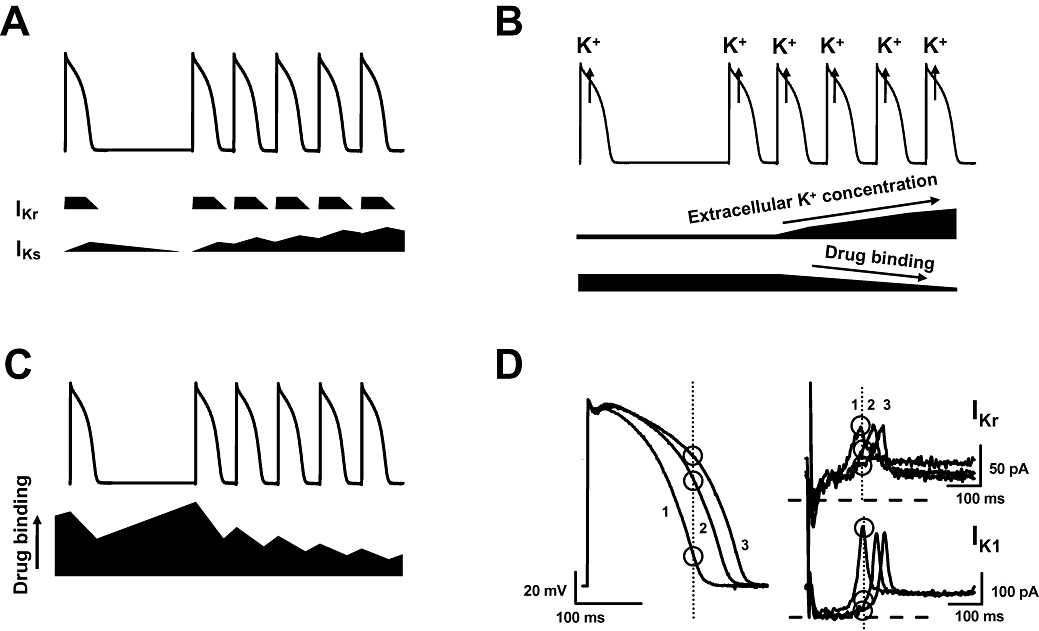
Three different hypotheses put forward earlier and the proposed present hypothesis to explain reverse rate-dependent repolarization lengthening: (A) Jurkiewicz and Sanguinetti hypothesis (1993), (B) Yang and Roden hypothesis (1996), (C) Modulated receptor hypothesis (1977) and (D) The hypothesis based on the intrinsic properties of IKr and IK1. See detailed explanation in the text. IK1, inward rectifier potassium current; IKr, rapid component of the delayed rectifier potassium current.
An alternative hypothesis was proposed by Yang and Roden (1996). In their voltage-clamp experiments performed in AT-1 cell lines expressing hERG current, they found that hERG current blockade by quinidine and dofetilide was attenuated by elevating extracellular potassium concentration. Based on this observation they speculated that, during fast rates, due to potassium accumulation in the sarcolemmal clefts, the effect of these drugs on IKr decreases and consequently, the drug-induced prolongation of APD also becomes diminished. The opposite effect could be expected at slow heart rates when the extracellular potassium concentration in the clefts was lower. This hypothesis still seems attractive but it has never been proven properly in experimental settings. Also, it does not account for the marked reverse rate-dependent nature of APD prolongation evoked by drugs not affecting IKr.
As a third proposal, the modulated receptor hypothesis, involving the sodium channel blockers, was put forward by Hondeghem and Katzung (1977). According to their suggestion, drug binding and dissociation are strongly dependent on the state of the ion channel that is dynamically changing during the action potential and diastole. If one assumes that potassium channel blockers or sodium and calcium channel activators were binding preferably during diastole and dissociating during the action potential, indeed a reverse rate-dependent APD prolongation could be expected. The validity of this hypothesis cannot be ignored, but it seems too general and it has never been experimentally proven convincingly for RRD.
At variance with the results of the present study, in guinea-pig ventricular myocytes, a moderate increase of peak IKr was induced by shorter and steeper repolarization (Rocchetti et al., 2001) but, as in our experiments, the rate of increase of IKr during long and less steep repolarization pulses was also decreased. In agreement with Gintant's study (2000) the present study showed that, in dog ventricular myocytes, the peak amplitude of IKr was independent of the rate of repolarization, but the slope of the development of IKr decreased by diminishing the steepness of the repolarizing ramp pulses.
In guinea-pig myocytes, IKr showed a rate dependency secondary to dependency on repolarization rate (Rocchetti et al., 2001). The latter was justified by the relation between activation and inactivation gating, peculiar to that species. IKr gating is reportedly different in canine and human myocytes; therefore, it was interesting to test whether IKr dependency on repolarization rate was preserved in these species. The results by Hua and Gilmour (2004) showed IKr rate dependency in canine myocytes, but with an entirely different mechanism, that is, incomplete channel deactivation during short diastolic intervals. Dependency of IKr on repolarization rate (or action potential shape), not systematically addressed in the study of Hua and Gilmour (2004), is the focus of the present work. The results obtained confirm that, albeit with potential differences from guinea-pig, explained by the peculiarity of channel gating, the course of IKr during repolarization is strictly driven by the course of membrane potential changes, also in canine and human myocytes. This leads to the relevant conclusion that, also in man, IKr and repolarization rate may form a positive feedback loop, suitable to support autoregenerative membrane potential changes.
The results indicating that IKr and IK1 densities at given isochronal time points during the action potential command pulses depend on the steepness and length of these pulses, can be related to the kinetic properties of both IKr and IK1 channels. These channels show strong inward going rectification. In the case of IKr, this was attributed to the faster time course of inactivation and recovery from inactivation than that of activation and deactivation (Shibasaki, 1987; Yang et al., 1997). In the case of IK1, the rapid binding of magnesium ion and polyamines to the channel was proposed to occur at less negative membrane potentials, which very rapidly occludes, and thereby inactivates the channel (Matsuda et al., 1987; Vandenberg, 1987; Ficker et al., 1994; Lopatin et al., 1994). Switching to positive potentials IKr current slowly activates but the channels rapidly inactivate. When repolarization proceeds with steeper slope, a larger amount of recovery from inactivation can occur per unit time than in the case of a less steep slope. Consequently, this results in more outward current at steep than at less steep repolarization, resulting in further acceleration of repolarization. Deactivation of the channels upon repolarization is a much slower process and therefore it may influence the developing current only to a limited extent. In the case of the IK1 channels, the fast block, induced by magnesium ion and polyamines at positive voltages, becomes relieved quickly upon increasing the slope of repolarization resulting in more IK1 current at steeper repolarization rates. Therefore, any factor decreasing the slope of repolarization, which can be independent of K+ channel block, such as the enhancement of the late sodium current after veratrine application observed in our experiments (Figure 1) or occurring in patients during heart failure (Maltsev and Undrovinas, 2008) and long QT (LQT)3 syndrome (Schwartz et al., 1995), would further diminish the repolarization force when comparison is made at the same time points between short and long action potentials.
Finally, it is important to emphasize that the above-described behaviour of IKr and IK1 currents may not be the only cause of the reverse rate-dependent repolarization lengthening, especially at higher frequencies. However, it may contribute to the RRD as an amplifier by magnifying and accelerating the consequences of a small drug-induced imbalance of inward and outward currents during the plateau.
Implications
The results showing that the steepness and duration of repolarization determine the reverse rate-dependent APD prolongation due to the intrinsic properties of IKr and IK1 channels have important implications. Accordingly, drugs lengthening repolarization by decreasing the repolarizing outward, or increasing the depolarizing inward, currents would cause reverse rate-dependent APD lengthening with high probability regardless of the channel type influenced. Combined ion channel modulators, blocking one type of potassium channel but activating another type of potassium channel, or blocking ion channels carrying inward current like INa or ICa may be of potential interest, as they can restore the decreased repolarization force during less steep, and therefore longer, repolarization process.
The positive feedback seen between IKr or IK1 and repolarization rate might play a role in the LQT syndrome, especially in LQT3 where the retarded repolarization due to an enhanced inward current may result in IKr/IK1 feedback suppression, thus leading to amplification of reverse rate-dependent APD prolongation. In LQT2 and in Anderson syndrome, due primarily to the mutation of IKr and IK1 channel genes that results in loss of function of these channels, however, such an amplification would be less pronounced than in LQT3 where the mutation leads to gain of function of the sodium channels. As a consequence, in LQT1 and LQT2 less reverse rate-dependent repolarization prolongation can be expected than in LQT3. In accordance with this, larger reverse rate-dependent QT lengthening was reported in LQT3 patients than in LQT1 or LQT2 patients (Schwartz et al., 1995). In these channelopathies the development of potassium channel activators may be of therapeutic value.
In conclusion, it can be suggested that the intrinsic rectifying properties of IKr and IK1 in an autogenerative manner amplify repolarization lengthening in the ventricle, and as such it may contribute to the reverse rate-dependent nature of drug-induced prolongation of repolarization. This should be taken into account in future drug development strategies and the interpretation of the rate-dependent QT changes observed in the different forms of LQT syndrome.
Acknowledgments
This work was supported by grants from the Hungarian Scientific Research Fund (OTKA K-68911, and NI-61902), Hungarian Ministry of Health (T-353/2006, T-542/2006), Hungarian Ministry of Education (Bio-37 KPI), National Research and Development Programmes (NKFP 1A/0011/2002 and NKFP 1A/046/2004), European Community (EU FP6 grant LSHM-CT-2005-018833, EUGeneHeart; EU FP6 grant LSHM-CT-2006-018676, NORMACOR; EU FP7 grant ICT-2008-224381, preDiCT).
Glossary
Abbreviations
- APD
action potential duration
- APD50 and APD90
action potential durations at 50% and 90% of repolarization
- ICa
L-type Ca2+ current
- IK1
inward rectifier potassium current
- IKr
rapid component of the delayed rectifier potassium current
- IKs
slow component of the delayed rectifier potassium current
- LQT
long QT syndrome
- RRD
reverse rate dependency
Conflict of interest
The authors state no conflict of interest.
References
- Boyett MR, Jewell BR. A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. J Physiol. 1978;285:359–380. doi: 10.1113/jphysiol.1978.sp012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther. 1992;262:809–817. [PubMed] [Google Scholar]
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Gintant GA. Two components of delayed rectifier current in canine atrium and ventricle. Does IKs play a role in the reverse rate dependence of class III agents? Circ Res. 1996;78(1):26–37. doi: 10.1161/01.res.78.1.26. [DOI] [PubMed] [Google Scholar]
- Gintant GA. Characterization and functional consequences of delayed rectifier current transient in ventricular repolarization. Am J Physiol Heart Circ Physiol. 2000;278(3):H806–817. doi: 10.1152/ajpheart.2000.278.3.H806. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Woosley RL. Sotalol. New Eng J Med. 1994;331:31–38. doi: 10.1056/NEJM199407073310108. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Katzung BG. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977;472(3–4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Snyders DJ. Class III antiarrhythmic agents have a lot of potential but a long way to go: reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- Hua F, Gilmour RF., Jr Contribution of IKr to rate-dependent action potential dynamics in canine endocardium. Circ Res. 2004;94(6):810–819. doi: 10.1161/01.RES.0000121102.24277.89. [DOI] [PubMed] [Google Scholar]
- Iost N, Virag L, Opincariu M, Szecsi J, Varro A, Papp JG. Delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 1998;40(3):508–515. doi: 10.1016/s0008-6363(98)00204-1. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz NK, Sanguinetti MC. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993;72(1):75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Undrovinas A. Late sodium current in failing heart: friend or foe? Prog Biophys Mol Biol. 2008;96(1–3):421–451. doi: 10.1016/j.pbiomolbio.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+ Nature. 1987;325:156–158. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Rocchetti M, Besana A, Gurrola GB, Possani LD, Zaza A. Rate dependency of delayed rectifier currents during the guinea-pig ventricular action potential. J Physiol. 2001;534(Pt 3):721–732. doi: 10.1111/j.1469-7793.2001.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengl M, Volders PG, Thomsen MB, Spatjens RL, Sipido KR, Vos MA. Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J Physiol. 2003;551(Pt 3):777–786. doi: 10.1113/jphysiol.2003.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci USA. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A, Balati B, Iost N, Takacs J, Virag L, Lathrop DA, et al. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol. 2000;523(Pt 1):67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A, Biliczki P, Iost N, Virag L, Hala O, Kovacs P, et al. Theoretical possibilities for the development of novel antiarrhythmic drugs. Curr Med Chem. 2004;11(1):1–11. doi: 10.2174/0929867043456296. [DOI] [PubMed] [Google Scholar]
- Virag L, Iost N, Opincariu M, Szolnoky J, Szecsi J, Bogats G, et al. The slow component of the delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 2001;49(4):790–797. doi: 10.1016/s0008-6363(00)00306-0. [DOI] [PubMed] [Google Scholar]
- Yang T, Roden DM. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93(3):407–411. doi: 10.1161/01.cir.93.3.407. [DOI] [PubMed] [Google Scholar]
- Yang T, Snyders DJ, Roden DM. Rapid inactivation determines the rectification and [K+]o dependence of the rapid component of the delayed rectifier K+ current in cardiac cells. Circ Res. 1997;80(6):782–789. doi: 10.1161/01.res.80.6.782. [DOI] [PubMed] [Google Scholar]


