Abstract
Background and purpose:
p-Coumaryl alcohol-γ-O-methyl ether (CAME) was isolated from Alpinia galanga and shown to contain a phenylpropanoid structure similar to p-coumaryl diacetate (CDA). CDA is known to have antioxidant and anti-inflammatory activity, but the biochemical activities of CAME are unknown. Inflammation is mediated by inflammatory cytokine production, in particular, by CD4+ T helper cells (Th cells), but it is unclear whether phenylpropanoids affect cytokine production in Th cells. In this study, we decided to investigate the functions of CAME and CDA in CD4+ Th cells.
Experimental approach:
Mouse CD4+ Th cells were isolated from C57BL6 mice and stimulated with an antibody against T cell receptors in the presence of phenylpropanoids. Cytokine production was measured by elisa and intracellular cytokine staining. Gene knockout mice and tetracycline-inducible transgenic mice were used to examine the molecular mechanisms of phenylpropanoids on modulation of cytokine production.
Key results:
CAME potently reduced intracellular reactive oxygen species in Th cells, as does CDA. However, although CDA was cytotoxic, CAME selectively and potently suppresses interferon-γ (IFNγ) production in CD4+ Th cells, without toxicity. This effect was caused by attenuated expression of the transcription factor, T-box protein expressed in T cells (T-bet), and T-bet was essential for CAME to inhibit IFNγ production in CD4+ Th cells.
Conclusions and implications:
CAME selectively and substantially suppresses IFNγ production in CD4+ Th cells by decreasing T-bet expression. As increased IFNγ production by CD4+ Th cells can mediate inflammatory immune responses, a selective IFNγ suppressor, such as CAME may be an effective, naturally occurring, compound for modulating inflammatory immune disorders.
Keywords: anti-inflammatory activity, p-coumaryl alcohol-γ-O-methyl ether, p-coumaryl diacetate, phenylpropanoids, antioxidant, CD4+ Th cells, IFNγ, T-bet
Introduction
Alpinia galanga (A. galanga) is a plant of the Zingiberaceae genus that is frequently used in traditional medicine as a stomachic, carminative and as an antibacterial agent (Janssen and Scheffer, 1985). Various chemical compounds including phenylpropanoids derived from plant extracts of A. galanga have been shown to possess biological activity. Structure–activity relationships of phenylpropanoids with respect to their antioxidant activity have already been reported (Ly et al., 2003; Matsuda et al., 2005; Morikawa et al., 2005), demonstrating that the p-[3-hydroxyprop-1-enyl] phenol structure is essential. In addition, Matsuda and colleagues have reported that phenylpropanoids isolated from the rhizomes of A. galanga inhibited allergic cytokine IL-4 production (Matsuda et al., 2003a) and markedly suppressed ethanol-induced gastric lesions (Matsuda et al., 2003b) in murine models. A principal compound derived from A. galanga, acetoxychavicol acetate (ACA) is known to have anti-tumour (Lee and Houghton, 2005), anti-inflammatory (Watanabe et al., 1995; Matsuda et al., 2003a) and anti-fungal activities (Janssen and Scheffer, 1985). p-Coumaryl alcohol-γ-O-methyl ether (CAME; Figure 1) was isolated from A. galanga as another phenylpropanoid (Nam et al., 2005) and differs in structure from p-coumaryl diacetate (CDA; Figure 1) by the 4-hydroxy and 3′-methoxy groups. The pharmacology of CAME is largely unknown.
Figure 1.
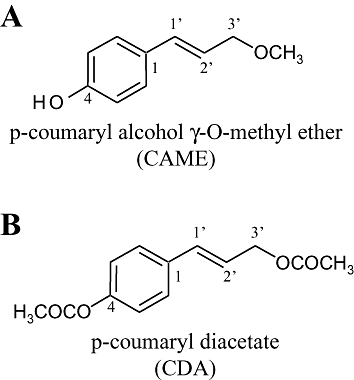
Structure of phenylpropanoids, p-coumaryl alcohol-γ-O-methyl ether (CAME) (A) and p-coumaryl diacetate (CDA) (B) isolated from Alpinia galanga.
Inflammation is mediated, in part, by an extensive production of pro-inflammatory cytokines derived from activated immune cells. CD4+ T helper (Th) cells modulate the activation of immune cells of both the innate and adaptive immune systems, indicating a crucial role of CD4+ Th cells in inflammatory responses. CD4+ Th cells express T cell receptors (TCR) that are stimulated by processed antigenic peptides in vivo and anti-CD3/anti-CD28 antibodies in vitro. TCR stimulation activates CD4+ Th cells to produce cytokines such as interleukin-2 (IL-2) and interferon-γ (IFNγ). Cytokines are also critical for CD4+ Th cell activation and differentiation into Th1, Th2 and Th17 cells, which produce characteristically different cytokines and mediate different immune responses to pathogens (Rengarajan et al., 2000; Glimcher, 2001; Bettelli et al., 2006). CD4+ Th cell-driven IFNγ production can potently promote Th1 cell differentiation and concomitantly activate cytotoxic CD8+ T cells and macrophages, which then mediate inflammatory immune responses. Thus, modulation of IFNγ production in CD4+ Th cells may be important for an anti-inflammatory effect. IFNγ production in CD4+ Th cells is mainly regulated at the transcription level, particularly by T-bet (T-box protein expressed in T cells), a T-box transcription factor (Szabo et al., 2000). As T-bet-deficient CD4+ Th cells fail to produce IFNγ (Szabo et al., 2002), T-bet is crucial for IFNγ expression (Hwang et al., 2005b; Mathur et al., 2006; Rangachari et al., 2006). T-bet also suppresses IL-2 production by blocking NF-κB-mediated IL-2 gene transcription, which, in turn, is mediated by specific serine phosphorylation of T-bet (Hwang et al., 2005a).
In this study, we have investigated the effects of CAME on cytokine production in murine CD4+ Th cells and compared its activity with that of CDA. CAME exhibited a potent antioxidant activity comparable with CDA. In addition, CAME, but not CDA substantially and selectively suppressed IFNγ production, an effect that was mediated by the suppression of T-bet in CD4+ Th cells. These results suggest that CAME may be useful as a naturally occurring anti-inflammatory compound for modulating inflammatory responses induced by IFNγ production.
Methods
Isolation and purification of CDA and CAME
p-coumaryl diacetate and CAME were isolated from the dried rhizomes of A. galanga (Zingiberaceae) as described by Nam et al. (2005), to give the compounds in 98% purity. The chemical structures of these samples of CAME and CDA were confirmed by MS and NMR analyses (Figure S1).
Animals
All handling of the animals and subsequent experimental protocols were in accordance with the Institutional Animal Care and Use Committee guidelines. Wild type C57BL6 mice were purchased from The Jackson Laboratory (Bar Harbor, MN, USA), and tetracycline-inducible T-bet transgenic (double transgenic-knockout, DTg-KO) mice were generated in T-bet KO background as previously described (Werneck et al., 2008). All mice were housed in specific pathogen-free conditions at Ewha Womans University
In vitro activation of CD4+ Th cell and incubation with phenylpropanoids
CD4+ Th cells (>95% purity) were isolated from the lymph nodes and spleens of mice by using mouse CD4 beads according to the commercial instructions (Miltenyi Biotech., Auburn, CA, USA). CD4+ Th cells (2 × 106 cells·mL−1) were incubated with plate-bound anti-CD3 (1 µg·mL−1, BD Pharmingen, San Diego, CA, USA) and anti-CD28 antibodies (1 µg·mL−1, BD Pharmingen) for the indicated time periods. CAME, and/or CDA was added to the cells during TCR stimulation, and supernatants were collected at 48 h after treatment for elisa (enzyme-linked immunosorbent assay).
Measurement of intracellular levels of reactive oxygen species (ROS) in Th cells
EL4 Th cell clones (from ATCC) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum and pretreated with either CDA or CAME for 24 h, followed by stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 2 h before harvest. Cells were immediately incubated with dichloro fluorescein diacetate (DCFDA; Sigma-Aldrich Inc., St. Louis, MO, USA) for 30 min. After washing twice with phosphate-buffered saline (PBS), cells were analysed by FACS Calibur and CellQuest programme (BD Biosciences, Mountain View, CA, USA).
Cell apoptosis assays
CD4+ Th cells were isolated and stimulated with TCR antibodies in the presence of either CDA or CAME for 24 h. After fixation in cold 70% ethanol, cells were resuspended in 200 µL of propidium iodide solution (50 µg·mL−1, Sigma-Aldrich Inc.), then analysed by FACS Calibur and CellQuest programme (BD Biosciences). Apoptotic cell populations were determined by ModFit programme (BD Biosciences).
Cell viability assay
CD4+ Th cells were activated in 96-well tissue culture plates and incubated with CDA or CAME for 24 h. Dimethylthiazol diphenyltetrazolium bromide solution was added to the cells according to the manufacturer's instructions (Biotium Inc., Hayward, CA, USA). Colorimetric changes were measured by elisa plate reader (Molecular Devices, Sunnyvale, CA, USA). Cell viability is given as a mean ± SD of three separate experiments and expressed as a percentage of the vehicle-treated control.
elisa for cytokines
Cell supernatants were collected from CD4+ Th cells treated with CAME for 24 h and incubated on the capture antibody-coated elisa plate. After washing the plates with PBST (PBS with 1% Tween-20), the plates were incubated with biotinylated anti-cytokine antibodies and subsequently phosphatase-conjugated streptavidin (BD Pharmingen). Plates were developed with a phosphatase substrate. Colorimetric changes were measured by elisa plate reader (Molecular Devices). Purified and known concentrations of mouse IL-2 and IFNγ were incubated in parallel with unknown samples, and standard curves were generated from assay of the purified cytokines.
Intracellular cytokine staining
CD4+ Th cells were treated with CAME for 72 h with TCR stimulation and pretreated with monensin for 3 h before harvesting. Paraformaldehyde-fixed cells were incubated with phycoerythrin-conjugated anti-IFNγ antibody (BD Pharmingen), washed with FACS buffer twice and run by FACS Calibur. Cells were analysed by CellQuest programme (BD Biosciences).
Reporter gene assay
EL4 Th cell clones (5 × 106 cells) were electroporated with IFNγ promoter-reporter gene (IFNγ promoter-luciferase) and pCMVβ (Clontech, Heidelberg, Germany) then maintained in RPMI1640 medium supplemented with 20% fetal bovine serum for an additional 24 h. Cell lysates were harvested and luciferase activity assayed in a Luminometer (Berthold, Wildbad, Germany). Relative luciferase activities were normalized by measuring β-galactosidase activity, which had been co-transfected as an internal control.
Doxycycline treatment in CD4+ Th cells of DTg-KO mice
CD4+ Th cells (^95% purity) were isolated from DTg-KO mice and stimulated with anti-CD3 and anti-CD28 antibodies. Cells were treated with or without the tetracycline derivative, doxycycline (Sigma-Aldrich Inc.) in the co-presence of CAME for 24 h. Supernatants were harvested for measuring IL-2 and IFNγ by elisa. Whole cell extracts were resolved by SDS-PAGE and blotted with anti-T-bet monoclonal antibody (Santa Cruz Biotech., Santa Cruz, CA, USA).
Data analysis
Data are expressed as mean values ± SD. Statistical significance between means was assessed by using the unpaired Student's t-test (P < 0.05 were considered to be statistically significant).
Results
Naturally occurring CAME demonstrates antioxidant activity similar to CDA
p-Coumaryl alcohol-γ-O-methyl ether has been isolated from the rhizomes of A. galanga as another phenylpropanoid (Ly et al., 2003) and found to have the chemical structure, shown in Figure 1B. CAME contains a 4-hydroxy and a 3′-methoxy group in the propenyl benzene ring, which is different from the 4-acetoxy and the 3′-acetoxy groups of CDA. However, the pharmacological properties of CAME have yet to be assessed. As the antioxidant activities of phenylpropanoids have already been well established in various systems (Matsuda et al., 2005; Morikawa et al., 2005; Korkina, 2007; Hosoya et al., 2008), we examined the antioxidant activity of CAME compared with that of CDA. Activation of Th cell clones (EL4 cell line) induced ROS generation, but CDA treatment significantly reduced intracellular ROS levels (Figure 2A). However, CAME was a more potent inhibitor of intracellular ROS generation (Figure 2B).
Figure 2.
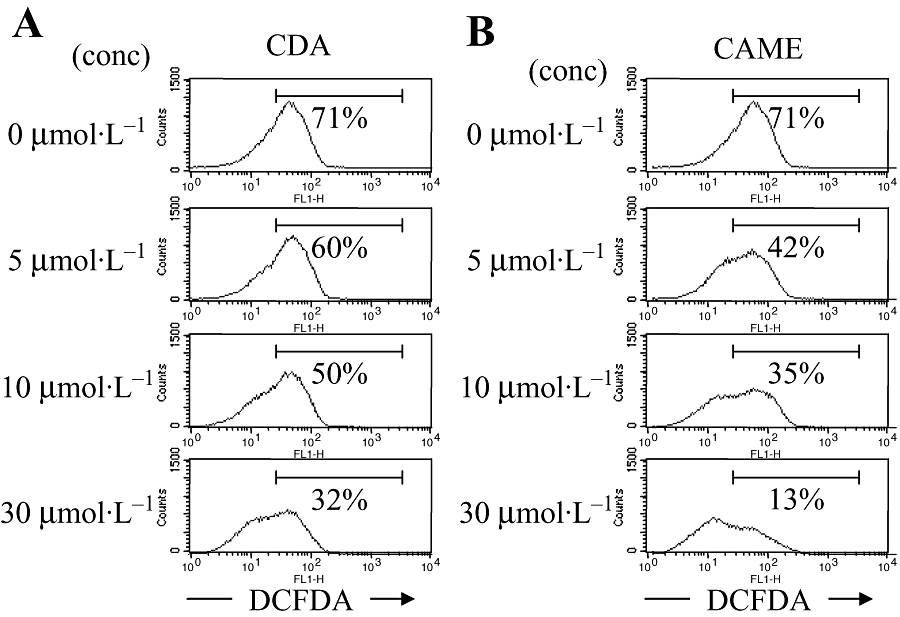
Antioxidant activity of CAME in Th cells. EL4 cells were incubated with either CDA (A) or CAME (B) for 24 h and additionally stimulated with PMA and ionomycin for 2 h. Cells were incubated with DCFDA and analysed by FACS Calibur. Numbers in the records show DCFDA-positive cells as % of live cells in the M1 gate (as shown; CellQuest software). Results from a representative experiment (of three) are shown. CAME, p-coumaryl alcohol-γ-O-methyl ether; CDA, p-coumaryl diacetate; conc, concentration; DCFDA, dichloro fluorescein diacetate; PMA, phorbol 12-myristate 13-acetate; Th cell, T helper cell.
CDA, not CAME reveals pro-apoptotic activity and decreases cell viability in CD4+ Th cells
In order to assess the antioxidant activity and other functions of CAME in primary CD4+ Th cells, we isolated mouse CD4+ Th cells and stimulated them with anti-CD3 and anti-CD28 antibodies in the presence of either CDA or CAME. While CAME had no effect on Th cell activation, CDA changed cell morphology of primary CD4+ Th cells upon TCR stimulation (data not shown). Further analysis confirmed that CDA dose-dependently increased cell apoptosis (Figure 3A) and decreased cell viability of primary CD4+ Th cells (Figure 3C), supporting a pro-apoptotic activity by acetoxylated phenylpropanoids (Muangnoi et al., 2007). However, CAME did not affect either cell apoptosis or cell viability of primary CD4+ Th cells (Figure 3B,C).
Figure 3.
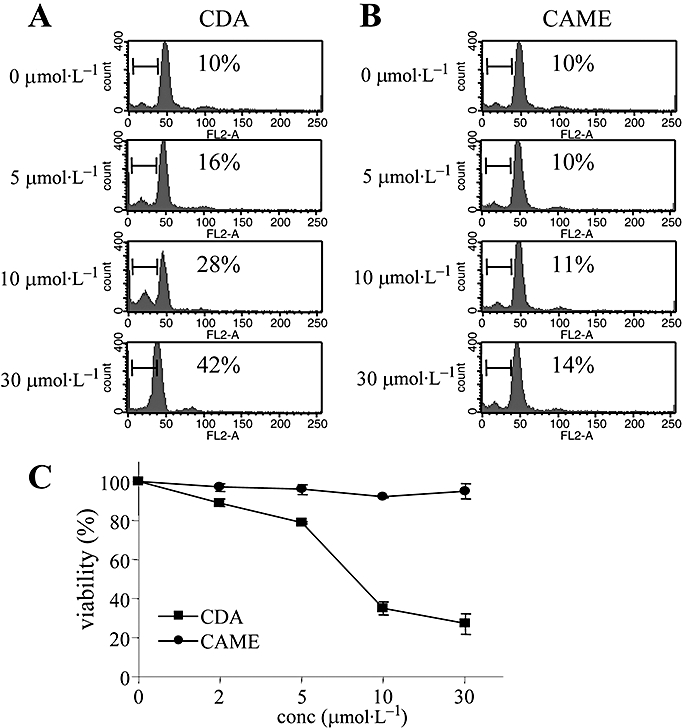
Effects of CDA and CAME on cell apoptosis and cell viability of primary Th cells. Single suspensions of CD4+ Th cells were stimulated with anti-CD3 (1 µg·mL−1) and anti-CD28 (1 µg·mL−1) for 24 h in the presence of different concentrations of CDA (A) or CAME (B); numbers in the records show the percentage of the cells in sub-G1 (using ModFit software). (C) Cells treated with either CDA or CAME for 24 h were incubated with MTT solution, and colorimetric changes were determined by elisa reader. Data are given as means ± SD, n= 3. CAME, p-coumaryl alcohol-γ-O-methyl ether; CDA, p-coumaryl diacetate; conc, concentration; elisa, enzyme-linked immunosorbent assay; MTT, dimethylthiazol diphenyltetrazolium bromide; Th cell, T helper cell.
CAME moderately but selectively suppresses IFNγ production by activated CD4+ Th cells
Stimulation of TCR in CD4+ Th precursor cells greatly increases cytokine production, in particular IL-2 and IFNγ (Rengarajan et al., 2000). We therefore examined the effects of CAME on cytokine production by CD4+ Th cells, stimulated with TCR antibodies. As CDA significantly inhibited the activation of CD4+ Th cells by inducing apoptosis, cytokine production was impaired in the presence of CDA (data not shown). However, different concentrations of CAME had no effect on the amounts of IL-2 cytokine produced by CD4+ Th cells (Figure 4A). Interestingly, IFNγ production was markedly suppressed by CAME over the same concentration range (Figure 4B). Intracellular cytokine staining by using anti-IFNγ antibodies also confirmed the moderate but selective reduction of IFNγ by CAME (Figure 4C), which is in accordance with cytokine levels measured by elisa (Figure 4B) and quantitative RT-PCR (data not shown). These results suggest that CAME is a moderate, but selective modulator of IFNγ in CD4+ Th cells.
Figure 4.
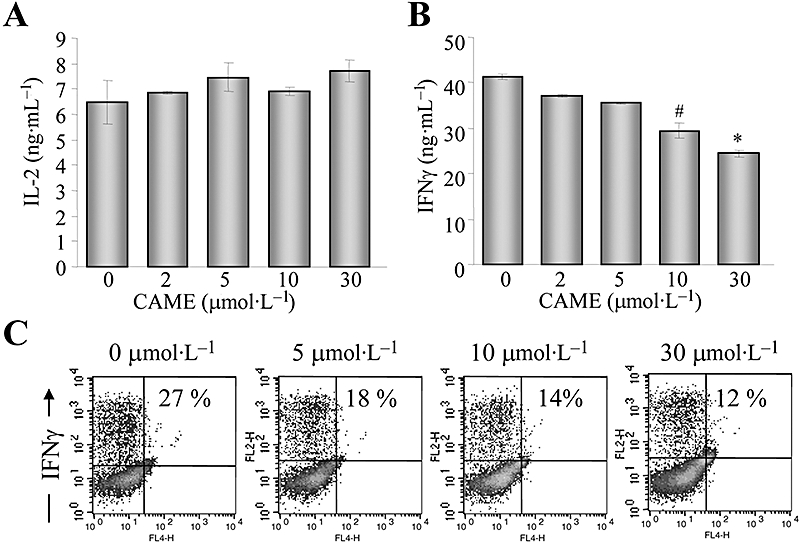
Selective suppression of IFNγ production by CAME during CD4+ Th cell activation. Isolated CD4+ Th cells were stimulated with antibodies to TCR in the presence of CAME for 24 h. Supernatants were collected and used for determining cytokines, IL-2 (A) and IFNγ (B) by elisa; data are given as means ± SD; n= 3. #P < 0.05; *P < 0.05; unpaired t-test. (C) CD4+ Th cells were incubated with CAME for 72 h and treated with monensin for 2 h prior to harvest. Cells were harvested and incubated with phycoerythrin-conjugated anti-IFNγ antibody, followed by flow cytometric analysis. Data acquisition and analysis were performed with CellQuest programme. Numbers in the records indicate percentage of live cells producing IFNγ. Representative data from three independent experiments. CAME, p-coumaryl alcohol-γ-O-methyl ether; elisa, enzyme-linked immunosorbent assay; IFNγ, interferon-γ; IL-2, interleukin-2; TCR, T cell receptor; Th cell, T helper cell.
CAME decreases T-bet protein expression and suppresses IFNγ gene transcription
We next attempted to elucidate the possible regulatory mechanisms of CAME-induced IFNγ suppression. In CD4+ Th cells, IFNγ production is mainly regulated at the transcription level by a specific transcription factor, T-bet (Szabo et al., 2000; 2002). Interestingly, CAME gradually decreased the expression of T-bet protein (Figure 5A) without affecting T-bet gene transcription (Figure 5B). In addition, we tested whether CAME suppressed T-bet-induced IFNγ gene transcription. EL4 cells were transfected with a IFNγ promoter-reporter gene T-bet expression vector and subsequently incubated with CAME for an additional 24 h. IFNγ promoter activity was increased by exogenous T-bet expression as reported (Szabo et al., 2000), but was gradually decreased by CAME treatment (Figure 6), suggesting that CAME suppresses T-bet-induced IFNγ gene transcription.
Figure 5.
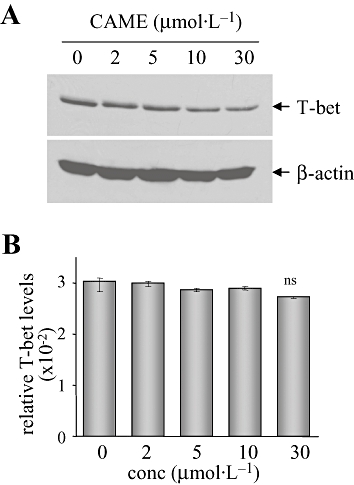
Diminished T-bet expression was induced by CAME in CD4+ Th cells. CD4+ Th cells were incubated in the presence of the indicated concentrations of CAME and stimulated with antibodies to TCR for 24 h. (A) Whole cell extracts were prepared and resolved by SDS-PAGE. Protein blot was incubated with anti-T-bet antibody. (B) Total RNA was extracted from the duplicate of Figure 5A and used for reverse transcription. Relative mRNA levels of T-bet were calculated after normalization to levels of β-actin by using real-time PCR. Data are given as means ± SD (n= 4); ns, not significant; unpaired t-test. CAME, p-coumaryl alcohol-γ-O-methyl ether; conc, concentration; T-bet, T-box protein expressed in T cells; TCR, T cell receptor; Th cell, T helper cell.
Figure 6.
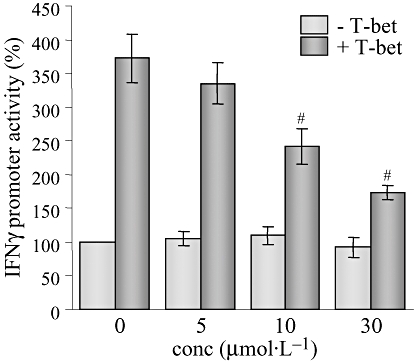
Suppression of T-bet-induced IFNγ gene transcription by CAME. EL4 cells were electroporated with IFNγ-promoter-linked reporter and pCMVβ with (+T-bet) or without (−T-bet) T-bet expression vector and subsequently incubated with CAME for 24 h. Luciferase activity was assayed by using luminometer and normalized to the β-galactosidase activity. Data are given as means ± SD; n= 3. #P < 0.05; unpaired t-test. CAME, p-coumaryl alcohol-γ-O-methyl ether; conc, concentration; IFNγ, interferon-γ; T-bet, T-box protein expressed in T cells.
T-bet is primarily required for CAME function to suppress IFNγ
As CAME moderately attenuated T-bet expression and suppressed IFNγ production in CD4+ Th cells, we tested whether T-bet expression is crucial for CAME to suppress IFNγ production. In order to confirm the T-bet-dependent CAME function, we used DTg-KO mice, which only express T-bet in T-bet-deficient mice in response to doxycycline and in a T cell-specific manner. We have obtained CD4+ Th cells from DTg-KO mice and stimulated them with anti-TCR antibodies in the absence or presence of doxycycline and determined doxycycline-induced T-bet restoration (Figure 7A). In addition, doxycycline -induced T-bet expression increased IFNγ production (Figure 7B) and concomitantly suppressed IL-2 production (Figure 7C). Increased IFNγ production in the presence of doxycycline was subsequently decreased by CAME in a dose-dependent manner, whereas IL-2 output was not affected by CAME (Figure 7B,C), suggesting that T-bet is primarily required for CAME to suppress IFNγ production.
Figure 7.
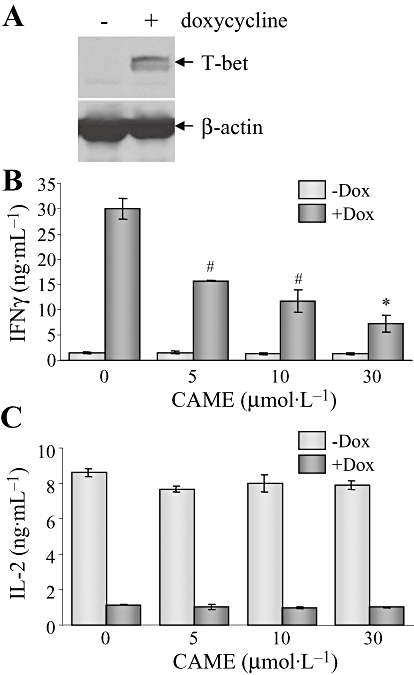
T-bet is essential for CAME-driven IFNγ suppression. CD4+ Th cells were isolated from the lymph nodes of DTg-KO mice and activated for 24 h. Cells were treated with CAME in the presence or absence of doxycycline (Dox; 0.1 µg·mL−1). Whole cell extracts were prepared from the cells and analysed by immunoblot analysis (A); results shown are representative of three independent experiments. Cell supernatants were used for measuring IFNγ (B) and IL-2 (C) by elisa; data are given as means ± SD; n= 3. #P < 0.05; *P < 0.05; unpaired t-test. CAME, p-coumaryl alcohol-γ-O-methyl ether; DTg-KO, double transgenic-knockout; elisa, enzyme-linked immunosorbent assay; IFNγ, interferon-γ; IL-2, interleukin-2; T-bet, T-box protein expressed in T cells; Th cell, T helper cell.
Discussion and conclusion
Our studies demonstrated novel functions and mechanisms of a naturally occurring compound, CAME, in CD4+ Th cells. CAME had potent antioxidant activity and selectively reduced IFNγ production by suppressing T-bet expression in CD4+ Th cells.
Structure–activity relationships between phenylpropanoids and antioxidant activity is well established (Murakami et al., 2000; Matsuda et al., 2005). Both CAME and CDA possess a p-[3-hydroxyprop-1-enyl] phenol in their structure, as do other phenylpropanoids, but have different functional groups at the 4- and 3′-positions. As both CAME and CDA potently suppressed intracellular ROS generation, it is likely that the p-[3-hydroxyprop-1-enyl] phenol moiety may function as an effective antioxidant in CD4+ Th cells. However, CDA, but not CAME induced apoptotic cell death in primary CD4+ Th cells, suggesting that the acetoxyl groups of phenylpropanoids may be responsible for the pro-apoptotic activity. This conclusion may be supported by the observation that another constituent of A galanga, ACA, which has two acetoxyl groups also induced cell apoptosis in myeloid leukemic cells (Ito et al., 2004).
Our studies suggest that the reduction of IFNγ production by CAME may be due to the attenuation of T-bet expression. Although it is well known that T-bet also modulates IL-2 production as a suppressor, IL-2 levels were not increased by attenuated T-bet expression in CAME-treated CD4+ Th cells. During CD4+ Th cell activation, T-bet proteins undergo post-translational modifications such as serine and tyrosine phosphorylation, which inhibit IL-2 and Th2 cytokine production respectively (Hwang et al., 2005a,b). Therefore, it would be useful to determine whether CAME can specifically decrease the serine phosphorylation of T-bet and thus prevent T-bet from suppressing IL-2 production.
Our studies suggest that CAME is another naturally occurring antioxidant and anti-inflammatory alkaloid, similar to ACA that is the principal component of A. galanga, (Matsuda et al., 2003a). However, there have been only limited studies on the mechanistic relationships between the antioxidant and anti-inflammatory activities of phenylpropanoids in CD4+ Th cells. Further studies are required to determine whether ROS generation is associated with pro-inflammatory cytokine production in CD4+ Th cells.
In these studies, we have examined the effects of CAME on cytokine production in CD4+ Th cells and have shown that CAME suppressed the generation of the pro-inflammatory cytokine IFNγ by inhibiting T-bet expression. In conclusion, our observations on the effects of CAME in CD4+ Th cells strongly suggest that CAME may be beneficial in modulating inflammatory immune disorders mediated by excess IFNγ production.
Acknowledgments
This work was supported by National R&D programme for Cancer Control of National Cancer Center (0620380, ESH) and by MEST and KOSEF (R15-2006-020 and R01-2006-000-10429-0) and Korea University Grant (2006, JHH).
Glossary
Abbreviations
- CAME
p-coumaryl alcohol-γ-O-methyl ether
- CDA
p-coumaryl diacetate
- DCFDA
dichloro fluorescein diacetate
- DTg-KO
double transgenic-knockout
- elisa
enzyme-linked immunosorbent assay
- IFNγ
interferon-γ
- IL-2
interleukin-2
- MTT
dimethylthiazol diphenyltetrazolium bromide
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- T-bet
T-box protein expressed in T cells
- TCR
T cell receptor
- Th cell
T helper cell
Conflict of interests
The authors state no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Chemical structure of CDA and CAME confirmed by NMR analysis. (A) Chemical structure of CDA and CAME. (B) Proton and carbon NMR of CDA and CAME. CAME, p-coumaryl alcohol-g-O-methyl ether; CDA, p-coumaryl diacetate.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Glimcher LH. Lineage commitment in lymphocytes: controlling the immune response. J Clin Invest. 2001;108:s25–s30. [PubMed] [Google Scholar]
- Hosoya T, Yun YS, Kunugi A. Antioxidant phenylpropanoid glycosides from the leaves of Wasabia japonica. Phytochemistry. 2008;69:827–832. doi: 10.1016/j.phytochem.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Hong JH, Glimcher LH. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med. 2005a;202:1289–1300. doi: 10.1084/jem.20051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005b;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- Ito K, Nakazato T, Murakami A, Yamato K, Miyakawa Y, Yamada T, et al. Induction of apoptosis in human myeloid leukemic cells by 1′-acetoxychavicol acetate through a mitochondrial- and Fas-mediated dual mechanism. Clin Cancer Res. 2004;10:2120–2130. doi: 10.1158/1078-0432.ccr-1142-03. [DOI] [PubMed] [Google Scholar]
- Janssen AM, Scheffer JJ. Acetoxychavicol acetate, an antifungal component of Alpinia galanga. Planta Med. 1985;51:507–511. [PubMed] [Google Scholar]
- Korkina LG. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol (Noisy-le-grand) 2007;53:15–25. [PubMed] [Google Scholar]
- Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100:237–243. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Ly TN, Shimoyamada M, Kato K, Yamauchi R. Isolation and characterization of some antioxidative compounds from the rhizomes of smaller galanga (Alpinia officinarum Hance) J Agric Food Chem. 2003;51:4924–4929. doi: 10.1021/jf034295m. [DOI] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, et al. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Morikawa T, Managi H, Yoshikawa M. Antiallergic principles from Alpinia galanga: structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem Lett. 2003a;13:3197–3202. doi: 10.1016/s0960-894x(03)00710-8. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Pongpiriyadacha Y, Morikawa T, Ochi M, Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. Eur J Pharmacol. 2003b;471:59–67. doi: 10.1016/s0014-2999(03)01785-0. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Ando S, Morikawa T, Kataoka S, Yoshikawa M. Structure-activity relationships of 1′S-1′-acetoxychavicol acetate for inhibitory effect on NO production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg Med Chem Lett. 2005;15:1949–1953. doi: 10.1016/j.bmcl.2005.01.070. [DOI] [PubMed] [Google Scholar]
- Morikawa T, Ando S, Matsuda H, Kataoka S, Muraoka O, Yoshikawa M. Inhibitors of nitric oxide production from the rhizomes of Alpinia galanga: structures of new 8-9′ linked neolignans and sesquineolignan. Chem Pharm Bull (Tokyo) 2005;53:625–630. doi: 10.1248/cpb.53.625. [DOI] [PubMed] [Google Scholar]
- Muangnoi P, Lu M, Lee J, Thepouyporn A, Mirzayans R, Le XC, et al. Cytotoxicity, apoptosis and DNA damage induced by Alpinia galanga rhizome extract. Planta Med. 2007;73:748–754. doi: 10.1055/s-2007-981542. [DOI] [PubMed] [Google Scholar]
- Murakami A, Toyota K, Ohura S, Koshimizu K, Ohigashi H. Structure-activity relationships of (1′S)-1′-acetoxychavicol acetate, a major constituent of a southeast Asian condiment plant Languas galanga, on the inhibition of tumor-promoter-induced Epstein-Barr virus activation. J Agric Food Chem. 2000;48:1518–1523. doi: 10.1021/jf990528r. [DOI] [PubMed] [Google Scholar]
- Nam J, Kim S, Han A, Lee S, Seo E. Cytotoxic phenylpropanoids from the rhizomes of Alpinia galanga. J Appl Pharmacol. 2005;13:263–266. [Google Scholar]
- Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21:479–483. doi: 10.1016/s0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Kataoka T, Tajika T, Uramoto M, Magae J, Nagai K. 1′-Acetoxychavicol acetate as an inhibitor of phagocytosis of macrophages. Biosci Biotechnol Biochem. 1995;59:1566–1567. doi: 10.1271/bbb.59.1566. [DOI] [PubMed] [Google Scholar]
- Werneck MB, Lugo-Villarino G, Hwang ES, Cantor H, Glimcher LH. T-bet plays a key role in NK-mediated control of melanoma metastatic disease. J Immunol. 2008;180:8004–8010. doi: 10.4049/jimmunol.180.12.8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


