Abstract
Background and purpose:
The kisspeptins are critical regulators of reproduction and a therapeutic target for reproductive disease. Intracerebroventricular (i.c.v.) or peripheral injection of kisspeptin potently stimulates the hypothalamic-pituitary gonadal (HPG) axis via gonadotrophin-releasing hormone (GnRH). However, little is known regarding the effects of kisspeptin administration on testicular function. We investigated the mechanism(s) of kisspeptin-induced testicular degeneration in the rat.
Experimental approach:
Kisspeptin-54 (50 nmol·day−1) was continuously administered subcutaneously (6 h to 3 days) to male Wistar rats and reproductive hormones and testicular histology analysed. We also investigated the effects of a single subcutaneous injection of 0.5, 5 or 50 nmol kisspeptin-54. In order to determine whether the testicular degeneration observed is peripherally or centrally mediated, we investigated effects of i.c.v. injections of 5 nmol kisspeptin-54 and pre-administered a GnRH-receptor antagonist (cetrorelix) to rats peripherally treated with kisspeptin-54.
Key results:
Continuous subcutaneous administration of kisspeptin-54 caused testicular degeneration after only 12 h, when gonadotrophins were still markedly raised, suggesting that the degeneration is independent of the desensitization of the HPG axis to kisspeptin-54. Furthermore, a single subcutaneous injection of kisspeptin-54 caused dose-dependent testicular degeneration. Continuous kisspeptin-54 administration is thus not required to cause testicular degeneration. Pretreatment with cetrorelix blocked kisspeptin-induced testicular degeneration, and a single i.c.v. injection of kisspeptin-54 caused testicular degeneration, suggesting it is GnRH-mediated.
Conclusions and implications:
Kisspeptin-induced testicular degeneration appears to be centrally mediated, and result from acute hyper-stimulation of the HPG axis. Doses must be carefully considered if kisspeptin is to be used therapeutically.
Keywords: metastin, GPR54, testis, seminiferous tubules
Introduction
The KiSS-1/G protein-coupled receptor-54 (GPR54) system has recently been identified as a critical component of the hypothalamic-pituitary gonadal (HPG) axis. Mice and humans lacking functional GPR54 or KiSS-1 genes do not undergo pubertal development and are infertile (Funes et al., 2003; de Roux et al., 2003; Seminara et al., 2003; Semple et al., 2005; d'Anglemont de Tassigny et al., 2007; Lapatto et al., 2007). The KiSS-1 gene encodes a 145 amino acid protein (Muir et al., 2001; Ohtaki et al., 2001) that is cleaved into kisspeptin-54, corresponding to residues 68–121 of the full length KiSS-1 product (Ohtaki et al., 2001). Shorter KiSS-1 derived peptides, 14, 13 and 10 amino acids in length have also been identified (Kotani et al., 2001; Bilban et al., 2004). All of the kisspeptins are reported to have a similar affinity and efficacy in vitro at GPR54 (Kotani et al., 2001). However, kisspeptin-54 is likely to be the most effective form of kisspeptin in vivo (Thompson et al., 2006).
The stimulatory effects of kisspeptin on the HPG axis are now well characterized. Acute intracerebroventricular (i.c.v.) or peripheral administration of kisspeptin to rodents or primates, and peripheral administration to humans, stimulates the HPG axis (Tena-Sempere, 2006; Mead et al., 2007a). This effect is likely to be mediated primarily via hypothalamic gonadotrophin-releasing hormone (GnRH) (Gottsch et al., 2004; Irwig et al., 2004; Matsui et al., 2004; Thompson et al., 2004; Kinoshita et al., 2005; Messager et al., 2005; Shahab et al., 2005). GPR54 is expressed in the pituitary (Kotani et al., 2001; Muir et al., 2001; Kinoshita et al., 2005), and in vitro evidence suggests that kisspeptin can have a direct effect on gonadotrophin release from cultured rat pituitary cells (Navarro et al., 2005a,b). However, other studies have shown no direct effect of kisspeptin on gonadotrophin secretion from cultured rat pituitary cells or pituitary fragments in vitro (Matsui et al., 2004; Thompson et al., 2004). In addition, it has recently been shown that hypothalamic-pituitary disconnected ewes maintained with pulsatile GnRH do not respond to exogenous intravenous administration of kisspeptin (Smith et al., 2007), suggesting that direct effects of kisspeptin on the pituitary are unlikely to play a major role in kisspeptin-induced stimulation of the HPG axis in vivo.
The kisspeptin-GPR54 system is a putative target for drugs to treat reproductive disorders and manipulate the reproductive axis. Administration of kisspeptin to humans potently stimulates the HPG axis (Dhillo et al., 2005; Dhillo et al., 2007). The kisspeptins may thus have utility in initiating puberty in adolescents with delayed puberty and in the treatment of adult infertility. Conversely, GPR54 antagonists may be useful in the treatment of precocious puberty and as contraceptive agents. Interestingly, continuous peripheral administration of kisspeptins to monkeys and rodents results in desensitization of the HPG axis to their effects (Seminara et al., 2006; Thompson et al., 2006; Ramaswamy et al., 2007). Furthermore, the chronic long-term (13 days) continuous s.c. [subcutaneous(ly)] administration of 50 nmol kisspeptin-54 per day to adult rats leads to significant degeneration of the testes, with loss of both germ and Sertoli cells (Thompson et al., 2006). The mechanism by which chronic kisspeptin-54 causes testicular degeneration is unknown. Understanding the mechanism(s) of kisspeptin-54-induced testicular degeneration is essential if the kisspeptins are to be used to manipulate the reproductive axis in humans.
In the present study we investigated the mechanism(s) of kisspeptin-54-induced testicular degeneration in the adult rat. We performed a time course analysis of the effects of continuous s.c. kisspeptin-54 administration on testicular histology and plasma reproductive hormone levels. Surprisingly, continuous s.c. kisspeptin-54 administration led to testicular degeneration as early as 12 h after commencing continuous administration. We therefore investigated the effects of a single s.c. injection of high-dose kisspeptin-54 (50 nmol) on testicular histology and compared them with the effects of a high-dose s.c. injection of GnRH (50 nmol). In order to determine whether the testicular degeneration observed following both continuous and single s.c. high-dose kisspeptin-54 administration is peripherally or centrally mediated, we investigated the effect of a single i.c.v. injection of kisspeptin-54 on testicular histology. We also pre-administered a GnRH-receptor (GnRH-R) antagonist, cetrorelix, to rats peripherally treated with kisspeptin-54 to further clarify whether the effects of kisspeptin-54 on the testes are centrally mediated. Finally, we investigated whether the testes show histological recovery from kisspeptin-54-induced testicular degeneration, and whether there were any prolonged effects on plasma hormone levels. Taken together, the results suggest that kisspeptin-induced testicular degeneration is centrally mediated and results from acute hyper-stimulation of the HPG axis. The present findings are potentially of clinical significance if the kisspeptins are to be used as a treatment in reproductive disorders and suggest the therapeutic doses of kisspeptins will need to be chosen with care.
Methods
Animals
All animal procedures were conducted under the British Home Office Animals Scientific Procedures Act 1986 (Project License 70/5516) and in accordance with accepted standards of the local ethical review committee. Adult male Wistar rats (specific pathogen free, Charles River) weighing 275–325 g were housed in individual cages for the continuous Alzet® pump kisspeptin-54 studies and the i.c.v. study, and in groups of five for the peripheral single injection studies. Animals were maintained under controlled temperature (21–23°C) and light conditions (12:12 h light/dark cycle, lights on at 0700 h) with ad libitum access to food (RM1 diet, SDS UK Ltd.) and water.
Alzet® mini-osmotic pump insertion and peptide delivery
Alzet® mini-osmotic pumps, model 2001D (6 h, 12 h and 1 day treatment), model 2001 (2 and 3 day treatment) were filled with either 0.9% saline (control) or kisspeptin-54 (dissolved in 0.9% saline to deliver at a rate of 50 nmol·day−1) under sterile conditions, following the manufacturers instructions. The Alzet® pumps were then primed in vials containing 0.9% saline at 37°C overnight (model 2001), or for 3 h (model 2001D), before implantation so that they would deliver their contents immediately upon implantation. The Alzet® model 2001D infuses solutions at a rate of 8.0 µL·h−1 (±1.0 µL·h−1) for 1 day, and model 2001 infuses solutions at a rate of 1.0 µL·h−1 (±0.15 µL·h−1) for 7 days.
Study A:Effect of continuous s.c. kisspeptin-54 administration (50 nmol·day−1) for 1, 2 or 3 days on testicular histology and plasma inhibin B
We investigated the time course of effects of continuous administration of kisspeptin-54 on testicular degeneration and on plasma inhibin B levels. Rats were randomized into six groups (n = 8 per group). On day 0, under deep inhalation anaesthesia (halothane, Concord Pharmaceuticals Ltd) the rats were implanted s.c. in the interscapular region with the preloaded, primed pumps, containing either 0.9% saline or kisspeptin-54 (to deliver at a rate of 50 nmol·day−1) (Alzet® models used are detailed above). The surgery was performed as previously described (Thompson et al., 2006).
From day −1, body weight and food intake were measured each morning between 0900 h and 1000 h. Behaviour was observed throughout the study. Rats were killed by decapitation on days 1, 2 or 3 of the study between 0900 h and 1100 h. Trunk blood was collected into lithium-heparin tubes containing 0.6 mg of aprotinin (Bayer, Haywards Heath, UK). Plasma was separated by centrifugation, frozen on dry ice and stored at −20°C until measurement of inhibin B.
Histological examination
Following careful removal to avoid mechanical trauma, the left testis from the 1, 2 and 3 day treated rats (n = 8 per group) was perforated with a fine gauge needle, immersion fixed in Bouin's fixative for 24 h and subsequently stored in 70% ethanol until processing and embedding in paraffin. Following embedding, testes were serially sectioned at the longitudinal plane at 4 µm and stained with haematoxylin and eosin (H&E) following standard techniques (Drury and Wallington, 1967) for histology. The tissue sections closest to the largest cross-sectional area of the organs were used in the microscopic assessment (light microscope: Nikon Eclipse 80i; digital camera: Nikon DXM1200F). An average number of 250 seminiferous tubules were examined per animal, and the criteria used for histological examination followed the principles described in standard methods of rodent testicular analysis (Russell et al., 1990). Testicular degeneration was qualitatively assessed and quantitatively scored. The percentage of seminiferous tubules degenerated per section was scored as in previously published studies (Leon et al., 1987; Kerr and Sharpe, 1989). In addition, the Alzet® pumps were removed from all groups and examined to verify delivery of their contents.
Study B:(i) Investigating the earliest time point at which testicular degeneration is present following continuous s.c. kisspeptin-54 administration, and (ii) examining the hormonal profile at these early continuous administration time points
The results from Study A demonstrated that continuous s.c. kisspeptin-54 treatment led to testicular degeneration following only 1 day of continuous administration, which was the earliest time point examined. We therefore investigated the effects of shorter-term continuous kisspeptin-54 treatment (6, 12, and 24 h continuous kisspeptin-54 administration, to deliver at a rate of 50 nmol·24 h−1) on testicular degeneration and plasma luteinising hormone (LH), follicle-stimulating hormone (FSH) and testosterone. Rats were randomized into six groups (n = 5 per group). At time zero (t = 0) under deep inhalation anaesthesia (halothane, Concord Pharmaceuticals Ltd) the rats were implanted s.c. in the interscapular region with the preloaded, primed pumps, containing either 0.9% saline or kisspeptin-54 (Alzet® model 2001D, detailed above). Rats received continuous s.c. administration of saline or kisspeptin-54 and were killed by decapitation 6, 12 or 24 h after Alzet® pump insertion. Trunk blood was collected and stored as described above until measurement of LH, FSH and free testosterone. The left testes (n = 5 per group) were immediately dissected, weighed and prepared for histological analysis and assessed as described above. In addition, the Alzet® pumps were removed from all groups and examined to verify content delivery.
(iii)Investigating the specificity of kisspeptin-54-induced testicular degeneration
The possibility that the damaging effects of kisspeptin-54 on the testes could be due to contaminants in the kisspeptin-54 preparation and/or non-specific tissue degenerative effects was investigated. Aliquots of the same batch of kisspeptin-54 used in these studies were sent for pyrogen analysis by the Limulus Amoebocyte Lysate assay test (Associates of Cape Cod, Liverpool, UK). In addition, following continuous s.c. administration of saline or 50 nmol kisspeptin-54 for 24 h the vas deferens, prostate, epididymis, liver, heart, lung, small intestine and spleen were quickly removed, immersion fixed in buffered 10% formalin and subsequently stored in 70% ethanol until processing and embedding in paraffin. Following embedding, tissues were serially sectioned at 4 µm and stained with H&E following standard techniques (Drury and Wallington, 1967), for histological analysis (n = 5 per group).
(iv)Investigating the effect of a single s.c. injection of kisspeptin-54 on testicular degeneration
Following the results from Study A showing that short-term s.c. continuous kisspeptin-54 administration led to testicular degeneration in adult male rats, the effects of a single s.c. 50 nmol kisseptin-54 injection on testicular degeneration was investigated to determine whether the continuous administration of kisspeptin-54 is necessary for these degenerative effects. The 50 nmol dose of kisspeptin-54 was chosen as this was the dose given over 24 h in the continuous administration studies, and allows the comparison of the testes at 24 h following a single injection with those from the 24 h continuously treated group. Rats were randomized into two groups (n = 5 per group). At t = 0, rats received a single s.c. injection of either 0.9% saline or 50 nmol kisspeptin-54 and were killed by decapitation 24 h following injection. The left testes (n = 5 per group) were immediately dissected, weighed and prepared for histological analysis and assessed as described above.
Study C:(i) Dose response of single s.c. injections of kisspeptin-54 on testicular degeneration and comparison with a single s.c. injection of GnRH
We performed a dose–response study of the effects of acute s.c. administration of kisspeptin-54 on the testes. Evidence suggests that kisspeptin acts upstream of hypothalamic GnRH (Gottsch et al., 2004; Irwig et al., 2004; Matsui et al., 2004; Thompson et al., 2004; Kinoshita et al., 2005; Messager et al., 2005; Shahab et al., 2005). Continuous administration of GnRH agonists can also lead to testicular degeneration (Sandow et al., 1978; Rivier et al., 1979; Lefebvre et al., 1984; Rajfer et al., 1984; van Kroonenburgh et al., 1986; Ward et al., 1989; Zheng and Fulu, 2006). However, the effects of acute administration of GnRH are less characterized. We thus also compared the effects of an equal high dose of a single injection of kisspeptin-54 (50 nmol) and GnRH (50 nmol) on testicular degeneration.
Rats were randomized into five groups (n = 5 per group). At t = 0, rats received a single s.c. injection of either 0.9% saline, 0.5 nmol, 5 nmol or 50 nmol kisspeptin-54 or 50 nmol GnRH and were killed by decapitation 24 h following injection. The left testes (n = 5 per group) were immediately dissected and weighed. Following careful removal, the testes were prepared for histological analysis and assessed as described above (n = 5 per group).
(ii)Comparison of the effects of a single high-dose s.c. injection of kisspeptin-54 or GnRH on plasma LH, FSH and testosterone
In separate rats, we compared the effects of the same dose of a single s.c. injection of kisspeptin-54 (50 nmol) and GnRH (50 nmol) on plasma LH, FSH and testosterone at 60 min following injection. Rats were randomized into six groups (n = 5 per group). At t = 0, rats received a s.c. injection of either 100 µL 0.9% saline, 50 nmol kisspeptin-54 or 50 nmol GnRH. Rats were killed by decapitation at 60 min following injection. Trunk blood was collected from the rats killed at 60 min post injection, as described above, and stored at −20°C until measurement of LH, FSH and free testosterone.
Study D:Investigation of the effect of i.c.v. administration of kisspeptin-54 on testicular histology in adult male rats
i.c.v. cannulation and injections
Animals were implanted with permanent 22-gauge stainless steel i.c.v. cannulae projecting to the third cerebral ventricle (coordinates; 0.8 mm posterior to bregma on the midline and implanted 6.5 mm below the outer surface of the skull) as previously described (Rossi et al., 1997). Following a 7 day recovery period, the animals were acclimatized to the injection procedure and to the guillotine apparatus to minimize the metabolic consequences of stress on the study day. Only animals with correct cannula placement, as confirmed by a sustained drinking response to i.c.v. angiotensin II (150 ng), were included in the studies. All studies were carried out in the early light phase (0800–1100 h). Kisspeptin-54 was dissolved in 0.9% saline and administered in a 5 µL volume via a stainless steel injector projecting 1 mm beyond the tip of the cannula. The injector was connected by polyethylene tubing (inner diameter, 0.5 mm; outer diameter, 1 mm) to a Hamilton syringe (Reno, NV, USA) in a syringe pump set to dispense 5 µL solution per minute.
i.c.v. study procedure
Rats were randomized into two groups (n = 5 per group) and i.c.v. injected with either 0.9% saline or 5 nmol kisspeptin-54. Rats were decapitated at 24 h following injection. The left testes (n = 5 per group) were immediately dissected and weighed, prepared for histological analysis and assessed as described above.
Study E:Effect of s.c. administration of kisspeptin-54 following pretreatment with a GnRH-R antagonist on testicular degeneration
(i) A study was first performed to confirm that cetrorelix, a GnRH-R antagonist, inhibits the kisspeptin-54-induced stimulation of the HPG axis.
In this study two groups of rats (n = 5 per group) were pretreated with s.c. injection of 0.9% saline (200 µL), and two separate groups of rats (n = 5 per group) were pretreated with s.c. injection of cetrorelix (200 nmol·200 µL−1 saline). Thirty min later each group of rats were treated with either s.c. injection of 0.9% saline (200 µL) or kisspeptin-54 (50 nmol·200 µL−1 saline) (Matsui et al., 2004). The animals were decapitated, and their trunk blood was collected 60 min after kisspeptin-54 administration and stored as described above until measurement of LH, FSH and free testosterone.
(ii) Following confirmation that pretreatment with cetrorelix prevented s.c. kisspeptin-54 (50 nmol)-induced HPG axis stimulation, adult male rats were s.c. injected with kisspeptin-54 (50 nmol·200 µL−1 saline) or 0.9% saline alone (200 µL) 30 min after pretreatment with either s.c. cetrorelix (200 nmol·200 µL−1 saline) or 0.9% saline (200 µL). Rats were decapitated at 24 h following injection. The left testes (n = 5 per group) were immediately dissected and weighed, prepared for histological analysis and assessed as described above.
Study F:Testicular degeneration recovery study
We investigated whether the testicular degeneration following a single peripheral injection of high-dose kisspeptin-54 is reversible. Rats were randomized into three groups (n = 5 per group). At time zero (t = 0) on day 0 rats received a s.c. injection of either 0.9% saline, 50 nmol kisspeptin-54 or 50 nmol GnRH. Rats were killed by decapitation 2 months following injection. Trunk blood was collected and stored as described above until measurement of LH, FSH, free testosterone and inhibin B. The left testes (n = 5 per group) were immediately dissected, weighed and prepared for histological analysis and assessed as described above.
Hormone assays
Luteinising hormone and FSH levels in plasma were assayed by using reagents and methods provided by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Hormone and Pituitary Program (Dr A Parlow, Harbor University, Los Angeles Medical Center, Los Angeles, CA), as previously described (Beak et al., 1998). The intra- and inter-assay coefficients of variation for the LH assay were 8.2% and 13.6% respectively, and 8.3% and 12.4% for the FSH assay. Free testosterone in plasma was measured by EIA (Diagnostic Systems Laboratories, Oxfordshire, UK). Inhibin B was measured by solid-phase sandwich elisa (Oxford Bio-Innovation, Oxfordshire, UK) (Harris and Levine, 2003). The intra- and inter-assay coefficients of variation for the commercial assays were <10%.
Statistical analysis
Results are shown as mean values ± SEM. Plasma hormone measurements for all studies were compared by anova with post hoc Tukey adjustment (Systat). The weights of the testes for all studies were compared by anova with post hoc Tukey adjustment. In all cases P < 0.05 was considered to be statistically significant.
Materials
The drug and molecular target nomenclature used in this study conforms to the BJP Guide to Receptors and Channels (Alexander et al., 2006). Kisspeptin-54 was synthesized by the Advanced Biotechnology Centre, Imperial College (London, UK). GnRH was purchased from Bachem (UK) Ltd. The GnRH-R antagonist cetrorelix was purchased from Serono Ltd (Middlesex, UK).
Results
Study A:Effect of continuous s.c. kisspeptin-54 administration (50 nmol·day−1) for 1, 2 or 3 days on testicular histology and plasma inhibin B
(i) Testes: The absolute and relative weights of the left testes were not significantly changed following short-term 1, 2 or 3 day continuous s.c. kisspeptin-54 administration, compared with saline-treated controls. All saline-treated controls had normal testicular histology, with no seminiferous cell degeneration observed. However, administration of 50 nmol·day−1 kisspeptin-54 for 1, 2 or 3 days led to focal damage of the seminiferous tubules, which was present as early as 1 day post initiation of treatment. Individual tubules showed varying degrees of degeneration. There was variable cell maturation arrest, generation of multinucleated spermatid giant cells formed from round spermatids, sloughing and apoptosis of germ cells, with complete focal atrophy of germ cells (Figure 1A–D). There were no evident differences in Leydig cell morphology or cell number (Figure 1E,F). The median percentage [interquartile range] of seminiferous tubules showing degeneration following 1 day of continuous s.c. kisspeptin-54 administration was 41.2% [19%–44%]. The percentage of tubules showing degeneration following 2 or 3 days administration was similar to that following 1 day administration (29% [12.8%–42.7%] and 28.4% [12.8%–42.7%] respectively). (ii) Plasma inhibin B: Continuous s.c. administration of 50 nmol·day−1 kisspeptin-54 for 1 day had no effect on plasma inhibin B levels compared with saline-treated controls. However, following 2 days of s.c. kisspeptin-54 administration, plasma inhibin B levels were approximately 50% lower than the saline-treated controls. The plasma inhibin B levels remained significantly suppressed following 3 days kisspeptin-54 treatment (Figure 2).
Figure 1.
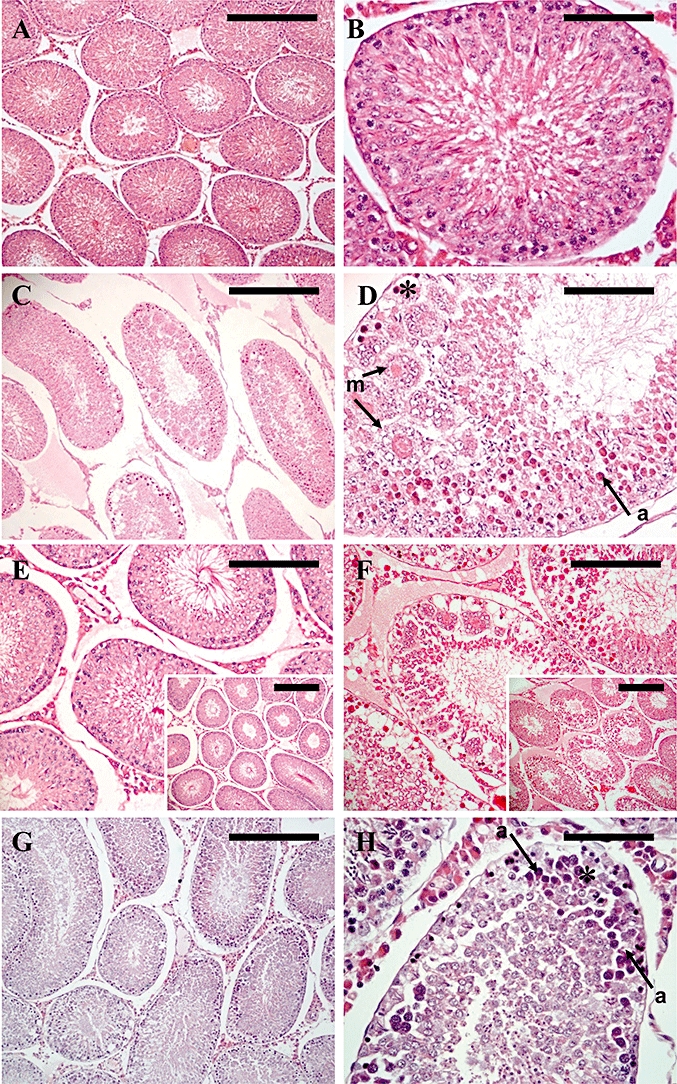
Testicular histology following continuous s.c. administration of kisspeptin-54 for 1 day (A–F) or 12 h (G & H). (A–F) Cross section of a seminiferous tubule assessed by light microscopy on H&E-stained sections from rats treated with continuous s.c. administration of (A, B & E) saline, or (C, D & F) 50 nmol·day−1 kisspeptin-54, for 1 day. (A) Representative section from a control animal showing tubules with normal maturation to late-stage elongated spermatids and a few spermatozoa. (B) Higher magnification of (A), to show a single tubule. (C) Representative section of an animal treated with continuous s.c. kisspeptin-54 administration for 1 day showing tubules with loss of seminiferous tubule structure with vacuolation, atrophy and formation of multinucleated giant cells. (D) Higher magnification of (C) to show a single tubule with severe necrotic degeneration of the germinal cells (asterisk), degeneration characterized by multinucleated giant cells (m, arrow) and apoptotic cells (a, arrow). (E & F) Comparison of histology between (E) a saline-treated animal and (F) a kisspeptin-54-treated animal showing comparable Leydig cell morphology (Original magnifications ×200. Scale bar represents 50 µm. Inset pictures, original magnifications ×100. Scale bar represents 100 µm). (G & H) Moderate testicular degeneration was present as early as following 12 h of continuous s.c. kisspeptin-54 administration. Cross section of a testicular tubule assessed by light microscopy on H&E-stained sections from adult rats treated with continuous s.c. administration of 50 nmol·day−1 kisspeptin-54, for 12 h. (G) Representative section of an animal treated with continuous s.c. kisspeptin-54 administration for 12 h showing seminiferous tubules with moderate degeneration. (H) Higher magnification of (G) showing a tubule with moderate degeneration of the germinal cells (asterisk) and apoptotic cells (a, arrow). (A, C & G: Original magnifications ×100. Scale bar represents 100 µm. B, D & H: Original magnifications ×400. Scale bar represents 25 µm). H&E, haematoxylin and eosin.
Figure 2.
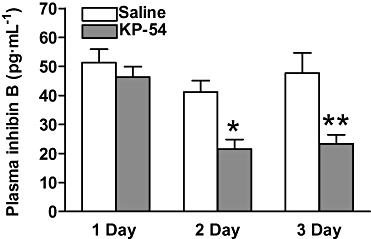
The effect of continuous, short-term (1, 2 and 3 day), s.c. administration of 50 nmol·day−1 kisspeptin-54 (KP-54) in adult male Wistar rats on plasma levels of inhibin B. All data are expressed as mean ± SEM. Significance is indicated by *P < 0.05, **P < 0.01 vs. saline-treated control (n = 8 per group).
Study B:(i) Testicular degeneration is present as early as 12 h following continuous s.c. kisspeptin-54 administration
The absolute and relative weights of the left testes were not significantly reduced following 6, 12 or 24 h continuous s.c. kisspeptin-54 administration, compared with saline-treated controls. All saline-treated controls had normal testicular histology, with no seminiferous tubule degeneration observed. Testicular degeneration was not observed following 6 h kisspeptin-54 administration, the earliest time point examined. Continuous s.c. administration of 50 nmol·day−1 kisspeptin-54 led to testicular degeneration following 12 h administration. In terms of histological features present, the degree of degeneration following 12 h of kisspeptin-54 administration was not as severe as that seen following administration for 24 h. Following 12 h of kisspeptin-54 administration there was moderate focal degeneration of the seminiferous tubules characterized by apoptosis and sloughing of germ cells (Figure 1G,H). Following 24 h of administration, the degeneration was as severe and showed the same features as that observed following 24 h continuous administration in Study A. The median percentage [interquartile range] of seminiferous tubules showing degeneration following 12 h and 24 h of continuous s.c. kisspeptin-54 administration were 12 h: 18.1% [10.1%–26.1%]; 24 h: 38.9 [24.3%–47.9%].
(ii)Early hormonal profile
Continuous s.c. administration of 50 nmol·day−1 kisspeptin-54 for 6 h significantly increased plasma LH almost 15-fold compared with saline-treated controls. Plasma LH was significantly increased following 12 h of continuous s.c. kisspeptin-54 administration. However, although the kisspeptin-54-stimulated increase in plasma LH following 12 h of continuous s.c. administration was almost 10-fold greater than saline-treated controls, it was significantly lower than the kisspeptin-54-stimulated plasma LH level following 6 h administration. Following 24 h of continuous kisspeptin-54 administration plasma LH was increased twofold compared with saline-treated controls; however, this increase did not reach statistical significance and was significantly lower than the plasma LH levels reached following 6 h and 12 h of kisspeptin-54 administration (Figure 3A).
Figure 3.
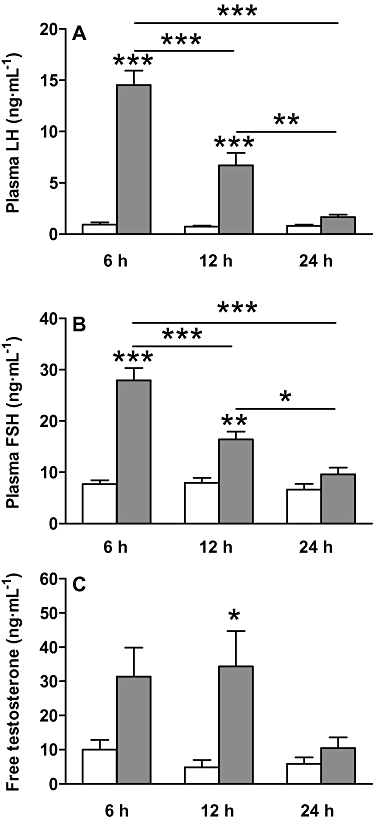
The effect of continuous, short-term (6, 12 and 24 h), s.c. administration of 50 nmol·day−1 kisspeptin-54 on the HPG axis. The effect of continuous, short-term (6, 12 and 24 h), s.c. administration of 50 nmol·day−1 kisspeptin-54 in adult male Wistar rats on plasma levels of (A) LH, (B) FSH and (C) free testosterone. All data are expressed as mean ± SEM. Significance is indicated by *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline-treated control, or a kisspeptin-54-treated group at a different time point (n = 5 per group). FSH, follicle-stimulating hormone; HPG, hypothalamic-pituitary gonadal; LH, luteinising hormone.
Continuous s.c. administration of 50 nmol·day−1 kisspeptin-54 for 6 h significantly increased plasma FSH approximately 3.5-fold compared with saline-treated controls. Plasma FSH remained significantly increased following 12 h of s.c. kisspeptin-54 administration, when it was approximately twofold greater than saline-treated controls. However, the kisspeptin-54-stimulated increase in plasma FSH following 12 h of s.c. administration was significantly lower than the kisspeptin-54-stimulated plasma FSH level following 6 h of administration. Following 24 h of kisspeptin-54 administration, plasma FSH was no longer significantly increased compared with saline-treated controls and was significantly lower that the plasma FSH levels reached following 6 h and 12 h of s.c. kisspeptin-54 administration (Figure 3B).
Continuous s.c. administration of 50 nmol·day−1 kisspeptin-54 for 6 h increased plasma free testosterone approximately threefold compared with saline-treated controls; however, this increase did not reach statistical significance (P = 0.1). Plasma free testosterone was significantly increased following 12 h of kisspeptin-54 administration compared with saline-treated controls and was approximately sevenfold greater than saline-treated controls. The absolute levels of plasma free testosterone following kisspeptin-54 administration for 12 h was similar to and not significantly different from the plasma free testosterone levels following 6 h administration (6 h: 31.4 ± 8.45 pg·mL−1, 12 h: 34.4 ± 10.31 pg·mL−1, P = 1.0). Following 24 h of kisspeptin-54 administration plasma free testosterone was no longer significantly increased compared with saline-treated controls (Figure 3C).
(iii)Kisspeptin-54 does not induce non-specific tissue degeneration
The Limulus Amoebocyte Lysate assay test (Associates of Cape Cod, Liverpool, UK) for pyrogen was negative, suggesting that the kisspeptin-54 used in these studies did not contain pyrogens. In addition, no other tissues histologically assessed [vas deferens, prostate, epididymis, liver, heart, lung, small intestine and spleen (n = 5 per group)] following continuous s.c. 50 nmol kisspeptin-54 administration for 24 h underwent degenerative changes and all were indistinguishable from saline-treated controls (data not shown).
(iv)A single s.c. injection of kisspeptin-54 leads to testicular degeneration
The absolute and relative weights of the left testes were not significantly reduced following a single s.c. injection of 50 nmol kisspeptin-54, compared with saline-treated controls, when assessed 24 h following injection. Interestingly, a single s.c. injection of 50 nmol kisspeptin-54 led to significant testicular degeneration compared with saline-treated controls, when observed 24 h after acute administration. All saline-treated animals had normal testicular histology, with no seminiferous tubule degeneration observed. The severity of degeneration seen 24 h after a single s.c. injection of 50 nmol kisspeptin-54 was comparable with that produced following continuous s.c. administration of 50 nmol of kisspeptin-54 over 24 h [Study A (i) and B (i) as described above]. The median percentage [interquartile range] of degenerated tubules following a single s.c. injection of 50 nmol kisspeptin-54 administration was similar to that produced by continuous s.c. administration of 50 nmol kisspeptin-54 over 24 h (continuous administration: 38.9% [24.3%–47.9%], single injection: 33.1% [28.8%–43.8%].
Study C:(i) Dose response of single s.c. kisspeptin-54 injections and comparison with a single s.c. GnRH injection on testicular degeneration
The absolute and relative weights of the left testes were not significantly reduced following a single s.c. injection of 0.5, 5 or 50 nmol kisspeptin-54, or 50 nmol GnRH compared with saline-treated control, when assessed 24 h following injection. All saline-treated animals had normal testicular histology, with no seminiferous cell degeneration observed (Figure 4A,B). A single s.c. injection of 0.5 nmol kisspeptin-54 did not lead to testicular degeneration at 24 h post injection. However, a s.c. injection of 5 nmol or 50 nmol kisspeptin-54 resulted in testicular degeneration. The median percentage [interquartile range] of seminiferous tubules showing signs of degeneration following 5 nmol s.c. kisspeptin-54 administration was 46% [12%–50.9%], and following 50 nmol kisspeptin-54 was 58.8% [40%–65.8%] (Figure 4C,D). The features were comparable to those described above [Study B (iv)].
Figure 4.
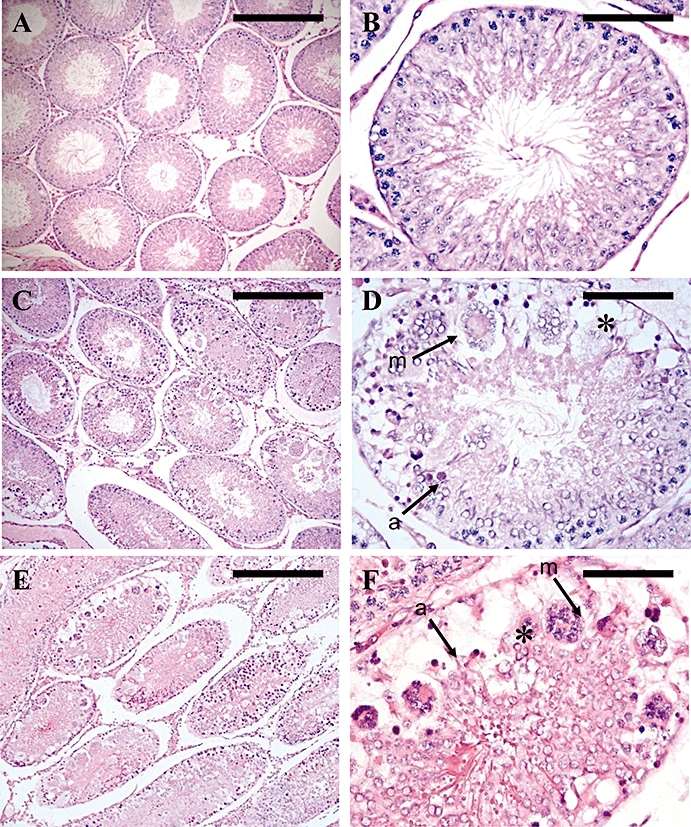
Testicular histology following a single s.c. injection of 50 nmol kisspeptin-54 or GnRH in the adult rat. Testicular histology was assessed 24 h following injection. Cross sections of testicular tubules assessed by light microscopy on H&E-stained sections from rats treated with single s.c. injection of (A & B) saline, (C & D) kisspeptin-54 (50 nmol) or (E & F) GnRH (50 nmol). (A) Representative section from a control animal showing tubules with normal maturation to late-stage elongated spermatids and a few spermatozoa. (B) Higher magnification of (A) to show a single tubule. (C) Representative section of an animal treated with a single s.c. injection of 50 nmol kisspeptin-54 showing severe testicular degeneration. (D) Higher magnification of (C) to show a tubule with severe necrotic degeneration and atrophy of the germinal cells (asterisk), degeneration characterized by multinucleated giant cells (m, arrow) and apoptotic cells (a, arrow). (E & F) Representative section of an animal treated with a single s.c. injection of 50 nmol GnRH showing the same features as described for kisspeptin-54 in C & D. (A, C & E: Original magnifications ×100. Scale bar represents 100 µm. B, D & F: Original magnifications ×400. Scale bar represents 25 µm). GnRH, gonadotrophin-releasing hormone; H&E, haematoxylin and eosin.
A single s.c. injection of 50 nmol GnRH also caused degeneration of the testes at 24 h post injection. Three animals showed severe testicular degeneration following acute GnRH administration, which was comparable, in terms of the histological features seen, with that induced by a single s.c. injection of 5 and 50 nmol kisspeptin-54 (Figure 4E,F). However, two animals did not experience testicular degeneration in any of the tubules assessed, reducing the median percentage of tubules showing degeneration. The percentage of tubules showing degeneration for the five animals injected with 50 nmol GnRH was 0%, 0%, 19.6%, 30% and 42.2%. The median percentage [interquartile range] of seminiferous tubules showing signs of degeneration following GnRH administration was 19.6% [0%–30%].
(ii)Circulating hormones following equal s.c. doses of kisspeptin-54 and GnRH
A single s.c. injection of 50 nmol kisspeptin-54 or 50 nmol GnRH significantly increased plasma LH at 60 min post injection approximately fivefold and sixfold respectively, compared with saline-treated control. The plasma LH levels following kisspeptin-54 or GnRH administration were not significantly different from each other (Figure 5A).
Figure 5.
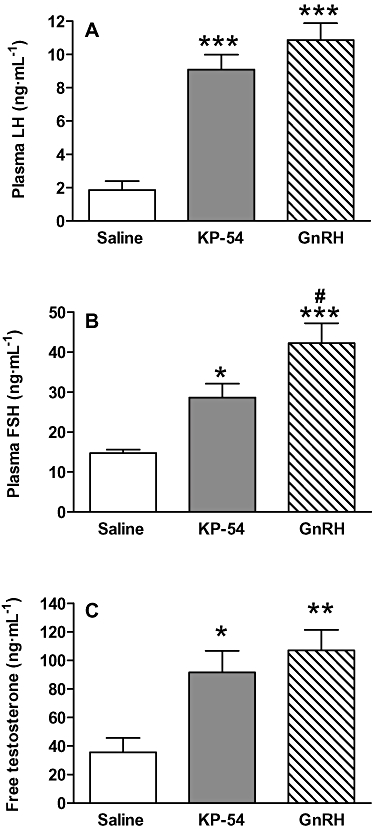
Comparison of s.c. kisspeptin-54 and GnRH on hormones of the HPG axis. Effect of s.c. administration of 50 nmol kisspeptin-54 (KP-54) and 50 nmol GnRH on plasma levels of (A) LH, (B) FSH and (C) free testosterone in adult male Wistar rats at 60 min post injection. All data are expressed as mean ± SEM. Significance is indicated by *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline-treated control, or #P < 0.05, GnRH vs. kisspeptin-54 (n = 5 per group). FSH, follicle-stimulating hormone; GnRH, gonadotrophin-releasing hormone; HPG, hypothalamic-pituitary gonadal; LH, luteinising hormone.
A single s.c. injection of 50 nmol kisspeptin-54 or 50 nmol GnRH significantly increased plasma FSH at 60 min post injection approximately twofold and threefold respectively, compared with saline-treated control. A s.c. injection of 50 nmol GnRH produced a significantly greater increase in plasma FSH than 50 nmol kisspeptin-54 at 60 min post injection (Figure 5B).
A single s.c. injection of 50 nmol kisspeptin-54 or 50 nmol GnRH significantly increased plasma free testosterone at 60 min post injection by approximately 2.5-fold and threefold respectively, compared with saline-treated control. The plasma free testosterone levels following kisspeptin-54 or GnRH were not significantly different from each other (Figure 5C).
The increases in plasma gonadotrophins induced by 50 nmol kisspeptin-54 or GnRH were diminished by 24 h following injection. At this time point there were no significant differences in plasma levels of these hormones between treatment groups (LH: saline 0.68 ± 0.25 ng·mL−1, kisspeptin-54 0.42 ± 0.10 ng·mL−1, GnRH 0.58 ± 0.26 ng·mL−1, no significance. FSH: saline 11.34 ± 0.74 ng·mL−1, kisspeptin-54 15.29 ± 1.24 ng·mL−1, GnRH 14.32 ± 2.06 ng·mL−1, no significance).
Study D:i.c.v. kisspeptin-54 on testicular degeneration
The absolute and relative weights of the left testes were not significantly reduced following i.c.v. administration of 5 nmol kisspeptin-54, compared with saline-treated controls, when assessed 24 h following injection. No saline-treated animals had abnormal testicular histology (Figure 6A,B). A single i.c.v. injection of 5 nmol kisspeptin-54 led to testicular degeneration in all animals, when the testes were assessed 24 h following i.c.v. injection. However, there was considerable variation in the number of tubules showing degeneration between animals. The histological features observed in the degenerated tubules were similar to those described above [Study A (i), B (i) and B (iv)] (Figure 6C,D). The median percentage [interquartile range] of degenerated tubules in the group that received 5 nmol kisspeptin-54 was 13.3% [4.7%–25%].
Figure 6.
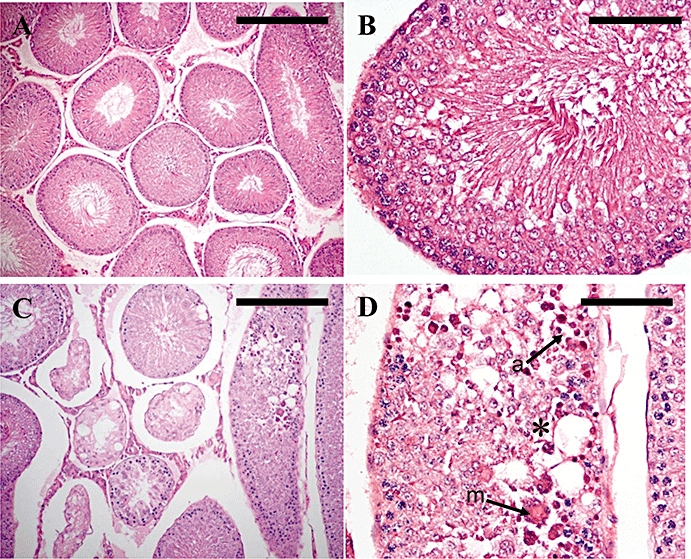
Testicular histology following a single i.c.v. injection of 5 nmol kisspeptin-54 in the adult rat. Testicular histology was assessed 24 h following injection. Cross sections of testicular tubules assessed by light microscopy on H&E-stained sections from rats treated with a single i.c.v. injection of (A & B) saline, or (C & D) 5 nmol kisspeptin-54. (A) Representative section from a control animal showing normal maturation to late-stage elongated spermatids and a few spermatozoa. (B) Higher magnification of (A) to show a single tubule. (C) Representative section from an animal that received 5 nmol i.c.v. kisspeptin-54, showing a severe testicular degeneration. (D) Higher magnification of (C) to show a tubule with severe necrotic degeneration and atrophy of the germinal cells (asterisk), degeneration characterized by multinucleated giant cells (m, arrow) and apoptotic cells (a, arrow). (A & C: Original magnifications ×100. Scale bar represents 100 µm. B & D: Original magnifications ×400. Scale bar represents 25 µm). H&E, haematoxylin and eosin; i.c.v., intracerebroventricular.
Study E:Pretreatment with a GnRH-R antagonist on kisspeptin-54-induced testicular degeneration
Subcutaneous pretreatment with cetrorelix (200 nmol) abolished the s.c. induced stimulation of the HPG axis by 50 nmol kisspeptin-54. A s.c. injection of 50 nmol kisspeptin-54 dramatically increased plasma LH (P < 0.001), FSH (P < 0.001) and free testosterone (P < 0.001) compared with saline-treated controls. Gonadotrophin and testosterone responses to 50 nmol kisspeptin-54 were blocked in rats pretreated with 200 nmol of GnRH-R antagonist cetrorelix (P < 0.001 cetrorelix plus kisspeptin-54 vs. kisspeptin-54 alone). There were no significant differences between the gonadotrophin or testosterone levels following treatment with cetrorelix alone, cetrorelix plus kisspeptin-54 or saline (Figure 7).
A single s.c. injection of 50 nmol kisspeptin-54 alone produced testicular degeneration in all animals at 24 h following administration. Histological features were consistent with those described above [Study B (iv) and Study C (i)], and, as previously seen following kisspeptin-54 administration, the number of seminiferous tubules showing degeneration varied between the animals. The percentage of tubules showing degenerative changes per testis section scored ranged from 2.7% to 50%. Pretreatment with the GnRH-R antagonist cetrorelix (200 nmol) completely prevented the kisspeptin-54-induced testicular degeneration in all animals, with none of the seminiferous tubules examined in this group showing signs of damage. No saline-treated rats or rats treated with cetrorelix alone showed any testicular degeneration (Figure 8).
Figure 7.
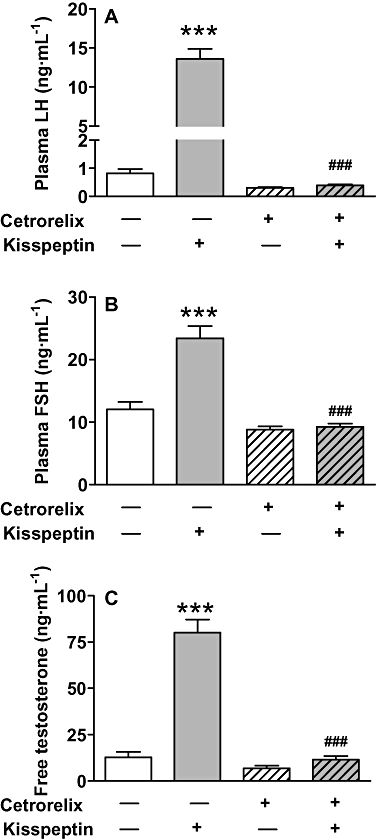
Plasma gonadotrophins and testosterone following a single s.c. injection of kisspeptin-54 following pretreatment with a GnRH-R antagonist in the adult male rat. The effects of s.c. administration of 50 nmol kisspeptin-54 30 min following s.c. pretreatment with a GnRH antagonist, cetrorelix (200 nmol) or saline alone in adult male Wistar rats on plasma levels of (A) LH, (B) FSH and (C) free testosterone at 60 min post injection of kisspeptin-54. All data are expressed as mean ± SEM. Significance is indicated by ***P < 0.001 vs. saline-treated control, or ###P < 0.001, cetrorelix plus kisspeptin-54 vs. kisspeptin-54 alone. There were no significant differences between cetrorelix- or cetrorelix plus kisspeptin-54-treated animals and animals treated with saline only (n = 8 per group). GnRH, gonadotrophin-releasing hormone; GnRH-R, GnRH-receptor; FSH, follicle-stimulating hormone; LH, luteinising hormone.
Figure 8.
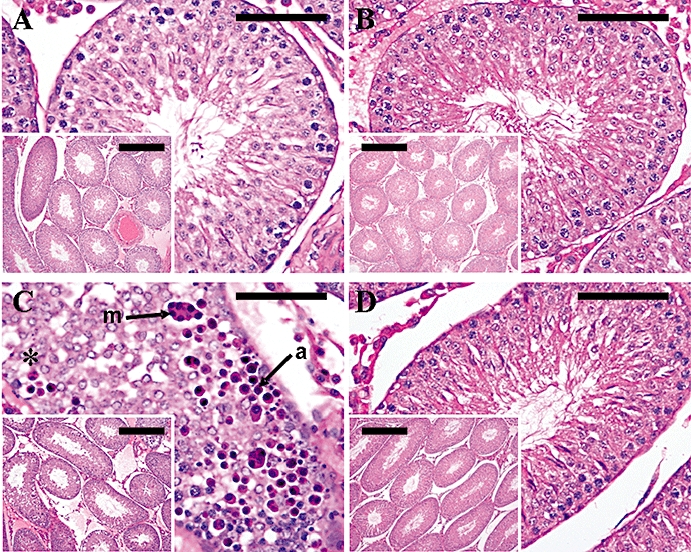
Testicular histology following a single s.c. injection of kisspeptin-54 following pretreatment with a GnRH-R antagonist in the adult male rat. The effect of s.c. administration of 50 nmol kisspeptin-54 30 min following s.c. pretreatment with a GnRH antagonist, cetrorelix (200 nmol) or saline alone in adult male Wistar rats on testicular histology. Testicular histology was assessed 24 h following injection of kisspeptin-54. Cross sections of testicular tubules assessed by light microscopy on H&E-stained sections. (A) Representative section from a control animal showing normal maturation to late-stage elongated spermatids and a few spermatozoa. (B) Representative section from an animal treated with cetrorelix alone showing normal maturation to late-stage elongated spermatids and a few spermatozoa. (C) Representative sections of an animal treated with s.c. kisspeptin-54 showing a tubule with severe necrotic degeneration and atrophy of the germinal cells (asterisk), degeneration characterized by multinucleated giant cells (m, arrow) and apoptotic cells (a, arrow). (D) Representative section from an animal pretreated with cetrorelix before a s.c. injection of kisspeptin-54, showing normal maturation to late-stage elongated spermatids and a few spermatozoa. (Original magnifications ×400. Scale bar represents 25 µm. Inset pictures, original magnifications ×100. Scale bar represents 100 µm). GnRH, gonadotrophin-releasing hormone; GnRH-R, GnRH-receptor; H&E, haematoxylin and eosin.
Study F:Testicular degeneration induced by high-dose s.c. single injection of kisspeptin-54 and GnRH persists 2 months after injection
(i)Testicular histology
A single s.c. injection of 50 nmol kisspeptin-54 led to a significant decrease in testis weight at 2 months following injection compared with saline-treated control. A single s.c. injection of 50 nmol GnRH caused a trend towards decreased testis weight at 2 months following injection, but this effect did not achieve statistical significance (left testis weight, saline 1.98 ± 0.08 g; kisspeptin-54 1.51 ± 0.13 g, P < 0.05 vs. saline; GnRH 1.70 ± 0.10 g, P = 0.06 vs. saline). There was no significant difference in testis weight between kisspeptin-54- and GnRH-treated rats.
In addition, the testicular degeneration produced by a single s.c. injection of 50 nmol kisspeptin-54 or 50 nmol GnRH was still present at 2 months following injection. The median percentage [interquartile range] of seminiferous tubules showing degeneration at 2 months following a single s.c. injection of high-dose kisspeptin-54 was 31% [13%–31%]. Kisspeptin-54 caused testicular degeneration in all animals treated. The median percentage [interquartile range] of seminiferous tubules showing degeneration at 2 months following a single s.c. injection of high-dose GnRH was 20% [0%–21%]. Two animals treated with GnRH did not experience testicular degeneration in any of the tubules assessed, reducing the median percentage of tubules showing degeneration. The percentage of tubules showing degeneration for the five animals injected with 50 nmol GnRH was 0%, 0%, 20%, 25.7% and 21.1%. The histological changes observed in the kisspeptin-54- and GnRH-treated animals were comparable. Histopathology revealed loss of seminiferous tubular structure, with vacuolation, atrophy and tubular hyalinization as common features (Figure 9). There were no evident differences in Leydig cell morphology or number (Figure 10).
Figure 9.
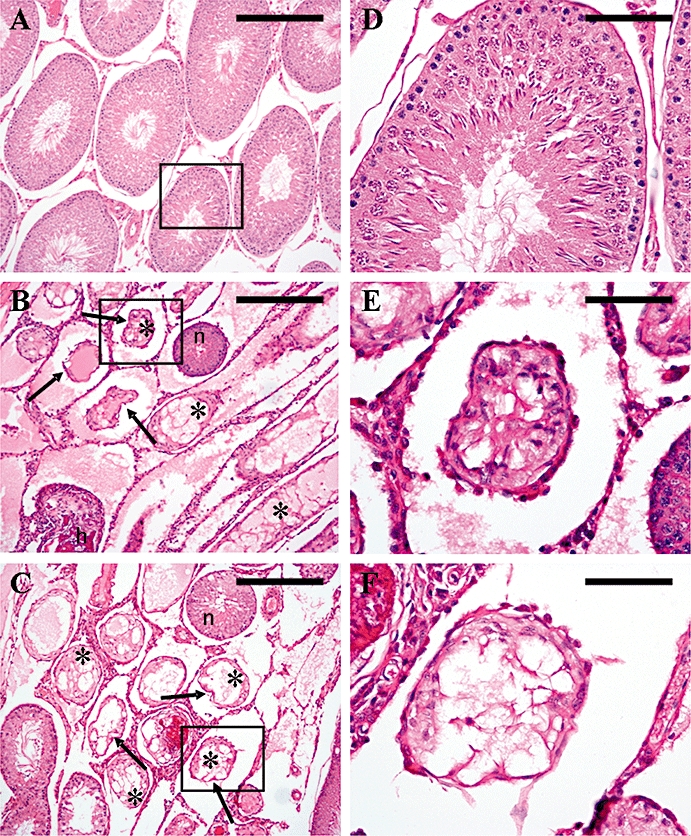
Seminiferous tubule histology at 2 months following a single s.c. injection of kisspeptin-54 (50 nmol) or GnRH (50 nmol) in the adult male rat. Cross sections of testicular tubules assessed by light microscopy on H&E-stained sections. (A) Representative section from a control animal showing tubules with normal maturation to late-stage elongated spermatids and a few spermatozoa. (B) Representative section of an animal treated with a single s.c. injection of 50 nmol kisspeptin-54 showing tubules with loss of seminiferous tubular structure (arrows) with vacuolation and atrophy (asterisk) and tubular hyalinization (h) as common features. The section also shows an apparently normal seminiferous tubule alongside the degenerated tubules (n). (C) Representative section of an animal treated with a single s.c. injection of 50 nmol GnRH showing the same features as described for kisspeptin-54 in (B). (Original magnifications ×100. Scale bar represents 100 µm). (D–F) Higher magnification of (A–C), to show a single tubule (boxed section) from each treatment group. (Original magnifications ×400. Scale bar represents 25 µm). GnRH, gonadotrophin-releasing hormone; H&E, haematoxylin and eosin.
Figure 10.
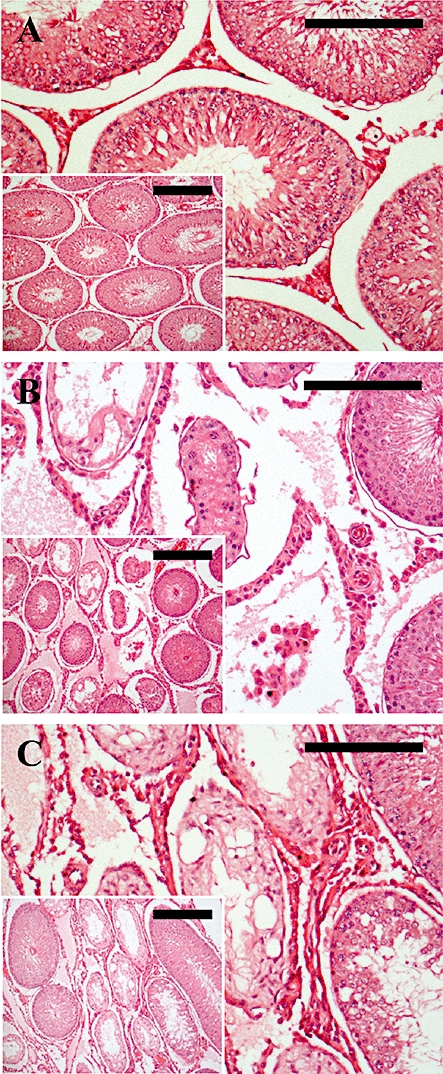
Leydig cell histology at 2 months following a single s.c. injection of kisspeptin-54 (50 nmol) or GnRH (50 nmol) in the adult male rat. Cross sections of testicular tubules assessed by light microscopy on H&E-stained sections. (A) Representative section from a control animal showing normal seminiferous tubules and Leydig cell morphology. (B) Representative section of an animal treated with a single s.c. injection of 50 nmol kisspeptin-54 showing normal Leydig cell morphology and cell number surrounding degenerated seminiferous tubules. (C) Representative section of an animal treated with a single s.c. injection of 50 nmol GnRH showing the same features as described for kisspeptin-54 in (B). (Original magnifications × 200. Scale bar represents 50 µm. Inset pictures, original magnifications × 100. Scale bar represents 100 µm). GnRH, gonadotrophin-releasing hormone; H&E, haematoxylin and eosin.
(ii)Circulating hormones at 2 months following a single s.c. injection of kisspeptin-54 or GnRH
A single s.c. injection of 50 nmol kisspeptin-54 or 50 nmol GnRH had no effect on circulating levels of LH or FSH at 2 months following injection (Figure 11A,B). The single s.c. injection of 50 nmol kisspeptin-54 or 50 nmol GnRH led to a trend towards decreased free testosterone at 2 months following injection, but this did not reach statistical significance (Figure 11C). The single s.c. injection of 50 nmol kisspeptin-54 led to a significant 2.5-fold decrease in plasma inhibin B (P < 0.05). There was also a trend towards decreased plasma inhibin B following a single s.c. injection of 50 nmol GnRH, but this effect did not reach statistical significance (P = 0.06) (Figure 11D).
Figure 11.
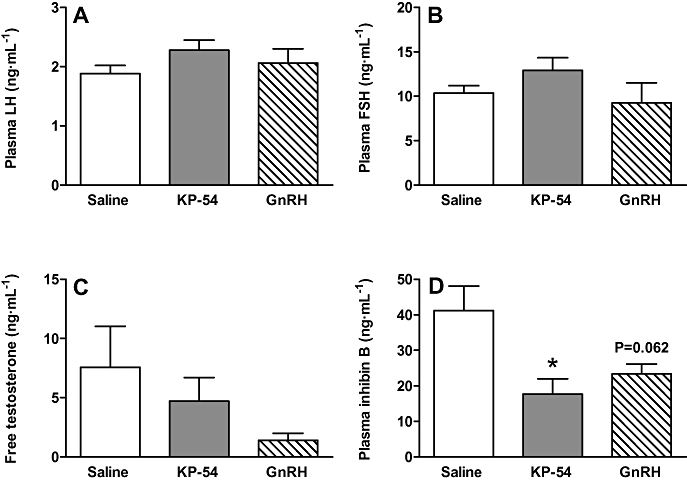
Plasma hormone levels at 2 months following a single injection of kisspeptin-54 or GnRH. The effects of a single s.c. injection of 50 nmol kisspeptin-54 (KP-54) or GnRH in adult male Wistar rats on plasma levels of (A) LH, (B) FSH, (C) free testosterone and (D) inhibin B at 2 months following injection. All data are expressed as mean ± SEM. Significance is indicated by *P < 0.05 vs. saline-treated control (n = 5 per group). FSH, follicle-stimulating hormone; GnRH, gonadotrophin-releasing hormone; LH, luteinising hormone.
Discussion
Our data suggest that acute administration of kisspeptin-54 can cause testicular degeneration via a GnRH-mediated effect. These results have important implications for targeting the kisspeptin system to treat reproductive disease.
We have previously shown that stimulation of the HPG axis following continuous s.c. administration of 50 nmol·day−1 kisspeptin-54 is completely abolished by 2 days of continuous treatment (Thompson et al., 2006). In the present study continuous s.c. administration of 50 nmol·day−1 kisspeptin-54 led to a dramatic increase in plasma LH, FSH and testosterone at 6 h, and while still largely increased at 12 h of continuous treatment there was a significant decrease in plasma gonadotrophins over time. These results suggest that, although stimulation of the HPG axis was still evident over a 24 h period, it was starting to become desensitized to the stimulatory effects of kisspeptin-54. However, the results of the current study suggest that kisspeptin-induced testicular degeneration in adult male rats is not due to the desensitization of the HPG axis to the effects of kisspeptin-54. In the present study the continuous s.c. administration of kisspeptin-54 led to testicular degeneration after only 12 h of administration. This effect was thus apparent before the HPG axis was completely desensitized to the stimulatory effects of kisspeptin-54. In addition, a single s.c. injection of high-dose kisspeptin-54 (50 nmol) also led to severe testicular degeneration. Therefore, the testicular degeneration seen following kisspeptin-54 administration is unlikely to be explained by the desensitization or down-regulation of the HPG axis. Importantly, s.c. kisspeptin-54 administration did not lead to degenerative changes in the vas deferens, prostate, epididymis, liver, heart, lung, small intestine or spleen. GPR54 mRNA has been detected in moderate levels in the rat small intestine (Lee et al., 1999; Terao et al., 2004) and liver (Lee et al., 1999), but has not been detected in the heart, lung or spleen (Lee et al., 1999; Terao et al., 2004). GPR54 expression has not been reported in the vas deferens, prostate and epididymis. The lack of kisspeptin-54-induced morphological changes in tissues that do or do not express GPR54 suggests that the degeneration caused by kisspeptin-54 administration in the testes is a specific effect.
The testicular degeneration following a single s.c. injection of kisspeptin-54 was dose-dependent. A s.c. injection of 0.5 nmol kisspeptin-54 has previously been shown to produce a submaximal increase in plasma LH (Thompson et al., 2006), but in the present study had no effect on the testicular histology. However, administration of 5 or 50 nmol kisspeptin-54, which produce a maximal increase in plasma LH (Thompson et al., 2006), did cause testicular degeneration. We hypothesized that the testicular degenerative effects of continuous or acute peripheral kisspeptin-54 administration may be a result of hyper-stimulation of the HPG axis. If this is the case, the effects are likely to be centrally mediated via an increase in hypothalamic GnRH release. Interestingly, in the present study we have shown that a single s.c. injection of 50 nmol GnRH can also cause testicular degeneration. Although not all animals were affected, three of the five GnRH-treated animals displayed the same histological features and severity of degenerative effects as those seen following a single s.c. injection of 50 nmol kisspeptin-54. To the best of our knowledge there are no reported studies on the acute effects of GnRH on the testes. However, in accord with our findings, the single injection of a potent GnRH super-agonist has previously been shown to cause severe damage to the rat testis within 24 h of administration (Habenicht et al., 1985). In the present study, the increase in plasma LH and testosterone were also comparable following a single s.c. injection of either 50 nmol kisspeptin-54 or 50 nmol GnRH at 60 min following administration. Therefore, at this dose and time point, kisspeptin-54 and GnRH are equipotent in stimulating LH and testosterone release and both cause comparable testicular degeneration. The stimulation of the HPG axis following a single injection of 50 nmol kisspeptin-54 or GnRH was lost by 24 h following injection. A single s.c. injection of a similar dose of kisspeptin-54 to that used in the present study (100 nmol·kg−1) to adult male rats has been shown to maximally stimulate plasma LH and FSH at 2 h after injection. However, by 4 h following injection, plasma LH was not significantly different from saline-treated controls (Matsui et al., 2004). The kisspeptin-54-induced hyper-stimulation of the HPG axis is therefore likely to be lost within 4 h. To further support the hypothesis that kisspeptin-54-induced testicular degeneration is gonadotrophin-mediated, we have demonstrated that a single i.c.v. injection of high-dose kisspeptin-54 (5 nmol) led to testicular degeneration. This dose of kisspeptin-54 is higher than the dose required for maximal stimulation of gonadotrophin and testosterone release (Thompson et al., 2004; Navarro et al., 2005a,b; Patterson et al., 2006). Although the percentage of damaged seminiferous tubules varied between animals, there was qualitative evidence of tubule degeneration following central administration of high-dose kisspeptin-54, in marked contrast to saline-treated animals that showed no degeneration. It therefore appears that the effects of kisspeptin-54 on testicular degeneration are likely to be centrally mediated, rather than a direct effect of kisspeptin-54 on the testes. Although there are no reported studies on the effects of acute LH administration on testicular histology, treating adult hypophysectomized male rats with LH twice daily for 2 weeks also dose-dependently disrupts the seminiferous tubules, with comparable histological features (Kerr and Sharpe, 1986). Interestingly, the single or repeated peripheral injection of pharmacological doses of human chorionic gonadotrophin (hCG), which acts via the LH-receptor, can lead to testicular degeneration in rats, with patchy necrosis and tubular atrophy (Leon et al., 1987; Kerr and Sharpe, 1989; Chatani, 2006; Karaman et al., 2006). Although hCG is used clinically to stimulate testicular descent and stimulate spermatogonia maturation in cryptorchid boys (Lala et al., 1997), similar testicular degenerative changes have been observed in follow-up of hCG-treated boys (Hjertkvist et al., 1993; Heiskanen et al., 1996). The time course of effects and histological features that are manifested following a single injection of hCG, are similar to those seen in the present study following peripheral kisspeptin-54 administration, leading to degeneration of germ cells in focal areas between 6 and 12 h following injection (Leon et al., 1987; Kerr and Sharpe, 1989). In the present study the high-dose s.c. kisspeptin-54-induced testicular degeneration was completely prevented by pretreatment with a GnRH-R antagonist, strongly supporting the hypothesis that the testicular degeneration seen in the present study following high doses of kisspeptin-54 is a result of hyper-stimulation of the HPG axis and is driven by the marked, and presumably supraphysiological, increase in plasma LH.
It is possible that the degeneration represents in part a direct testicular effect of kisspeptin-54. GPR54 has been reported to be expressed in the testis, although the cell type and physiological significance has not been determined (Kotani et al., 2001; Ohtaki et al., 2001; Funes et al., 2003; Terao et al., 2004). One possibility is that the testicular degeneration seen following kisspeptin administration may represent changes in testicular blood flow. Decreases in testicular blood flow result in focal damage of the seminiferous tubules (Markey et al., 1994; Bergh et al., 2001). GPR54 mRNA is expressed in the smooth muscle of the aorta, coronary artery and umbilical vein (Mead et al., 2007b), but its expression in the blood vessels of the testis has not been investigated. Kisspeptins have been shown to act as constrictors of isolated rings of coronary artery in vitro (Mead et al., 2007b). One study has detected kisspeptin mRNA in the human testis by quantitative reverse transcriptase polymerase chain reaction (Ohtaki et al., 2001), but it has not been detected in the testis of the rat (Terao et al., 2004). Thus it is possible that kisspeptins may play an autocrine or paracrine role in the testis. However, our finding that pretreatment with a GnRH-R antagonist prevented the testicular degeneration caused by kisspeptin-54 suggests that it is unlikely that a direct effect of kisspeptin-54 on the testes plays any major role in inducing testicular degeneration.
The testicular degeneration following continuous kisspeptin-54 administration was accompanied by a significant decrease in circulating inhibin B after 2 days of continuous administration. Plasma inhibin B, produced predominantly by the Sertoli cells, usually correlates with Sertoli cell number (Ramaswamy et al., 1999; Sharpe et al., 1999). Inhibin B has been proposed as a sensitive endocrine marker reflecting the state of spermatogenesis, with low levels reflecting a disturbance of spermatogenesis (Pierik et al., 2003). Interestingly, the kisspeptin-54- and GnRH-induced testicular degeneration following a single 50 nmol s.c. injection remained visible 2 months after administration. This degeneration was associated with a trend towards decreased plasma testosterone. There was no obvious change in the morphology or cell number of the Leydig cells between treatment groups. However, it is possible that the trend towards a decrease in testosterone may indicate a defect in the steroidogenic potential of the Leydig cells. It would be interesting to investigate this possibility further. There was a significant decrease in plasma inhibin B at 2 months following injection suggesting that the visible damage may reflect a persistent disruption of spermatogenesis (Pierik et al., 2003). It would be useful to investigate whether fertility is disturbed and whether the testes may recover from the degenerative effects at a later time point after cessation of treatment. A single oral dose of the indenopyridine, CDB-4022, which is in development as a male contraceptive, leads to testicular degeneration and a progressive decrease in testes weight and plasma inhibin B levels that reach non-detectable levels by 14 days (Koduri et al., 2008). The long-term suppression of plasma inhibin B may be attributable to a lasting effect on the Sertoli cell and/or the lack of differentiating germ cells in the seminiferous tubules (Koduri et al., 2008). Sustained testicular degeneration has also been observed in the adult rat following a single injection of hCG at 3 months following injection (Leon et al., 1987). Leon et al. suggest that the extreme germ cell depletion, tubular hyalinization and loss of cellular content were likely to represent the late evolution of the acute damage. The mechanism by which an acute hyper-stimulation of the HPG axis is responsible for sustained testicular degeneration is unclear, but may result from the acutely raised plasma LH. The mechanism by which hCG causes testicular damage appears to involve induction of local testicular production of inflammatory mediators and vascular regulators, resulting in testis inflammation that impairs circulation and results in hypoxia (Chatani, 2006; Bergh and Soder, 2007).
Thus in summary, pharmacological doses of kisspeptin-54 can cause testicular degeneration in the adult male rat, which is likely to be a result of central hyper-stimulation of the HPG axis. Importantly, the effects of acute kisspeptin-54 on the testes were dose-dependent. It is possible to stimulate the HPG axis with kisspeptin-54 without causing damage to the testes. The kisspeptins therefore represent a potential tool for HPG axis manipulation in humans, but our results suggest that the doses used must be chosen with considerable care.
Acknowledgments
ELT is a supported by a Biotechnology and Biological Sciences Research Council-GlaxoSmithKline case studentship. MP is supported by the Biotechnology and Biological Sciences Research Council. JHC is supported by the MRC. WSD is supported by a Department of Health Clinician Scientist Award. KGM is supported by a Biotechnology and Biological Sciences Research Council New Investigator Award. The department is funded by programme grants from the MRC (G7811974) and Wellcome Trust (072643/Z/03/Z) and by an EU FP6 Integrated Project Grant LSHM-CT-2003-503041. We are also grateful for support from the NIHR Biomedical Research Centre funding scheme and an IMB Capacity building award.
Glossary
Abbreviations:
- FSH
follicle-stimulating hormone
- GnRH
gonadotrophin-releasing hormone
- GnRH-R
GnRH-receptor
- H&E
haematoxylin and eosin
- hCG
human chorionic gonadotrophin
- HPG
hypothalamic-pituitary gonadal
- LH
luteinising hormone
Conflict of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (2nd edn) 2006;147(Suppl.)(3):S1–S180. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beak SA, Heath MM, Small CJ, Morgan DG, Ghatei MA, Taylor AD, et al. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J Clin Invest. 1998;101:1334–1341. doi: 10.1172/JCI610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh A, Soder O. Studies of cryptorchidism in experimental animal models. Acta Paediatr. 2007;96:617–621. doi: 10.1111/j.1651-2227.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- Bergh A, Collin O, Lissbrant E. Effects of acute graded reductions in testicular blood flow on testicular morphology in the adult rat. Biol Reprod. 2001;64:13–20. doi: 10.1095/biolreprod64.1.13. [DOI] [PubMed] [Google Scholar]
- Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- Chatani F. Possible mechanism for testicular focal necrosis induced by hCG in rats. J Toxicol Sci. 2006;31:291–303. doi: 10.2131/jts.31.291. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92:3958–3966. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- Drury RAB, Wallington EA. Carleton's Histological Techniques. London: Oxford University Press; 1967. [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Habenicht UF, el Etreby MF, Neumann F. Possible vascular effects of an LH-RH agonist on the peripheral circulation of the rat testes. Andrologia. 1985;17:440–443. doi: 10.1111/j.1439-0272.1985.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Levine JE. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology. 2003;144:163–171. doi: 10.1210/en.2002-220767. [DOI] [PubMed] [Google Scholar]
- Heiskanen P, Billig H, Toppari J, Kaleva M, Arsalo A, Rapola J, et al. Apoptotic cell death in the normal and cryptorchid human testis: the effect of human chorionic gonadotropin on testicular cell survival. Pediatr Res. 1996;40:351–356. doi: 10.1203/00006450-199608000-00026. [DOI] [PubMed] [Google Scholar]
- Hjertkvist M, Lackgren G, Ploen L, Bergh A. Does HCG treatment induce inflammation-like changes in undescended testes in boys? J Pediatr Surg. 1993;28:254–258. doi: 10.1016/s0022-3468(05)80288-x. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Karaman IM, Kaya C, Ozturk M, Pirincci N, Yimazgumrukcu G, Tuken M. The effects of human chorionic gonadotrophin on normal testicular tissue of rats: dose-dependence and reversibility. BJU Int. 2006;97:1116–1118. doi: 10.1111/j.1464-410X.2006.06139.x. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Sharpe RM. Effects and interactions of LH and LHRH agonist on testicular morphology and function in hypophysectomized rats. J Reprod Fertil. 1986;76:175–192. doi: 10.1530/jrf.0.0760175. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Sharpe RM. Focal disruption of spermatogenesis in the testis of adult rats after a single administration of human chorionic gonadotrophin. Cell Tissue Res. 1989;257:163–169. doi: 10.1007/BF00221647. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Koduri S, Hild SA, Pessaint L, Reel JR, Attardi BJ. Mechanism of action of l-CDB-4022, a potential nonhormonal male contraceptive, in the seminiferous epithelium of the rat testis. Endocrinology. 2008;149:1850–1860. doi: 10.1210/en.2007-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- van Kroonenburgh MJ, Beck JL, Vemer HM, Rolland R, Thomas CM, Herman CJ. Effects of a single injection of a new depot formulation of an LH-releasing hormone agonist on spermatogenesis in adult rats. J Endocrinol. 1986;111:449–454. doi: 10.1677/joe.0.1110449. [DOI] [PubMed] [Google Scholar]
- Lala R, Matarazzo P, Chiabotto P, Gennari F, Cortese MG, Canavese F, et al. Early hormonal and surgical treatment of cryptorchidism. J Urol. 1997;157:1898–1901. [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, et al. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Lefebvre FA, Belanger A, Pelletier G, Labrie F. Recovery of gonadal functions in the adult male rat following cessation of five-month daily treatment with an LHRH agonist. J Androl. 1984;5:181–192. doi: 10.1002/j.1939-4640.1984.tb02391.x. [DOI] [PubMed] [Google Scholar]
- Leon MD, Chiauzzi VA, Calvo JC, Charreau EH, Chemes HE. Acute hCG administration induces seminiferous tubule damage in the adult rat. Acta Physiol Pharmacol Latinoam. 1987;37:277–288. [PubMed] [Google Scholar]
- Markey CM, Jequier AM, Meyer GT, Martin GB. Testicular morphology and androgen profiles following testicular ischaemia in rams. J Reprod Fertil. 1994;101:643–650. doi: 10.1530/jrf.0.1010643. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br J Pharmacol. 2007a;151:1143–1153. doi: 10.1038/sj.bjp.0707295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels. Endocrinology. 2007b;148:140–147. doi: 10.1210/en.2006-0818. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005a;146:1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005b;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Patterson M, Murphy KG, Thompson EL, Patel S, Ghatei MA, Bloom SR. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. J Neuroendocrinol. 2006;18:349–354. doi: 10.1111/j.1365-2826.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- Pierik FH, Burdorf A, de Jong FH, Weber RF. Inhibin B: a novel marker of spermatogenesis. Ann Med. 2003;35:12–20. doi: 10.1080/07853890310004084. [DOI] [PubMed] [Google Scholar]
- Rajfer J, Swerdloff RS, Heber DM. Testicular histology following chronic gonadotropin-releasing hormone agonist treatment. Fertil Steril. 1984;42:765–771. doi: 10.1016/s0015-0282(16)48205-1. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, McNeilly AS, Plant TM. Evidence that in a physiological setting Sertol cell number is the major determinant of circulating concentrations of inhibin B in the adult male rhesus monkey (Macaca mulatta) J Androl. 1999;20:430–434. [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Pohl CR, Dipietro MJ, Crowley WF, Jr, Plant TM, et al. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364–3370. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivier J, Vale W. Chronic effects of [D-Trp6,Pro9-NEt]luteinizing hormone-releasing factor on reproductive processes in the male rat. Endocrinology. 1979;105:1191–1201. doi: 10.1210/endo-105-5-1191. [DOI] [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O'Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Hikim AP, Clegg ED. Histopathology of the testis- quantitative evaluation of testis histopathology. In: Russel LD, Ettlin RA, Sinha Hikim AP, Clegg ED, editors. Histological and Histopathological Evaluation of the Testis. St Louis: Cache River Press; 1990. pp. 240–264. [Google Scholar]
- Sandow J, Von RW, Jerzabek G, Stoll W. Pituitary gonadotropin inhibition by a highly active analog of luteinizing hormone-releasing hormone. Fertil Steril. 1978;30:205–209. doi: 10.1016/s0015-0282(16)43461-8. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, et al. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–1855. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Turner KJ, McKinnell C, Groome NP, Atanassova N, Millar MR, et al. Inhibin B levels in plasma of the male rat from birth to adulthood: effect of experimental manipulation of Sertoli cell number. J Androl. 1999;20:94–101. [PubMed] [Google Scholar]
- Smith JT, Pereira A, Rao A, Morgan K, Millar R, Clarke IJ. Evidence that Pituitary Gonadotropes are not a Major Target of Kisspeptin. The Endocrine Society's 89th Annual Meeting. 2-6-2007. Ref Type: Abstract.
- Tena-Sempere M. GPR54 and kisspeptin in reproduction. Hum Reprod Update. 2006;12:631–639. doi: 10.1093/humupd/dml023. [DOI] [PubMed] [Google Scholar]
- Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, et al. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta. 2004;1678:102–110. doi: 10.1016/j.bbaexp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Murphy KG, Patterson M, Bewick GA, Stamp GW, Curtis AE, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab. 2006;291:E1074–E1082. doi: 10.1152/ajpendo.00040.2006. [DOI] [PubMed] [Google Scholar]
- Ward JA, Furr BJ, Valcaccia B, Curry B, Bardin CW, Gunsalus GL, et al. Prolonged suppression of rat testis function by a depot formulation of Zoladex, a GnRH agonist. J Androl. 1989;10:478–486. doi: 10.1002/j.1939-4640.1989.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Zheng JY, Fulu MY. Decrease of genital organ weights and plasma testosterone levels in rats following oral administration of leuprolide microemulsion. Int J Pharm. 2006;307:209–215. doi: 10.1016/j.ijpharm.2005.10.007. [DOI] [PubMed] [Google Scholar]


