Abstract
Background and purpose:
Cyclo-L-glycyl-L-2-allylproline (NNZ-2591), a modified diketopiperazine, is neuroprotective and improves long-term function after hypoxic-ischaemic brain injury in rats. The present studies were designed to examine both the neuroprotective and neurotrophic actions of NNZ-2591 on neurochemical and behavioural changes in a rat model of Parkinson's disease.
Experimental approach:
To examine its protective effect, either NNZ-2591 (20 ng·day−1) or saline was given intracerebroventricularly for 3 days starting 2 h after 6-hydroxydopamine (6-OHDA) induced unilateral striatal lesion. In a subsequent experiment either NNZ-2591 (0.2, 1 and 5 mg·day−1, s.c.) or saline was administered daily for 14 days starting 2 weeks after the lesion. Behavioural and neurochemical outcomes were examined using the adjusting step test and immunohistochemical staining.
Key results:
Cyclo-L-glycyl-L-2-allylproline given 2 h after the lesion reduced the degree of motor deficit compared with the saline-treated group. Delayed treatment with NNZ-2591, initiated after the onset of motor deficit, significantly improved motor function from week 7 onwards compared with the saline-treated group. Neither treatment regime altered nigrostriatal dopamine depletion. NNZ-2591 significantly enhanced the expression of doublecortin-positive neuroblasts in the sub-ventricular zone.
Conclusions and implications:
These studies reveal that early treatment with NNZ-2591 protects against the motor deficit induced by 6-OHDA and that treatment initiated after the establishment of motor impairment significantly improves long-term motor function. These effects of NNZ-2591 on functional recovery were independent of dopamine depletion and also appeared not to be symptomatic as the improved motor function was long-lasting. NNZ-2591 has potential as a therapeutic agent for neurodegenerative disorders.
Keywords: diketopiperazine, functional recovery, forelimb akinesia, Parkinson's disease, rats and 6-OHDA lesion
Introduction
Parkinson's disease (PD) is a common neurodegenerative disorder characterized by severe motor disabilities such as bradykinesia, tremor and rigidity. Degeneration of dopaminergic neurons begins long before the motor deficit emerge, with >80% striatal dopamine depletion occurring in advance of the onset of symptoms (Hodaie et al., 2007). Endogenous neurogenesis and plasticity have been considered to play a key role in maintaining motor function during the pre-symptomatic period (Bezard and Gross, 1998). Because of dissatisfaction with current treatments, the search for new interventions continues, particularly for agents with the potential to improve motor function.
Naturally occurring diketopiperazines can be derived from neurotrophic factors and have been reported to be neuroprotective and to improve long-term function (Ikegami and Prasad, 1990; Faden et al., 2003; Guan et al., 2007). Cyclo-L-glycyl-L-2-allylproline (NNZ-2591) is a modified diketopiperazine (Guan et al., 2007) based on the endogenous diketopiperazine cyclo-glycyl-proline (cyclic GP) (Samonina et al., 2002). Both cyclic GP and NNZ-2591 have been shown to be neuroprotective after hypoxic-ischaemic (HI) brain injury in rats (Guan et al., 2007). NNZ-2591 crosses the blood–brain barrier independent of HI brain injury and remains detectable in the cerebrospinal fluid (CSF) several hours after a single administration (Guan et al., 2007). Peripheral administration of NNZ-2591 improves long-term somatosensory-motor function after HI injury in rats. Prevention of both initial and progressive neuronal loss has been suggested as the mechanism underlying its long-term neuroprotection (Guan et al., 2007). Given the progressive nature of PD and the massive loss of dopamine prior to its diagnosis, we examined the potential protective and reparative effects of NNZ-2591 on dopaminergic neurons and its effect on functional recovery in a rat model of PD.
Methods
This study was approved by the Animal Ethics Committee of Auckland University. All efforts were made to minimize pain and discomfort during experimental manipulation and the number of animals used. The experiments were conducted in accordance with international standards on animal welfare and compliant with local regulations. Wistar rats (male, 280–310 g) were supplied from the Animal Resources Unit, Auckland University, New Zealand.
Intrastriatal 6-OHDA lesion
Stereotaxic surgical procedures for 6-hydroxydopamine (6-OHDA) lesions were carried out as described previously (Krishnamurthi et al., 2004). Briefly, 6-OHDA solution was made by dissolving 7 µg 6-OHDA in 2 µL of 0.2% ascorbic acid. The solution was made fresh on the day of surgery, protected from exposure to light and kept at 4°C until surgery. An infusion pump connected to a 40 cm polyethylene catheter (internal diameter 0.35 mm) and a 25 G needle was used for the administration of 6-OHDA. The rats were administered with two (7 µg in 2 µL) 6-OHDA injections into the right striatum under halothane (3%) anaesthesia. The co-ordinates for the double striatal lesions were (i) R 3.0 mm, V −6.0 mm, AP +8.0 mm from the interaural zero plane; and (ii) R 3.5 mm, V −6.5 mm AP +7.0 mm from the interaural zero plane (Paxinos and Watson, 1982), respectively. This partial striatal lesion induces a moderate and more progressive dopamine depletion compared with the more severe and rapid lesion induced by a single injection to the medial forebrain bundle (Krishnamurthi et al., 2004). After recovery the rats were housed under standard conditions with free access to rat chow and water.
Adjusting steps test
All animals were habituated to this test for three consecutive days prior to the 6-OHDA lesion. Data from the third habituation were used as pre-lesion control. The slowness of initiating movement of the forelimb was examined using the adjusting steps test, which has been used widely for the evaluation of 6-OHDA-induced unilateral Parkinsonian motor deficit (Olsson et al., 1995; Kirik et al., 1998; Pinna et al., 2007). It is one of few tests that can detect motor deficit from moderate dopamine depletion, which does not respond to apomorphine-induced rotational behaviour effectively (Kirik et al., 1998). The procedure was performed as described previously (Krishnamurthi et al., 2004). Briefly, both forepaws were tested consecutively. During the test, the rat was held by both hind limbs and the untested forepaw. The test forepaw was placed on a flat surface and dragged in the forehand direction for 90 cm distance over 5 s. The number of adjusted steps was counted and averaged over two consecutive runs on each forepaw. The expected deficit caused by 6-OHDA lesion in the right side striatum would be in the left forepaw; thus, the ratio of left/right (%) adjusted steps was calculated and used for indicating forelimb akinesia. The tests were then carried out over 12 weeks after the lesion. The following three experiments were conducted for assessing the treatment effect and the central uptake of NNZ-2591.
Experiment 1: early central administration of NNZ-2591
To facilitate i.c.v. administration, a guide cannula (21 G × 6 mm) was stereotaxically placed on the top of the dura at the co-ordinates of R 1.5 mm and AP +7.5 mm from the interaural zero plane (Paxinos and Watson, 1982) under halothane (3%) anaesthesia (Guan et al., 1993) immediately after the completion of 6-OHDA lesion. The rats were re-anaesthetized 2 h after 6-OHDA lesion and either NNZ-2591 (20 ng in 20 µL of 0.9% saline, n = 8) or saline alone (n = 8) was infused slowly into the right lateral ventricle, then the treatment procedure was repeated daily for the next 2 days. The infusion was controlled by a microdialysis infusion pump and was at the rate of 1 µL·min−1. The adjusting step test was performed at 2, 4, 6 and 12 weeks after the lesion. The brains were then collected in week 12 for immunohistochemical staining.
Experiment 2: central uptake of NNZ-2591
Rats were treated with a single dose of NNZ-2591 (either 1 or 10 mg·mL−1 saline) s.c. 2 h after 6-OHDA lesion. Blood and CSF were collected simultaneously 2 h after the administration of NNZ-2591. For CSF collection, the rat was deeply anaesthetized with an overdose of pentobarbital and its head was held in a stereotaxic frame. CSF was collected from the cisterna magna (Guan et al., 2004) following blood sampling via cardiac puncture. NNZ-2591 concentration in plasma and CSF were determined by high-performance liquid chromatography (HPLC) mass spectrometry.
Experiment 3: delayed peripheral administration of NNZ-2591
This experiment examined the effects of NNZ-2591 on an established 6-OHDA lesion following delayed subcutaneous administration. The rats were habituated to the adjusting step test prior to the lesion. To detect motor deficit before treatment, the adjusting steps test was performed 2 weeks after 6-OHDA lesion. The rats were then assigned to four different treatment groups depending on the degree of the deficit, such that the degree of the deficit was similar across all treatment groups. Either NNZ-2591 (0.2, 1 and 5 mg in 1 mL saline) or saline (1 mL) was administered subcutaneously once daily for the next 14 days, starting 2 weeks after 6-OHDA lesion. Adjusting steps were then examined 7, 8, 10 and 12 weeks after the lesion (or 3, 4, 6 and 8 weeks after the completion of treatments). Brain tissues from the groups treated with either saline or 5 mg·day−1 NNZ-2591 were collected 12 weeks after the lesion and processed for immunohistochemistry. Brains from six normal/untreated rats were also collected and used as normal controls for immunohistochemistry.
Tissue preparation and immunohistochemistry
The brains were perfused with normal saline followed by 10% formalin. The brains were further fixed in the same fixative for 48 h before being processed and paraffin embedded as described previously (Guan et al., 2000). Coronal sections (6 µm) were cut and mounted on chrome-alum coated slides for immunohistochemical analysis. Representative sections of the striatum (level 1: AP 8.70 mm and level 2: 8.60 mm), the globus pallidus (AP 8.08 mm) and the substantia nigra pars compacta (SNc, level 1: AP 4.20 mm; level 2: AP 3.80 mm and level 3: AP 3.40 mm) were used for immunohistochemical staining. Three adjacent sections from each level were stained.
Immunohistochemistry was performed as described previously (Guan et al., 2000; Krishnamurthi et al., 2004). Briefly, the sections were deparaffinized in xylene and dehydrated in a series of ethanol and 0.1 mol·L−1 phosphate-buffered saline (PBS) solutions. For antigen unmasking the sections were microwave boiled in 10 mmol·L−1 sodium citrate buffer (pH 6.0) for 1 min. All sections were pretreated with 1% H2O2 in 50% methanol for 30 min to quench endogenous peroxidase activity and then incubated with either 1.5% normal horse serum or 2.5% normal sheep serum/PBS at room temperature to block non-specific staining. The sections were then incubated with the following primary antibodies: rabbit anti-tyrosine-hydroxylase (TH, 1:200 for striatum, 1:500 for SNc) for labelling dopaminergic neurons in the SNc and dopamine terminals in the striatum, mouse anti-proliferating cell nuclear antigen (PCNA, 1:200) for labelling proliferating cells in the sub-ventricular zone (SVZ), goat anti-doublecortin (DCX, 1:200) for labelling the neurobloast in the SVZ, goat anti-cholineacetyltransferase (ChAT, 1:50) for labelling cholinergic neurons in the striatum, rabbit anti-calbindin (1:100 for striatum, 1:200 for SNc) for labelling GABA projection neurons and rabbit anti-L-enkephalin (1:1000) for labelling GABA neurotransmitter in the globus pallidus at 4°C for 48 h. Sections were incubated with either biotinylated horse anti-mouse or goat anti-rabbit or rabbit anti-goat secondary antibodies accordingly (1:200) at 4°C overnight. ExtrAvidin (1:200) was applied for 3 h at room temperature and then 0.05% 3,3-diaminobenzidine was added to produce a brown reaction product.
Data acquisition
The numbers of neurons and terminal density were assessed in three adjacent sections of each level. The average densities of TH in the striatum and enkephalin in the striatum and the globus pallidius were measured using a light microscope and computerized image analyser. The density reading was corrected with the background readings chosen from nearby tissue with no specific staining. The right/left (R/L) ratio of density was calculated for each section to indicate the increase/decrease in density as a percentage of the contralateral (left) side (Krishnamurthi et al., 2004). The numbers of TH-positive cells in the A9 area of the SNc, as well as calbindin and ChAT-positive cells in the striatum, were counted under a light microscope using Axiovision software. Cells identified as positively stained were selected with the computer mouse and the software calculated the total number of cells selected for each image.
The number of PCNA- and DCX-positive cells in both sides of the SVZ was measured using a high throughput image analysis assay developed by Professor M Dragunow in the High Content Screening Laboratory, Department of Pharmacology, The University of Auckland (http://www.health.auckland.ac.nz/pharmacology/discovery-1) using the Metamorph v.6.2.6 Image analysis software (Molecular Devices) (Svedin et al., 2007). Brightfield images of positive cells were acquired at 10× objective and stored as JPEGs. The journal/assay then automatically opened each image in the folder and performed the Findspots application in Metamorph on each image. Total cell numbers were then automatically logged into an Excel spreadsheet.
NNZ-2591 assay by HPLC mass spectrometry
The method has been previously described (Guan et al., 2007). Briefly, chromatography conditions consisted of a Synergy 4 µm MAX-RP 80A column 1 × 50 mm with a mobile phase of 30% methanol, 0.05% formic acid in water flowing at 50 µL·min−1 with a column temperature of 25°C. The mass spectrometry conditions consisted of electrospray ionization in positive mode with a voltage of 5000 V, a sheath gas flow of 50 psi, an auxiliary gas flow of 5 psi, a capillary temperature of 245°C, collision gas of argon at 0.7 m Torr at a voltage of 40 V, with selective reaction monitoring transition of 195.15 → 96 m/z. The standard curve was prepared in plasma and was linear from 50–15 000 pg on the column.
Materials
6-OHDA, biotinylated horse anti-mouse, goat anti-rabbit and rabbit anti-goat secondary antibodies and ExtrAvidin were obtained from Sigma; rabbit anti-TH, Protos Biotech; PCNA, Dako; DCX, Santa Cruz; ChAT and rabbit anti-calbindin, Chemicon; rabbit anti-L-enkephalin, Immunostar. The light microscope and computerized image analyser (AxioVision) were from Carl Zeiss Vision (Germany) and the Synergy 4 µm MAX-RP 80A column from Phenomenex. NNZ-2591 was a gift from Neuren Pharmaceuticals Limited (Auckland, New Zealand).
Statistical analysis
The data from three adjacent sections were averaged for statistical analysis. Statistical analysis was carried out using Graphpad Prism 5 software and all data are presented as means ± s.e.mean. Two-way anova repeated measure and Bonferroni post tests were used to compare treatment effects in two or more representative levels of brain tissues, as well as in different time points of behavioural tests.
Results
Experiment 1: early central administration of NNZ-2591
Adjusting steps test
The two-way anova analysis suggested significant differences between two treatment groups (F[1,4] = 7.5, P = 0.0075) and across all the time points examined (F[1,4] = 74.2, P < 0.001, n = 8, Figure 1A). Compared with pre-lesion/treatment, the L/R ratio in the adjusting steps was reduced in both the saline- and NNZ-2591-treated groups examined 2, 4, 6 and 12 weeks after the lesion (P < 0.001, Figure 1A). The treatment effect of NNZ-2591 was analysed using the data between weeks 2 and 12 post-lesion/treatment (i.e. excluding pre-lesion/treatment data). Two-way anova analysis showed an overall increase in the L/R ratio of the adjusting steps in the groups treated with NNZ-2591 compared with the saline-treated groups (F[1,3] = 5.1, P = 0.02, Figure 1B), but no effect across the time point examined between weeks 2 and 12. The post-hoc test did not suggest any difference between the two treatment groups in the individual time points examined.
Figure 1.
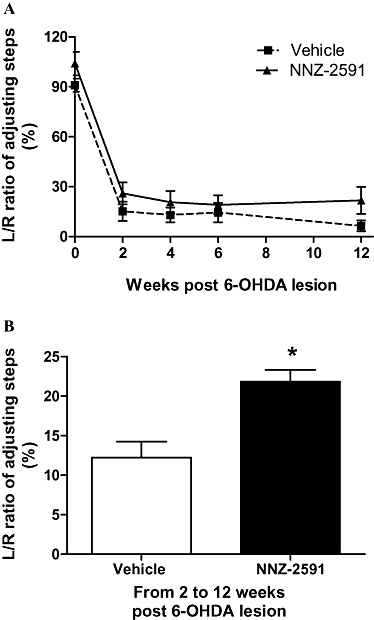
NNZ-2591 reduced motor deficit after central administration. Either NNZ-2591 or saline was given 2 h after 6-OHDA lesion, then daily for 2 days. The treatment did not prevent the development of deficit examined 2 weeks after the lesion (A, F[1,4] = 74.2, P < 0.001, n = 8). However, two-way anova suggested that NNZ-2591 treatment improved the L/R ratio of the adjusting steps between weeks 2–12 compared with the saline-treated group (B, F[1,3] = 5.1, *P < 0.05, n = 8); there was no difference between the two groups at each individual time point examined. 6-OHDA, 6-hydroxydopamine; L, left; NNZ-2591, cyclo-L-glycyl-L-2-allylproline; R, right.
Immunohistochemistry of TH
Striatal 6-OHDA lesion resulted in more than 90% loss of TH-positive neurons in the ipsilateral SNc and the complete loss of TH density in ipsilateral striatum in 15/16 rats examined 12 weeks after the lesion (Figure 2A,B). Given that the double striatal injections of 6-OHDA induced a rather moderate lesion, the severe loss of TH-positive neurons in the SNc and the terminals in the striatum examined 12 weeks after the lesion is probably due to the long-term effect of progressive dopamine depletion. The number of TH-positive neurons was assessed in the level 1 and level 2. NNZ-2591 treatment did not alter the number of TH-positive neurons in the SNc compared with the saline-treated group (Figure 2A,B).
Figure 2.
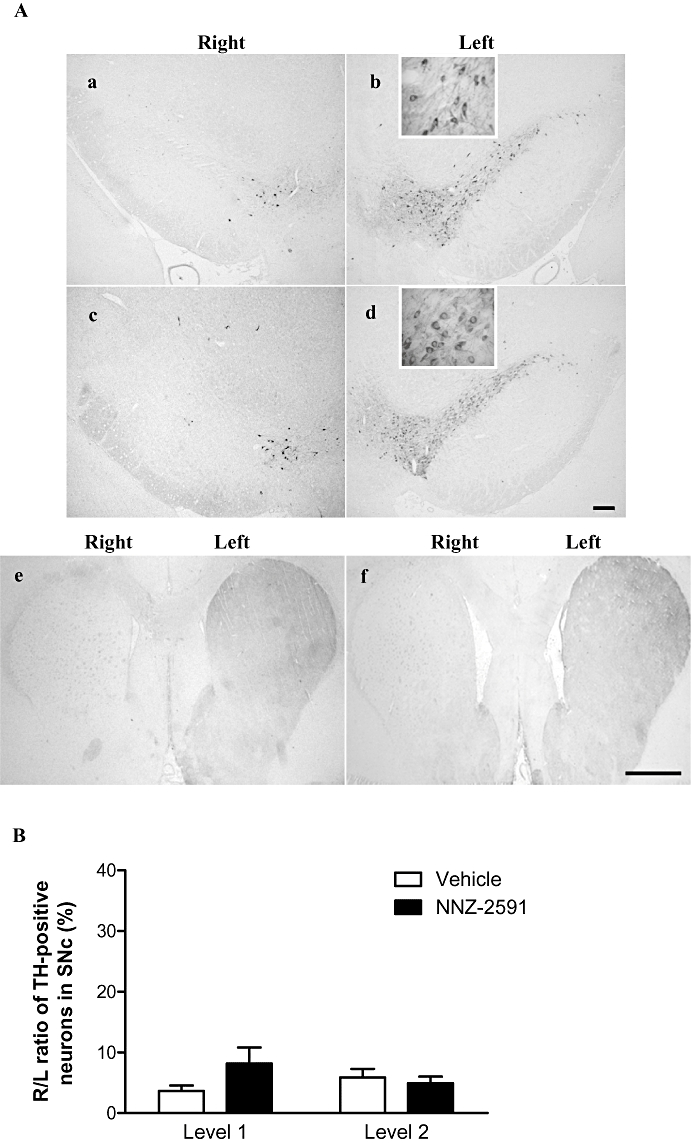
(A) The bilateral distribution (a–d) and morphology (the inserts of b and d) of TH-positive neurons in the SNc. There was more than 90% loss of TH-positive neurons in the ipsilateral (right) SNc in both saline (a) and NNZ-2591 (c) treated groups (×4, scale bar = 100 µm). Photos (e) and (f) show the complete loss of the TH terminal staining in the ipsilateral (right) striatum of both treatment groups (×1, scale bar = 1 mm). (B) Shows that the central administration of NNZ-2591 (20 ng, i.c.v.) did not alter the R/L ratio of TH-positive neurons in the SNc (n = 8). L, left; NNZ-2591, cyclo-L-glycyl-L-2-allylproline; R, right; SNc, substantia nigra pars compacta; TH, tyrosine-hydroxylase.
Experiment 2: central penetration of NNZ-2591
The data generated from the HPLC ms did not indicate a significant difference in NNZ-2591 concentration between the CSF (1 mg: 819.0 ± 30.0; 10 mg: 9112 ± 268.5) and the plasma (1 mg: 849.0 ± 63.7; 10 mg: 12655 ± 1923) examined 2 h after a single dose administration. Plasma and CSF concentrations were also proportional to the two doses administered (n = 4–6).
Experiment 3: delayed peripheral administration of NNZ-2591
Adjusting steps tests
Compared with pre-lesion, the L/R ratio in the adjusting steps was significantly reduced by approximately 85% when examined 2 weeks after the lesion. The degree of the deficit was similar across all groups before the treatments (Figure 3). Two-way anova analysis suggested significant treatment (F[3,5] = 24.1, P < 0.0001) and time (F[3,5] = 158.4, P < 0.0001) effects on the L/R ratio in adjusting steps, with significant interaction between treatment and time point (F[3,15] = 3.1, P = 0.0001, n = 8–9, Figure 3). While the deficit remained in the saline-treated groups, all three NNZ-2591 (0.2, 1 and 5 mg·day−1, s.c.)-treated groups made significant recovery by showing increased L/R ratio of the adjusting steps from weeks 7 to 8 onwards. The improved function was highly significant and well maintained (post-tests, P < 0.01, P < 0.0001, Figure 3). There were no differences between the groups treated with different doses of NNZ-2591.
Figure 3.
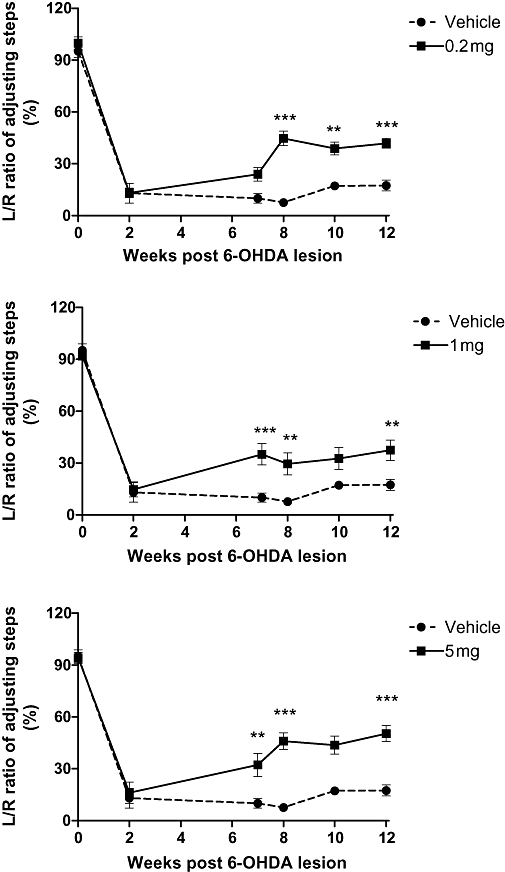
The treatment effect of NNZ-2591 after peripheral administration. Three different doses of NNZ-2591 or saline were administered daily for 14 days starting 2 weeks after lesion and motor function was examined for 12 weeks. 6-OHDA lesion resulted in approximately 85% reduction in the number of adjusting steps in all groups examined 2 weeks post-lesion compared with pre-lesion. Two-way anova analysis suggested treatment (F[3,5] = 24.1, P < 0.0001, n = 8–9) and time effects (F[3,5] = 158.4, P < 0.0001) on the L/R ratio in the adjusting steps, with significant interaction between the treatments and time points (F[3,15] = 3.1, P = 0.0001). Compared with the saline-treated group, treatment with 0.2, 1 and 5 mg·day−1 significantly improved the L/R ratio in the adjusting steps over weeks 7–12 (**P < 0.001, ***P < 0.0001). There was no difference between the groups treated with different doses of NNZ-2591. 6-OHDA, 6-hydroxydopamine; L, left; NNZ-2591, cyclo-L-glycyl-L-2-allylproline; R, right.
Immunohistochemistry
The 6-OHDA lesion resulted in an approximately 80% loss of TH density in the ipsilateral striatum and approximately 90% loss of TH-positive neurons in the ipsilateral SNc examined 12 weeks after the lesion (Figure 4). Treatment with NNZ-2591 (5 mg·day−1 for 14 days) did not alter either the TH density in the striatum or the number of TH-positive neurons in the SNc compared with the saline-treated group (Figure 4).
Figure 4.
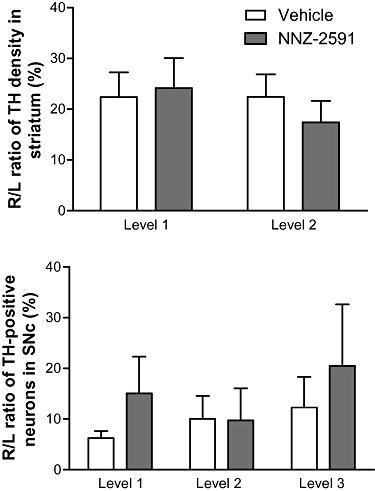
The effect of NNZ-2591 on dopaminergic neurons 12 weeks after the lesion. Treatment with NNZ-2591 (5 mg·day−1) did not alter either the R/L ratio of average density of TH in the striatum or the number of TH-positive neurons in the SNc compared with the saline-treated group at all levels examined. L, left; NNZ-2591, cyclo-L-glycyl-L-2-allylproline; R, right; SNc, substantia nigra pars compacta; TH, tyrosine-hydroxylase.
Figure 5A shows the bilateral distribution (a, c and e) and the morphology (b, d and f) of PCNA-positive cells of the ipsilateral SVZ. Two-way anova analysis suggested a difference in the number of PCNA-positive cells across the groups (F[2,1] = 3.2, P = 0.047, Figure 5B), but not between the hemispheres. Compared with normal control brains (Figure 5Aa,b), the group with 6-OHDA lesion and saline-treatment (Figure 5Ac,d) showed more PCNA-positive cells in level 1 (anterior) but not at level 2 (posterior) of the SVZ. The increased PCNA-positive cells in the SVZ were bilateral (Figure 5A,B). There was no difference between the groups treated with NNZ-2591 (Figure 5Ae,d) and saline after the lesion at either level (Figure 5B).
Figure 5.
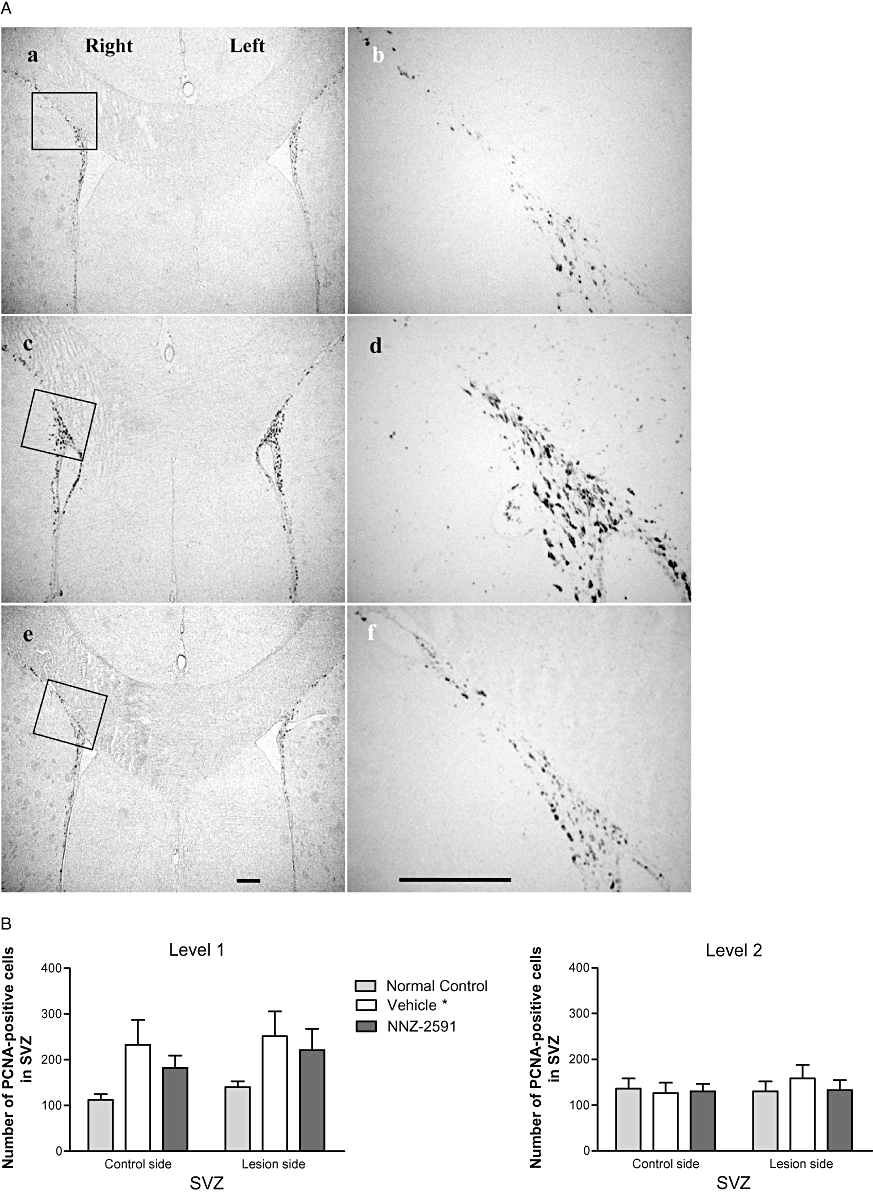
(A) The bilateral distribution (a, c and e, ×4) and the morphology (b, d and f, ×20) of PCNA-positive cells of the SVZ (level 1) in a normal control rat (a and b), and in the rats with 6-OHDA lesion and treated with either saline (c and d) or NNZ-2591 (e and f, scale bar = 100 µm). The squares in photos (a, c and e) indicate where photos (b, d and f) were taken. (B) The effect of 6-OHDA lesion and treatment with NNZ-2591 on cell proliferation in the SVZ. Two-way anova suggested a difference between the groups (F[2,1] = 3.2, overall, *P = 0.047, n = 8–9), but not between the hemispheres. Compared with normal controls, lesioned + saline-treated animals showed a bilateral increase in PCNA-positive cells in level 1 (anterior) but not level 2 (posterior) of the SVZ. A similar trend towards increased PCNA-positive cells was also seen in the group treated with NNZ-2591 after the lesion compared with normal controls. There was no difference in cell proliferation between the groups treated with NNZ-2591 and saline. 6-OHDA, 6-hydroxydopamine; NNZ-2591, cyclo-L-glycyl-L-2-allylproline; PCNA, proliferating cell nuclear antigen; SVZ, sub-ventricular zone.
Figure 6A shows the bilateral distribution (a, c and e) and the morphology (b, d and f) of DCX-positive cells in level 1 (anterior) of the ipsilateral SVZ. DCX-immunopositive neuroblasts show a characteristic morphology of chain-like clusters. Two-way anova suggested a significant difference between the groups (F[2,1] = 15.2, P < 0.0001), but no differences between the hemispheres. Compared with the normal control rats (Figure 6Aa,b), there was an increase in DCX-positive cells on both sides of the SVZ particularly at level 2 (posterior) of the group with 6-OHDA lesion + saline treatment (post-test, P < 0.01, Figure 6Ac–d,B). NNZ-2591 treatment caused a further increase in the number of DCX-positive cells in the SVZ compared with the saline-treated groups (P < 0.05, Figure 6Ae–f,B); this occurred bilaterally and predominantly at level 1.
Figure 6.
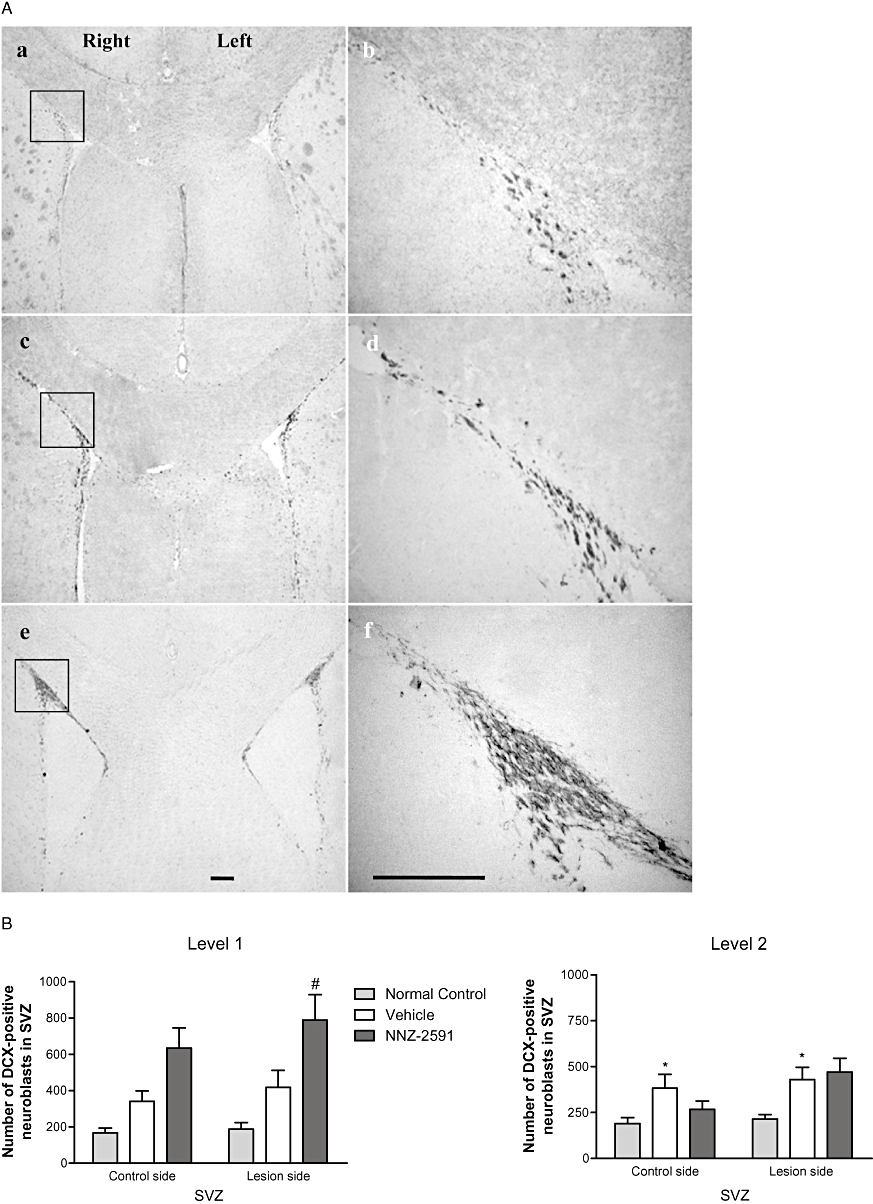
(A) The bilateral distribution (a, c and e, ×4) and the morphology (b, d and f, ×20) of DCX-positive neurons of the SVZ (level 1) in a normal control rat (a and b), and in rats with a 6-OHDA lesion and treated either with saline (c and d) or NNZ-2591 (e and f, scale bar = 100 µm). The squares in photos (a, c and e) indicate where photos (b, d and f) were taken. (B) The effect of NNZ-2591 on neurogenesis in the SVZ. Two-way anova suggested a difference between the groups (F[2,1] = 15.2, *P < 0.001, n = 8–9), but no effect between the hemispheres. Compared with the normal controls, the number of DCX-positive neurons in the SVZ was increased bilaterally in lesioned + saline-treated animals, particularly in level 2 (*P < 0.01). Treatment with NNZ-2591 significantly, further increased the number of DCX-positive neurons in level 1, predominantly in the ipsilateral SVZ, compared with the saline-treated groups (#P < 0.05, n = 8–9). 6-OHDA, 6-hydroxydopamine; DCX, doublecortin; NNZ-2591, cyclo-L-glycyl-L-2-allylproline; SVZ, sub-ventricular zone.
The number of ChAT-positive neurons was reduced in the ventral-medial striatum following 6-OHDA lesion compared with normal control animals. NNZ-2591 treatment did not change the number of ChAT-positive neurons in the striatum (data not shown). Neither injury nor treatment with NNZ-2591 altered the number of calbindin-positive neurons in the striatum and the SNc (data not shown). There was also no change in the average density of L-enkephalin in both the striatum and globus pallidus (data not shown).
Discussion
The results obtained in the present study with the 6-OHDA-induced rat model of PD suggest that an earlier intervention with NNZ-2591 during the acute phase of neuronal loss reduces the degree of motor deficit compared with the saline-treated group. The delayed treatment of NNZ-2591 improved functional recovery when administered after the onset of dyskinesia. The functional improvement was independent of nigrostriatal dopamine depletion. The mechanism of action underlying this functional recovery is not clear, but does not appear to be symptomatic given that the improved motor function induced by NNZ-2591 was long-lasting.
In PD, dopamine depletion begins long before the onset of motor deficits such as bradykinesia and slowness in initiating movement (Schwarting and Huston, 1996). During this pre-symptomatic period, enhanced endogenous neuronal plasticity and neurogenesis play a key role in compensating for this early stage of dopamine depletion, thus maintaining brain function (Schwarting and Huston, 1996; Blandini et al., 2000). In addition to preventing further progressive neuronal loss, treatments that improve motor function may be highly desirable.
The majority of dopamine depletion and neuronal loss occur due to neurotoxicity during the first few weeks after 6-OHDA lesion (Blandini et al., 2007). To test whether NNZ-2591 can prevent 6-OHDA-induced dopamine depletion, the compound was given during the first 3 days after the lesion, a window prior to the peak of dopamine depletion. Unexpectedly, treatment with NNZ-2591 failed to prevent dopamine depletion (Figure 2A,B) and the deficit in motor co-ordination examined 2 weeks post-lesion by the adjusting steps test (Figure 1A). However, the degree of motor deficit was less in the group treated with NNZ-2591 compared with the saline-treated group (Figure 1B). Without improving neuronal outcome, the difference in motor function remained between the two groups and lasted for 12 weeks, a probable consequence of further deterioration in the saline-treated group. Our data also suggest that this improved motor function was not mediated by preventing dopamine depletion, thus indicating a possible reparative role for NNZ-2591. We have recently found that NNZ-2591 prevents HI brain injury in rats (Guan et al., 2007). The most obvious explanation for this would be that there are differences between the mechanisms underlying ischaemic neuronal injury and 6-OHDA-induced neuronal toxicity (Hanrott et al., 2006; Xu et al., 2007).
Delayed treatment with NNZ-2591, initiated after the onset of motor deficit at 2 weeks post-lesion, caused a recovery in motor function from week 7 onwards, demonstrating its reparative action. The neuroprotective effect of NNZ-2591 has been demonstrated to be dose-dependent after central administration (Guan et al., 2007), and in the present study NNZ-2591 showed a broad effective dose range on functional improvement after peripheral administration as the three doses tested were all effective (Figure 3). A similar finding has been obtained in a rat model with HI brain injury (Guan et al., 2007). Functional recovery after delayed treatment with NNZ-2591 was also independent of nigrostriatal dopamine depletion. Nevertheless, we found that NNZ-2591 treatment enhanced the expression of neuroblasts in the SVZ, which may be associated with the mechanisms underlying the observed functional recovery.
The SVZ contains highly undifferentiated, mitotically active stem cells that have extensive self-renewal capacity and nigrostriatal dopamine projection can mediate neurogenesis in the SVZ (Baker et al., 2004; Borta and Hoglinger, 2007). In our study, 6-OHDA lesion enhanced both cell proliferation and neuroblast expression bilaterally in the SVZ. This injury-induced potential neurogenesis also has been suggested to occur in the SNc after 1-methyl-4-pheny-1,2,3,6-tetrahydropyridine (MPTP) intoxication in mice (Zhao et al., 2003). In addition, it is known that environmental enrichment and treatment with neurotrophic factors can further enhance 6-OHDA lesion-induced neural progenitors and as a result improve motor function (Mohapel et al., 2005; Urakawa et al., 2007). Thus, improving endogenous neurogenesis is seen as a potential intervention for improving functional recovery in PD (Borta and Hoglinger, 2007; Geraerts et al., 2007; Okano et al., 2007). Interestingly, the effect of NNZ-2591 on neuroblast expression appeared to be unrelated to the enhancement of cell proliferation. The non-proliferative neurogenesis could result from the inhibition of apoptosis of stem cells and/or the promotion of neuronal orientated proliferation in the SVZ. Without further study, we do not have direct evidence that the increased neuroblast numbers are associated with improved motor function. Even though the mechanism underlying the improved motor function is not known, the finding that the functional recovery was long-lasting suggests that the treatment effect was not symptomatic.
Cyclic histidyl-proline, another diketopiperazine generated from thyrotropin-releasing hormone, interacts with dopamine receptors (Mizuma et al., 1994) and dopamine transporters (Ikegami and Prasad, 1990); yet it is not clear whether effects on dopamine plasticity at the synaptic level play a role in the functional recovery promoted by NNZ-2591. Given that the majority of dopamine neurons are lost in the SNc during the pre-symptomatic period, nigro-striatal dopamine depletion could be viewed as an initial stage in the pathology of PD, which progresses far beyond dopamine depletion when motor deficit becomes clinically detectable. Several research groups have demonstrated that enhancing the neuronal and synaptic plasticity of non-dopamine circuits could be equally important in improving motor function in PD (Kase et al., 2003; Whone et al., 2003). Thus, we also examined the possible role of non-dopamine neurotransmission in the functional recovery promoted by NNZ-2591, but found no changes in striatal acetylcholine synthesis or in GABA neurotransmission in the striatum and the SNc. Without further examining other non-dopamine neurotransmission in the entire basal ganglia-cortical circuits, we are unable to suggest a suitable explanation, at this stage, for the improved motor function that does not involve the prevention of striatal dopamine depletion.
Several neuropeptides, including growth factors, have been shown to improve motor function in various models of PD (Aoi et al., 2000; Kirik et al., 2001). However, the identification of an effective route of administration for these peptides has been and still is problematic because of their large molecular size and potential mitogenic effects (Partridge, 2002; Guan et al., 2003; 2004). At 2 h after a single subcutaneous injection, the concentration of NNZ-2591 in the CSF was similar to that in the plasma, and both concentrations were proportional to the dose administered, indicating an effective central uptake of NNZ-2591. Results from our previous study suggested that central uptake of NNZ-2591 was independent of concurrent HI injury (Guan et al., 2007). This effective central uptake and repair-promoting activity of NNZ-2591 suggest its possible utility as a therapeutic agent for neurodegenerative disorders.
In summary, treatment with NNZ-2591 after the onset of motor deficit improved long-term functional recovery in a rat model of PD. Whether administered during or after the active phase of neuronal degeneration, the treatment effect was independent of dopamine depletion. The long-lasting effect on motor co-ordination suggests the treatment is not simply symptomatic. Thus, NNZ-2591 is likely to provide a reparative intervention rather than a preventive and symptomatic treatment. Given its good central uptake, NNZ-2591 may have potential as a desirable therapeutic agent for neurodegenerative disorders.
Acknowledgments
The study was funded by the Foundation for Research Science and Technology (FRST), New Zealand.
Glossary
Abbreviations:
- 6-OHDA
6-hydroxydopamine
- ChAT
cholineacetyltransferase
- CSF
cerebrospinal fluid
- cyclic GP
cyclo-glycyl-proline
- DCX
doublecortin
- HI
hypoxic-ischaemic
- NNZ-2591
cyclo-L-glycyl-L-2-allylproline
- PCNA
proliferating cell nuclear antigen
- PD
Parkinson's disease
- SNc
substantia nigra pars compacta
- SVZ
sub-ventricular zone
- TH
tyrosine-hydroxylase
Conflicts of interest
J.G., the principal investigator, has equity interests in Neuren Pharmaceuticals Limited but has not been associated with Neuren Pharmaceuticals as an employee.
References
- Aoi M, Date I, Tomita S, Ohmoto T. The effect of intrastriatal single injection of GDNF on the nigrostriatal dopaminergic system in hemiparkinsonian rats: behavioural and histological studies using two different dosages. Neurosci Res Suppl. 2000;36:319–325. doi: 10.1016/s0168-0102(00)00097-3. [DOI] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Blandini F, Levandis G, Bazzini E, Nappi G, Armentero MT. Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old model. Eur J Neurosci. 2007;25:397–405. doi: 10.1111/j.1460-9568.2006.05285.x. [DOI] [PubMed] [Google Scholar]
- Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- Faden AI, Knoblach SM, Cernak I, Fan L, Vink R, Araldi GL, et al. Novel diketopiperazine enhances motor and cognitive recovery after traumatic brain injury in rats and shows neuroprotection in vitro and in vivo. J Cereb Blood Flow Metab. 2003;23:342–354. doi: 10.1097/01.WCB.0000046143.31247.FD. [DOI] [PubMed] [Google Scholar]
- Geraerts M, Krylyshkina O, Debyser Z, Baekelandt V. Concise review: therapeutic strategies for Parkinson disease based on the modulation of adult neurogenesis. Stem Cells. 2007;25:263–270. doi: 10.1634/stemcells.2006-0364. [DOI] [PubMed] [Google Scholar]
- Guan J, Williams C, Gunning M, Mallard C, Gluckman P. The effects of IGF-1 treatment after hypoxic-ischemic brain injury in adult rats. J Cereb Blood Flow Metab. 1993;13:609–616. doi: 10.1038/jcbfm.1993.79. [DOI] [PubMed] [Google Scholar]
- Guan J, Krishnamurthi R, Waldvogel HJ, Faull RL, Clark R, Gluckman P. N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH positive neurons after 6-OHDA induced nigral lesion in rats. Brain Res. 2000;859:286–292. doi: 10.1016/s0006-8993(00)01988-0. [DOI] [PubMed] [Google Scholar]
- Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol. 2003;70:443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Guan J, Thomas GB, Lin H, Mathai S, Bachelor DC, George S, et al. Neuroprotective effects of the N-terminal tripeptide of insulin-like growth factor-1, glycine-proline-glutamate (GPE) following intravenous infusion in hypoxic-ischemic adult rats. Neuropharmacology. 2004;47:892–903. doi: 10.1016/j.neuropharm.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Guan J, Mathai S, Harris AP, Wen JY, Zhang R, Brimble MA, et al. Peripheral administration of a novel diketopiperazine, NNZ-2591, prevents brain injury and improves somatosensory-motor funcion following hypoxia-ischemia in adult rats. Neuropharmacology. 2007;53:749–762. doi: 10.1016/j.neuropharm.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Hanrott K, Gudmunsen L, O'Neill MJ, Wonnacott S. 6-hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cdelta. J Biol Chem. 2006;281:5373–5382. doi: 10.1074/jbc.M511560200. [DOI] [PubMed] [Google Scholar]
- Hodaie M, Neimat JS, Lozano AM. The dopaminergic nigrostriatal system and Parkinson's disease: molecular events in development, disease, and cell death, and new therapeutic strategies. Neurosurgery. 2007;60:17–28. doi: 10.1227/01.NEU.0000249209.11967.CB. [DOI] [PubMed] [Google Scholar]
- Ikegami H, Prasad C. Neuropeptide-dopamine interactions. V. Cyclo(His-Pro) regulation of striatal dopamine transporter complex. Peptides. 1990;11:145–148. doi: 10.1016/0196-9781(90)90123-m. [DOI] [PubMed] [Google Scholar]
- Kase H, Aoyama S, Ichimura M, Ikeda K, Ishii A, Kanda T, et al. Progress in pursuit of therapeutic A2A antagonists: the adenosine A2A receptor selective antagonist KW6002: research and development toward a novel nondopaminergic therapy for Parkinson's disease. Neurology. 2003;61:S97–S100. doi: 10.1212/01.wnl.0000095219.22086.31. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioural and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Rosenblad C, Bjorklund A. Delayed infusion of GDNF promotes recovery of motor function in the partial lesion model of Parkinson's disease. Eur J Neurosci. 2001;13:1589–1599. doi: 10.1046/j.0953-816x.2001.01534.x. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi R, Stott S, Maingay M, Faull RL, McCarthy D, Gluckman P, et al. N-terminal tripeptide of IGF-1 improves functional deficits after 6-OHDA lesion in rats. Neuroreport. 2004;15:1601–1604. doi: 10.1097/01.wnr.0000127461.15985.07. [DOI] [PubMed] [Google Scholar]
- Mizuma H, Shimizu I, Hilton C, Prasad C. Does cyclo(His-Pro) act like amphetamine? Life Sci. 1994;54:1625–1633. doi: 10.1016/0024-3205(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102:1459–1465. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15(2):3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- Pinna A, Pontis S, Borsini F, Morelli M. Adenosine A2A receptor antagonists improve deficits in initiation of movement and sensory motor integration in the unilateral 6-hydroxydopamine rat model of Parkinson's disease. Synapse. 2007;61:606–614. doi: 10.1002/syn.20410. [DOI] [PubMed] [Google Scholar]
- Samonina G, Ashmarin I, Lyapina L. Glyproline peptide family: review on bioactivity and possible origins. Pathophysiology. 2002;8:229–234. doi: 10.1016/s0928-4680(02)00018-4. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioural brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Svedin P, Guan J, Mathai S, Zhang R, Wang X, Gustavsson M, et al. Delayed peripheral administration of a GPE analogue induces astrogliosis and angiogenesis and reduces inflammation and brain injury following hypoxia-ischemia in the neonatal rat. Dev Neurosci. 2007;29:393–402. doi: 10.1159/000105480. [DOI] [PubMed] [Google Scholar]
- Urakawa S, Hida H, Masuda T, Misumi S, Kim TS, Nishino H. Environmental enrichment brings a beneficial effect on beam walking and enhances the migration of doublecortin-positive cells following striatal lesions in rats. Neuroscience. 2007;144:920–933. doi: 10.1016/j.neuroscience.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Whone AL, Moore RY, Piccini PP, Brooks DJ. Plasticity of the nigropallidal pathway in Parkinson's disease. Ann Neurol. 2003;53:206–213. doi: 10.1002/ana.10427. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zheng XX, Zhou Q, Li H. Inhibition of excitatory amino acid efflux contributes to protective effects of puerarin against cerebral ischemia in rats. Biomed Environ Sci. 2007;20:336–342. [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, et al. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci USA. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]


