Abstract
In the aorta of spontaneously hypertensive rats (SHR), the endothelial dysfunction is due to the release of endothelium-derived contracting factors (EDCFs) that counteract the vasodilator effect of nitric oxide, with no or minor alteration of its production. The endothelium-dependent contractions elicited by acetylcholine (ACh) involve an increase in endothelial [Ca2+]i, the production of reactive oxygen species, the activation of endothelial cyclooxygenase-1, the diffusion of EDCF and the subsequent stimulation of smooth muscle cell TP receptors. The EDCFs released by ACh have been identified as PGH2 and paradoxically prostacyclin. Prostacyclin generally acts as an endothelium-derived vasodilator, which, by stimulating IP receptors, produces hyperpolarization and relaxation of the smooth muscle and inhibits platelet aggregation. In the aorta of SHR and Wistar-Kyoto rats, prostacyclin is the principal metabolite of arachidonic acid released by ACh. However, in SHR aorta, prostacyclin does not produce relaxations but activates the TP receptors on vascular smooth muscle cells and produces contraction. The IP receptor is not functional in the aortic smooth muscle cells of SHR as early as 12 weeks of age, but its activity is not reduced in platelets. Therefore, prostacyclin in the rule protects the vascular wall, but in the SHR aorta it can contribute to endothelial dysfunction. Whether or not prostacyclin plays a detrimental role as an EDCF in other animal models or in human remains to be demonstrated. Nevertheless, because EDCFs converge to activate TP receptors, selective antagonists of this receptor, by preventing endothelium-dependent contractions, curtail the endothelial dysfunction in diseases such as hypertension and diabetes.
Keywords: nitric oxide, prostaglandins, EDCF, oxidative stress, superoxide anion, hypertension, endothelium, smooth muscle, platelets, cyclooxygenase
Introduction
Endothelial cells synthesize and release various factors that modulate vascular tone as well as angiogenesis, inflammatory responses, haemostasis and permeability. As a major regulator of local vascular homeostasis, the endothelium maintains the balance between vasodilatation and vasoconstriction, inhibition and promotion of the proliferation and migration of smooth muscle cells, prevention and stimulation of the adhesion and aggregation of platelets as well as thrombogenesis and fibrinolysis. Upsetting this tightly regulated balance leads to endothelial dysfunction. A reduced bioavailability of nitric oxide (NO), an alteration in the production of prostanoids (including prostacyclin, thromboxane A2 and/or isoprostanes), an impairment of endothelium-dependent hyperpolarization as well as an increased release of endothelin-1 can individually or in association contribute to endothelial dysfunction (Félétou and Vanhoutte, 2006a). The present review focuses on the endothelial function observed in spontaneously hypertensive rats (SHR).
Endothelium-dependent contractions in SHR aorta
The endothelium-dependent relaxations are impaired in the aorta of hypertensive rats (Lockette et al., 1986; Luscher and Vanhoutte, 1986). Thus, in contracted aortic rings of SHR, acetylcholine (ACh) induces endothelium-dependent relaxations, but the concentration–response curve to the muscarinic agonist is biphasic and at concentrations higher than 100 nmol·L−1the relaxations become smaller. In quiescent aortic rings of SHR, ACh produces endothelium-dependent contractions that are amplified in the presence of inhibitor of NO synthases (NOS; Luscher and Vanhoutte, 1986; Auch-Schwelk et al., 1992; Iwama et al., 1992; Yang et al., 2002) (Fig. 1). These endothelium-dependent contractions are larger in aortae from male than female SHR (Kauser and Rubanyi, 1995), are positively correlated with the severity of hypertension and the aging process and occur in aging normotensive Wistar-Kyoto rats (WKY) (Koga et al., 1988; 1989; Iwama et al., 1992; Ibarra et al., 1995).
Figure 1.
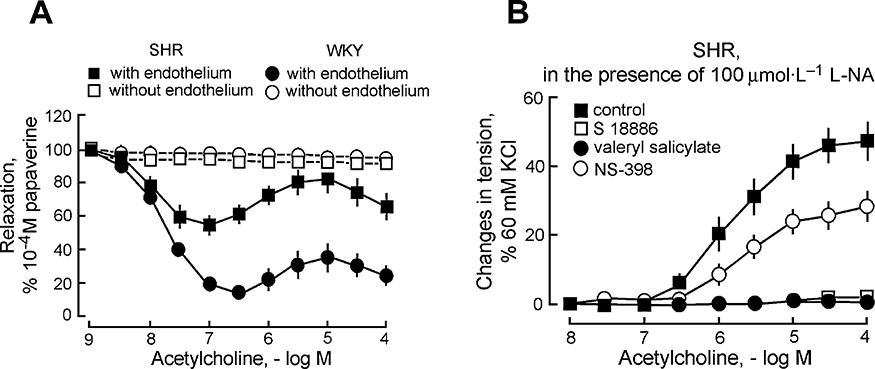
Endothelial dysfunction and endothelium-dependent contractions in SHR aorta. (A) In contracted rings of WKY and SHR aorta, acetylcholine induces endothelium-dependent relaxations, which at higher concentrations are blunted in the arteries of the hypertensive strain. (B) In quiescent aortic rings of SHR, acetylcholine induces endothelium-dependent contractions (in the presence of the nitric oxide synthase inhibitor L-nitro-arginine, L-NA) that are blocked by the antagonist of the TP receptor, S18886, the preferential COX-1 inhibitor, valeryl salicylate, but only partially affected by the preferential COX-2 inhibitor, NS-398. Modified from Yang et al.(Br J Pharmacol, 2002). COX, cyclooxygenase; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats.
Inhibitors of cyclooxygenase (COX) inhibit the endothelium-dependent contractions and fully restore the impaired endothelium-dependent relaxations, indicating that there is no or little alteration in NO production (Luscher and Vanhoutte, 1986), a conclusion strengthened by perfusion-superfusion bioassay studies (Hoeffner and Vanhoutte, 1989). Further bioassay studies using layered ‘sandwich’ preparations demonstrated that endothelium-dependent contractions to ACh involve the endothelial release of diffusible contractile COX derivatives, which oppose the relaxing effect of NO (Yang et al., 2003a) (Fig. 2).
Figure 2.
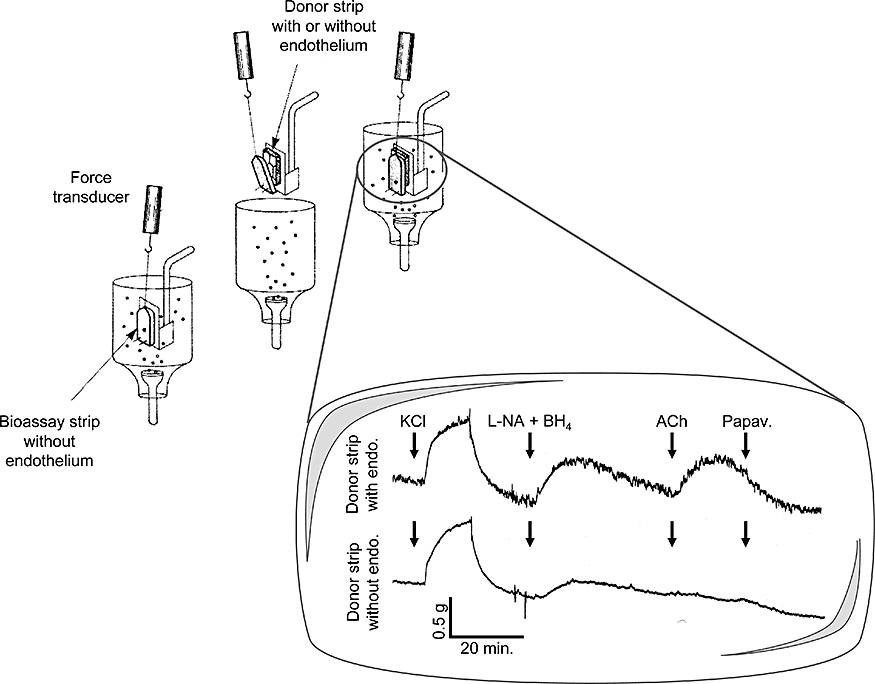
Bioassay of endothelium-derived contracting factor in SHR aortic rings: layered or ‘sandwich’ preparation. Acetylcholine (ACh) produces contraction of the bioassay strip (without endothelium) only if endothelial cells are present on the donor aortic strip. L-nitro-arginine (L-NA) and tetrahydrobiopterin (BH4) are present to optimize endothelium-derived contracting factor-mediated responses. Papaverine (Papav.) produces complete relaxation of the bioassay strip. Modified from Vanhoutte et al.(Br J Pharmacol, 2005).
The generation of endothelium-derived contracting factor (EDCF) is observed not only in response to endothelial muscarinic M3 receptor stimulation (Boulanger et al., 1994) but also in response to ATP (Koga et al., 1989; Mombouli and Vanhoutte, 1993;Yang et al., 2004), VEGF (Liu et al., 2001), as well as in response to receptor-independent stimuli, for instance the calcium ionophore, A 23187 (Yang et al., 2004; Tang et al., 2007). EDCF contributes to the contractile responses of endothelin (Taddei and Vanhoutte, 1993a,b) and in the presence of inhibitor of NOS, a tonic generation of EDCF is observed in SHR aorta and in that of aging WKY (Abeywardena et al., 2002).
Mechanisms underlying endothelium-dependent contractions
Endothelium-dependent contractions can be elicited by receptor-dependent mechanisms and by A 23187, which allows the free entry of extracellular calcium into endothelial cells, indicating that an increase in intracellular calcium is necessary for the production of endothelium-dependent contractions (Yang et al., 2004; Gluais et al., 2006; Tang et al., 2007). Indeed, ACh causes a rapid increase in cytosolic calcium concentration in endothelial cells of SHR and to a lesser extent in that of WKY. This rise of calcium was not affected by inhibiting COX or by the combination of tiron (a superoxide scavenger) plus diethyldithiocarbamate acid (DETCA; a superoxide dismutase inhibitor) (Tang et al., 2007). However, endothelium-dependent contractions are reduced by the acute exposure to the combination of tiron plus DETCA or by a chronic treatment with dimethylthiourea (an in vivo depletor of free radicals) (Yang et al., 2002). Furthermore, the production of superoxide anions selectively enhances endothelium-dependent contractions (Yang et al., 2003b) and under bioassay conditions, the transfer of EDCF from the donor tissue to the bioassay preparation is diminished by the combination of superoxide dismutase plus catalase (Yang et al., 2003a). Confocal microscopy shows that ACh causes a rapid increase in reactive oxygen species in endothelial cells of SHR aorta but not in that of WKY. This burst in reactive oxygen species generation is prevented by COX inhibition or by the combination of tiron plus DETCA (Tang et al., 2007). In contrast to ACh, the increase in endothelial intracellular calcium, the generation of reactive oxygen species and the amplitude of endothelium-dependent contractions elicited by A 23187 are of similar amplitude in WKY and SHR aorta (Gluais et al., 2006; Tang et al., 2007).
These results indicate that an abnormal accumulation of calcium in SHR endothelial cells is a prerequisite to initiate the release of EDCF, and this can be mimicked in that of WKY when stimulated by the calcium ionophore. The sequence of events occurring during endothelium-dependent contractions firstly requires the accumulation of calcium, which then most likely induces the phospholipase A2-dependent mobilization of arachidonic acid (Luscher and Vanhoutte, 1986), COX activation and the production of reactive oxygen species along with that of EDCF(s) (Tang et al., 2007). Reactive oxygen species can diffuse towards the vascular smooth muscle cells and produce contraction (Auch-Schwelk et al., 1989; Katusic and Vanhoutte, 1989; Suzuki and Ford, 1992; Yang et al., 2002) and be involved in a positive feedback loop on the endothelial cells by further activating COX (Harlan and Callahan, 1984).
Identification of EDCFs
Cyclooxygenases and prostaglandin synthases
COX are the first enzymes involved in the biosynthetic pathway leading to prostanoid formation. A constitutive (COX-1) and an inducible isoforms (COX-2) have been cloned and characterized (De Witt, 1988; Merlie et al., 1988; Hla and Neilson, 1992; O'Banion et al., 1992; Yokoyama et al., 2002). COX-2 can be induced by several stimuli associated with cell activation and inflammation. In endothelial cells, COX-1 is expressed constitutively but can also be over-expressed, for instance by shear stress (Vane et al., 1998; Doroudi et al., 2000; Davidge, 2001). Both endothelial and vascular smooth muscle cells contain COX, however endothelial cells contain 20 times more of the enzyme than smooth muscle cells (DeWitt et al., 1983). Endothelial cells express preferentially COX-1 versus COX-2 (Onodera et al., 2000; Kawka et al., 2007). In SHR endothelial cells the mRNA and protein expression of COX-1 are enhanced when compared with that of WKY, and in both strains they are augmented by aging (Ge et al., 1995; Tang and Vanhoutte, 2008). Endothelium-dependent contractions to ACh are blocked by specific inhibitors of COX-1 and minimally affected by specific inhibitors of COX-2 (Ge et al., 1995; Yang et al., 2002; 2003a,b; Gluais et al., 2006) (Fig. 1). In agreement with a preponderant role for COX-1 in endothelium-dependent contractions, these responses are abolished in aorta taken from COX-1 knockout mice while they are maintained in aortic rings of COX-2 knockout animals (Tang et al., 2005). However, in some instances, COX-2-derived contractile prostanoids are produced by WKY and SHR aortic endothelial cells (Camacho et al., 1998; Zerrouk et al., 1998; Garcia-Cohen et al., 2000; Alvarez et al., 2005; Blanco-Rivero et al., 2005).
Various biologically active eicosanoids are formed from the short lasting but biologically active endoperoxide (PGH2), through the action of a set of synthases namely PGD, PGE, PGF, PGI and thromboxane synthases (Tsuboi et al., 2002). The expression of prostacyclin synthase (PGIS) is by far the most abundant of these synthases expressed in the rat aortic endothelial cells (Tang and Vanhoutte, 2008), and a greater co-distribution of PGIS with COX-1 is observed when compared with COX-2 (Kawka et al., 2007) explaining why the majority of the endothelial COX-1-derived endoperoxides are transformed into prostacyclin (Gluais et al., 2005; 2006; Tang and Vanhoutte, 2008) (Fig. 3). The expression of PGIS and thromboxane synthase is higher in SHR aortic endothelial cells than in those of WKY (Tang and Vanhoutte, 2008).
Figure 3.
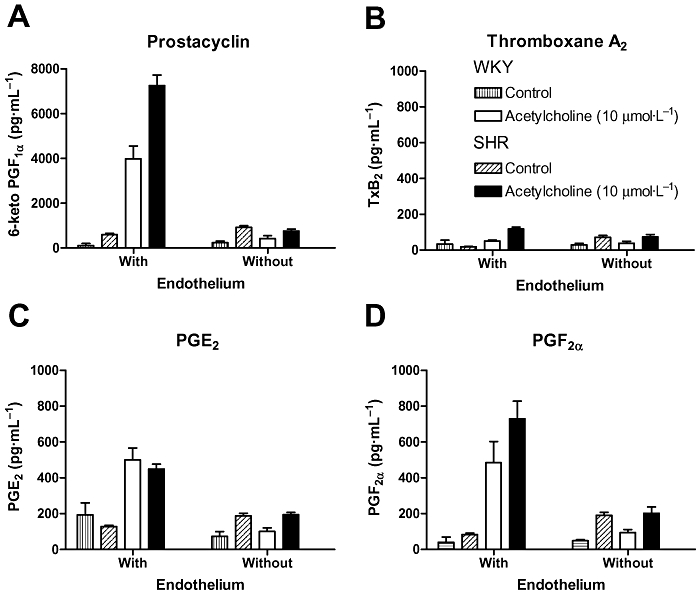
Acetylcholine-induced prostaglandin release in WKY and SHR aorta. Acetylcholine produces the endothelium-dependent release of prostacyclin (A, as measured as its stable metabolite 6-keto-PGF1α), thromboxane A2 (B, as measured as its stable metabolite thromboxane B2), PGE2 (C) and PGF2α (D). Prostacyclin is by far the most abundant prostaglandin released, and its generation is markedly higher in SHR than in WKY aortic rings. Modified from Gluais et al.(Br J Pharmacol, 2005). SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats.
Acetylcholine-induced endothelium-dependent contractions and thromboxane A2
Prostaglandins interact with specific seven transmembrane, G protein-coupled receptors, which are classified in five subtypes DP, EP, FP, IP and TP in function of their sensitivity to the five primary prostanoids, prostaglandins D2, E2, F2α, I2and thromboxane A2 respectively (Tsuboi et al., 2002; Alexander et al., 2008). These receptors are all expressed in WKY and SHR aortae, although at low levels (Tang and Vanhoutte, 2008). ACh-induced endothelium-dependent contractions are blocked by antagonists of the TP receptors. However, inhibitors of thromboxane synthase do not affect these endothelium-dependent contractions indicating that thromboxane A2is not the EDCF released following muscarinic receptor activation (Luscher and Vanhoutte, 1986; Koga et al., 1989; Auch-Schwelk et al., 1990; Kato et al., 1990; Ge et al., 1995; Tesfamariam and Ogletree, 1995; Yang et al., 2002; 2003a,b; 2004; Gluais et al., 2005; 2006) (Fig. 4).
Figure 4.
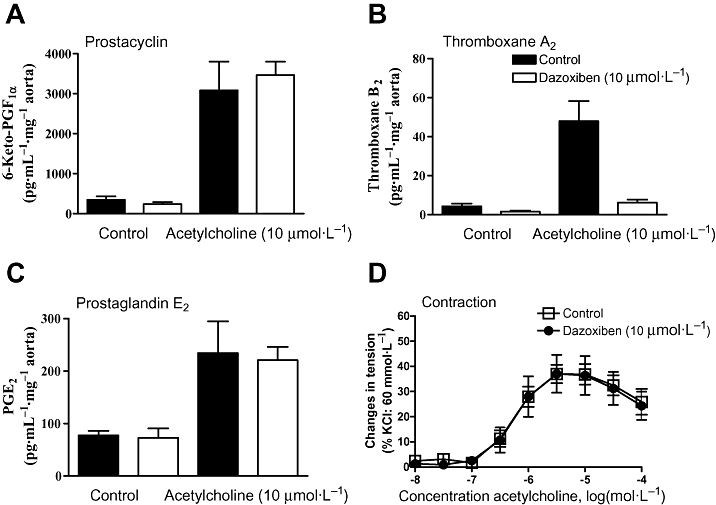
Thromboxane A2 generation and acetylcholine-induced endothelium-dependent contractions. Dazoxiben, the thromboxane synthase inhibitor, does not affect acetylcholine-induced release of prostacyclin (A), PGE2 (C), PGF2α (data not shown), but abolishes that to thromboxane A2 (B). However, acetylcholine-induced endothelium-dependent contraction in SHR aortic rings is not influenced by dazoxiben (D). Modified from Gluais et al.(Br J Pharmacol, 2005).
Acetylcholine-induced endothelium-dependent contractions and PGH2
Thromboxane A2is the most potent agonist at TP receptors but is not its exclusive ligand. In fact, in WKY and SHR aorta numerous prostanoids produce contraction by activating TP receptors, with the following order of potency 9,11-dideoxy-9α,11α-epoxymethano prostaglandin F2α (U 46619) >> 8-isoprostane = PGF2α = PGH2 > PGE2 = PGD2 > PGI2. Among those agonists, only PGH2 and prostacyclin evoke transient contractions, possibly because of their short half-life in aqueous solutions (3–4 min, Dickinson and Murphy, 2002), that mimic endothelium-dependent contractions (Gluais et al., 2005). PGH2is the second most potent agonist at TP receptors, and there is an augmented sensitivity of the SHR smooth muscle cells towards this endoperoxide when compared with that of WKY (Ge et al., 1995; 1999). Therefore, PGH2 has been considered as a suitable candidate for an EDCF (Auch-Schwelk et al., 1990; Kato et al., 1990; Ge et al., 1995; Gluais et al., 2005; 2006; 2007). However, in SHR aortic endothelial cells, the massive expression of PGIS (Tang and Vanhoutte, 2008) and its close association with COX-1 (Kawka et al., 2007) are not in favour of a large PGH2spillover. The levels of PGH2 are difficult to measure and still need to be better assessed in order to evaluate more precisely the contribution of this endoperoxide to endothelium-dependent contractions.
PGH2 is spontaneously or enzymatically transformed in the more stable isomer PGE2 and in presence of mild reducing agent or enzymatically into PGF2α. Although PGE2, via EP receptor activation, is an endothelium-derived contractile factor in a rat model of diabetes (Shi et al., 2007), in the SHR aorta the involvement of this prostaglandin either via EP or TP receptor activation has been ruled out (Tang et al., 2008). However, when PGIS is inhibited, a compensatory increase in the production of PGE2 and PGF2α is observed, and then these prostaglandins act as EDCF (Gluais et al., 2005).
Acetylcholine-induced endothelium-dependent contractions and 8-isoprostane
8-isoprostane (8-epiPGF2α) is generally produced from the oxidative modification of polyunsaturated fatty acids via a free radical-catalysed mechanism (Morrow et al., 1990). However, under some circumstances, 8-isoprostane could be a direct product of COX or an indirect consequence of superoxide anion production by COX-mediated metabolism (Watkins et al., 1999). In both WKY and SHR aortic rings, 8-isoprostane is a potent constrictor (Gluais et al., 2005), supporting the hypothesis that an isoprostane could contribute to EDCF-mediated responses (Janssen, 2002). However, proper measurement of 8-isoprostane generation failed to detect significant ACh-stimulated and endothelium-dependent release of this prostanoid (Gluais et al., 2005). Therefore, in the SHR aorta, 8-isoprostane is unlikely to be an EDCF released by ACh.
Acetylcholine-induced endothelium-dependent contractions and PGI2
ACh produces the endothelium-dependent release of prostacyclin, PGE2, PGF2α and thromboxane A2 in the aorta of both WKY and SHR. The release of prostacyclin is 10 to 100 times larger than that of the other prostaglandins, while the generation of thromboxane A2 is the smallest. Furthermore, the release of prostacyclin is much larger in the aorta of SHR than in that of WKY (Gluais et al., 2005). In the SHR aorta and that of aging WKY, prostacyclin paradoxically is not a relaxing but a contracting prostaglandin (Gluais et al., 2005; Gomez et al., 2008) (Figs 5 and 6). Whether or not, the reduction in the relaxing response to prostacyclin in SHR and the decrease of this response during aging is associated with parallel changes in the expression of the IP receptor gene remains controversial (Numaguchi et al., 1999; Tang and Vanhoutte, 2008). Nevertheless, the IP receptor dysfunction is specific of vascular smooth muscle cells because IP receptor-dependent inhibition of platelet activation is not altered in SHR or by aging (Gomez et al., 2008). Therefore, endothelium-dependent contractions elicited by ACh in the aorta of SHR and aging WKY are likely to involve at least in part the release of prostacyclin.
Figure 5.
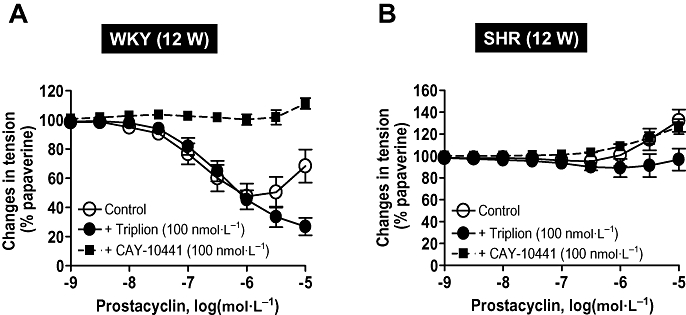
Prostacyclin induces relaxation in WKY aortic rings but not in those of SHR. (A) In aortic rings with endothelium of young 12-week-old WKY, prostacyclin induces relaxations that are inhibited by the specific IP receptor antagonist CAY 10441. The presence of the TP receptor antagonist, Triplion®, enhances the relaxations to prostacyclin. (B) In aortic rings with endothelium of young 12-week-old SHR, prostacyclin does not provoke relaxations but only contractions at higher concentrations. These contractions are blocked by the TP receptor antagonist, Triplion®. SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats. Modified from Gomez et al. (AM J Physiol 2008).
Figure 6.
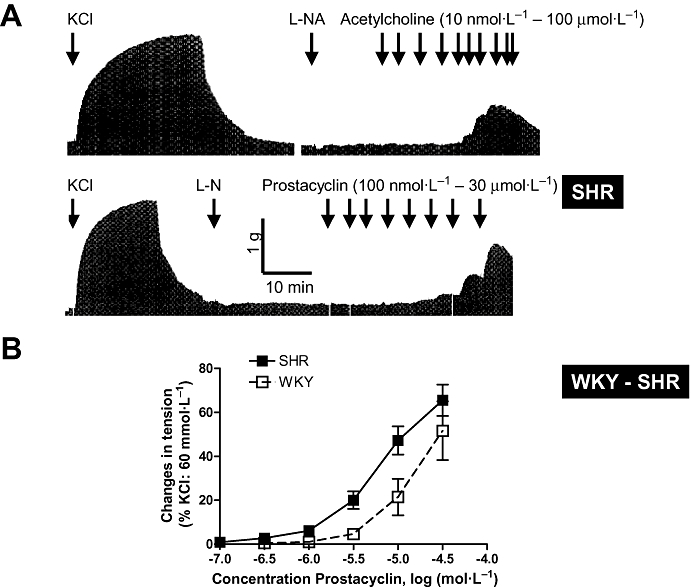
Prostacyclin-induced contractions and acetylcholine-induced endothelium-dependent contractions. (A) Original trace showing acetylcholine-induced concentration and endothelium-dependent contractions in SHR aortic rings (presence of L-nitro-arginine, L-NA). The modest amplitude of the contractions and their transient nature can be visualized in comparison with the reference contraction produced by 60 mmol·L−1 KCl (upper trace). In aortic rings with endothelium and in the presence of L-NA (or in rings without endothelium, data not shown), prostacyclin induces transient concentration-dependent contractions (lower trace). (B) SHR aortic rings are more sensitive than that of WKY to the contractile effect of prostacyclin. In both strains, these contractions are blocked by TP receptor antagonists (data not shown). Modified from Gluais et al.(Br J Pharmacol, 2005). SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats.
This conclusion is based on the following observations: (i) in WKY and SHR, prostacyclin is a contracting but not a relaxing factor (Figs 5 and 6); (ii) prostacyclin is a more potent contracting agent in SHR than in WKY (Fig. 6); (iii) the contractions evoked by prostacyclin mimic the endothelium-dependent contractions produced by ACh both in term of duration and amplitude (Fig. 5); (iv) prostacyclin- and the endothelium-dependent contractions both involve activation of TP receptors (Figs 1 and 5); (v) prostacyclin is the most abundant prostaglandin released by ACh and is of endothelial origin (Fig. 3); (vi) the release of prostacyclin is two times larger in SHR than in WKY (Fig. 3); (vii) the time course of the release of prostacyclin is compatible with the time course of the observed endothelium-dependent contractions; (viii) the release of prostacyclin correlates with the amplitude of the endothelium-dependent contractions over the full concentration range of ACh in both WKY and SHR (Fig. 7); (ix) the endothelium-dependent contractions and the release of prostacyclin are affected similarly by COX inhibitors; (x) The expression of PGIS is by far the most abundant of the prostaglandin synthases expressed in the rat aortic endothelial cells and PGIS and COX-1 co-segregate; and finally (xi) the inhibition of prostacyclin synthesis enhances the ACh-induced endothelium-dependent contractions. Paradoxically, this latter observation also supports the hypothesis that prostacyclin contribute to endothelium-dependent contractions because the inhibition of PGIS may enhance PGH2spillover, a more potent TP receptor agonist than prostacyclin itself (Rapoport and Williams, 1996; Gluais et al., 2005). The hypothesis that prostacyclin is an EDCF is in agreement with the conclusion that prostacyclin is the main factor accounting for endothelial dysfunction in the aorta of WKY and SHR treated with aldosterone (Blanco-Rivero et al., 2005).
Figure 7.
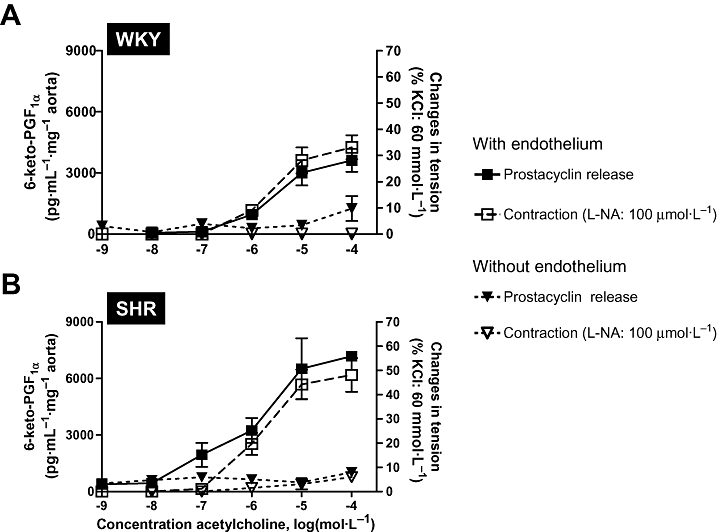
Acetylcholine-induced concentration-dependent and endothelium-dependent prostacyclin release and contractions. (A) WKY aortic rings with and without endothelium. (B) SHR aortic rings with and without endothelium. Prostacyclin release is represented on the left Y-scale and endothelium-dependent contractions on the right Y-scale (presence of L-nitro-arginine, L-NA). Modified from Gluais et al.(Br J Pharmacol, 2005). SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats.
EDCFs released in response to other stimuli
In response to other stimuli, such as ATP or the calcium ionophore A 23187, thromboxane synthase inhibitors partially inhibit endothelium-dependent contractions indicating that thromboxane A2contributes to these responses, prostacyclin and/or PGH2 being the other contributors (Gluais et al., 2006; 2007) (Fig. 8). Similarly, in response to endothelin, the endothelial generation of thromboxane A2 contributes to the contractile response (Taddei and Vanhoutte, 1993a,b).
Figure 8.
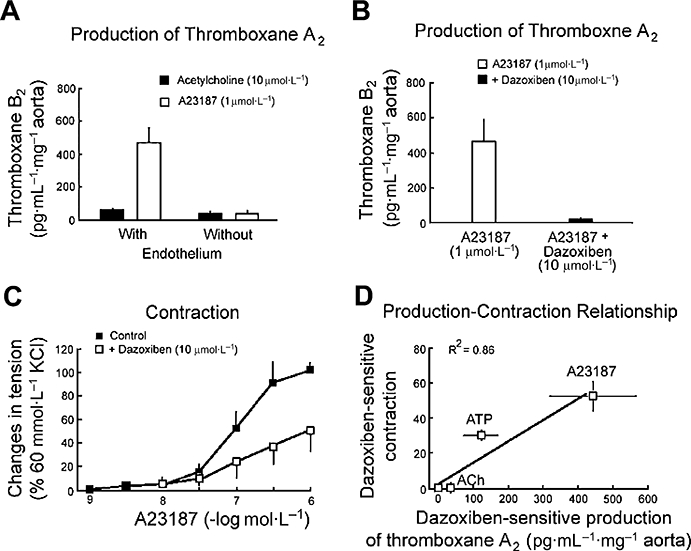
Thromboxane A2 and endothelium-dependent contractions in SHR aortic rings. (A) The calcium ionophore, A23187, produces a significantly larger endothelial release of thromboxane A2 than acetylcholine. (B) The endothelial release of thromboxane A2 by A23187 is blocked by dazoxiben, the thromboxane synthase inhibitor. (C) Dazoxiben partially inhibits the endothelium-dependent contraction to A23187 (in the presence of L-nitro-arginine), indicating the contribution of thromboxane A2 in the endothelium-dependent contractions elicited by the calcium ionophore. (D) In SHR aortic rings with endothelium, the dazoxiben-sensitive production of thromboxane A2 is significantly correlated to the dazoxiben-sensitive component of the endothelium-dependent contractions. SHR, spontaneously hypertensive rats.
Different stimuli, that is, ATP, ACh and A 23187, induce a very different pattern in prostaglandin release. Subsequently, the endothelium-dependent contractions do not involve the same COX derivatives, although the final effector remains the prostanoid TP receptor on the vascular smooth muscle cells (Yang et al., 2004). These differences are not linked to agonist stimulating G protein-coupled receptors versus receptor-independent mechanism because thromboxane A2 contributes to both ATP- and A 23187-induced endothelium-dependent contractions but not in those evoked by ACh. These differences are unexplained at present but could be linked to differences in the dynamic of the increase in endothelial intracellular calcium evoked by these different stimuli (Gordon and Martin, 1983; Carter et al., 1988). (Fig. 9)
Figure 9.
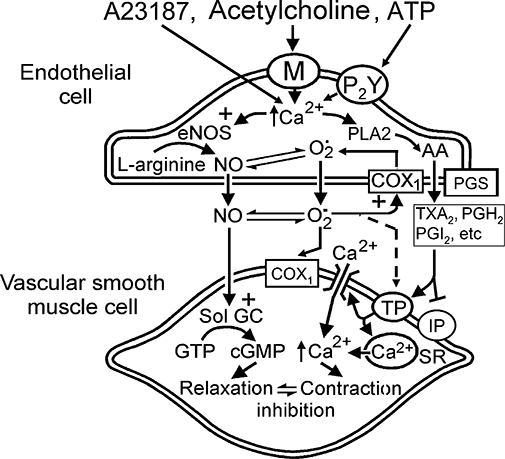
Mechanisms of endothelium-dependent contractions in WKY and SHR aortic rings. The sequence of events occurring during endothelium-dependent contractions firstly requires the intracellular accumulation of calcium, which then most likely induces the phospholipase A2-dependent mobilization of arachidonic acid, COX-1 activation and the production of reactive oxygen species along with that of EDCF(s). Reactive oxygen species inactivate NO, can be involved in a positive feedback loop on the endothelial cells by further activating COX-1 and can diffuse towards the vascular smooth muscle cells and produce contraction. PGH2 along with the various prostaglandins produced by the PG-synthases, prostacyclin (PGI2), thromboxane A2 (TXA2) and possibly under some circumstances PGE2 or PGF2α, converge towards the TP receptors located on the vascular smooth muscle cells and produce contraction. In the SHR, a functional impairment of the smooth muscle IP receptors contributes to the endothelial dysfunction. COX, cyclooxygenase; EDCF, endothelium-derived contracting factor; NOS, nitric oxide synthase; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats.
Endothelial dysfunction in other SHR vascular beds
The endothelial dysfunction observed in the mesenteric (Lüscher et al., 1990; Fu-Xiang et al., 1992; Takase et al., 1994; Lang et al., 1995; Hutri-Kahonen et al., 1997; Dantas et al., 1999; Xavier et al., 2008), renal (Lüscher et al., 1988; Dai et al., 1992; Fu-Xiang et al., 1992; Ito and Carretero, 1992; Dohi et al., 1996; Kagota et al., 1999) and skeletal muscle (Huang et al., 1993; Lübbe et al., 1993; Huang and Koller, 1996; Mori et al., 2006) vascular beds is qualitatively similar to that reported for the aorta. The generation of EDCFs similar to those identified in the aorta contributes to the altered endothelium-dependent relaxations/vasodilatations.
In these resistance arteries, in contrast to the aorta, endothelium-dependent hyperpolarizations (EDHF-mediated responses) participate to endothelium-dependent relaxations (Félétou and Vanhoutte, 2006b). Most studies show a marked attenuation of the EDHF-mediated component in SHR arteries (Fujii et al., 1992; 1993; Hayakawa et al., 1993; 1995; Mantelli et al., 1995; Dohi et al., 1996; Hutri-Kahonen et al., 1997; Bussemaker et al., 2003). The decrease in EDHF-mediated response has been associated with, but not yet causally linked, to a change in the expression profile of gap junctions in endothelial cells (Busse et al., 2002; Félétou and Vanhoutte, 2004; Griffith, 2004). Indeed, the expression of connexins 37 and 40 is lower in arteries of the SHR than in that of the WKY (Kansui et al., 2004; Rummery and Hill, 2004). Additionally, alterations in the expression or function of endothelial calcium-activated potassium channels may lead to the preferential activation of calcium-activated chloride channels and endothelium-dependent depolarization instead of endothelium-dependent hyperpolarization (Corriu et al., 1996; Coleman et al., 2001; Goto et al., 2007). The production of NO is generally not altered, although in the mesenteric artery, a decrease in its bioavailibity due to the generation of oxidative stress may occur (Tschudi et al., 1996; DeLano et al., 2006; Macarthur et al., 2008). In renal arteries of the WKY, inhibitors of EDHF-mediated responses favour endothelium-dependent contractions (Michel et al., 2008b).
EDCF-mediated responses are not ubiquitous in SHR arteries. Thus, in the carotid and cerebral arteries of that strain, the endothelium-dependent relaxations to ACh are attenuated, but this involves an impairment of the NO component without generation of EDCF and without alteration of the EDHF-mediated responses (Hongo et al., 1988; Lüscher et al., 1988; Mayhan, 1990; Sobey et al., 1999; Dina et al., 2004; Iaccarino et al., 2004). Likewise, in SHR coronary arteries when compared with those of WKY, the endothelium-dependent relaxations are not or minimally affected and are not associated with the production of EDCF (Tschudi et al., 1994; 1995; Nava et al., 1995; Bund, 1998; Garcia and Bund, 1998).
Conclusions and perspectives
In the SHR, prostacyclin, PGH2, thromboxane A2and depending on the circumstances, PGE2 and PGF2α can act as EDCFs and all converge towards the TP receptor (Fig. 9). The generation of EDCFs has been demonstrated in human essential hypertension and also in various other animal models of cardiovascular diseases, in particular diabetes (Taddei et al., 2001; Vanhoutte et al., 2005; Verbeuren, 2006a,b; Xu et al., 2006; Cheng et al., 2007; Matsumoto et al., 2007; 2008; Shi et al., 2007; Michel et al., 2008a). In apo E-deficient mice blockade of TP receptors but not aspirin inhibits atherogenesis (Cayatte et al., 2000). In patients with coronary artery disease, a TP receptor blocker improves endothelial function beyond the simple inhibition of COX (Belhassen et al., 2003), indicating that eicosanoids other that the above-mentioned arachidonic acid metabolites, possibly isoprostanes, activate TP receptor and are involved in these pathologies. In the SHR, a functional impairment of IP receptors of the vascular smooth muscle is likely to contribute to the endothelial dysfunction. Mice knockout for the IP receptor (Xiao et al., 2001; Cheng et al., 2002) and human patients with a dysfunctional prostacyclin IP receptor mutation (Arehart et al., 2008) show accelerated atherothrombosis indicating that an imbalance between vasoconstrictor/relaxing and thrombogenic/anti-thrombogenic prostaglandins is of major importance in the generation of cardiovascular disease.
Glossary
Abbreviations:
- ACh
acetylcholine
- COX
cyclooxygenase
- DETCA
diethyldithiocarbamate acid
- EDCF
endothelium-derived contracting factor
- NOS
nitric oxide synthase
- PGIS
prostacyclin synthase
- SHR
spontaneously hypertensive rats
- WKY
Wistar-Kyoto rats
Conflicts of interest
MF and TJV are employees of the Institut de Recherches Servier, a pharmaceutical company that is currently involved in the clinical development of a TP receptor antagonist (S 18886 or Terutroban, or Triplion®). PMV is a consultant for the group Servier and a former employee of this company.
References
- Abeywardena MY, Jablonskis LT, Head RJ. Age- and hypertension-induced changes in abnormal contractions in rat aorta. J Cardiovasc Pharmacol. 2002;40:930–937. doi: 10.1097/00005344-200212000-00015. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Y, Briones AM, Balfagón G, Alonso MJ, Salaices M. Hypertension increases the participation of vasoconstrictor prostanoids from cyclooxygenase-2 in phenylephrine responses. J Hypertens. 2005;23:767–777. doi: 10.1097/01.hjh.0000163145.12707.63. [DOI] [PubMed] [Google Scholar]
- Arehart E, Stitham J, Asselbergs FW, Douville K, MacKenzie T, Fetalvero KM, et al. Acceleration of cardiovascular disease by a dysfunctional prostacyclin receptor mutation: potential implications for cyclooxygenase-2 inhibition. Circ Res. 2008;102:986–993. doi: 10.1161/CIRCRESAHA.107.165936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–864. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Nitric oxide inactivates endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:442–445. doi: 10.1161/01.hyp.19.5.442. [DOI] [PubMed] [Google Scholar]
- Belhassen L, Pelle G, Dubois-Rande J-L, Adnot S. Improved endothelial function by the thromboxane A2 receptor antagonist S 18886 in patients with coronary artery disease treated with aspirin. J Am Coll Cardiol. 2003;41:1198–1204. doi: 10.1016/s0735-1097(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Márquez-Rodas I, Salaices M, et al. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–112. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- Boulanger CM, Morrison KJ, Vanhoutte PM. Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br J Pharmacol. 1994;112:519–524. doi: 10.1111/j.1476-5381.1994.tb13104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bund SJ. Influence of mode of contraction on the mechanism of acetylcholine-mediated relaxation of coronary arteries from normotensive and spontaneously hypertensive rats. Clin Sci (Lond) 1998;94:231–238. doi: 10.1042/cs0940231. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. Endothelium-dependent hyperpolarization, bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Bussemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, et al. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- Camacho M, Lopez-Belmonte J, Vila L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circ Res. 1998;83:353–365. doi: 10.1161/01.res.83.4.353. [DOI] [PubMed] [Google Scholar]
- Carter TD, Hallam TJ, Cusack NJ, Pearson JD. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988;95:1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayatte AJ, Du Y, Oliver-Krasinski J, Lavielle G, Verbeuren TJ, Cohen RA. The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apo E-deficient mice: evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:1724–1728. doi: 10.1161/01.atv.20.7.1724. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Jiang YF, Ding H, Severson D, Triggle CR. Vascular dysfunction in type 2 diabetic TallyHo mice: role for an increase in the contribution of PGH2/TxA2 receptor activation and cytochrome P450 products. Can J Physiol Pharmacol. 2007;285:404–412. doi: 10.1139/y07-010. [DOI] [PubMed] [Google Scholar]
- Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol. 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriu C, Félétou M, Canet E, Vanhoutte PM. Endothelium-derived factors and hyperpolarization of the carotid artery of the guinea-pig. Br J Pharmacol. 1996;119:959–964. doi: 10.1111/j.1476-5381.1996.tb15765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai FX, Skopec J, Diederich A, Diederich D. Prostaglandin H2 and thromboxane A2 are contractile factors in intrarenal arteries of spontaneously hypertensive rats. Hypertension. 1992;19:795–798. doi: 10.1161/01.hyp.19.6.795. [DOI] [PubMed] [Google Scholar]
- Dantas AP, Scivoletto R, Fortes ZB, Nigro D, Carvalho MH. Influence of female sex hormones on endothelium-derived vasoconstrictor prostanoid generation in microvessels of spontaneously hypertensive rats. Hypertension. 1999;34:914–919. doi: 10.1161/01.hyp.34.4.914. [DOI] [PubMed] [Google Scholar]
- Davidge ST. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650–660. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- DeLano FA, Parks DA, Ruedi JM, Babior BM, Schmid-Schönbein GW. Microvascular display of xanthine oxidase and NADPH oxidase in the spontaneously hypertensive rat. Microcirculation. 2006;13:551–566. doi: 10.1080/10739680600885152. [DOI] [PubMed] [Google Scholar]
- DeWitt DL, Smith WL. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci USA. 1988;85:1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt DL, Day JS, Sonnenburg WK, Smith WL. Concentrations of prostaglandin endoperoxide synthase and prostaglandin I2 synthase in the endothelium and smooth muscle of bovine aorta. J Clin Invest. 1983;72:1882–1888. doi: 10.1172/JCI111151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson JS, Murphy RC. Mass spectrometric analysis of leukotriene A4 and other chemically reactive metabolites of arachidonic acid. J Am Soc Mass Spectrom. 2002;13:1227–1234. doi: 10.1016/S1044-0305(02)00456-7. [DOI] [PubMed] [Google Scholar]
- Dina JP, Feres T, Paiva AC, Paiva TB. Role of membrane potential and expression of endothelial factors in restenosis after angioplasty in SHR. Hypertension. 2004;43:131–135. doi: 10.1161/01.HYP.0000105300.74809.4F. [DOI] [PubMed] [Google Scholar]
- Dohi Y, Kojima M, Sato K. Benidipine improves endothelial function in renal resistance arteries of hypertensive rats. Hypertension. 1996;28:58–63. doi: 10.1161/01.hyp.28.1.58. [DOI] [PubMed] [Google Scholar]
- Doroudi R, Gan LM, Selin Sjögren L, Jern S. Effects of shear stress on eicosanoid gene expression and metabolite production in vascular endothelium as studied in a novel biomechanical perfusion model. Biochem Biophys Res Commun. 2000;269:257–264. doi: 10.1006/bbrc.2000.2279. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. EDHF: new therapeutic targets? Pharmacol Res. 2004;49:565–580. doi: 10.1016/j.phrs.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006a;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006b;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Fu-Xiang D, Jameson M, Skopec J, Diederich A, Diederich D. Endothelial dysfunction of resistance arteries of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1992;20:S190–S192. doi: 10.1097/00005344-199204002-00053. [DOI] [PubMed] [Google Scholar]
- Fujii K, Tominaga M, Ohmori S, Kobayashi K, Koga T, Takata Y, et al. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res. 1992;70:660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- Fujii K, Ohmori S, Tominaga M, Abe I, Takata Y, Ohya Y, et al. Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am J Physiol. 1993;265:H509–H516. doi: 10.1152/ajpheart.1993.265.2.H509. [DOI] [PubMed] [Google Scholar]
- Garcia SR, Bund SJ. Nitric oxide modulation of coronary artery myogenic tone in spontaneously hypertensive and Wistar-Kyoto rats. Clin Sci (Lond) 1998;94:225–229. doi: 10.1042/cs0940225. [DOI] [PubMed] [Google Scholar]
- Garcia-Cohen EC, Marin J, Diez-Picazo LD, Baena AB, Salaices M, Rodriguez-Martinez MA. Oxidative stress induced by tert-butyl hydroperoxide causes vasoconstriction in the aorta from hypertensive and aged rats: role of cyclooxygenase-2 isoform. J Pharmacol Exp Ther. 2000;293:75–81. [PubMed] [Google Scholar]
- Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- Ge T, Vanhoutte PM, Boulanger CM. Increased response to prostaglandin H2 precedes changes in PGH synthase-1 expression in the SHR aorta. Zhongguo Yao Li Xue Bao. 1999;20:1087–1092. [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Félétou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Paysant J, Badier-Commander C, Verbeuren T, Vanhoutte PM, Félétou M. In SHR aorta, calcium ionophore A-23187 releases prostacyclin and thromboxane A2 as endothelium-derived contracting factors. Am J Physiol Heart Circ Physiol. 2006;291:H2255–H2264. doi: 10.1152/ajpheart.01115.2005. [DOI] [PubMed] [Google Scholar]
- Gluais P, Vanhoutte PM, Félétou M. Mechanisms underlying ATP-induced endothelium-dependent contractions in the SHR aorta. Eur J Pharmacol. 2007;556:107–114. doi: 10.1016/j.ejphar.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Gomez E, Schwendemann C, Roger S, Simonet S, Paysant J, Courchay C, et al. Aging and prostacyclin responses in aorta and platelets from WKY and SHR rats. Am J Physiol Heart Circ Physiol. 2008;295:H2198–H2211. doi: 10.1152/ajpheart.00507.2008. [DOI] [PubMed] [Google Scholar]
- Gordon JL, Martin W. Endothelium-dependent relaxation of the pig aorta, relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983;79:531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Edwards FR, Hill CE. Depolarization evoked by acetylcholine in mesenteric arteries of hypertensive rats attenuates endothelium-dependent hyperpolarizing factor. J Hypertens. 2007;25:345–359. doi: 10.1097/HJH.0b013e328010d616. [DOI] [PubMed] [Google Scholar]
- Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JM, Callahan KS. Role of hydrogen peroxide in the neutrophil-mediated release of prostacyclin from cultured endothelial cells. J Clin Invest. 1984;74:442–448. doi: 10.1172/JCI111440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa H, Hirata Y, Suzuki E, Sugimoto T, Matsuoka H, Kikuchi K, et al. Mechanisms for altered endothelium-dependent vasorelaxation in isolated kidneys from experimental hypertensive rats. Am J Physiol. 1993;264:H1535–H1541. doi: 10.1152/ajpheart.1993.264.5.H1535. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hirata Y, Suzuki E, Kakoki M, Kikuchi K, Nagano T, et al. Endothelium-derived relaxing factors in the kidney of spontaneously hypertensive rats. Life Sci. 1995;56:L401–L408. doi: 10.1016/0024-3205(95)00157-2. [DOI] [PubMed] [Google Scholar]
- Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffner U, Vanhoutte PM. Increases in flow reduce the release of endothelium-derived relaxing factor in the aorta of normotensive and spontaneously hypertensive rats. Am J Hypertens. 1989;2:762–767. doi: 10.1093/ajh/2.10.762. [DOI] [PubMed] [Google Scholar]
- Hongo K, Nakagomi T, Kassell NF, Sasaki T, Lehman M, Vollmer DG, et al. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke. 1988;19:892–897. doi: 10.1161/01.str.19.7.892. [DOI] [PubMed] [Google Scholar]
- Huang A, Koller A. Both nitric oxide and prostaglandin-mediated responses are impaired in skeletal muscle arterioles of hypertensive rats. J Hypertens. 1996;14:887–895. doi: 10.1097/00004872-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Koller A. Endothelial dysfunction augments myogenic arteriolar constriction in hypertension. Hypertension. 1993;22:913–921. doi: 10.1161/01.hyp.22.6.913. [DOI] [PubMed] [Google Scholar]
- Hutri-Kahonen N, Kahonen M, Tolvanen JP, Wu X, Sallinen K, Porsti I. Ramipril therapy improves arterial dilation in experimental hypertension. Cardiovasc Res. 1997;33:188–195. doi: 10.1016/s0008-6363(96)00197-6. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Ciccarelli M, Sorriento D, Cipolletta E, Cerullo V, Iovino GL, et al. AKT participates in endothelial dysfunction in hypertension. Circulation. 2004;109:2587–2593. doi: 10.1161/01.CIR.0000129768.35536.FA. [DOI] [PubMed] [Google Scholar]
- Ibarra M, Meneses A, Ransanz V, Castillo C, Hong E. Changes in endothelium-dependent vascular responses associated with spontaneous hypertension and age in rats. Arch Med Res. 1995;26:S177–S183. [PubMed] [Google Scholar]
- Ito S, Carretero OA. Impaired response to acetylcholine despite intact endothelium-derived relaxing factor/nitric oxide in isolated microperfused afferent arterioles of the spontaneously hypertensive rat. J Cardiovasc Pharmacol. 1992;20:S187–S189. doi: 10.1097/00005344-199204002-00052. [DOI] [PubMed] [Google Scholar]
- Iwama Y, Kato T, Muramatsu M, Asano H, Shimizu K, Toki Y, et al. Correlation with blood pressure of the acetylcholine-induced endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:326–332. doi: 10.1161/01.hyp.19.4.326. [DOI] [PubMed] [Google Scholar]
- Janssen LJ. Are endothelium-derived hyperpolarizating and contracting factors isoprostanes? Trends Pharmacol Sci. 2002;23:59–62. doi: 10.1016/s0165-6147(02)01890-4. [DOI] [PubMed] [Google Scholar]
- Kagota S, Tamashiro A, Yamaguchi Y, Nakamura K, Kunitomo M. Excessive salt or cholesterol intake alters the balance among endothelium-derived factors released from renal arteries in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1999;34:533–539. doi: 10.1097/00005344-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Kansui Y, Fujii K, Nakamura K, Goto K, Oniki H, Abe I, et al. Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol. 2004;287:H216–H224. doi: 10.1152/ajpheart.00915.2003. [DOI] [PubMed] [Google Scholar]
- Kato T, Iwama Y, Okumura K, Hashimoto H, Ito T, Satake T. Prostaglandin H2 may be the endothelium-derived contracting factor released by acetylcholine in the aorta of the rat. Hypertension. 1990;15:475–481. doi: 10.1161/01.hyp.15.5.475. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25:517–523. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- Kawka DW, Ouellet M, Hétu PO, Singer II, Riendeau D. Double-label expression studies of prostacyclin synthase, thromboxane synthase and COX isoforms in normal aortic endothelium. Biochim Biophys Acta. 2007;1771:45–54. doi: 10.1016/j.bbalip.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayashi K, Takeshita S, Yamashita Y, Fuljishima M. Ageing suppresses endothelium-dependent relaxation and generates contraction mediated by the muscarinic receptors in vascular smooth muscle of normotensive Wistar Kyoto and spontaneously hypertensive rats. J Hypertens. 1988;6:S243–S245. doi: 10.1097/00004872-198812040-00073. [DOI] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14:542–548. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- Lang MG, Noll G, Lüscher TF. Effect of aging and hypertension on contractility of resistance arteries: modulation by endothelial factors. Am J Physiol. 1995;269:H837–H844. doi: 10.1152/ajpheart.1995.269.3.H837. [DOI] [PubMed] [Google Scholar]
- Liu MH, Jin HK, Floten HS, Yang Q, Yim AP, Furnary A, et al. Vascular endothelial growth factor-mediated endothelium-dependent relaxation is blunted in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2001;296:473–477. [PubMed] [Google Scholar]
- Lockette W, Otsuka Y, Carretero O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension. 1986;8:II-61-6. doi: 10.1161/01.hyp.8.6_pt_2.ii61. [DOI] [PubMed] [Google Scholar]
- Lübbe AS, Harris PD, Alsip NL. Hypertension decreases small arteriole responses to acetylcholine in skeletal muscle. Clin Exp Hypertens. 1993;15:479–487. doi: 10.3109/10641969309041623. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Diederich D, Weber E, Vanhoutte PM, Bühler FR. Endothelium-dependent responses in carotid and renal arteries of normotensive and hypertensive rats. Hypertension. 1988;11:573–578. doi: 10.1161/01.hyp.11.6.573. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Aarhus LL, Vanhoutte PM. Indomethacin improves the impaired endothelium-dependent relaxations in small mesenteric arteries of the spontaneously hypertensive rat. Am J Hypertens. 1990;3:55–58. doi: 10.1093/ajh/3.1.55. [DOI] [PubMed] [Google Scholar]
- Macarthur H, Westfall TC, Wilken GH. Oxidative stress attenuates NO-induced modulation of sympathetic neurotransmission in the mesenteric arterial bed of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H183–H189. doi: 10.1152/ajpheart.01040.2007. [DOI] [PubMed] [Google Scholar]
- Mantelli L, Amerini S, Ledda F. Roles of nitric oxide and endothelium-derived hyperpolarizing factor in vasorelaxant effect of acetylcholine as influenced by aging and hypertension. J Cardiovasc Pharmacol. 1995;25:595–602. doi: 10.1097/00005344-199504000-00013. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;293:H1480–H1490. doi: 10.1152/ajpheart.00229.2007. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008;295:H1165–H1176. doi: 10.1152/ajpheart.00486.2008. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Impairment of endothelium-dependent dilatation of basilar artery during chronic hypertension. Am J Physiol. 1990;259:H1455–H1462. doi: 10.1152/ajpheart.1990.259.5.H1455. [DOI] [PubMed] [Google Scholar]
- Merlie JP, Fagan D, Mudd J, Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase) J Biol Chem. 1988;263:3550–3553. [PubMed] [Google Scholar]
- Michel F, Simonet S, Vayssettes-Courchay C, Bertin F, Sansilvestri-Morel P, Bernhardt F, et al. Altered TP receptor function in isolated, perfused kidneys of nondiabetic and diabetic ApoE-deficient mice. Am J Physiol Renal Physiol. 2008a;294:F120–F129. doi: 10.1152/ajprenal.00111.2007. [DOI] [PubMed] [Google Scholar]
- Michel FS, Man GS, Man RY, Vanhoutte PM. Hypertension and the absence of EDHF-mediated responses favour endothelium-dependent contractions in renal arteries of the rat. Br J Pharmacol. 2008b;155:217–226. doi: 10.1038/bjp.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Purinergic endothelium-dependent and -independent contractions in rat aorta. Hypertension. 1993;22:577–583. doi: 10.1161/01.hyp.22.4.577. [DOI] [PubMed] [Google Scholar]
- Mori Y, Ohyanagi M, Koida S, Ueda A, Ishiko K, Iwasaki T. Effects of endothelium-derived hyperpolarizing factor and nitric oxide on endothelial function in femoral resistance arteries of spontaneously hypertensive rats. Hypertens Res. 2006;29:187–195. doi: 10.1291/hypres.29.187. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Hill KE, Burk RF, Nannour TM, Badr KF, Roberts LJ., II A series of prostaglandins F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical catalysed mechanism. Proc Natl Acad Sci USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava E, Noll G, Lüscher TF. Increased activity of constitutive nitric oxide synthase in cardiac endothelium in spontaneous hypertension. Circulation. 1995;91:2310–2313. doi: 10.1161/01.cir.91.9.2310. [DOI] [PubMed] [Google Scholar]
- Numaguchi Y, Harada M, Osanai H, Hayashi K, Toki Y, Okamura K, et al. Altered gene expression of prostacyclin synthase and prostacyclin receptor in the thoracic aorta of spontaneously hypertensive rats. Cardiovasc Res. 1999;41:682–688. doi: 10.1016/s0008-6363(98)00239-9. [DOI] [PubMed] [Google Scholar]
- O'Banion MK, Winn VD, Young DA. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci USA. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera M, Morita I, Mano Y, Murota S. Differential effects of nitric oxide on the activity of prostaglandin endoperoxide H synthase-1 and -2 in vascular endothelial cells. Prostaglandins Leukot Essent Fatty Acids. 2000;62:161–167. doi: 10.1054/plef.2000.0136. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Williams SP. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1996;28:64–75. doi: 10.1161/01.hyp.28.1.64. [DOI] [PubMed] [Google Scholar]
- Rummery NM, Hill CE. Vascular gap junctions and implications for hypertension. Clin Exp Pharmacol Physiol. 2004;31:659–667. doi: 10.1111/j.1440-1681.2004.04071.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Félétou M, Ku DD, Man RY, Vanhoutte PM. The calcium ionophore A23187 induces endothelium-dependent contractions in femoral arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007;150:624–632. doi: 10.1038/sj.bjp.0706999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Moffatt JD, Cocks TM. Evidence for selective effects of chronic hypertension on cerebral artery vasodilatation to protease-activated receptor-2 activation. Stroke. 1999;30:1933–1940. doi: 10.1161/01.str.30.9.1933. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Ford GD. Superoxide stimulates IP3-induced Ca2+ release from vascular smooth muscle sarcoplasmic reticulum. Am J Physiol. 1992;262:H114–H116. doi: 10.1152/ajpheart.1992.262.1.H114. [DOI] [PubMed] [Google Scholar]
- Taddei S, Vanhoutte PM. Endothelium-dependent contractions to endothelin in the rat aorta are mediated by thromboxane A2. J Cardiovasc Pharmacol. 1993a;22:S328–S331. doi: 10.1097/00005344-199322008-00086. [DOI] [PubMed] [Google Scholar]
- Taddei S, Vanhoutte PM. Role of endothelium in endothelin-evoked contractions in the rat aorta. Hypertension. 1993b;21:9–15. doi: 10.1161/01.hyp.21.1.9. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A. Endothelial dysfunction in hypertension. J Cardiovasc Pharmacol. 2001;38:S11–S14. doi: 10.1097/00005344-200111002-00004. [DOI] [PubMed] [Google Scholar]
- Takase H, Dohi Y, Kojima M, Sato K. Changes in the endothelial cyclooxygenase pathway in resistance arteries of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1994;23:326–330. [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Tang EH, Ku DD, Tipoe GL, Félétou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2-/- knockout but not COX1-/- knockout mice. J Cardiovasc Pharmacol. 2005;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- Tang EH, Leung FP, Huang Y, Félétou M, So KF, Man RY, et al. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EH, Jensen BL, Skott O, Leung GP, Félétou M, Man RY, et al. The role of prostaglandin E and thromboxane-prostanoid receptors in the response to prostaglandin E2 in the aorta of Wistar Kyoto rats and spontaneously hypertensive rats. Cardiovasc Res. 2008;78:130–138. doi: 10.1093/cvr/cvm112. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Ogletree ML. Dissociation of endothelial cell dysfunction and blood pressure in SHR. Am J Physiol. 1995;269:H189–H194. doi: 10.1152/ajpheart.1995.269.1.H189. [DOI] [PubMed] [Google Scholar]
- Tschudi MR, Lüscher TF. Age and hypertension differently affect coronary contractions to endothelin-1, serotonin, and angiotensins. Circulation. 1995;91:2415–2422. doi: 10.1161/01.cir.91.9.2415. [DOI] [PubMed] [Google Scholar]
- Tschudi MR, Criscione L, Novosel D, Pfeiffer K, Lüscher TF. Antihypertensive therapy augments endothelium-dependent relaxations in coronary arteries of spontaneously hypertensive rats. Circulation. 1994;89:2212–2218. doi: 10.1161/01.cir.89.5.2212. [DOI] [PubMed] [Google Scholar]
- Tschudi MR, Mesaros S, Lüscher TF, Malinski T. Direct in situ measurement of nitric oxide in mesenteric resistance arteries. Increased decomposition by superoxide in hypertension. Hypertension. 1996;27:32–35. doi: 10.1161/01.hyp.27.1.32. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sugimoto Y, Ichikawa A. Prostanoid receptor subtypes. Prostaglandins Other Lipid Mediat. 2002;68–69:535–556. doi: 10.1016/s0090-6980(02)00054-0. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Félétou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeuren TJ. Experimental models of thrombosis and atherosclerosis. Therapie. 2006a;61:379–387. doi: 10.2515/therapie:2006069. [DOI] [PubMed] [Google Scholar]
- Verbeuren TJ. Terutroban and endothelial TP receptors in atherogenesis. Med Sci (Paris) 2006b;22:437–443. doi: 10.1051/medsci/2006224437. [DOI] [PubMed] [Google Scholar]
- Watkins MT, Patton GM, Soler HM, Albadawi H, Humphries DE, Evans JE, et al. Synthesis of 8-epi-prostaglandinF2α by human endothelial cells, role of prostaglandin H2 synthase. Biochem J. 1999;344:747–775. [PMC free article] [PubMed] [Google Scholar]
- Xavier FE, Aras-López R, Arroyo-Villa I, Campo LD, Salaices M, Rossoni LV, et al. Aldosterone induces endothelial dysfunction in resistance arteries from normotensive and hypertensive rats by increasing thromboxane A(2) and prostacyclin. Br J Pharmacol. 2008;154:1225–1235. doi: 10.1038/bjp.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao CY, Hara A, Yuhki K, Fujino T, Ma H, Okada Y, et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001;104:2210–2215. doi: 10.1161/hc4301.098058. [DOI] [PubMed] [Google Scholar]
- Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, et al. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes. 2006;55:110–119. [PubMed] [Google Scholar]
- Yang D, Félétou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Félétou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003a;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- Yang D, Levens N, Zhang JN, Vanhoutte PM, Félétou M. Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension. 2003b;41:136–142. doi: 10.1161/01.hyp.0000047669.93078.a7. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Félétou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A 23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol. 2004;18:321–326. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Yabuki T, Shimonishi M, Wada M, Hatae T, Ohkawara S, et al. Prostacyclin-deficient mice develop ischemic renal disorders, including nephrosclerosis and renal infarction. Circulation. 2002;106:2397–2403. doi: 10.1161/01.cir.0000034733.93020.bc. [DOI] [PubMed] [Google Scholar]
- Zerrouk A, Auguet M, Chabrier PE. Augmented endothelium-dependent contraction to angiotensin II in the SHR aorta: role of an inducible cyclooxygenase metabolite. J Cardiovasc Pharmacol. 1998;31:525–533. doi: 10.1097/00005344-199804000-00009. [DOI] [PubMed] [Google Scholar]


