Abstract
Background and purpose:
α4 and β2 nicotinic acetylcholine (ACh) receptor subunits expressed heterologously in Xenopus oocytes assemble into a mixed population of (α4)2(β2)3 and (α4)3(β2)2 receptors. In order to express these receptors separately in heterologous systems, we have engineered pentameric concatenated (α4)2(β2)3 and (α4)3(β2)2 receptors.
Experimental approach:
α4 and β2 subunits were concatenated by synthetic linkers into pentameric constructs to produce either (α4)2(β2)3 or (α4)3(β2)2 receptors. Using two-electrode voltage-clamp techniques, we examined the ability of the concatenated constructs to produce functional expression in Xenopus oocytes. Functional constructs were further characterized in respect to agonists, competitive antagonists, Ca2+ permeability, sensitivity to modulation by Zn2+ and sensitivity to up-regulation by chaperone protein 14-3-3.
Key results:
We found that pentameric concatamers with a subunit arrangement of β2_α4_β2_α4_β2 or β2_α4_β2_α4_α4 were stable and functional in Xenopus oocytes. By comparison, when α4 and β2 were concatenated with a subunit order of β2_β2_α4_β2_α4 or β2_α4_α4_β2_α4, functional expression in Xenopus oocytes was very low, even though the proteins were synthesized and stable. Both β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 concatamers recapitulated the ACh concentration response curve, the sensitivity to Zn2+ modulation, Ca2+ permeability and the sensitivity to up-regulation by chaperone protein 14-3-3 of the corresponding non-linked (α4)2(β2)3 and (α4)3(β2)2 receptors respectively. Using these concatamers, we found that most α4β2-preferring compounds studied, including A85380, 5I-A85380, cytisine, epibatidine, TC2559 and dihydro-β-erythroidine, demonstrate stoichiometry-specific potencies and efficacies.
Conclusions and implications:
We concluded that the α4β2 nicotinic ACh receptors produced with β2_α4_β2_α4_β2 or β2_α4_β2_α4_α4 pentameric constructs are valid models of non-linked (α4)2(β2)3 and (α4)3(β2)2 receptors respectively.
Keywords: concatenated receptors, nicotinic acetylcholine receptors
Introduction
The α4β2 nicotinic acetylcholine receptors (nAChR) are signalling proteins that belong to the Cys-loop superfamily of ligand-gated ion channels (LGIC). They are distributed widely in the brain of mammals and are involved in nicotine addiction, in nociception and in cognitive processes such as attention, learning and memory (Picciotto et al., 2001; Gotti et al., 2006). Their pathologies include autosomal nocturnal frontal epilepsy, depression, autism, Parkinson's disease and Alzheimer's disease (Cassels et al., 2006; Gotti et al., 2006). Understanding the functional and molecular properties of the α4β2 nAChR is thus of great interest for aiding the elucidation of the functions of α4β2 nAChRs in the brain and for the development of new drug therapies.
Heterologous co-expression of α4 and β2 nAChR subunits produces high- and low-affinity receptor populations as shown by biphasic acetylcholine (ACh) concentration-response curves. High- and low-affinity α4β2 nAChR result from the assembly of receptors with two distinct stoichiometries: (α4)2(β2)3 (high-affinity subtype) and (α4)3(β2)2 (low-affinity subtype) (Nelson et al., 2003; Moroni et al., 2006; Zwart et al., 2006), although pharmacological studies of α4β2 nAChR expressed heterologously in Xenopus oocytes suggest that other stoichiometric arrangements may also occur (Zwart and Vijverberg, 1998; López-Hernández et al., 2004). (α4)2(β2)3 and (α4)3(β2)2 receptors differ in sensitivity to activation by agonists, desensitization kinetics, unitary conductance (Nelson et al., 2003; Moroni et al., 2006), Ca2+ permeability (Tapia et al., 2007) and sensitivity to both Zn2+ modulation (Moroni et al., 2008) and chronic exposure to nicotine (Nelson et al., 2003; Kuryatov et al., 2005; Moroni et al., 2006). Both (α4)2(β2)3 and (α4)3(β2)2 are likely to be present in the brain (Marks et al., 1999; Butt et al., 2002; Gotti et al., 2008) and it has been suggested that their relative ratio may influence basal behaviours influenced by α4β2 receptors as well as sensitivity to the acute effects of nicotine (Stitzel et al., 2001; Tritto et al., 2002; Kim et al., 2003). Hence, the separate expression of (α4)2(β2)3 and (α4)3(β2)2 in heterologous systems has become an increasingly attractive approach for aiding the characterization of the properties of these receptors and for the development of drugs acting on stoichiometry-specific α4β2 nAChR.
There have been several attempts at expressing (α4)2(β2)3 and (α4)3(β2)2 nAChRs separately in heterologous systems. These include injection of 1 : 10 or 10 : 1 ratios of α4 and β2 subunit cDNAs into the nucleus of Xenopus oocytes (Moroni et al., 2006), growing human embryonic kidney 293T cells stably transfected with α4 and β2 subunits at 29°C or chronically exposed to nicotine (Nelson et al., 2003). Although, as suggested by monophasic ACh concentration-response curves, these approaches yield very homogeneous populations of (α4)2(β2)3 or (α4)3(β2)2 receptors, it is not conclusive that they abolish concurrent expression of multiple forms of the α4β2 receptor. Tandem subunit constructs with two subunits attached together by synthetic AGS linkers have also been used to constrain the stoichiometry of α4β2 nAChRs. However, this approach can lead to the formation of dipentamers (Zhou et al., 2003) or linked subunits may not be fully incorporated into the pentameric structure of the receptor (Groot-Kormelink et al., 2004). An alternative approach that circumvents these problems is to bridge the subunits by synthetic linkers into pentameric concatamers. This strategy has been used to produce functional expression of (α3)2(β4)3 nAChR (Groot-Kormelink et al., 2006) and (α1)2(β2)2γ2 GABAA receptors (Baur et al., 2006). As suggested by functional studies, pentameric (α3)2(β4)3 and (α1)2(β2)2γ2 GABAA concatamers are similar to their non-linked counterparts, which shows that concatenation of Cys-loop subunit receptors to pentamers does not alter receptor functions. Here, we show that linking the α4 and β2 subunits covalently into pentameric concatamers with a subunit order of β2_α4_β2_α4_β2 or β2_α4_β2_α4_α4 produce receptors that express well in Xenopus oocytes. These concatenated receptors reproduced the functional properties of non-linked (α4)2(β2)3 and (α4)3(β2)2 receptors. In addition, the functional expression of both β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 receptors was up-regulated by co-expression with the chaperone protein 14-3-3. This shows that subunit concatenation does not impair the ability of α4β2 nAChRs to interact with protein 14-3-3. The functional expression of constructs with a subunit order similar to that used to produce functional α3β4 pentameric concatamers (i.e. β3_β3_α4_β3_α4) was also tested (Groot-Kormelink et al., 2006). We found that these constructs, although synthesized and stable in Xenopus oocytes, expressed poorly and did not replicate the pharmacological properties of the corresponding non-linked receptors. We discuss these findings in terms of the effects of subunit arrangements on receptor functionality and possibly receptor maturation.
Methods
Construction of pentameric (α4)2(β2)3 and (α4)3(β2)2 concatamers
Three types of α4β2 receptor concatamers were constructed. The first type was engineered by the procedure described by Groot-Kormelink et al. (2006) to construct α3β4 pentameric concatamers. Briefly, the subunit order for the (α4)2(β2)3 was β2_β2_α4_β2_α4, and for the (α4)3(β2)2 was β2_α4_α4_β2_α4. The signal peptides were maintained in all subunits which were linked from the C- to the N-terminal by (Q)8 linkers. For clarity, these constructs are referred to as β2Q8β2Q8α4Q8β2Q8α4 and β2Q8α4Q8α4Q8β2Q8α4 respectively. The second type of constructs maintained the subunit order of the previous constructs. However, the signal peptide was removed from all the subunits but the first and the subunits were bridged by AGS linkers of variable length to compensate for differences in the length of the C-terminus of the α4 and β2 subunits. Thus, an (AGS)6 linker joined β2 to the α4 subunit, whereas an (AGS)9 linker bridged α4 to the β2 subunit. These constructs are referred to as β2AGSβ2AGSα4AGSβ2AGSα4 and β2AGSα4AGSα4AGSβ2AGSα4 respectively. The third type of constructs used a subunit order of β2_α4_β2_α4_β2 for the (α4)2(β2)3 and β2_α4_β2_α4_α4 for the (α4)3(β2)2 receptor. In these constructs, the signal peptide was removed from all the subunits but the first and the subunits were bridged by AGS linkers of variable length, as in the β2AGSβ2AGSα4AGSβ2AGSα4 and β2AGSα4AGSα4AGSβ2AGSα4 constructs.
Two consecutive PCR steps were used to prepare the subunits for concatenation. Briefly, the first PCR step eliminated stop codons (for all constructs) and signal peptides (for the second and third type of constructs) and inserted the kozac sequence GCCACC immediately before the signal peptide of the first subunit. Half of the length of the linkers [i.e. Q4, (AGS)3 or (AGS)4/5] was added with the first PCR step upstream and downstream from the 5' and 3' coding regions of each subunit. The second PCR step introduced unique restriction sites upstream and downstream of the linkers to allow successive subcloning into a modified pcDNA3.1 Hygro (−) plasmid vector (Invitrogen, UK). This plasmid was also used to assemble the concatamers. To facilitate assembly and subcloning AscI, XbaI and AgeI restriction sites were inserted by oligonucleotide hybridization between the NheI and XhoI sites in the multiple cloning site of the plasmid. For all constructs, the enzyme restriction sites introduced were: 1st subunit AscI/XbaI; 2nd subunit XbaI/AgeI; 3rd subunit AgeI/XhoI; 4th subunit XhoI/NotI; 5th subunit NotI/EcoRV.
Ligation of the subunits
Subunits were ligated sequentially into pentameric constructs using the T4 ligase enzyme as specified by the manufacturer (NEB Biolabs, UK). Following assembly, pentameric concatamers were subcloned into the vector pCI (Promega, UK). The multiple cloning site of this vector was modified by oligo hybridization to contain an AscI and EcoRV restriction sites. In addition a SwaI site was inserted downstream of the SV40 late region for linearization prior to RNA in vitro transcription.
Expression of α4β2 receptors
The care and use of Xenopus laevis toads in this study were approved by the Oxford Brookes University Animal Research Committee and comply with the guidelines of the Scientific Procedures Act, 1986 (UK). The α4β2 receptors were expressed in defolliculated stage V or VI X. laevis oocytes, which were dissected from adult female X. laevis (Horst Kaehler, Hamburg, Germany). Non-linked human α4 or β2 subunit cDNAs or cRNAs were injected into the nucleus or cytoplasm, respectively, of oocytes in a volume of 18.4 nL per oocyte using a Nanoject Automatic Oocyte Injector (Drummond, Broomall, USA). To favour the expression of (α4)2(β2)3 nAChRs, α4 and β2 subunit cDNAs or cRNAs were combined in a ratio of 1 : 10, whereas a subunit ratio of 10 : 1 was used to produce the expression of (α4)3(β2)2 nAChRs (Moroni et al., 2006). The receptors produced in this manner are referred henceforth as non-linked (α4)2(β2)3 and (α4)3(β2)2 receptors respectively. The total amount of cDNA or cRNA injected per oocyte was kept constant at 2 ng. Each oocyte was injected with 50 nL of RNA at appropriate concentrations (up to 150 ng for constructs β2Q8β2Q8α4Q8β2Q8α4, β2Q8α4Q8α4Q8β2Q8α4, β2AGSβ2AGSα4AGSβ2AGSα4 and β2AGSα4AGSα4AGSβ2AGSα4, 10 ng for β2_α4_β2_α4_β2 and 5 ng for β2_α4_β2_α4_α4). For studies of the effects of chaperone protein 14-3-3 on the functional expression of the pentameric concatamers, 2 ng of cRNA coding for rat 14-3-3 was co-injected with concatamer-cRNA. The cDNA for this protein was kindly provided by Dr R. Anand (Ohio State University, USA). Capped RNAs were synthesized using the mMessage mMachine T7 kit (Ambion, UK) from the linearized pCI vectors containing the non-linked or linked subunits. After injection, oocytes were incubated at 18°C for 2–6 days in a modified Barth's solution containing 88 mmol·L−1 NaCl, 1 mmol·L−1 KCl, 2.4 mmol·L−1 NaHCO3, 0.3 mmol·L−1 Ca(NO3)2, 0.41 mmol·L−1 CaCl2, 0.82 mmol·L−1 MgSO4, 15 mmol·L−1 HEPES and 50 µg·mL−1 neomycin (pH 7.6).
Electrophysiology and data analysis
Recordings were performed 2–6 days post injection. Oocytes were placed in a 0.1 mL recording chamber and perfused at a rate of 15 mL·min−1 with modified Ringer solution (in mmol·L−1: NaCl 150, KCl 2.8, HEPES 10, BaCl2 1.8 and adjusted to pH 7.2 with 5 mmol L−1 NaOH), unless otherwise stated. We chose a nominally Ca2+-free solution in order to minimize the contribution to the response of Ca2+-gated chloride channels which are endogenous to the Xenopus oocyte and may be activated by Ca2+ entry through the heterologously expressed nAChRs. Oocytes were impaled by two agarose-cushioned microelectrodes filled with 3 mol·L−1 KCl (Ω= 0.5–1.0 MΩ) and voltage-clamped at −60 mV, except for the determination of the reversal potential of the ACh currents under different concentrations of external Ca2+, using a Geneclamp 500B amplifier and PCLAMP 8 software (Molecular Devices, USA). Typically traces were filtered at 1 kHz during recording and digitized at 10 kHz using the DigiData 1200 interface (Molecular Devices, USA). All experiments were carried out at room temperature (approx. 20°C). Agonist concentration-response curves were obtained by normalizing agonist-induced responses to the control responses induced by 1 mmol·L−1 ACh [a near-maximum effective concentration at receptors obtained with non-linked or linked (α4)3(β2)2 but an EC100 concentration at non-linked or linked (α4)2(β2)3 receptors]. A minimum interval of 4 min was allowed between agonist applications as this procedure ensured reproducible recordings. The sensitivity of the receptors to inhibition by the nAChR antagonist dihydro-β-erythroidine (DhβE) was tested by first superfusing the antagonist for 2 min and then co-applying it with the appropriate ACh EC50. Antagonist concentration response data were normalized to the appropriate ACh EC50. The sensitivity to Zn2+ was assessed by co-applying a range of Zn2+ concentrations with 1 µmol·L−1 ACh, the ACh EC20 at linked and non-linked (α4)2(β2)3 nAChRs, or 10 µmol·L−1 ACh, the ACh EC10 at linked and non-linked (α4)3(β2)2 receptors. For Zn2+ to attain equilibrium around impaled oocytes, Zn2+ was pre-applied for 30 s to the cell prior to co-application of ACh and Zn2+. Concentration–response relationships for Zn2+ were obtained using this protocol. Peak responses elicited by ACh + Zn2+ were normalized to the peak response of the appropriate ACh alone. The Ca2+ permeability of the concatamers were determined by constructing current-voltage plots relationships and measuring the reversal potential of ACh EC50 currents in the presence of 1.8 mmol·L−1 Ca2+ or 18 mmol·L−1 Ca2+ in the perfusing Ringer solution and measuring the shift in the reversal potential.
Western blot analysis
Western blot assays were carried out on total oocyte membrane homogenates prepared from oocytes microinjected with cRNA coding for the pentameric constructs. Oocytes were homogenized 6 days after microinjection with concatamer cRNAs. Four batches of oocytes (50 oocytes per batch) were used. Oocytes were homogenized using an ice-cold homogenization buffer (150 mmol·L−1 NaCl, 2 mmol·L−1 CaCl2, 2% Triton-X100, 20 mmol·L−1 Tris-HCl, pH 7.4, supplemented with 1 µmol·L−1 pepstatin, 1 mg·mL−1 leupeptin, 2 mmol·L−1 PMSF) at a ratio of 10 µL buffer per oocyte. Homogenates were centrifuged twice at 1000×g for 5 min at 4°C to remove the yolk and the supernatants were then recentrifuged at 10 000×g for 10 min at 4°C. Aliquots of the supernatants containing 30 µg of protein were separated by SDS-PAGE electrophoresis (Novex, 7% Tris-acetate gels; Invitrogen, UK). The proteins were subsequently transferred onto nitrocellulose membranes (Optitran BA-S83, Schleider & Schuell, Germany) by electro-blotting (2 h transfer at room temperature at 25 mV). Membranes were blocked for 2 h at room temperature with 5% non-fat dry milk in phosphate buffered saline containing 0.1% Tween 20 (Sigma-Aldrich Co, UK). Membranes were incubated overnight at 4°C with 0.7 µg·mL−1 of primary antibody [AChRα4 (H-133); Santa Cruz Biotechnology, USA] and further incubated for 3 h at room temperature with 2 µg·mL−1 of secondary antibody (Cy-5-goat anti-rabbit IgG conjugate; Invitrogen, UK). Bound antibodies were visualized on a Typhoon variable mode imager (GE Healthcare, UK).
Drugs
Acetylcholine, A85380, epibatidine, cytisine, DhβE and ZnCl2 were purchased from Sigma-Aldrich (St Louis, MO, USA). 5I-A85380 was purchased from Tocris (UK). TC2559 was a gift from Targacept Inc. (Winston-Salem, NC, USA) and sazetidine-A from Eli Lilly (Surrey, UK). 5-Br-cytisine (5-Br-Cys) was a gift from Prof. Bruce Cassels (University of Chile, Santiago, Chile).
Data analysis and statistics
The concentration of agonist that evokes 50% of maximum response (EC50, mean and 95% confidence interval, CI), the maximum observed normalized response (I/Imax, mean ± SEM), nHill coefficient (nHill, mean ± SEM) or the concentration of antagonist that inhibits 50% of the responses (IC50, mean and 95% CI) were determined by non-linear regression (fitting to one component Hill equation: I = Imax/[1 + (EC50/x)nH], where x is the agonist or antagonist concentration). Biphasic agonist data were fitted to the sum of two Hill equations as previously reported (Houlihan et al., 2001). For analysis of Zn2+ effects where a single component concentration–response relationship was evident, data were fitted to the one component Hill equation shown above. When Zn2+ produced both a potentiating and inhibiting effect, data were fitted to the equation designed to account for potentiating and inhibitory effects of Zn2+ on α4β2 receptors, assuming that this cation binds to two distinct sites on the receptor: I = {1 + ((Imax− 1)/(1 + 10∧[(LogEC50− X)*nHpot])}/(1 + 10∧[(LogIC50− X)*nHinh]), where I represents the current response at a given Zn2+ concentration (X), Imax represents the maximally potentiated peak, EC50 and IC50 are the respective concentrations of Zn2+ inducing half-maximal potentiation or inhibition, and nHpot and nHinh are the Hill coefficients for potentiation and inhibition respectively. F tests were used to assess the curve fitting of all concentration-response data. The one-component model was preferred unless the extra sum-of-squares F test had a value of P < 0.05. Fits to full concentration-response curves for individual oocytes were made independently using Prism 4 (GraphPad Software, CA, USA) and then averaged in order to compare significant differences between groups. Statistical significance was assessed using one-way analysis of the variance (anova) or Student's t-test as appropriate. Values of P < 0.05 were considered statistically significant.
Results
We engineered pentameric concatenated α4β2 cDNA constructs to produce either (α4)2(β2)3 or (α4)3(β2)2 receptors. For each stoichiometry, concatenated receptors with two different subunit orders were produced (Figure 1). For the (α4)2(β2)3 stoichiometry, the subunit order was β2_β2_α4_β2_α4 or β2_α4_β2_α4_β2. For the stoichiometry (α4)3(β2)2, the subunit orders were β2_α4_α4_β2_α4 and β2_α4_β2_α4_α4. The function of each construct was assessed using two-electrode voltage-clamping 3–6 days after microinjection of Xenopus oocytes with appropriate concatamer cRNAs. The criteria for normal receptor function were defined as levels of functional expression and pharmacological profiles comparable to those of (α4)2(β2)3 or (α4)3(β2)2 receptors assembled from single α4 and β2 subunits, that is, the non-linked receptors. These criteria have been used previously (Baur et al., 2006) to assess the functional expression of GABAAα1_β2_α1_γ_β2 pentameric concatenated receptors.
Figure 1.
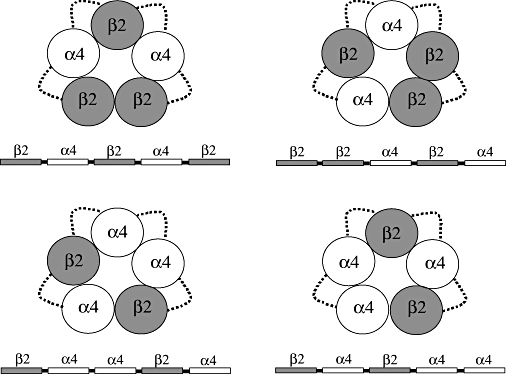
Diagram representing the (α4)2(β2)3 and (α4)3(β2)2 nAChR thought to be formed by the pentameric concatenated constructs shown underneath the diagrams. Dotted lines represent the synthetic linkers bridging the subunits.
β2Q8β2Q8α4Q8β2Q8α4 and β2Q8α4Q8α4Q8β2Q8α4 constructs were examined to see if they produced functional expression in Xenopus oocytes. These constructs were engineered using the same approaches reported to produce functional expression of α3β4 pentameric concatamers [i.e. subunits were linked from the C-terminus to the N-terminus by (Q)8 linkers and the signal peptide was maintained in all the five subunits of the concatamer] (Groot-Kormelink et al., 2006). Figure 2A shows that 5 days after microinjection with 150 ng or 100 ng of cRNA coding for β2Q8β2Q8α4Q8β2Q8α4 or β2Q8α4Q8α4Q8β2Q8α4 receptors functional expression was significantly lower than that obtained by injection of 2 ng of non-linked α4 and β2 cRNAs at a 1:10 ratio or 10:1 ratio. Furthermore, although concentration-response curves for ACh and other ligands were monophasic (F test; P < 0.05; n= 6), suggesting the presence of only one type of receptor population, the pharmacological profile of β2Q8β2Q8α4Q8β2Q8α4 or β2Q8α4Q8α4Q8β2Q8α4 receptors was significantly different from that of the corresponding non-linked receptor (Table 1). Analysis by Western blot showed concatamer proteins of appropriate size (β2Q8β2Q8α4Q8β2Q8α4, 290 kD, and β2Q8α4Q8α4Q8β2Q8α4, 310 kD) but also small amounts of fragments of various sizes that included monomeric and intermediate-sized by-products. These findings suggest proteolytic cleavage of β2Q8β2Q8α4Q8β2Q8α4 and β2Q8α4Q8α4Q8β2Q8α4 proteins. Proteolytic cleavage could have occurred because of the presence of signal peptides between the linked subunits. Signal peptides between tethered receptor subunits favour degradation of concatenated LGIC subunits, which subsequently might produce reduced levels of functional expression and/or incorporation of the breakdown products into receptors of complex and unknown subunit composition (Nicke et al., 2003; Boileau et al., 2005; Baur et al., 2006; Sigel et al., 2006; Ericksen and Boileau, 2007) and possibly novel pharmacological profiles, as found with the β2Q8β2Q8α4Q8β2Q8α4 or β2Q8α4Q8α4Q8β2Q8α4 receptors.
Figure 2.
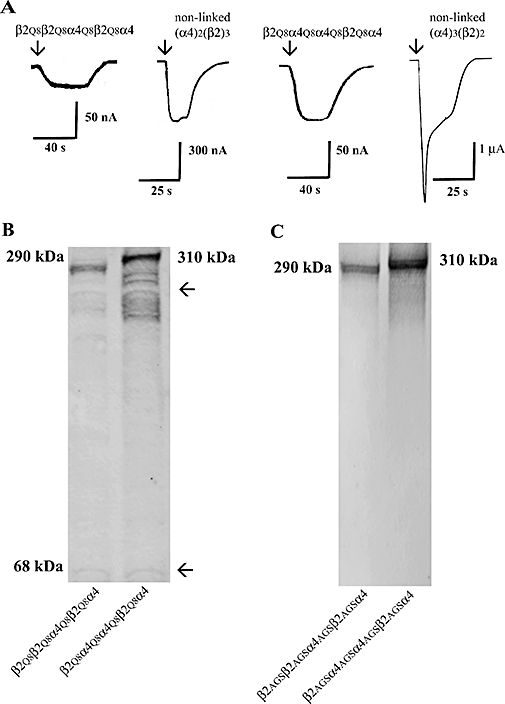
Expression of pentameric concatamers with subunit order of β2_β2_α4_β2_α4 or β2_α4_α4_β2_α4 in Xenopus oocytes. (A) ACh evoked small inward currents in oocytes microinjected with 150 ng of β2Q8β2Q8α4Q8β2Q8α4 or β2Q8α4Q8α4Q8β2Q8α4 cRNA. For comparison the ACh EC100 current responses of the corresponding non-linked receptors are included. Arrows indicate the start of the application of ACh. (B) Molecular mass of β2Q8β2Q8α4Q8β2Q8α4 or β2Q8α4Q8α4Q8β2Q8α4 proteins. Membrane homogenates prepared from oocytes injected with β2Q8β2Q8α4Q8β2Q8α4 or β2Q8α4Q8α4Q8β2Q8α4 cRNAs were resolved and then blotted and immunostained as described in Methods. Note the bands underneath the 310 and 290 kDa bands, which suggest cleavage of the pentameric constructs. (C) Western blot analysis of β2AGSβ2AGSα4AGSβ2AGSα4 and β2AGSα4AGSα4AGSβ2AGSα4 proteins indicated that the pentameric constructs were not cleaved. Total protein from oocytes injected with β2AGSβ2AGSα4AGSβ2AGSα4 and β2AGSα4AGSα4AGSβ2AGSα4 cRNAs was resolved by reducing SDS-PAGE on a NuPage 7% Tris Acetate gel. Immunoblot analysis was carried as described in Methods. The molecular mass of the concatenated constructs are shown next to the blots.
Table 1.
Functional properties of glutamine (Q)-linked (α4)2(β2)3 and (α4)3(β2)2 receptors
| β2Q8β2Q8α4Q8β2Q8α4 | Non-linked (α4)2(β2)3 | |||||
|---|---|---|---|---|---|---|
| I/Imax±SEM | EC50µmol·L−1 (95% CI) | nHill±SEM | I/Imax±SEM | EC50µmol·L−1 (95% CI) | nHill±SEM | |
| ACh | 0.99 ± 0.01 | 3.8 (2.7–5.3)* | 0.57 ± 0.06 | 0.99 ± 0.02 | 2.8 (2.1–3.7) | 0.64 ± 0.05 |
| A85380 | 1.62 ± 0.03* | 0.022 (0.02–0.03)* | 0.92 ± 0.05 | 1.42 ± 0.07 | 0.047 (0.03–0.07) | 0.77 ± 0.11 |
| TC2559 | 0.39 ± 0.04* | 1.57 (0.8–3) | 0.73 ± 0.14 | 2.43 ± 0.1 | 1.84 (1.3–2.6) | 0.89 ± 0.10 |
| β2Q8α4Q8α4Q8β2Q8α4 | Non-linked (α4)3(β2)2 | |||||
| I/Imax ± SEM | EC50µmol·L−1 (95% CI) | nHill ± SEM | I/Imax ± SEM | EC50µmol·L−1 (95% CI) | nHill ± SEM | |
| ACh | 1.04 ± 0.01 | 150 (128–178)* | 0.78 ± 0.05 | 1.1 ± 0.01 | 88 (76–94) | 0.93 ± 0.08 |
| A85380 | 4.02 ± 0.2* | 11.70 (8–17)* | 0.78 ± 0.08 | 1.52 ± 0.06 | 3.34 (2.3–4.8) | 0.63 ± 0.06 |
| Cytisine | 0.10 ± 0.03* | 2.8* (2.2–3.4) | 0.89 ± 0.06* | 0.24 ± 0.04 | 15.80 (13–88) | 0.52 ± 0.1 |
| Epibatidine | 2.8 ± 0.08* | 12 (6–17)* | 1.2 ± 0.15* | 2.6 ± 0.26 | 2.3 (0.8–7) | 0.50 ± 0.07 |
| TC2559 | 0.39 ± 0.04* | 1.57 (0.8–3) | 0.73 ± 0.20 | 0.22 ± 0.02 | 2.73 (1.1–7.1) | 0.85 ± 0.30 |
| DhβE | – | −0.12 −(0.1–0.15)* | 1.1 ± 0.20 | – | −0.4 −(0.3–0.5) | 1.0 ± 0.10 |
All values are means ± SEM from 5 to 10 cells. Statistical analysis was performed by comparing the agonist EC50, I/Imax and nHill of the concatenated receptors to the non-linked receptor using one-way analysis of the variance (anova) to assess significance.
P < 0.05; significantly different from corresponding value for non-linked (α4)2(β2)3 or (α4)3(β2)2 receptors as appropriate.
In an attempt to circumvent the problem of concatamer degradation we removed the signal peptide from all the subunits except the first one and constructed concatamers β2AGSβ2AGSα4AGSβ2AGSα4 and β2AGSα4AGSα4AGSβ2AGSα4. Previous studies on pentameric concatamers of GABAA receptors (Baur et al., 2006), dimers of α1β glycine receptor (Grudzinska et al., 2005) and dimers of α4β2 nAChR (Zhou et al., 2003) have shown that linkers lengths between 35 and 50 amino acids generally produce good functional expression. Therefore, in concatamers β2AGSβ2AGSα4AGSβ2AGSα4 and β2AGSα4AGSα4AGSβ2AGSα4, the overall length of the linker (length of synthetic linker plus added restriction enzyme sites and the C-terminal of each subunit) was 43-amino-acid residues when bridging β2 to α4 and 37-amino-acid residues when bridging α4 to β2 or α4 to α4. The linkers were of different lengths to compensate for differences in the length of the C-terminus and N-terminal sequence prior to the first conserved secondary structure element (α-helix A; Brejc et al., 2001) of the α4 and β2 subunits. In addition, to minimize possible amino acid depletion (Sigel et al., 2006), AGS linkers were used, which have been previously used to construct α4β2 receptor dimers (Zhou et al., 2003). Three to six days after microinjection of up to 150 ng of cRNAs coding for β2AGSβ2AGSα4AGSβ2AGSα4 and β2AGSα4AGSα4AGSβ2AGSα4 concatamers, functional expression amounted to less than 20 nA (not shown). Because of the low levels of expression, we did not characterize the pharmacological profile of these receptors. Although functional expression was poor, Western blots showed full-length pentameric concatamers without apparent degradation products (Figure 2C).
β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 concatamers recapitulate non-linked (α4)2(β2)3 or (α4)3(β2)2 properties
Thus far, we have shown that even though β2_β2_α4_β2_α4 and β2_α4_α4_β2_α4 concatamers are synthesized and stable in Xenopus oocytes, they express very poorly. Previous studies have shown that β2-α4 dimers favour the expression of (α4)2(β2)3 or (α4)3(β2)2 receptors when co-expressed with free β2 or α4 subunits respectively (Zhou et al., 2003). Therefore, we engineered concatamers with two consecutive β2_α4 interfaces that were followed by a α4 or β2 subunit, that is, β2_α4_β2_α4_β2 or β2_α4_β2_α4_α4 to produce (α4)2(β2)3 or (α4)3(β2)2 receptors respectively. In these constructs, only the first subunit included the signal sequence at the N-terminus and the overall length of the linkers was as described above. We first tested whether expression of the pentameric concatamers resulted in the synthesis of full-length proteins. Western blot analysis revealed proteins of appropriate size with no apparent breakdown products, suggesting that the pentameric concatamers do not break into fragments or single subunits that could potentially assemble into functional receptors (Figure 3A). Functional expression of both constructs was achieved in Xenopus oocytes (Figure 3B). After 4 days, maximal ACh (1 mmol·L−1) evoked up to 200 nA of inward currents in Xenopus oocytes microinjected with 10 ng of β2_α4_β2_α4_β2 cRNA. In the case of the β2_α4_β2_α4_α4 construct, 72 h after injection with 5 ng of cRNA, functional expression was approximately 2 µA. These expression levels were significantly lower than those obtained by microinjection of non-linked subunit cDNAs [β2_α4_β2_α4_β2/non-linked (α4)2(β2)3= 66 ± 2% and β2_α4_β2_α4_α4/non-linked (α4)3(β2)2= 77 ± 1%; n= 10; P < 0.05] but markedly higher than the levels achieved with the β2_β2_α4_β2_α4 or β2_α4_α4_β2_α4 constructs.
Figure 3.
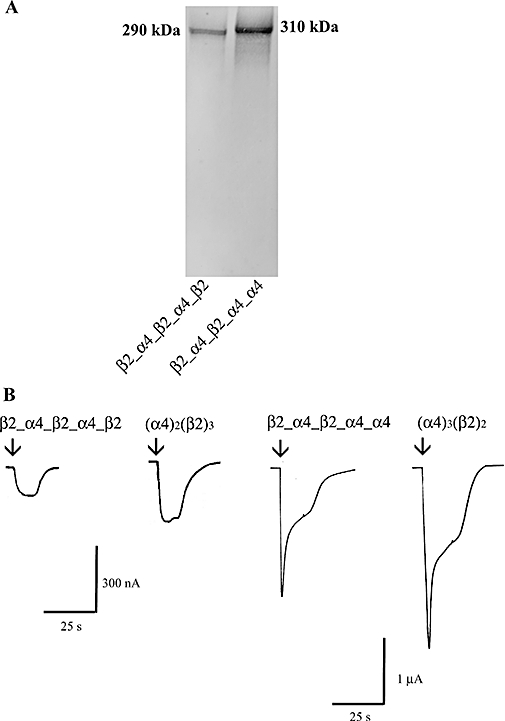
Expression of β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 in Xenopus oocytes. (A) Western blot analysis indicated that pentameric concatamers β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 are synthesized and stable when expressed heterologously in Xenopus oocytes. Total protein from oocytes injected with either β2_α4_β2_α4_β2 or β2_α4_β2_α4_α4 cRNAs was separated by reducing SDS-PAGE on a NuPage 7% Tris Acetate gel. (B) 1 mmol·L−1 ACh (EC100) evoked inward currents whose amplitude was concentration-dependent in oocytes injected with 10 ng of β2_α4_β2_α4_β2 cRNA (left/right panel) or 5 ng of β2_α4_β2_α4_α4 cRNA (right/left panel). For comparison the ACh EC100 current responses of the corresponding non-linked receptors are included. Arrows indicate the start of the application of ACh.
The effects of a range of α4β2-preferring compounds were examined to characterize the functional pharmacology of β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 receptors in comparison to their non-linked counterparts. Functional pharmacological properties of β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 receptors were very similar to those we have previously shown for non-linked (α4)2(β2)3 or (α4)3(β2)2 receptors (Moroni et al., 2006; Zwart et al., 2006; 2008). The value of the EC50, I/Imax and nHill parameters estimated from concentration-response curves are shown in Tables 2 and 3. Like the corresponding non-linked receptors, β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 receptors were activated by ACh, A85380, 5I-A85380, epibatidine and TC2559 in a concentration-dependent manner. In contrast, cytisine and 5-Br-Cys evoked responses only in oocytes expressing β2_α4_β2_α4_α4 or non-linked (α4)3(β2)2 receptors. At these two types of receptors, cytisine and 5-Br-Cys behaved as partial agonists. Sazetidine-A was a full agonist at both β2_α4_β2_α4_β2 and non-linked (α4)2(β2)3 receptors, whereas at β2_α4_β2_α4_α4 receptors or non-linked (α4)3(β2)2 receptor it behaved as a poor partial agonist. Epibatidine was as potent at β2_α4_β2_α4_β2 as at non-linked (α4)2(β2)3 receptors. However, A85380, 5I-A85380 and TC2559 were significantly more potent at β2_α4_β2_α4_β2 than at non-linked (α4)2(β2)3 receptors (P < 0.001, n= 7–10) (Figure 4A). The potency of A85380 and TC2559 was lower at β2_α4_β2_α4_α4 than at non-linked (α4)3(β2)2 receptors (P < 0.001, n= 7–10) (Figure 4B). It is likely that these differences may be due to mixed expression of both forms of the α4β2 receptor in the case of the non-linked receptors. All agonists produced concentration-response curves that were best fit to a one-component sigmoidal equation (P < 0.001, F test, n= 5–10). 5I-A85380 produced a monophasic effect on both β2_α4_β2_α4_β2 and non-linked (α4)2(β2)3 receptors. However, whereas the effects of 5I-A85380 were monophasic at β2_α4_β2_α4_α4 receptors, at non-linked (α4)3(β2)2 receptors they were clearly biphasic (P < 0.001, F test, n= 5–10) (Figure 4B). The EC50 for activation of β2_α4_β2_α4_α4 receptors by 5I-A85380 was comparable to the low-affinity EC50 displayed by this compound at non-linked (α4)3(β2)2, while the high-affinity EC50 was comparable to that displayed at both β2_α4_β2_α4_β2 and non-linked (α4)2(β2)3 receptors. The effect of 5I-A85380 on non-linked (α4)3(β2)2 receptors is similar to the effects that are observed when 5I-A85380 activates a mixed population of high- and low-sensitivity α4β2 receptors expressed in Xenopus or mammalian clonal cells (Zwart et al., 2006). This observation suggests that functional expression of non-linked (α4)3(β2)2 is contaminated with a small population, about 10%, of non-linked (α4)2(β2)3 receptors or other possible stoichiometric combinations (Zwart and Vijverberg, 1998; López-Hernández et al., 2004). Therefore, overall, the findings on the effects of A85380, 5I-A85380 and TC2559 on linked and non-linked α4β2 receptors show that microinjection of oocytes with extreme ratios of α4 and β2 cDNAs to produce either (α4)2(β2)3 or (α4)2(β2)3 receptors does not fully prevent the assembly and expression of multiple forms of the α4β2 nAChR. Inward currents elicited by EC50 of ACh at either β2_α4_β2_α4_β2 or β2_α4_β2_α4_α4 receptors were inhibited by the α4β2 receptor antagonist DhβE in a concentration-dependent and monophasic manner (P < 0.0001, F test, n= 6) (Figure 4A,B). The IC50 for DhβE at β2_α4_β2_α4_β2 was 12 (9–15) nmol·L−1 and at β2_α4_β2_α4_α4 receptors was 40 (30–50) µmol L−1. These values are very similar to those of the corresponding non-linked receptors [(α4)2(β2)3, 17 (14–19) nmol·L−1; (α4)3(β2)2, 0.4 (0.36–0.49) µmol·L−1].
Table 2.
Functional properties of β2_α4_β2_α4_β2 and non-linked (α4)2(β2)3 nAChR
| β2_α4_β2_α4_β2 | Non-linked (α4)2(β2)3 | |||||
|---|---|---|---|---|---|---|
| I/Imax±SEM | EC50µmol·L−1 (95% CI) | nHill±SEM | I/Imax±SEM | EC50µmol·L−1 (95% CI) | nHill±SEM | |
| ACh | 1.02 ± 0.01 | 2.37 (2.1–2.7) | 1.04 ± 0.06 | 0.99 ± 0.02 | 2.8 (2.1–3.7) | 0.64 ± 0.05 |
| A85380 | 1.86 ± 0.1* | 0.26 (0.2–0.4) | 0.88 ± 0.05 | 1.42 ± 0.07 | 0.047 (0.03–0.07) | 0.77 ± 0.11 |
| 5I-A85380 | 2.40 ± 0.1* | 0.14 (0.09–0.2) | 0.71 ± 0.10 | 2.01 ± 0.09 | 0.24 (0.2–0.3) | 0.89 ± 0.09 |
| Cytisine | ND | – | – | ND | – | – |
| 5-Br-Cys | ND | – | – | ND | – | – |
| Epibatidine | 0.6 ± 0.014 | 0.16 (0.1–0.3) | 0.82 ± 0.10 | 0.59 ± 0.02 | 0.19 (0.1–0.4) | 0.86 ± 0.12 |
| TC2559 | 4.18 ± 0.1* | 1.88 (1.6–2.3) | 1.0 ± 0.14 | 2.43 ± 0.1 | 1.84 (1.3–2.6) | 0.89 ± 0.10 |
| Sazetidine | 1.01 ± 0.01 | 0.0069 (5–8 nmol·L−1) | 1.01 ± 0.4 | 0.98 ± 0.09 | 0.0065 (4–8 nmol·L−1) | 1.03 ± 0.07 |
All values are means ± SEM from 5 to 10 cells. Statistical comparisons between the concentration-response curve parameters of the concatenated receptors and those of the non-linked receptors were carried out using one-way analysis of the variance (anova).
P < 0.05; significantly different from corresponding value for non-linked (α4)2(β2)3 receptors.
ND, not determined; 5-Br-Cys, 5-Br-cytisine.
Table 3.
Functional properties of β2_α4_β2_α4_α4 and non-linked (α4)3(β2)2 nAChR
| β2_α4_β2_α4_α4 | Non-linked (α4)3(β2)2 | |||||
|---|---|---|---|---|---|---|
| I/Imax±SEM | EC50µmol·L−1 (95% CI) | nHill±SEM | I/Imax±SEM | EC50µmol·L−1 (95% CI) | nHill±SEM | |
| ACh | 1.06 ± 0.01 | 111 (82–151) | 0.92 ± 0.09 | 1.1 ± 0.04 | 88 (76–94) | 0.93 ± 0.08 |
| A85380 | 1.32 ± 0.06* | 2.7 (1.7–3.9) | 0.80 ± 0.1 | 1.52 ± 0.06 | 3.34 (2.3–4.8) | 0.63 ± 0.06 |
| 5I-A85380 | 0.99 ± 0.06* | 28.20 (17–48) | 0.73 ± 0.1 | 0.22 ± 0.04* | 0.14 (0.1–0.2)* | 0.4 ± 0.03 |
| 0.88 ± 0.1* | 22 (14–35) | 1.2 ± 0.4 | ||||
| Cytisine | 0.27 ± 0.04 | 55 (11–150) | 0.43 ± 0.05 | 0.24 ± 0.04 | 15.80 (13–88) | 0.52 ± 0.1 |
| 5-Br-Cys | 0.28 ± 0.05 | 11 (10–12) | 1.3 ± 0.12 | 0.29 ± 0.01 | 14 (10–19) | 0.9 ± 0.1 |
| Epibatidine | 2.7 ± 0.01 | 0.30 (0.2–0.6) | 0.50 ± 0.1 | 2.4 ± 0.26 | 2.3 (0.8–7) | 0.62 ± 0.07 |
| TC2559 | 0.13 ± 0.1* | 0.91 (0.63–1.3)* | 1.3 ± 0.3 | 0.22 ± 0.02 | 2.73 (1.1–7.1) | 0.85 ± 0.30 |
| Sazetidine | 0.008 ± 0.0004 | ND | ND | 0.0062 ± 0.0004 | ND | ND |
One-way analysis of variance (anova) compared the level of significance between the values of the parameters of the agonist concentration-response curves of concatenated receptors and those of the non-linked receptors. Note that, with the non-linked (α4)3(β2)2 receptors, 5I-A85380 produces both high-sensitivity [EC50 0.14 (0.1–0.2) µmol L−1] and low-sensitivity [EC50 22 (14–35) µmol·L−1] current responses.
P < 0.05; significantly different from corresponding value for non-linked (α4)3(β2)2 receptor.
ND, not determined; 5-Br-Cys, 5-Br-cytisine.
Figure 4.
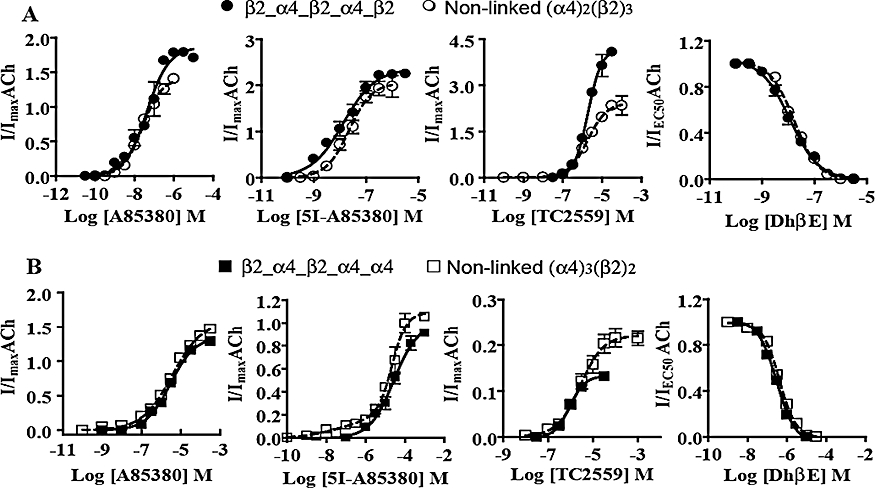
Functional sensitivity of concatenated and non-linked (α4)2(β2)3 and (α4)3(β2)2 nAChR to α4β2-preferring ligands. Concentration-response curves were obtained for the effects of A85380, 5I-A8530, TC2559 and DhβE on (A) β2_α4_β2_α4_β2 and (B) β2_α4_β2_α4_α4 nAChR expressed heterologously in Xenopus oocytes as described in Methods. Averaged parameters of best fits to agonist or concentration-response data are given in Tables 2 and 3. For comparison the concentration-response curve constructed for the corresponding non-linked receptors have been included in (A) and (B).
Further studies examined the sensitivity of the β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 receptors to Zn2+ modulation as well as their Ca2+ permeability. Non-linked (α4)2(β2)3 and (α4)3(β2)2 receptors differ significantly in their sensitivity to modulation by Zn2+ (Moroni et al., 2008) and Ca2+permeability (Tapia et al., 2007). These differences reflect stoichiometry-specific structural signatures. Concentration-dependent modulation by Zn2+ of currents evoked by ACh revealed that the sensitivity of the α4β2 concatamers was comparable to that of the corresponding non-linked α4β2 receptors (Figure 5A) (Moroni et al., 2008). Zn2+ inhibited the ACh responses of both β2_α4_β2_α4_β2 and (α4)2(β2)3 receptors monophasically and with similar IC50 values, that is, 32 (16–67) and 19 (10–36) µmol·L−1 respectively. The ACh responses of both β2_α4_β2_α4_α4 and (α4)3(β2)2 receptors were modulated biphasically by Zn2+ (P= 0.002; F test; n= 3). Zn2+ concentrations ranging from 1 to 100 µmol L−1 potentiated ACh responses at β2_α4_β2_α4_α4 or (α4)3(β2)2 receptors. Zn2+ (100 µmol·L−1) increased ACh elicited current to 1.82 ± 0.3 and 1.51 ± 0.5 for β2_α4_β2_α4_α4 or (α4)3(β2)2 receptors respectively. The EC50 for potentiation was 32 (18–44) µmol·L−1 for β2_α4_β2_α4_α4 receptors and 19 (17–24) µmol·L−1 for non-linked (α4)3(β2)2 receptors. None of these values were significantly different. Higher concentrations of Zn2+ decreased the degree of potentiation until at concentrations greater than 800 µmol·L−1 Zn2+ the amplitudes of the ACh responses elicited in the presence of Zn2+ were smaller than those mediated by applications of ACh alone (Figure 5A). Zn2+ inhibited β2_α4_β2_α4_α4 and non-linked (α4)3(β2)2 nAChR with a similar IC50 values of 810 (800–819) and 803 (799–812) µmol·L−1 respectively.
Figure 5.
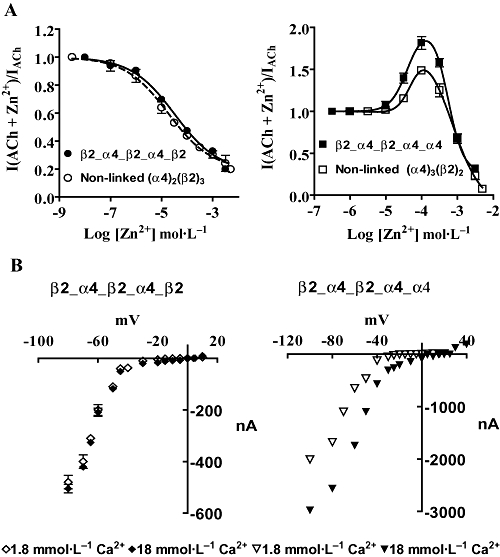
Zn2+ sensitivity and Ca2+ permeability of concatenated (α4)2(β2)3 and (α4)3(β2)2 nAChR expressed heterologously in Xenopus oocytes. (A) Averaged concentration-response for the effects of Zn2+ at concatenated (α4)2(β2)3 and (α4)3(β2)2 nAChRs. The effects of Zn2+ on currents activated by EC20 or EC10 ACh concentrations on concatenated and non-linked (α4)2(β2)3 and (α4)3(β2)2 nAChRs, respectively, were determined as detailed in the methods. (B) Current-voltage relationship of β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 nAChR in the presence of 1.8 or 18 mmol·L−1 extracellular Ca2+. β2_α4_β2_α4_α4 were more permeable to Ca2+ as judged by the positive shift of the reversal potential when the external Ca2+ was increased by 10-fold.
Ca2+ permeability was examined by measuring the reversal potential of IACh in the presence of 1.8 or 18 mmol·L−1 extracellular Ca2+ (Tapia et al., 2007). β2_α4_β2_α4_α4 receptors were most permeable to Ca2+ than β2_α4_β2_α4_β2 receptors (Figure 5B), which would be expected if these receptors replicated the structure and functional properties of non-linked (α4)3(β2)2 and (α4)2(β2)3 receptors respectively. A 10-fold increase in Ca2+ concentration shifted the reversal potential of IACh in the positive direction by 4 ± 0.1 mV in β2_α4_β2_α4_β2 receptors and 21 ± 5 mV in β2_α4_β2_α4_α4 receptors.
Chaperone 14-3-3 increases functional expression of β2_α4_β2_α4_β2(α4) constructs
Chaperone protein 14-3-3 interacts with the native (Jeancloss et al., 2001) and recombinant α4 subunit (Jeancloss et al., 2001; Exley et al., 2006) following activation of protein kinase A (PKA). The interaction is dependent on phosphorylation of a serine residue within a PKA consensus sequence (RSLSV; PKA target underlined) in the large cytoplasmic domain of the subunit, which is also a binding motif recognized by protein 14-3-3 (Jeancloss et al., 2001; O'Kelly et al., 2002; Exley et al., 2006). The interaction significantly increases the steady state levels of α4 subunit alone and α4β2 nAChRs by masking of a dibasic retention signal within the large cytoplasmic domain of α4 subunit (O'Kelly et al., 2002). To investigate the effects of protein 14-3-3 on functional expression of α4β2 nAChR concatamers, this protein was co-expressed with β2_α4_β2_α4_β2 or β2_α4_β2_α4_α4 receptors and was found to significantly increase the functional expression of both α4β2 concatamers (Figure 6A,B). These results indicate that subunit concatenation does not impair the ability of the α4 subunit to interact with chaperone 14-3-3 protein. Interestingly, it was observed that protein 14-3-3 was more effective at increasing the functional expression of the β2_α4_β2_α4_α4 receptor than that of the β2_α4_β2_α4_β2 receptors. Thus, in the case of the β2_α4_β2_α4_β2 receptor, functional expression was increased by 1.7-fold (P= 0.06; n= 6) whereas functional expression of β2_α4_β2_α4_α4 increased by fourfold (P= 0.0001; n= 6).
Figure 6.
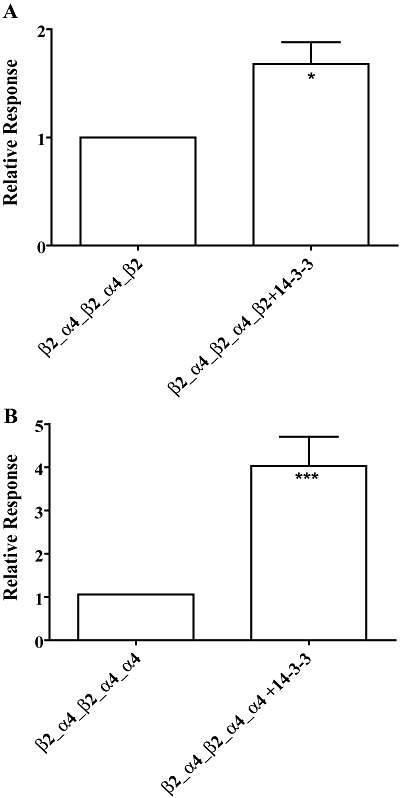
Chaperone 14-3-3 increases functional expression of concatenated (α4)2(β2)3 and (α4)3(β2)2 nAChR. Bargraph of normalized ACh responses at concatenated (A) β2_α4_β2_α4_β2 or (B) β2_α4_β2_α4_α4 nAChR receptors expressed on their own or co-expressed with chaperone protein 14-3-3. Data are given as means ± SEM from five to seven oocytes per column. *P < 0.05 and ***P < 0.001, statistically significant difference from corresponding concatenated receptors expressed on their own (unpaired Student's t-test).
Discussion
We show that the pentameric constructs β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 express well and functionally, in Xenopus oocytes and that these receptors reproduce the sensitivity to activation by ACh, Ca2+ permeability and ability to interact with chaperone protein 14-3-3 of the corresponding non-linked (α4)2(β2)3 or (α4)3(β2)2 receptors respectively. Using these concatenated receptors, we examined the pharmacological properties of the alternate stoichiometries of the α4β2 nAChR and, from the findings, we concluded that the pentameric concatamers β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 are valid models of the corresponding non-linked (α4)2(β2)3 and (α4)3(β2)2 receptors respectively.
The sensitivity of β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 receptors to activation by ACh, sazetidine-A, cytisine, 5-Br-Cys and inhibition by DhβE were comparable to those of the corresponding non-linked α4β2 nAChRs. Exceptions were the agonist effects of TC2559, A85380 and 5I-A85380, which activated the concatenated receptors and their non-linked counterparts with significantly different potency and/or efficacy. In addition, 5I-A85380 produced a biphasic concentration response curve at non-linked (α4)3(β2)2 receptors comprising a high- and a low-affinity components whose respective EC50 values were similar to those of the concatenated β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 receptors respectively. A straightforward explanation for these results is that one or more linkers may affect the pharmacological properties of the receptors. However, because the pharmacological profile of non-linked (α4)3(β2)2 receptors resembles that of a mixed population of high- and low-sensitivity α4β2 receptor stoichiometries (Zwart et al., 2006), a more likely explanation is that non-linked α4 and β2 subunits produce multiple receptor stoichiometries, even when the relative abundance of the subunits is manipulated to favour the assembly of only one type of α4β2 receptor, as in our experimental conditions. From the biphasic concentration-response curve produced by 5I-A85380 at α4β2 receptors expressed following microinjection of oocytes with single α4 and β2 cDNAs at a ratio of 10 : 1, it is clear that approximately 10% of the receptors produced are of the (α4)2(β2)3 or other possible stoichiometric arrangements. The low levels of receptor contamination in the linked (concatenated) receptors are revealed by compounds with exceptionally high stoichiometry-selectivity, such as 5I-A85380. The presence of more than one stoichiometric arrangement may still confound functional assays and obscure stoichiometry-specific receptor properties (see Zwart et al., 2006). These results indicate that caution should be applied when interpreting functional data produced by non-linked α4β2 receptors expressed heterologously in surrogate cells.
The concentration-response curves obtained with ACh and α4β2-preferring agonists indicate that most established α4β2 nAChR ligands distinguish, with varying degrees, the different stoichiometries of the α4β2 nAChR. In addition, the receptors clearly differ in their sensitivity to modulation by Zn2+ and Ca2+ permeability. These findings suggest stoichiometry-specific structural signatures as determinants of the functional behaviour of the (α4)2(β2)3 and (α4)3(β2)2 nAChR. What may be the structural basis for the stoichiometry-dependent properties of the α4β2 nAChR? By analogy to the muscle α1γα1δβ1 nAChR (Unwin, 2005), it is thought that the α4β2 nAChR harbours two functional agonist binding sites, which must be located at the α4(+)/β2(−) interfaces (one per interface). This implies that the subunit order around the channel is α4β2α4β2(α4/β2). This subunit arrangement is supported by the present reported studies of concatenated (α4)2(β2)3 and (α4)3(β2)2 nAChRs. On both stoichiometries, the agonist sites are both located at the α4(+)/β2(−) interfaces and suggest identical properties. However, one α4(+)/β2(−) interface on both stoichiometries is flanked by non-ACh binding β2(+)/α4(−) interfaces, whereas the other is flanked by a β2(+)/α4(−) interface and depending on the stoichiometry, by a non-agonist binding α4(+)/α4(−) or β2(−)/β2(+) interface. The latter interfaces are stoichiometry-specific and therefore likely candidates for conferring stoichiometry-specific properties to (α4)2(β2)3 and (α4)3(β2)2 nAChR. This view is supported by our recent studies on the effect of Zn2+ on the alternate forms of the α4β2 receptor, which show that the signature α4/α4 interface of the (α4)3(β2)2 receptor harbours a Zn2+ potentiating site that is absent in the (α4)2(β2)3 stoichiometry (Moroni et al., 2008). Additional support for our findings come from recent studies that have shown that accessory subunits influence the function of neuronal nAChRs (Kuryatov et al., 2008) and that conserved hydrophobic amino acid residues contribute to an allosteric site on heteromeric nAChRs (Hansen and Taylor, 2007).
Functional expression of both concatamers was increased by co-expression with protein 14-3-3, indicating that concatenation does not obliterate the binding site for protein 14-3-3 within the large intracellular loop of α4. The effects of protein 14-3-3 on the functional expression of β2_α4_β2_α4_α4 and β2_α4_β2_α4_β2 receptors were strikingly similar to the effects that we previously observed when the subunits expressed were non-assembled α4 and β2 (Exley et al., 2006). In that case, protein 14-3-3 favoured expression of low sensitivity [e.g. (α4)3(β2)2 receptors]. We showed that protein 14-3-3 increased the steady-state levels of the α4 subunit and hypothesized that this effect possibly resulted into greater incorporation of α4 subunits into receptor complexes. This possibility could not account for the differential effects of protein 14-3-3 on the α4β2 pentameric concatamers, because the subunit composition of these receptors is fixed. A possible explanation is that the higher α4 content of the β2_α4_β2_α4_α4 concatamer enhances the stabilizing and up-regulating effects of protein 14-3-3 in comparison to its actions on β2_α4_β2_α4_β2 receptors. This implies that subunit composition may confer stoichiometry-specific ‘receptor maturation’ patterns. This view is supported by previous studies that have shown that α4β2 nAChR matures inefficiently in comparison to α4β4 receptors (Sallette et al., 2004), possibly because β2 weakens the process of receptor maturation through inefficient subunit interactions and/or assembly (Sallette et al., 2004; 2005). Thus, (α4)2(β2)3 receptors may mature less efficiently than (α4)3(β2)2 receptors, which would lower the functional expression of (α4)2(β2)3 relative to that of (α4)3(β2)2 receptors. We have found in this and previous (Moroni et al., 2006; 2008; Zwart et al., 2006) studies that the heterologous functional expression of (α4)3(β2)2 receptors in Xenopus oocytes is about 30-fold higher than that of (α4)2(β2)3 receptors.
Interestingly, neither β2_β2_α4_β2_α4 nor β2_α4_α4_β2_α4 expressed well in Xenopus oocytes, even though both constructs were synthesized and stable. This implies that the constructs were trafficked inefficiently to the cell surface and/or that the constructs did not assemble into properly functional α4β2 receptors. What may influence the functionality of these concatamers? In comparison to β2_α4_β2_α4_β2 or β2_α4_β2_α4_α4 concatamers, the β2-α4 interfaces in the β2_β2_α4_β2_α4 and β2_α4_α4_β2_α4 receptors are preceded by a β2 or are separated by an α4 subunit respectively. Thus, a possible explanation for the low functional expression of β2_β2_α4_β2_α4 and β2_α4_α4_β2_α4 is that their subunit arrangement does not facilitate the subunit interactions that drive the assembly and maturation of α4β2 pentamers (Sallette et al., 2005). Little is known about the elementary steps leading to assembly of pentameric α4β2 complexes, which occurs within the endoplasmic reticulum. By analogy to the assembly of the muscle nAChR (Green and Claudio, 1993), it is likely that the subunits incorporate into pentamers through sequential steps driven by specific subunit–subunit or subunit–chaperone interactions. Thus, when those subunit interactions are impaired, which might occur if the subunits were not oriented properly or did not acquire appropriate three-dimensional structures, oligomerization and/or maturation may be inefficient, producing ultimately low expression levels or receptors with altered function. Interestingly, although β4_β4_α3_β4_α3 pentameric concatamers produce functional receptors in Xenopus oocytes, the levels of expression were very poor in comparison to the functional expression of non-linked α3β4 receptors (Groot-Kormelink et al., 2006). This suggests that positioning β_α interfaces prior to a β or α subunit may be a strategy that could be applied across the nAChR family to produce concatamers with good functional expression.
A subunit domain that may play a critical role in functional expression is the C-terminus. Insertion of fluorescent proteins in the C-terminus of β2 nAChR subunit (Nashmi et al., 2003), ε or γ nAChR subunits (Gensler et al., 2001) or γ2 GABAA receptor subunit (Kittler et al., 2000) results in partial or complete abolition of function. Although there is evidence that green fluorescent-tagged C-terminus may affect the function of nAChR (Fucile et al., 2002), recent studies suggest that the effects of the C-terminus on the functional expression of Cys-loop LGIC are likely to reflect its contribution to the process of receptor maturation (Butler et al., 2008). Thus, C-terminus single-point mutants of the 5-HT3A receptor reduce specific radioligand binding and membrane expression, both of which can be partially restored by growing cells expressing the mutant receptors at temperatures lower than 37°C (Butler et al., 2008). In the case of pentameric concatenated LGIC, poor functional expression could well reflect the fact that the C-terminus of all but one subunit (the fifth) of the concatenated subunits is linked to the N-terminus of the subsequent subunit (Baur et al., 2006; Groot-Kormelink et al., 2006; this report).
In summary, we have demonstrated that pentameric concatamers β2_α4_β2_α4_β2 and β2_α4_β2_α4_α4 have pharmacological signatures comparable to those of non-linked (α4)2(β2)3 and (α4)3(β2)2 nAChRs respectively. Thus, this study provides a diagnostic tool for the different forms of the α4β2 nAChR. In addition, (α4)2(β2)3 and (α4)3(β2)2 concatamers in combination with mutational and functional experimental approaches can be used to aid the characterization of other possible stoichiometric arrangements of the α4β2 nAChR. Concatamers with a subunit order of β2_β2_α4_β2_α4 or β2_α4_α4_β2_α4 do not express well in Xenopus oocytes nor do they reproduce the pharmacological properties of non-linked receptors. This may be because the subunit arrangement of these constructs hinders interactions between subunits or between subunits and chaperone proteins that are required for receptor assembly and maturation. We are presently unable to distinguish between these possibilities because the processes that drive the genesis of functional α4β2 nAChRs are essentially unknown. However, future studies that address this issue may benefit from the availability of pentameric concatenated (α4)2(β2)3 and (α4)3(β2)2 nAChRs whose assembly and maturation as judged by their functional properties and sensitivity to the chaperone protein 14-3-3, may be comparable to that of the corresponding non-linked α4β2 nAChR.
Acknowledgments
The research was supported by the Royal Society and Oxford Brookes University PhD Studentship funds (A.L.C. and M.M.). A.L.C. and M.M. contributed equally to the work. The authors thank Dr Erwin Sigel (University of Berne, Switzerland) for helpful advice on concatamer design. The authors also thank Dr Lucia Sivilotti (University College London, UK) for helpful comments on the work reported here, Dr Mike Franklin (Oxford Brookes University) and Prof Bruce Cassels (University of Chile) for comments on the manuscript.
Glossary
Abbreviations:
- ACh
acetylcholine
- DhβE
dihydro-β-erythroidine
- LGIC
ligand-gated ion channels
- nAChR
nicotinic acetylcholine receptor
- PKA
protein kinase A
Conflicts of interest
The authors state no conflict of interest.
References
- *.Baur R, Minier F, Sigel E. A GABAA receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 2006;580:1616–1620. doi: 10.1016/j.febslet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Pearce RA, Czajkowski C. Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABA receptor-assocaiated protein. J Neurosci. 2005;25:11219–11230. doi: 10.1523/JNEUROSCI.3751-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurman M, van Der Oost J, Smit AB, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Butler AS, Lindsey SA, Dover TJ, Kennedy MD, Hope AG, Barnes NM. The importance of the C-terminus of the human 5-HT3A receptor subunit. Neuropharmacology. doi: 10.1016/j.neuropharm.2008.08.017 [Epub ahead of print. [DOI] [PubMed]
- Butt CM, Hutton SR, Marks MJ, Collins AC. Bovine serum albumin enhances nicotinic acetylcholine receptor function in mouse thalamic synaptosomes. J Neurochem. 2002;83:48–56. doi: 10.1046/j.1471-4159.2002.01135.x. [DOI] [PubMed] [Google Scholar]
- Cassels BK, Bermudez I, Dajas F, Abin-Carriquiry A, Wonnacott S. From ligand design to therapeutic efficacy: the challenge for nicotinic receptor research. Drug Discov Today. 2006;10:1657–1665. doi: 10.1016/S1359-6446(05)03665-2. [DOI] [PubMed] [Google Scholar]
- Ericksen S, Boileau AJ. Cys-loop receptor concatamer insights and caveat. Mol Neurobiol. 2007;35:113–127. [PMC free article] [PubMed] [Google Scholar]
- Exley R, Moroni M, Sasdelli F, Houlihan LM, Lukas RJ, Sher E, et al. Chaperone protein 14-3-3 and protein kinase A increase the relative abundance of low agonist sensitivity human α4β2 nicotinic acetylcholine receptors in Xenopus oocytes. J Neurochem. 2006;98:876–885. doi: 10.1111/j.1471-4159.2006.03915.x. [DOI] [PubMed] [Google Scholar]
- Fucile S, Palma E, Martinez-Torres A, Miledi R, Eusebi F. The single-channel properties of human acetylcholine alpha 7 receptors are altered by fusing alpha 7 to the green fluorescent protein. Proc Natl Acad Sci USA. 2002;99:3956–3961. doi: 10.1073/pnas.052699599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensler S, Sander A, Korngreen A, Traina G, Giese G, Witzemann V. Assembly and clustering of acetylcholine receptors containing GFP-tagged ε or γ subunits: selective targeting to the neuromuscular junction in vivo. Eur J Biochem. 2001;268:2209–2217. doi: 10.1046/j.1432-1327.2001.02093.x. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, et al. Partial deletion of the nicotinic cholinergic receptor α4 or β2 subunit genes change the acetylcholine sensitivity of receptor mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of subunits. Mol Pharmacol. 2008;74:56–69. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- Green WN, Claudio T. Acetylcholine receptor assembly: subunit folding and oligomerization occur sequentially. Cell. 1993;74:57–69. doi: 10.1016/0092-8674(93)90294-z. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Broadbent SD, Boorman JP, Sivilotti LG. Incomplete incorporation of tandem subunits in recombinant neuronal nicotinic receptors. J Gen Physiol. 2004;123:697–708. doi: 10.1085/jgp.200409042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Broadbent SD, Beato M, Sivilotti LG. Constraining the expression of nicotinic acetylcholine receptors using pentameric constructs. Mol Pharmacol. 2006;69:558–563. doi: 10.1124/mol.105.019356. [DOI] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, et al. The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Hansen B, Taylor P. Galanthamine and non-competitive inhibitor binding to ACh-binding protein: evidence for a binding site on non-alpha-subunit interfaces of heteromeric neuronal nicotinic receptors. J Mol Biol. 2007;369:895–901. doi: 10.1016/j.jmb.2007.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan LM, Slater Y, Guerra DL, Peng JH, Kuo YP, Lukas RJ, et al. Activity of cytisine and its brominated isosteres on recombinant human α7, α4β2 and α4β4 nicotinic acetylcholine receptors. J Neurochem. 2001;78:1029–1043. doi: 10.1046/j.1471-4159.2001.00481.x. [DOI] [PubMed] [Google Scholar]
- Jeancloss EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R. The chaperone protein 14-3-3η interacts with the nicotinic acetylcholine receptor α4 subunit. J Biol Chem. 2001;276:28281–28290. doi: 10.1074/jbc.M011549200. [DOI] [PubMed] [Google Scholar]
- Kim H, Flanagin BA, Qin C, Macdonald RL, Stitzel JA. The mouse Chrna4 A529T polymorphism alters the ratio of high to low affinity α4β2 nAChRs. Neuropharmacology. 2003;45:345–354. doi: 10.1016/s0028-3908(03)00167-9. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Connolly CN, Vicini S, Smart TG, Moss SJ. Analysis of GABAA receptor assembly in mammalian cell lines and hippocampal neurons using γ2 subunit fluorescent protein chimeras. Mol Cell Neurosci. 2000;16:440–452. doi: 10.1006/mcne.2000.0882. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human α4β2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in α4β2* nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- López-Hernández GY, Sánchez-Padilla J, Ortiz-Acevedo A, Lizardi-Ortiz JE, Salas-Vincenty J, Rojas LV, et al. Nicotine-induced up-regulation of desensitisation of α4β2 neuronal nicotinic receptors depend on subunit ratio. J Biol Chem. 2004;279:38007–38015. doi: 10.1074/jbc.M403537200. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, et al. Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the β2 subunit. J PET. 1999;289:1090–1103. [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. α4β2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Moroni M, Vijayan R, Carbone A, Zwart R, Biggin PC, Bermudez I. Non-agonist binding subunit interfaces confer distinct functional signatures to the alternate stoichiometries of the α4β2 nicotinic receptor: a α4-α4 interface is required for Zn2+ potentiation. J Neurosci. 2008;28:6884–6894. doi: 10.1523/JNEUROSCI.1228-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Dickinson ME, McKinney S, Jareb M, Labarca C, Fraser SE, et al. Assembly of α4β2 nicotinic acetylcholine receptors assessed with functional fluorescently labelled subunits: effect of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci. 2003;23:11554–11567. doi: 10.1523/JNEUROSCI.23-37-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Nicke A, Rettinger J, Schmalzing G. Monomeric and dimeric byproducts are the principal functional elements of higher order P2X1 concatamers. Mol Pharmacol. 2003;63:243–252. doi: 10.1124/mol.63.1.243. [DOI] [PubMed] [Google Scholar]
- O’Kelly I, Butler MH, Ziberberg N, Goldstein SAN. Forward transport: 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioural phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Sallette J, Bohler S, Benoit P, Soudant M, Pons S, Le Novere N, et al. An extracellular protein microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. J Biol Chem. 2004;279:18767–18775. doi: 10.1074/jbc.M308260200. [DOI] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillier-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Boulineau N, Minier F. Impact of subunit positioning on GABAA receptor function. Biochem Soc Trans. 2006;34:868–871. doi: 10.1042/BST0340868. [DOI] [PubMed] [Google Scholar]
- Stitzel JA, Dobelis P, Jimenez M, Collins AC. Long sleep and short sleep mice differ in nicotine-stimulated 86Rb+ efflux and α4 nicotinic receptor subunit cDNA sequence. Pharmacogenetics. 2001;11:331–339. doi: 10.1097/00008571-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (α4)3(β2)2 stoichiometry exceeds that of (α4)2(β2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Tritto T, Stitzel JA, Marks MJ, Romm E, Collins AC. Variability in response to nicotine in the LSXSS RI strains: potential role of polymorphism in alpha4 and alpha6 nicotinic receptor genes. Pharmacogenetics. 2002;12:197–208. doi: 10.1097/00008571-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human α4β2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]
- Zwart R, Broad LM, Xi Q, Lee M, Moroni M, Bermudez I, Sher E. 5-I A-85380 and TC-2559 differentially activate heterologously expressed α4β2 nicotinic receptors. Eur J Pharmacol. 2006;539:10–17. doi: 10.1016/j.ejphar.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, et al. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]


