Abstract
Background and purpose:
We evaluated the effects of 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid (CHF5074), a new γ-secretase modulator, on brain β-amyloid pathology and spatial memory in transgenic mice expressing the Swedish and London mutations of human amyloid precursor protein (hAPP).
Experimental approach:
Sixty 6-month-old hAPP mice were treated for 6 months with CHF5074 or ibuprofen (375 ppm in the diet) or standard diet. Twenty-one wild-type mice received standard diet.
Key results:
Compared with transgenic controls, CHF5074 treatment significantly reduced the area occupied by plaques in cortex (P = 0.003) and hippocampus (P = 0.004). The number of plaques were also reduced by CHF5074 in both cortex (P = 0.022) and hippocampus (P = 0.005). Plaque-associated microglia in CHF5074-treated animals was lower than in transgenic controls in cortex (P = 0.008) and hippocampus (P = 0.002). Ibuprofen treatment significantly reduced microglia area in cortex and hippocampus but not β-amyloid burden. On the last day of the Morris water maze, transgenic controls performed significantly worse than the non-transgenic animals and the CHF5074-treated transgenic mice, on the swimming path to reach the hidden platform. Ibuprofen-treated animals did not perform significantly better than transgenic controls.
Conclusions and implications:
Chronic CHF5074 treatment reduced brain β-amyloid burden, associated microglia inflammation and attenuated spatial memory deficit in hAPP mice. This novel γ-secretase modulator is a promising therapeutic agent for Alzheimer's disease.
Keywords: γ-secretase modulators, β-amyloid, spatial memory
Introduction
Alzheimer's disease (AD) is the most common form of dementia. The basic pathological abnormalities in AD brains are amyloid plaques, neurofibrillary tangles and neuronal loss. Amyloid plaques are composed of β-amyloid peptides (Aβ) that are proteolytically produced from amyloid precursor protein (APP). APP is initially cleaved by β-secretase to generate a 99-residue carboxy-terminal fragment of APP (CTFβ or C99) that is subsequently cleaved by γ-secretase to generate Aβ. Proteolysis by γ-secretase is heterogeneous and generates several Aβ species with different length. The most abundant species is a 40-residue peptide (Aβ40). A 42-residue variant (Aβ42) is also formed and, although is much less abundant than Aβ40, is more prone to fibril formation and is the initially and predominantly deposited Aβ species in AD brains. γ-Secretase is a complex of four different membrane proteins: presenilin (PS), nicastrin, anterior pharynx (Aph-1) and PS enhancer 2 (Pen-2). PSs are of exceptional pathophysiological importance as more than 150 autosomal dominant point mutations are known in these proteins, all of which cause aggressive early-onset AD. Many APP and PS mutations result in increased production of Aβ42 and, according to the ‘amyloid hypothesis’, the oligomeric forms of Aβ42 are the main cause of neuronal death in AD (Lesnéet al., 2006; Shankar et al., 2008). Thus, inhibition or modulation of γ-secretase appears to be a logical strategy to decrease Aβ42 accumulation in AD patients.
While a number of highly potent inhibitors of γ-secretase have been identified (Olson and Albright, 2008), serious concerns about their toxicity have been raised as γ-secretase can cleave several other membrane proteins other than APP (Lleo 2008), the most pharmacologically relevant being the Notch receptor. Indeed, γ-secretase inhibitors block proteolysis of Notch by inhibiting cleavage at site 3 (Lewis et al., 2003). Physiological cleavage of Notch leads to release of the Notch intracellular domain (NICD), a protein fragment that is translocated to the nucleus where it regulates transcription of target genes involved in cell development and in differentiation of adult self-renewing cells. The inhibitory effects of γ-secretase inhibitors on Notch activation in embryonic and fetal development may not be of concern for the treatment of AD patients. However, it is known that Notch signalling plays an important role in the ongoing differentiation processes of the immune system (Maillard et al., 2003) and of the gastrointestinal tract (Stanger et al., 2005). Indeed, treatment of mice with γ-secretase inhibitors can cause severe gastrointestinal toxicity and compromise the proper maturation of B-and T-lymphocytes (Searfoss et al., 2003; Wong et al., 2004). Thus, the ability to selectively block APP proteolysis by γ-secretase without affecting the proteolysis of Notch is a major goal towards identifying safe and effective therapeutics for AD. This can be realized by modulating rather than inhibiting γ-secretase.
The first compounds of this type were certain non-steroidal anti-inflammatory drugs (NSAIDs) that were capable of altering the cleavage properties of γ-secretase to lower the production of Aβ42 and increase the formation of a shorter 38-residue species (Aβ38) (Weggen et al., 2001). These compounds include ibuprofen, sulindac sulfide, indomethacin, flurbiprofen and others. The ability of these NSAIDs to modulate Aβ production is not related to their inhibitory activity on cyclooxygenase (COX). Binding of these NSAIDs to APP is more efficient than to Notch and thus their effects on Notch processing are minor (Kukar and Golde, 2008). These NSAIDs are not very potent towards lowering Aβ production and structural modifications of these molecules have been proposed (Peretto and La Porta, 2008). We have synthesized new γ-secretase modulators endowed with selective Aβ42-lowering properties but devoid of COX inhibitory activity, thus suitable for chronic use in AD patients (Peretto et al., 2005). Within this new chemical series, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid (CHF5074) proved to be of major interest. In human neuroglioma cells over-expressing the Swedish mutated APP [human neuroglioma cell line expressing APPswe (H4swe)], CHF5074 preferentially lowers Aβ42 secretion with an IC50 of 3.6 µmol·L−1 (Imbimbo et al., 2007a). CHF5074 does not display inhibitory activity on COX-1 and COX-2 enzymes when employed at concentrations up to 100 µmol·L−1 and 300 µmol·L−1 respectively (Imbimbo et al., 2007b). At 5 µmol·L−1, no effects were observed on Notch intracellular cleavage in human embryonic kidney 293 cells expressing APPswe (HEK293swe) (Imbimbo et al., 2007a). At 100 µmol·L−1, CHF5074 does not alter the expression profile of several NICD-responsive genes (Imbimbo et al., 2007b). In rats, CHF5074 is orally well absorbed (50%) and is slowly eliminated from plasma (t1/2 ≈ 20 h) (Peretto et al., 2005). In Tg2576 transgenic mice, chronic treatment with CHF5074 markedly reduces brain Aβ burden without histological signs of peripheral Notch-mediated toxicity (Imbimbo et al., 2007a).
We evaluated the effects of chronic treatment with CHF5074 in an aggressive transgenic mouse model of AD (hAPP mice) and found that the drug attenuates brain β-amyloid pathology and associated behavioural deficit in these animals. We also evaluated the effects of the drug on APP processing in the brain homogenates of these transgenic animals. Studies were carried out including a transgenic group of mice treated with ibuprofen because this compound has been shown to reduce brain Aβ pathology in transgenic mice models of AD (Lim et al., 2000; Yan et al., 2003).
Methods
Animals and treatments
All animal care and handling were performed according to the European guidelines. hAPP transgenic mice expressing human APP751 with the Swedish (K670N/M671L) and London (V717I) mutations under the regulatory control of the neuron-specific murine (m)Thy-1 promoter, heterozygous with respect to the transgene, on a C57BL/6 F3 background (Rockenstein et al., 2001) were used. The hAPP colony was sustained by crossing transgenic APP751 with C57BL/6 (Harlan Winkelmann, Germany). Littermates were used as non-transgenic controls. The transgenic status of each animal was confirmed by real-time PCR of tail snips using specific primers and the appropriate hybridization probe. hAPP transgenic mice exhibit plaques starting at 3–4 months of age in the frontal cortex and at 5–6 months in the hippocampus (Rockenstein et al., 2001) and develop cognitive deficits starting at 6 months of age (Hutter-Paier et al., 2004). Mice were housed in single ventilated cages on standardized rodent bedding (Abedd, Wien, Austria) in a pathogen-free environment at JSW-CNS Research. Each cage contained a maximum of five mice of the same gender. The room temperature during the study was maintained at 24°C and the relative humidity was maintained at 40–70%. Animals were maintained on a 12: 12 h light/dark cycle with unrestricted access to food and water.
Both male and female animals were included in the study. Treatment of the animals started at 6 months ± 2 weeks of age and lasted for 6 months up to 12 months of age. hAPP transgenic mice were randomly allocated to one of three treatment groups: CHF5074 (375 ppm in the diet, n = 21), ibuprofen (375 ppm in the diet, n = 21) and vehicle (standard diet, n = 18). The dose of ibuprofen (375 ppm) is the same used in other studies that have shown a positive effect of the drug in counteracting brain Aβ deposition in APP transgenic mice models of AD (Lim et al., 2000; Lim et al., 2001; Yan et al., 2003). Non-transgenic mice were treated with standard diet (wild-type, n = 21). Mice were individually identified by ear markings. All animals had dark eyes and their visual abilities were controlled in the Morris water maze (MWM) pre-test with visible platform. Investigators performing behavioural testing, biochemical and immunohistochemistry analyses were unaware of the treatments allocated to the groups of mice.
Behavioural testing
Behavioural testing of the animals was performed with the MWM paradigm after 6 months of treatment (12 months of age). During behavioural testing, animals continued to receive assigned treatments with the diet. The MWM is a standardized behavioural task to evaluate spatial learning and memory in rodents (Morris, 1984). During the test the mouse swims to find a hidden platform, using visual cues. The task is based on the principle that rodents are highly motivated to escape from water environment by the quickest, most direct route. We used a black circular pool of a diameter of 100 cm virtually divided into four sectors (quadrants) and filled with tap water (22 ± 1°C). The test was carried out during five consecutive days under dimmed light conditions. On day 1, animals performed a pre-test consisting of two trials with a visible platform (8 cm diameter) to check visual and motor abilities. During days 1–4, the platform was placed about 0.5 cm beneath the water surface in the southwest quadrant of the pool (target quadrant). Animals were allowed to reach the hidden platform in a maximum time of 60 s starting from a randomly chosen quadrant. On each testing day, animals performed three trials separated by a 10 min interval. If the animal could not find the platform, it was guided to or placed on the platform. After each trial, mice were allowed to rest on the platform for 10–15 s. During this time, the mice had the possibility to orientate looking at the black, bold geometric symbols placed on the walls surrounding the pool. On day 4, 1 h after the last trial, the mice performed a so-called ‘probe trial’ during which the platform was removed from the pool and animals were allowed to swim for 60 s to verify if they remembered the original position of the platform. Swimming tracks of the mice were recorded by a camera placed above the centre of the pool detecting the signal of a light emitting diode fixed with a little hairgrip on the mouse tail. During each trial, the time (escape latency) and the length of the trajectory (swimming path) to reach the hidden platform were recorded by the computerized tracking system. The average of the escape latencies and of the swimming paths recorded during the three trials of each of the four study day sessions represented the efficacy variables of the study. During the probe trial, the percentage of the time spent in the target quadrant was recorded.
Tissue sampling and preparation
After behavioural testing, mice were anaesthetised with isoflurane (Baxter, Unterschleissheim, Germany) and exsanguinated. Cerebrospinal fluid (CSF) was then obtained by blunt dissection and exposure of the foramen magnum. Upon exposure, a Pasteur pipette was inserted for 0.3–1 mm into the cisterna magna. CSF was suctioned by capillary action until flow fully ceased. Samples were immediately frozen and kept at −80°C until analysis. Blood was collected via heart puncture into EDTA vials. To get plasma, blood samples were centrifuged at 700× g. Plasma samples were divided into two aliquots and frozen at −80°C. One aliquot was used for determination of Aβ40 and Aβ42. The other aliquot was used for measurement of drug levels. After blood sampling, mice were transcardially perfused with 0.9% sodium chloride until all the blood was washed out. Brains were rapidly removed and the right hemisphere was fixed by immersion in fresh 4% paraformaldehyde for 1 h and transferred to a 15% sucrose PBS solution for 24 h to ensure cryoprotection. On the next day, hemispheres were frozen in liquid isopentane and stored at −80°C until used for histological investigations. The left hemispheres were frozen on dry ice and then stored at −80°C until measurement of Aβ peptides and drug levels.
Aβ measurements
For Aβ measurements, left brain hemispheres were handled as described elsewhere (Kawarabayashi et al., 2001). In brief, frozen hemispheres were homogenized in TBS-buffer (5 mL) containing protease inhibitor cocktail. After centrifugation at 74 200 g for 1 h at 4°C, the supernatants (TBS fraction) were aliquoted and kept at −20°C. The pellets were suspended in Triton X-100 (5 mL), centrifuged (74 200 g for 1 h at 4oC) and the supernatants (Triton X-100 fraction) were aliquoted and kept at −20°C. These steps were repeated with sodium dodecyl sulphate (SDS) (5 mL). The pellets out of the SDS fraction were suspended in 70% formic acid (FA; 1 mL) prior to subsequent centrifugation (74 200 g for 1 h at 4oC). Supernatants were neutralized with 1 M Tris (19 mL), aliquoted and kept at −20°C. Aβ0 and Aβ2 levels in CSF, plasma and SDS and FA brain homogenate fractions were measured with commercial ELISA kits (The Genetics Company, Zurich, Switzerland). Absorption data were read at 450 nm (reference filter 620–650 nm) within 15 min using a microtiter plate reader. Aβ0 and Aβ2 concentrations were calculated by comparing the colour intensity to a standard curve made of synthetic Aβ0 and Aβ2 peptides provided by the manufacturer of the ELISA kits.
Immunohistochemistry analyses
For Aβ plaque measurements, right brain hemispheres were sagittally cut (10 µm sections) using a cryotome (CM3050S, Leica Microsystems, Wetzlar, Germany). Staining of Aβ plaques was performed by double immunohistochemistry using murine anti-human primary antibodies (clone 6E10; Signet Laboratories, Dedham, MA, USA) raised against the human amyloid peptide combined with a fluorescent Cy3-labelled secondary detection antibody (Dianova, Hamburg, Germany). For determination of microglial activation, immunohistochemistry was performed with anti-mouse CD11b (Mac-1 α chain) antibodies (BD Biosciences, San Jose, CA, USA) as primary antibody combined with a fluorescent Cy2-labelled secondary detection antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA). All staining were performed in slices of five different sagittal layers from the right brain hemisphere, corresponding to figures 105 to 115 of the morphology atlas ‘The mouse brain in stereotaxic coordinates’ (Paxinos and Franklin, 2001). For quantifications, tiled images at 100-fold (6E10) or 200-fold (CD11b) magnification were recorded using a PixelFly camera (PCO, Kelheim, Germany) mounted on a Nikon E800 microscope (Nikon, Tokyo, Japan) under control of an automated software-controlled table (StagePro, Version 5) and evaluated with the Image-Pro Plus software (Version 6.2, Media Cybernetics, Silver Spring, MD, USA). Pixel resolution of one image depended on tiling but was always around 50 × 106 pixels. Each image was recorded under the same camera settings in terms of colour profile, exposure time, as well as microscopic settings.
Image analyses
During Aβ plaque analysis, the region area was interactively drawn to delineate the cortex and hippocampus. An interactive drawn area of interest (AOI) was used to exclude flaws, folding or any other kind of bias. All measurement parameters like threshold, contrast and counting restrictions were rater-independent and kept constant for all images of the study. Minimal object size was set to 8.75 µm2 referring to the maximal size of non-specific staining in negative controls (omitted primary antibody). Extracted variables were the region size, the mean plaque size (=‘sum area of plaques/number of plaques’), the relative plaque number (=‘number of plaques/region area’) and the plaque area fraction (=‘sum area of plaques × 100/region area’). Criteria for excluding images were missing parts of the slice, wrinkles, dominant flaws or staining inconsistencies (e.g. due to bulges, which can impede the full reaction of the blocking reagent).
To determine CD11b immunoreactive area visualizing microglial activation, the area of stained objects deriving from blood vessel staining and fluorescent erythrocytes, respectively, was corrected. Therefore, the green intensity channel was extracted from the original image. This extracted image was evaluated separately in the same AOIs used in the original CD11b image, counting only most intensive and small round objects, a feature of non-specific immunoreactivity of erythrocytes in blood vessels and capillaries. The final corrected CD11b immunoreactive area and number of objects was calculated by reducing the count in the original image by the count in the green intensity channel image. All other steps were similar to plaque quantifications.
Pharmacokinetics
CHF5074 levels in plasma and in brain samples were measured by liquid chromatography coupled to mass spectrometry as previously described (Imbimbo et al., 2007a). Briefly, samples were prepared by adding 300 µL acetonitrile and 40 µL phosphoric acid 40% to 100 µL of plasma or brain homogenate and placing the mixture in a vortex for 5 s. Plasma and brain samples were then centrifuged at 14 000× g for 5 min and the supernatants (15 and 50 µL respectively) were injected into the high pressure liquid chromatography (HPLC) system with mass spectrometer detector (API 2000, Applied Biosystems, Foster City, CA, USA). The chromatographic conditions were adapted to each compound to obtain good peak separation and detection sensitivity. A mixture of ammonium formate (pH 2.7, 20 mmol·L−1) buffer-acetonitrile-methanol was used as mobile phase. The assay was linear between 400–20 000 ng·g−1 in the brain and 100–8500 ng·mL−1 in plasma with lower limits of quantitation of 400 ng·g−1 in brain and 100 ng·mL−1 in plasma.
Western blot and co-immunoprecipitation analyses
To investigate the effects of CHF5074 and ibuprofen treatments on APP processing, Western blot and co-immunoprecipitation analyses were carried out in total proteins taken from transgenic mice hemispheres homogenized in TBS-buffer and containing a protease inhibitor cocktail (Sigma, Oakville, Ontario, Canada). Protein extracts (50 µg proteins per sample) were resolved by 12% SDS/polyacrylamide gel. The proteins were transferred electrophoretically onto nitrocellulose membrane. Immunodetection was performed by incubating the membrane overnight at 4°C, with the following primary antibodies: β-amyloid 1–16 (clone 6E10) monoclonal antibody (1:700) (Covance, Philadelphia, PA, USA) and anti-β-tubulin antibody (1:1500) (NeoMarkers, Vancouver, WA, USA). The immunoreaction was revealed by 1 h incubation at 37°C with secondary antibodies coupled to horseradish peroxidase (1:1500) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and chemoluminescence detection using ECL Western blotting reagents (Amersham, Cologno Monzese, Italy). Quantification of immunoblots from separate protein preparations was performed by densitometric scanning of exposed film using GelPro Analyser software (Media Cibernetics, Bethesda, MD, USA). Data from densitometry analysis were expressed as the ratio of full length APP (FL-APP) or CTFβ to β-tubulin. Parallel co-immunoprecipitation studies were carried out in 50 µg of proteins from transgenic mice treated with vehicle, CHF5074 or ibuprofen. The samples were diluted in radio immuno precipitation assay (RIPA) buffer (10 mmol·L−1 Tris-HCl pH 8, 140 mmol·L−1 NaCl, 0.5% (v/v) Nonidet −40, 1 mmol·L−1 Na orthovanadate, 0.1% SDS, 1 mmol·L−1 phenylmethylsulphonyl fluoride, 1% protease inhibitor cocktail). Proteins were incubated at 4°C overnight with mouse anti-PS-1 antibody (1:50). Thereafter, 25 µL of protein A/G (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were added and the reaction mixture was rotated for 2 h at 4°C. Immunoprecipitates were collected by centrifuging at 77× g for 5 min followed by washing four times with the RIPA buffer. Following the final wash, all liquids that adhered to the protein A/G beads were removed. Samples were then resuspended in the sample loading buffer, subjected to SDS-polyacrylamide gel electrophoresis [4–12% gel (w/v)] and co-immunoprecipitated APP was detected by Western blotting with a rabbit anti-APP (Aβ-N-terminus) antibody (1:100) (eBioscience, San Diego, CA, USA) and secondary anti-rabbit IgG. Finally, the immunoblot membranes were reprobed with anti-PS1 antibody to detect immunoprecipitated PS1. Data from densitometry analysis are expressed as the ratio of APP to PS1 band in templates from controls, CHF5074-and ibuprofen-treated mice.
Statistics
For the behavioural data, the average of the escape latencies and of the swimming paths of the three different trials was calculated for each of the four test sessions. For the immunohistochemistry data (brain plaques and microglial activation), the averages of the five single measurements per animal were calculated separately for the cortex and hippocampus. Behavioural data were analysed with three-way analysis of variance (anova) for repeated measures with ‘treatment’, ‘time’ and ‘gender’ and their interactions as fixed factors. As immunohistochemistry analyses were carried out during three separate sessions, these data were analysed with three-way anova with ‘treatment’, ‘gender’ and ‘assay session’ and their interactions as fixed factors. Biochemistry data were analysed with two-way anova with ‘treatment’ and ‘gender’ and their interactions as fixed factors. Post hoc comparisons were directed only versus the transgenic control group (Vehicle) to reduce the loss of power due to multiple testing and were carried out with the Holm-Sidak's test that uses the t statistics. Calculations were performed with the statistical software SYSTAT™ (Version 12, Systat Software Inc., Chicago, IL, USA) and SigmaStat™ (Version 3.5, SPSS, Chicago, IL, USA). Results were presented as mean ± standard error of mean.
Results
Tolerability
Twenty transgenic mice and one wild-type littermate were found dead before completion of the 6-month treatment period. The cause of death was not investigated. In the vehicle, ibuprofen and CHF5074 transgenic groups, the number of dead animals (six, eight and six animals respectively), their gender distribution (two, four and three female animals respectively) and the median time to death (72, 65 and 66 days respectively) were similar. The good tolerability of CHF5074 was confirmed by the time-courses of the mean body weight gain that was very similar to those of transgenic controls (data not shown). Gender distribution of the animals completing the 6-month treatment period was not homogeneous across transgenic and non-transgenic animals with more female animals in the wild-type group completing the study (24%, 14%, 22% and 62% in the vehicle, ibuprofen, CHF5074 and wild-type groups respectively, P = 0.052). Thus, data were analysed with the appropriate anova model for simultaneously testing treatment, gender and, when appropriate, time effects.
Brain plaque burden
β-amyloid peptides immunoreactivity in representative sections of cortex and hippocampus of vehicle ibuprofen-and CHF5074-treated transgenic mice are shown in Figure 1. Quantification of plaque burden in cortex and hippocampus is reported in terms of brain area fraction occupied by plaques and number of plaques in Figure 2. Three-way anova revealed significant differences between treatment groups in plaque area fraction of cortex (F = 10.60, dfs = 2,23, P = 0.003) and hippocampus (F = 7.10, dfs = 2,23, P = 0.004) as well as in number of plaques in cortex (F = 4.50, dfs = 2, 23, P = 0.022) and hippocampus (F = 9.72, dfs = 2,23, P = 0.005). Compared with vehicle, CHF5074 treatment significantly reduced the area occupied by plaques in cortex (by 32 ± 6%, P = 0.010) and hippocampus (42 ± 6%, P = 0.005). The number of plaques were also significantly reduced by CHF5074 (by 28 ± 6%, P = 0.022 and 34 ± 7%, P = 0.014, in cortex and hippocampus respectively). Ibuprofen did not significantly reduce either plaque area fraction (18 ± 11% and 25 ± 10% in cortex and hippocampus respectively) or number of plaques (10 ± 12% and 18 ± 12% in cortex and hippocampus respectively).
Figure 1.
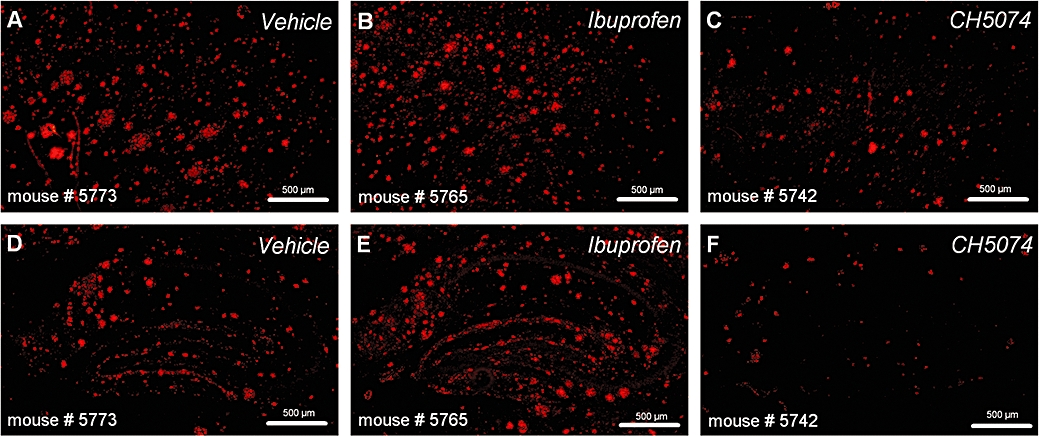
Immunohistochemical labelling of plaques with 6E10 antibody in representative images taken from the frontal cortex (A–C) and hippocampus (D–F) of hAPP transgenic mice treated with CHF5074 (375 in the diet), ibuprofen (375 ppm) or standard diet (vehicle) for 6 months. CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid; hAPP, transgenic mice expressing human APP751 with the Swedish and London mutations.
Figure 2.
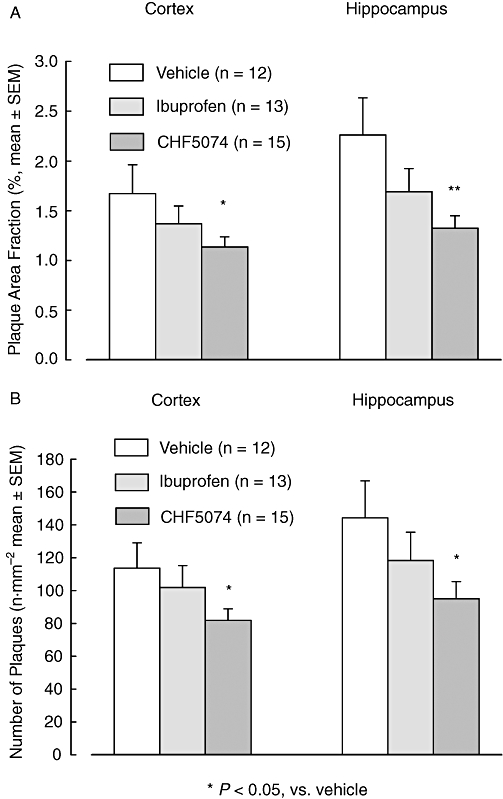
Mean (±SEM) brain area fraction occupied by plaques (A) and number of plaques (B) of hAPP transgenic mice treated with CHF5074 (375 in the diet), ibuprofen (375 ppm) or standard diet (vehicle) for 6 months. There was a significant reduction in the CHF5074-treated group compared with controls (vehicle) in plaque area fraction and number of plaques of cortex and hippocampus (anova, *P < 0.05, **P < 0.01, n = 12–15). CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid; hAPP, transgenic mice expressing human APP751 with the Swedish and London mutations.
The mean size of remaining plaques was not significantly affected by drug treatments in either cortex (reductions of 1 ± 5% and 5 ± 5%, after CHF5074 and ibuprofen respectively) or hippocampus (reduced by 10 ± 4% and 8 ± 4%, after CHF5074 and ibuprofen respectively).
There were significant gender differences in both plaque area fraction (F = 22.95, dfs = 1,23, P < 0.001 in the cortex and F = 19.55, dfs = 1,23, P < 0.001) and number of plaques (F = 4.66, dfs = 1,23, P = 0.042 in the cortex and F = 22.32, dfs = 1,23, P < 0.001). In the vehicle group, female animals (n = 5) presented higher plaque burden than males (n = 7) in terms of both plaque area fraction (2.50 ± 0.42 vs. 1.08 ± 0.19%, P < 0.001 in the cortex and 3.28 ± 0.47 vs. 1.54 ± 0.35%, P = 0.002 in the hippocampus) and plaque number (152 ± 24 vs. 86 ± 13 plaques·mm−2, P = 0.021 in the cortex and 200 ± 32 vs. 105 ± 22 plaques·mm−2, P = 0.007 in the hippocampus). Compared with the corresponding transgenic controls, plaque area reduction by CHF5074 was more robust in female (n = 4) than in male (n = 11) animals in both cortex (by36 ± 6% vs. 11 ± 7% in females and males respectively) and hippocampus (by 44 ± 6% vs. 26 ± 7% in females and males respectively). A similar phenomenon was observed for number of plaques (reduction by 29 ± 12% vs. 16 ± 6% in cortex and 36 ± 15% vs. 21 ± 7% in hippocampus).
Brain microglia
CD11b immunoreactivity in representative sections of cortex and hippocampus of vehicle-, ibuprofen-, CHF5074-treated transgenic mice as well in wild-type mice are shown in Figure 3. Quantification of brain immunoreactive microglia is reported in Figure 4. Two-way anova revealed significant differences between treatment groups in microglia area fraction of both cortex (F = 7.05, dfs = 3,52, P < 0.001) and hippocampus (F = 11.29, dfs = 3,52, P < 0.001). Compared with transgenic controls, wild-type animals had much lower activated microglia in both cortex (by 76 ± 4%, P < 0.001) and hippocampus (83 ± 3%, P < 0.001). Plaque-associated microglia in CHF5074-treated animals was significantly lower than in transgenic controls in both cortex (by 54 ± 10%, P = 0.008) and hippocampus (59 ± 8%, P = 0.002). Similarly, ibuprofen significantly reduced microglia area in cortex and hippocampus (by 57 ± 13%, P = 0.018) and −54 ± 11%, P = 0.003 respectively).
Figure 3.
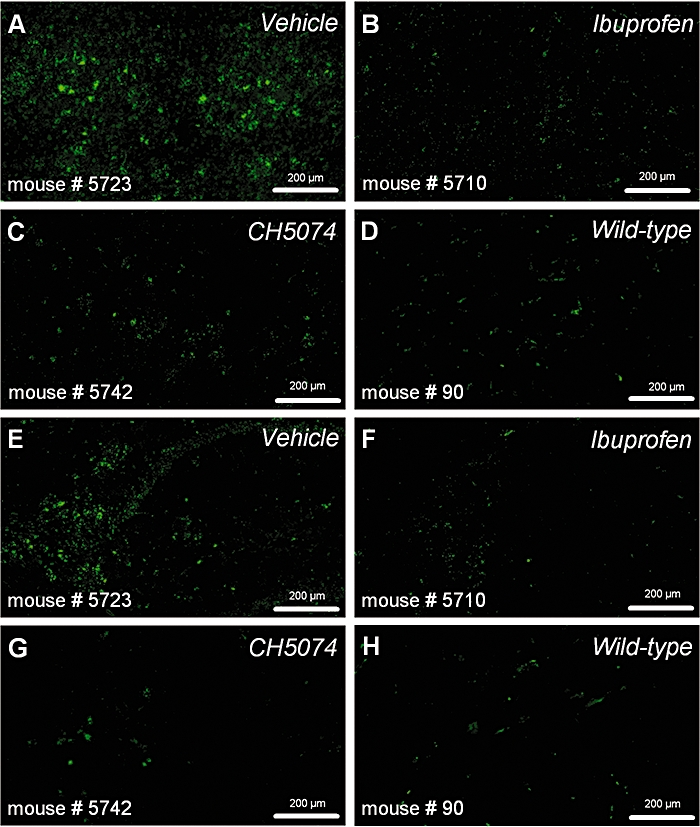
Immunohistochemical labelling of reactive microglia with CD11b antibody in representative images taken from the frontal cortex (A–D) and the subiculum of the hippocampus (E–H) in hAPP transgenic mice treated with standard diet (vehicle), ibuprofen (375 ppm) or CHF5074 (375 ppm) in the diet or in non-transgenic (wild-type) mice treated with standard diet for 6 months. CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid; hAPP, transgenic mice expressing human APP751 with the Swedish and London mutations.
Figure 4.
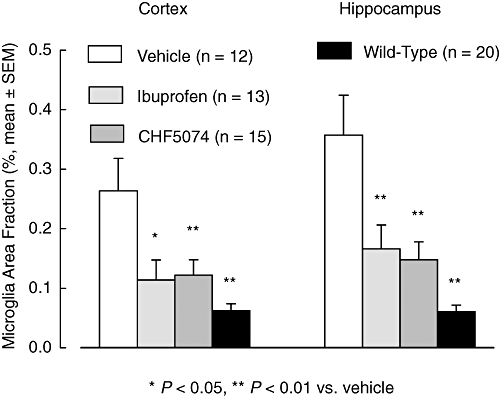
Mean (±SEM) brain area fraction occupied by immunoreactive microglia in hAPP transgenic mice treated with standard diet (vehicle), ibuprofen (375 ppm) or CHF5074 (375 ppm) in the diet or in non-transgenic (wild-type) mice treated with standard diet for 6 months. There was a significant reduction in CHF5074-and ibuprofen-treated groups and in wild-type mice compared with transgenic controls (vehicle) in activated microglia of cortex and hippocampus (anova, *P < 0.05, **P < 0.01, n = 12–20). CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid; hAPP, transgenic mice expressing human APP751 with the Swedish and London mutations.
Aß40 and Aß42 levels
One animal in the vehicle group and three animals in the ibuprofen group had abnormal values for plasma, CSF or formic acid (FA)-extracted brain Aβ40 and Aβ42 levels (from four-to 19-fold higher than group averages) and were excluded from the analysis.
At the end of the treatment, mean levels of Aβ40 and Aβ42 in brains from the transgenic animals receiving vehicle in SDS and FA fractions, are shown in Figure 5. Two-way anova did not show significant differences between treatment groups for either Aβ40 or Aβ42 brain levels. However, there was a significant effect of gender on brain SDS (F = 16.53, dfs = 1,39, P < 0.001 for Aβ40 and F = 20.36, dfs = 1,39, P < 0.001 for Aβ42) and FA (F = 16.07, dfs = 1,38, P < 0.001 for Aβ40 and F = 5.30, dfs = 1,38, P = 0.010 for Aβ42) extracted fractions. In the vehicle group, female animals (n = 5) had significantly higher brain Aβ40 and Aβ42 levels than male animals (n = 7). This was observed for SDS extracts (both Aβ40 and Aβ42, P = 0.023) and FA extracts (Aβ40, P = 0.002 and Aβ42, P = 0.003) (Figure 6). Interestingly, FA-extracted Aβ42 levels in female animals of the ibuprofen-and CHF5074-treated groups were significantly lower compared with those of the vehicle group (P = 0.004 and P = 0.028 respectively, Figure 7).
Figure 5.
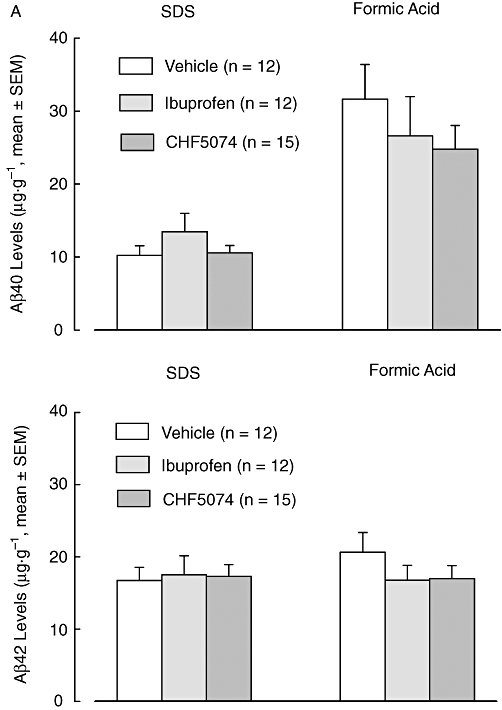
Mean (±SEM) brain Aβ40 (A) and Aβ42 (B) levels of SDS and formic acid (FA) fractions in hAPP transgenic mice treated with CHF5074 (375 ppm) in the diet or ibuprofen (375 ppm) or standard diet (vehicle) for 6 months. There were no significant differences between treatment groups (n = 12–15). Aβ, β-amyloid peptides; CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid; hAPP, transgenic mice expressing human APP751 with the Swedish and London mutations; SDS, sodium dodecyl sulphate.
Figure 6.
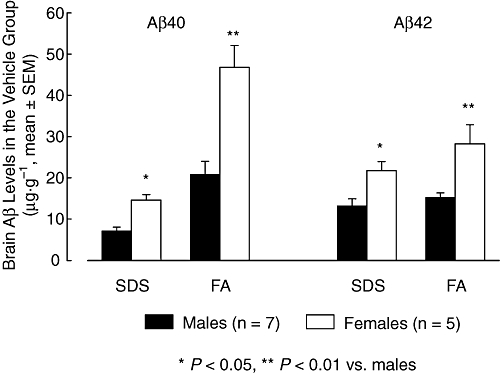
Mean (±SEM) brain Aβ40 and Aβ42 levels of SDS and FA fractions in male and female animals of the transgenic control group (vehicle). Female mice had significantly higher β40 and Aβ42 levels than male animals (anova, *P < 0.05, **P < 0.01, n = 5–7). Aβ, β-amyloid peptides; FA, formic acid; SDS, sodium dodecyl sulphate.
Figure 7.
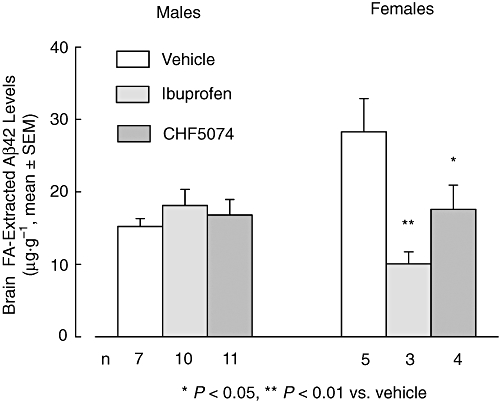
Mean (±SEM) brain Aβ42 levels extracted with formic acid (FA) of male and female hAPP transgenic mice treated with CHF5074 (375 ppm) or ibuprofen (375 ppm) in the diet or standard diet (vehicle) for 6 months. In female animals, there was a significant reduction in Aβ42 levels of the CHF5074-and ibuprofen-treated groups compared with transgenic controls (vehicle) (anova, *P < 0.05, **P < 0.01, n = 3–5). Aβ, β-amyloid peptides; CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid; hAPP, transgenic mice expressing human APP751 with the Swedish and London mutations.
Mean CSF Aβ40 (3121 ± 382, 3206 ± 487 and 2567 ± 489 pg·mL−1 in the vehicle, ibuprofen and CHF5074 group respectively) and Aβ42 (2819 ± 118, 2927 ± 154 and 2615 ± 98 pg·mL−1 in the vehicle, ibuprofen and CHF5074 groups respectively) levels did not differ significantly between treatment groups. Mean plasma Aβ40 (31.8 ± 0.5, 33.8 ± 1.4 and 36.8 ± 2.9 pg·mL−1 in the vehicle, ibuprofen and CHF5074 groups respectively) and Aβ42 (39.7 ± 0.2, 39.9 ± 0.3 and 40.5 ± 1.2 pg·mL−1 in the vehicle, ibuprofen and CHF5074 groups respectively) were also similar between treatment groups.
Behaviour
Mean escape latency and mean swimming path to reach the hidden platform during each of the three trials of the four testing days of the MWM are shown in Figure 8. Three-way anova for repeated measures showed that performance of all four groups improved significantly over the four testing days both in terms of escape latency (F = 31.08, dfs = 3,156, P < 0.001) and swimming path (F = 22.70, dfs 3,156, P < 0.001). Overall, there were no significant differences between groups in the time to reach the platform or in the distance travelled. However, on the last testing day (day 4), there was a significant difference between treatment groups in the swimming path (F = 2.872, dfs = 2.872, P = 0.045) with transgenic controls performing significantly worse than both wild-type animals (P = 0.007) and CHF5074-treated transgenic mice (P = 0.025) (Figure 9). Animals in the ibuprofen group did not perform significantly better than transgenic controls (P = 0.100).
Figure 8.
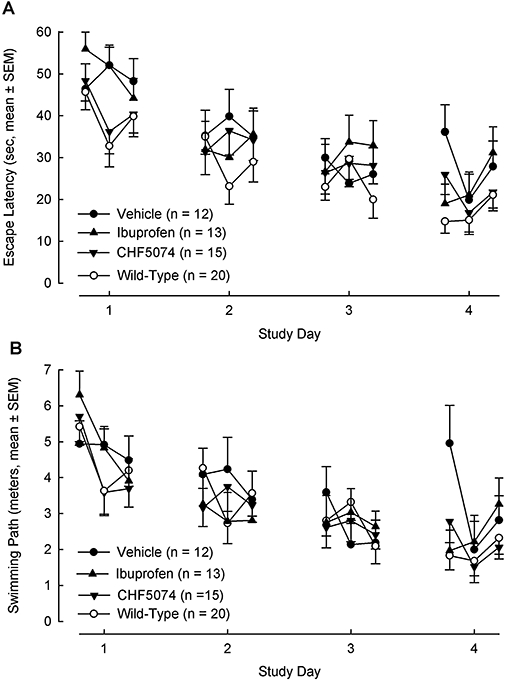
Mean (±SEM) escape latency (A) and swimming path (B) to reach the hidden platform during each trials of the four testing days of the Morris water maze test. Overall, there were no significant differences between treatment groups (n = 12–20). CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid.
Figure 9.
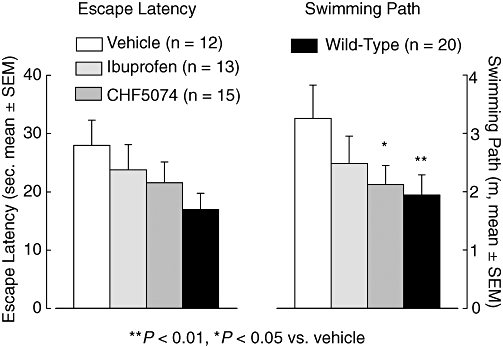
Mean (±SEM) escape latency (left) and swimming path (right) to reach the hidden platform at day 4 of the Morris water maze test in hAPP transgenic mice treated with standard diet (vehicle), ibuprofen (375 ppm) or CHF5074 (375 ppm) in the diet or in non-transgenic (wild-type) mice treated with standard diet for 6 months. There was a significant reduction in the swimming path of wild-type animals and CHF5074-treated transgenic mice compared with transgenic controls (vehicle) (anova, *P < 0.05, **P < 0.01, n = 12–20). CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid; hAPP, transgenic mice expressing human APP751 with the Swedish and London mutations.
In the probe trial, two-way anova did not reveal significant differences between groups in the per cent time spent in the target quadrant (34.3 ± 6.4%, 45.8 ± 4.4%, 38.0 ± 3.7% and 45.9 ± 3.3% in the vehicle, ibuprofen, CHF5074 and wild-type groups respectively).
Brain APP processing
The effects produced in vivo by CHF5074 and ibuprofen on the APP processing were evaluated in the brain extracts of the transgenic mice treated for 6 months with the drugs and with standard diet (vehicle group). Western blot analyses revealed no differences between treatment groups in the cellular content of CTFβ or FL-APP (Figure 10A–C). Equal amounts of proteins were immunoprecipitated using the anti-PS-1 antibody and probed for APP. Templates from mice treated with CHF5074 or ibuprofen revealed a statistically significant decrease, compared with vehicle, of the association between APP and PS1 (Figure 10D,E). These data suggest that both CHF5074 and ibuprofen may modulate the γ-secretase activity in vivo by reducing the interaction between APP and PS1. Long-term treatment with CHF5074 or ibuprofen did not decrease, compared with controls, the brain levels of FL-APP or PS1 or the generation of CTFβ (C99).
Figure 10.
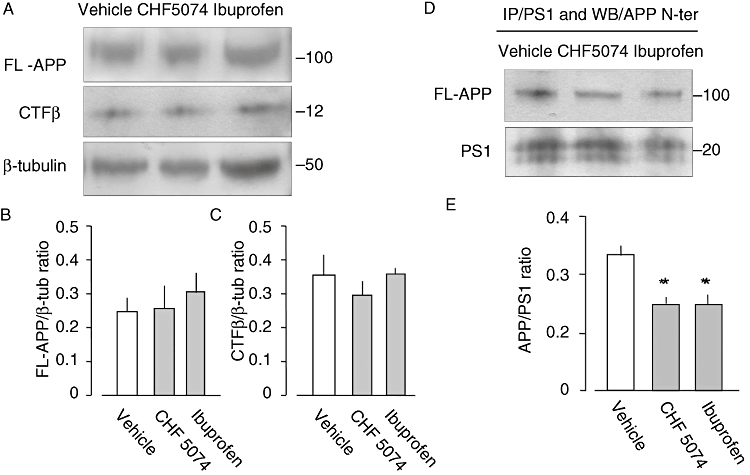
(A) Western blot analyses were performed using 6E10 antibody in brain protein extracts from transgenic mice treated with CHF5074 (375 ppm) or ibuprofen (375 ppm) in the diet or standard diet (vehicle) for 6 months. (A) Representative immunoblots of extracts from mouse no. 5723 (vehicle), mouse no 5729 (CHF5074) and mouse 5755 (ibuprofen). Data from densitometry analyses of FL-APP and CTFβ immunoblots are expressed as ratio of FL-APP (B) and CTFβ (C) to β-tubulin. Brain proteins from the same mice were immunoprecipitated with the anti-presenilin-1 antibody and probed for APP using APP N-terminal antibody (D). Densitometry analysis of PS1-APP interaction is expressed as ratio of APP to PS1 band in templates from vehicle, CHF5074 or ibuprofen-treated mice (E) (anova, *P < 0.05, n = 3). APP, amyloid precursor protein; CHF5074, 1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid; CTFβ, 99-residue carboxy-terminal fragment of APP; PS, presenilin.
Drug levels
At the end of the 6-month treatment, we measured mean plasma levels of 281.1 ± 16.0 µmol·L−1 in the CHF5074 group and 13.4 ± 2.0 µmol·L−1 in the ibuprofen-treated animals. The mean drug levels in the brain were 3.0 ± 0.6 µmol·L−1 and 0.9 ± 0.1 µmol·L−1 in the CHF5074 and ibuprofen group respectively.
Discussion and conclusions
This study shows that a 6-month treatment with CHF5074 in the diet (375 ppm) leads to a significant decrease in β-amyloid burden in transgenic hAPP mice expressing hAPP with the Swedish and London mutations. CHF5074 treatment reduced both the area occupied by amyloid deposits and the number of plaques in cortex and hippocampus. The results of this study further extend previous findings (Imbimbo et al., 2007a) showing that a 4-month treatment with CHF5074 (375 ppm in the diet) significantly reduced brain pathology in transgenic mice expressing hAPP with the Swedish mutations (Tg2576 mice). The degree of plaque load inhibition observed in the present study in hAPP mice (32–42%) appears to be lower than that observed in the Tg2576 mice (52–77%). This could be due to the fact that Tg2576 mice express hAPP with mutations at the β-secretase cleavage site (K670N/M671L) while hAPP mice express hAPP with mutations at both β-secretase (K670N/M671L) and γ-secretase (V717I) cleavage sites. Transgenic mice with human mutated APP at the γ-secretase site (like the London mutation) may be more resistant to the action of γ-secretase modulators. This hypothesis seems to be confirmed by the weak effects observed with ibuprofen on plaque deposition in the present study (18–25% inhibition). Similar weak effects on Aβ plaque load (11–19% inhibition) were observed with ibuprofen at the same dose (375 ppm in the diet) in transgenic mice expressing hAPP with London mutation only (Heneka et al., 2005) while in other studies using mice expressing the Swedish APP mutations, ibuprofen produced a robust and significant inhibition (45%–59%) of plaque amyloid burden (Lim et al., 2000, Yan et al., 2003, Morihara et al., 2005).
The effects of CHF5074 and ibuprofen on brain Aβ42 levels in the present study in hAPP mice were also weak (−18% and −19% respectively) being significant only in female animals (FA extracts). Conversely, robust inhibitions of brain Aβ42 levels were observed in Tg2576 mice with both CHF5074 (−44%, Imbimbo et al., 2007a) and ibuprofen (−54%, Lim et al., 2001, −39%, Lim et al., 2000). Resistance to the brain Aβ42 lowering properties of the γ-secretase modulator GSM-1 has been also observed in mice with double APP (Swedish) and PS2 (N141I) mutations while the drug was found to be fully effective in Tg2576 mice (Page et al., 2008), again suggesting that transgenic mouse models with certain PS or APP mutations may not respond to γ-secretase modulators when the point mutations concern specific APP or PS regions (Czirr et al., 2007) involved in the interaction between the γ-secretase modulator and the APP-PS complex (Lleo et al., 2004).
The question why a significant reduction of brain plaque load has been observed in this study with CHF5074 without a significant effect on total brain Aβ levels remains to be answered. The apparent disagreement may not simply be due to higher variability of the two measures. In the vehicle group, coefficient of variation of plaque area fraction was 60% in cortex and 57% in hippocampus while coefficient of variation of FA-extractable Aβ42 and Aβ40 levels were 46% and 52% respectively. It is worth noticing that CHF5074-treated female mice, unlike males, showed a significant reduction in brain Aβ42 levels. It is known that brain plaque load is increased in APP-transgenic female mice when compared with males (Callahan et al., 2001), an observation confirmed also by this study (Figure 6). Therefore, the Aβ42 lowering effects of CHF5074 may be more easily revealed in females because they accumulate Aβ more rapidly than males.
The immunoblot analyses of brain extracts from transgenic mice show that both CHF5074 and ibuprofen may modulate APP cleavage by γ-secretase, without inducing an accumulation of the APP carboxy-terminal fragment CTFβ (C99) or the brain content of FL-APP. The modulatory effects of CHF5074 and ibuprofen on γ-secretase activity is associated with a reduction of APP and PS1 interaction, a finding consistent with the Aβ lowering mechanism previously described for ibuprofen (Lleo et al., 2004). Further studies are needed to characterize the mode and the site of interaction of CHF5074 with the γ-secretase/APP complex. It is not clear why CHF5074 and ibuprofen treatment appear to decrease similarly the APP-PS1 interaction in the brain but seems to have different efficiencies in antagonizing brain plaque deposition. It may be possible that either the γ-secretase modulation or the direct inhibition of the Aβ aggregation process contribute to the interference of CHF5074 on brain plaque deposition. This is suggested by a recent paper on the mechanism of action of small-molecule γ-secretase modulators (Kukar et al., 2008) in which R-flurbiprofen (tarenflurbil) was shown to bind to residues 28–36 of Aβ, a region critical for β-amyloid aggregation process. Thus, it appears that some γ-secretase modulators have a dual mechanism of action (reduction of Aβ42 production and inhibition of Aβ aggregation) that may work synergistically to prevent brain Aβ deposition.
Plaque formation and maturation are normally accompanied by inflammatory processes. In the present study, CD11b immunostaining showed a four-to sixfold increase in microglia activation in hAPP transgenic animals compared with wild-type controls. CHF5074-treated animals showed a marked reduction of activated microglia compared with transgenic controls both in cortex (−54%) and hippocampus (−59%). A similar marked decrease in activated microglia (−54–57%) was also observed in the ibuprofen-treated animals but this seems to be more linked to anti-inflammatory activity rather than to its weak effects on plaque burden. Indeed, the IC50 of ibuprofen on COX-1 and COX-2 activity is around 1 µmol·L−1 (Tegeder et al., 2001), a value compatible with mean brain ibuprofen concentrations measured in our study (0.9 ± 0.1 µmol·L−1). The concentrations reached in the brain by CHF5074 (3.0 ± 0.6 µmol·L−1) is in the range of its in vitro inhibiting activity on Aβ42 secretion (IC50 = 3.6 µmol·L−1, Imbimbo et al., 2007a). As the IC50 of CHF5074 for inhibition of COX-1 and COX-2 is above 100 µmol·L−1 (Imbimbo et al., 2007b), at the doses used in this study, the drug can not affect COX-mediated inflammation in the brain. On the other hand, the suppressive effects of CHF5074 on amyloid deposition may down-regulate microglia activation as Aβ oligomers and fibrils can trigger the innate immunity system, in particular microglial cells, and induce inflammatory responses in human brain (Salminen et al., 2008). Taken together, our data are consistent with the hypothesis that the observed reduction of microglial immunoreactivity is associated with the anti-amyloidogenic properties of CHF5074.
Behavioural testing in the present study indicated that hAPP transgenic mice performed significantly worse than wild-type controls on the swimming path in the last day (day 4) of the MWM paradigm. On day 4, CHF5074 treatment also significantly shortened the swimming path to reach the platform compared with transgenic controls. To our knowledge, this is one of the few published studies (Kukar et al., 2007) demonstrating the ability of a γ-secretase modulator in attenuating spatial memory deficits of transgenic mice in the MWM paradigm. To rule out the possibility that improved performance in the water maze could be attributed to potential effects on motor activity, we compared swim speeds in the probe trials and found no significant effects of CHF5074 compared with vehicle. To confirm potential cognitive effects of this drug, further studies need to be performed in cognitive paradigms more sensitive to γ-secretase inhibition like the contextual fear conditioning test (Comery et al., 2005). In the present study, we did not detect a cognitive effect of ibuprofen on spatial memory, while a significant ameliorating effect of ibuprofen was recently described in Tg2576 mice expressing only the Swedish APP mutation (Kotilinek et al., 2008). This could probably reflect the different transgenic mice model used in the two studies.
CHF5074 and ibuprofen were well tolerated in hAPP transgenic mice when administered in the diet at 375 ppm for 6 months (estimated assumed dose of approximately 60 mg·kg−1·day−1). The number of deaths occurring during the 6-month treatment period was similar to that in the transgenic controls. Similarly, body weight and body weight gain over time was not different from that of the vehicle-treated group. Previous studies in Tg2576 mice have indicated that chronic treatment with CHF5074 attenuates brain Aβ pathology without causing histopathological changes in peripheral organs that could be associated to the inhibition of processing of alternative substrates of γ-secretase (Imbimbo et al., 2007a). Different reports have shown that the subset of NSAIDs acting as γ-secretase modulators can distinguish between the proteolytic activities on APP and Notch (Weggen et al., 2001; Weggen et al.2003). This feature differentiates γ-secretase modulators from conventional γ-secretase inhibitors that in addition to APP may affect processing of alternative substrates for γ-secretase, particularly the Notch family of receptors. Genetic deletion of the Notch pathway in mice results in defects in the gastrointestinal tract with a phenotype similar to that observed using a γ-secretase inhibitor (van Es et al., 2005). Intestinal goblet cell metaplasia and cell population changes in the ileum have been observed with several γ-secretase inhibitors (Searfoss et al., 2003; Milano et al., 2004; Wong et al., 2004). In addition, γ-secretase inhibitors have been associated to alteration of lymphopoiesis (Wong et al., 2004) and atrophy of the thymus (Hadland et al., 2001). Previous studies in HEK293swe indicated that CHF5074 concentrations inhibiting APP processing (5 µmol·L−1) do not affect Notch processing (Imbimbo et al., 2007a). Studies in H4swe cells have shown that CHF5074, at the concentration of 100 µmol·L−1, did not inhibit the expression of twenty NICD-responsive genes (Imbimbo et al., 2007b). The lack of overt toxicity in the present study seems to confirm the good tolerability of CHF5074 after prolonged treatment. Further formal chronic toxicological studies are ongoing to confirm these preliminary findings.
In conclusion, this study shows that 6-month treatment with CHF5074 in the diet (375 ppm) appears to be safe and well tolerated in hAPP transgenic mice. CHF5074 treatment significantly reduced plaque burden in cortex and hippocampus and attenuates spatial memory impairment. CHF5074 is therefore a novel γ-secretase modulator which has the potential to be a safe and promising therapeutic agent for AD treatment.
Acknowledgments
A part of this study was presented at the 11th International Conference on Alzheimer Disease (ICAD 2008), Chicago, 26–31 July 2008. The study was sponsored by Chiesi Farmaceutici, Parma, Italy.
Glossary
Abbreviations:
- Aβ
β-amyloid peptides
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- APPswe
Swedish mutated form of APP
- CHF5074
1-(3′,4′-dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid
- CSF
cerebrospinal fluid; CTFβ or C99, 99-residue carboxy-terminal fragment of APP, full length APP
- FA
formic acid
- H4swe
human neuroglioma cell line expressing APPswe
- hAPP
transgenic mice expressing human APP751 with the Swedish and London mutations
- HEK293swe
human embryonic kidney 293 cells expressing APPswe
- MWM
Morris water maze
- NICD
Notch intracellular domain
- NSAIDs
non-steroidal anti-inflammatory drugs
- PS
presenilin
- SDS
sodium dodecyl sulphate
Conflict of interest
Bruno P Imbimbo, Gino Villetti, Fabrizio Facchinetti, Valentina Cenacchi and Roberta Volta are employees of Chiesi Farmaceutici.
References
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female β-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–1177. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, et al. Acute γ-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2005;25:8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirr E, Leuchtenberger S, Dorner-Ciossek C, Schneider A, Jucker M, Koo EH, et al. Insensitivity to Aβ42-lowering nonsteroidal anti-inflammatory drugs and γ-secretase inhibitors is common among aggressive presenilin-1 mutations. J Biol Chem. 2007;282:24504–24513. doi: 10.1074/jbc.M700618200. [DOI] [PubMed] [Google Scholar]
- Hadland BK, Manley NR, Su D, Longmore GD, Moore CL, Wolfe MS, et al. γ-Secretase inhibitors repress thymocyte development. Proc Natl Acad Sci USA. 2001;98:7487–7491. doi: 10.1073/pnas.131202798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, et al. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1–42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, et al. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP, Del Giudice E, Colavito D, D'Arrigo A, Dalle Carbonare M, Villetti G, et al. 1-(3′,4′-Dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid (CHF5074), a novel γ-secretase modulator, reduces brain β-amyloid pathology in a transgenic mouse model of Alzheimer's disease without causing peripheral toxicity. J Pharmacol Exp Ther. 2007a;323:822–830. doi: 10.1124/jpet.107.129007. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP, Del Giudice E, Cenacchi V, Volta R, Villetti V, Facchinetti F, et al. In vitro and in vivo profiling of CHF5022 and CHF5074, two β-amyloid1–42 lowering agents. Pharmacol Res. 2007b;55:318–328. doi: 10.1016/j.phrs.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (β) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, et al. Cyclooxygenase-2 inhibition improves amyloid-β-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar T, Golde TE. Possible mechanisms of action of NSAIDs and related compounds that modulate γ-secretase cleavage. Curr Top Med Chem. 2008;8:47–53. doi: 10.2174/156802608783334042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar T, Prescott S, Eriksen JL, Holloway V, Murphy MP, Koo EH, et al. Chronic administration of R-flurbiprofen attenuates learning impairments in transgenic amyloid precursor protein mice. BMC Neurosci. 2007;8:54. doi: 10.1186/1471-2202-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar TL, Ladd TB, Bann MA, Fraering PC, Narlawar R, Maharvi GM, et al. Substrate-targeting γ-secretase modulators. Nature. 2008;453:925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lewis HD, Perez Revuelta BI, Nadin A, Neduvelil JG, Harrison T, Pollack SJ, et al. Catalytic site-directed γ-secretase complex inhibitors do not discriminate pharmacologically between Notch S3 and beta-APP cleavages. Biochemistry. 2003;42:7580–7586. doi: 10.1021/bi034310g. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, et al. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol Aging. 2001;22:983–991. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Lleo A. Activity of γ-secretase on substrates other than APP. Curr Top Med Chem. 2008;8:9–16. doi: 10.2174/156802608783334060. [DOI] [PubMed] [Google Scholar]
- Lleo A, Berezovska O, Herl L, Raju S, Deng A, Bacskai BJ, et al. Nonsteroidal anti-inflammatory drugs lower Aβ42 and change presenilin 1 conformation. Nat Med. 2004;10:1065–1066. doi: 10.1038/nm1112. [DOI] [PubMed] [Google Scholar]
- Maillard I, Adler SH, Pear WS. Notch and the immune system. Immunity. 2003;19:781–791. doi: 10.1016/s1074-7613(03)00325-x. [DOI] [PubMed] [Google Scholar]
- Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, et al. Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- Morihara T, Teter B, Yang F, Lim GP, Boudinot S, Boudinot FD, et al. Ibuprofen suppresses interleukin-1β induction of pro-amyloidogenic α1-antichymotrypsin to ameliorate beta-amyloid (Aβ) pathology in Alzheimer's models. Neuropsychopharmacology. 2005;30:1111–1120. doi: 10.1038/sj.npp.1300668. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Olson RE, Albright CF. Recent progress in the medicinal chemistry of γ-secretase inhibitors. Curr Top Med Chem. 2008;8:17–33. doi: 10.2174/156802608783334088. [DOI] [PubMed] [Google Scholar]
- Page RM, Baumann K, Tomioka M, Pérez-Revuelta BI, Fukumori A, Jacobsen H, et al. Generation of Aβ38 and Aβ42 is independently and differentially affected by familial Alzheimer disease-associated presenilin mutations and gamma-secretase modulation. J Biol Chem. 2008;283:677–683. doi: 10.1074/jbc.M708754200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Second. New York: Academic Press; 2001. [Google Scholar]
- Peretto I, La Porta E. γ-Secretase modulation and its promise for Alzheimer's disease: a medicinal chemistry perspective. Curr Top Med Chem. 2008;8:38–46. doi: 10.2174/156802608783334097. [DOI] [PubMed] [Google Scholar]
- Peretto I, Radaelli S, Parini C, Zandi M, Raveglia LF, Dondio G, et al. Synthesis and biological activity of flurbiprofen analogues as selective inhibitors of β-amyloid1–42 secretion. J Med Chem. 2005;48:5705–5720. doi: 10.1021/jm0502541. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-β protein deposits in a mutant APP transgenic model depends on levels of Aβ1–42. J Neurosci Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-β oligomers set fire to inflammasomes and induce Alzheimer's pathology. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00496.x. Sep 13 [Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searfoss GH, Jordan WH, Calligaro DO, Galbreath EJ, Schirtzinger LM, Berridge BR, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J Biol Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci USA. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, et al. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid β42 production by direct modulation of γ-secretase activity. J Biol Chem. 2003;278:31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, et al. Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits Aβ production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, et al. Anti-inflammatory drug therapy alters β-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


