Abstract
Background and purpose:
Ezetimibe, a selective inhibitor of intestinal cholesterol absorption, might also suppress inflammatory components of atherogenesis. We have studied the effects of ezetimibe on two characteristics of atherosclerotic plaques (infiltrate and fibrosis) and on expression of inflammatory genes in a rabbit model of accelerated atherosclerosis.
Experimental approach:
Femoral atherosclerosis was induced by a combination of endothelial desiccation and atherogenic diet. Animals were randomized to ezetimibe (0.6 mg·kg−1·day−1), simvastatin (5 mg·kg−1·day−1), ezetimibe plus simvastatin or no treatment, still on atherogenic diet. A control group of rabbits received normolipidemic diet.
Key results:
Rabbits fed the normolipidemic diet showed normal plasma lipid levels. Either the normolipidemic diet or drug treatment reduced the intima/media ratio (normolipidemic diet: 22%, ezetimibe: 13%, simvastatin: 27%, ezetimibe + simvastatin: 28%), compared with rabbits with atherosclerosis. Ezetimibe also decreased macrophage content and monocyte chemoattractant protein-1 expression in atherosclerotic lesions. Furthermore, ezetimibe reduced the increased activity of nuclear factor κB in peripheral blood leucocytes and plasma C-reactive protein levels in rabbits with atherosclerosis. In THP-1 cells, ezetimibe decreased monocyte chemoattractant protein-1-induced monocyte migration. Importantly, the combination of ezetimibe with simvastatin was associated with a more significant reduction in plaque monocyte/macrophage content and some proinflammatory markers than observed with each drug alone.
Conclusions and implications:
Ezetimibe had beneficial effects both on atherosclerosis progression and plaque stabilization and showed additional anti-atherogenic benefits when combined with simvastatin. Its effect on monocyte migration provides a potentially beneficial action, in addition to its effects on lipids.
Keywords: ezetimibe, anti-atherogenic, inflammation, statin
Introduction
Chronic inflammation is well known to play a key role both in the development and progression of the atherosclerotic plaque and its thrombotic complications, and it has been proposed as a mechanistic link between hyperlipidaemia and atherogenesis (Ross, 1999). Macrophages are key players in this process by secreting and/or being activated by cytokines, adhesion molecules or growth factors (Ross, 1999; Libby and Aikawa, 2002). Many of these genes implicated in inflammation are controlled by the transcription factor, nuclear factor κB (NF-κB).
Ezetimibe is the first example of a new class of lipid-lowering compounds that selectively inhibits the intestinal absorption of cholesterol. Although ezetimibe significantly lowered total cholesterol, low-density lipoprotein cholesterol (LDL-c) and triglycerides, and increased high-density lipoprotein cholesterol (HDL-c) (Van Heek et al., 1997; Sudhop et al., 2002; Al-Shaer et al., 2004), its greatest effectiveness results from its use in combination with statins. Thus, the addition of ezetimibe to statins produced further lowering of LDL-c of around 20% and had a more favourable effect on HDL-c and triglycerides when compared with statin therapy alone, even after doubling the dose of the statins (Al-Shaer et al., 2004). This synergistic effect was seen with all of the statins (simvastatin, atorvastatin, lovastatin, pravastatin). In apolipoprotein E knockout (apoE−/−) mice, Davis et al. (2001) demonstrated that ezetimibe reduced plasma cholesterol levels and inhibited atherogenesis. In addition to favourable effects on lipid levels, an anti-inflammatory effect of ezetimibe, similar to that seen after simvastatin, has been recently reported in rheumatoid arthritis patients (Mäki-Petäjä et al.,2007). Interestingly, the addition of ezetimibe to ongoing statin therapy led to a statistically significant reduction in C-reactive protein (CRP) (Sager et al., 2005; Kastelein et al., 2008), suggesting a potential synergistic anti-inflammatory and/or anti-atherosclerotic action of ezetimibe and a statin in the treatment of atherosclerosis that need to be explored.
Although the anti-inflammatory signalling mechanisms of ezetimibe are unknown at present, this compound interacts with the aminopeptidase N/CD13 (APN/CD13) (Kramer et al., 2005), a transmembrane protein implicated in adhesion and cell–cell interactions (Firla et al., 2002; Bauvois and Dauzonne, 2006). It has been recently reported that ezetimibe decreased the surface expression of APN/CD13 and of CD16, CD64 and the scavenger receptor CD36 in monocyte/macrophages, leading to an impaired macrophage differentiation and lipid uptake (Orsóet al., 2006). The question, therefore, arises as to whether ezetimibe could also suppress the inflammatory components of atherogenesis.
In this study, we have investigated whether ezetimibe can, alone or in combination with simvastatin, modify two characteristics of the atherosclerotic plaque (cellular infiltrates and fibrosis) and the expression of some genes potentially involved in these processes, as well as the inflammatory phenotype of peripheral blood leucocytes (PBLs) in a model of accelerated atherosclerosis in rabbits. In vitro, we have studied whether ezetimibe could alter monocyte migration, a hallmark of atherogenic disease.
Methods
Animals
All animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the European Union. Male New Zealand rabbits were obtained from Granja Cunicular San Bernardo, Navarra, Spain. Hyperlipidemic diet was purchased from Letica, Barcelona, Spain.
Experimental model
Femoral atherosclerosis was induced in 34 male New Zealand rabbits weighing 2.5–3 kg by a combination of hyperlipidemic diet (containing 2% cholesterol and 6% peanut oil) and vascular injury as previously described with minimal modifications (Hernández-Presa et al., 1997). After a 1 week adaptation period, 20 mL of fasting blood was collected from the ear vein, and rabbits fed a hyperlipidemic diet. After 1 week, rabbits were bled from the ear vein, anaesthetized with 6 mg·kg−1 xylazine, 15 mg·kg−1 ketamine and 0.2 mg·kg−1 atropine, and endothelial damage was induced in the femoral arteries by the passage of desiccated nitrogen gas at a rate of 140 mL·min−1 for 1 min. One week after surgery, animals were randomized to receive ezetimibe (0.6 mg·kg−1·day−1) (n = 7), simvastatin (5 mg·kg−1·day−1) (n = 10), ezetimibe plus simvastatin (n = 10) or no treatment (n = 7), while still on the hyperlipidemic diet. Vascular injury was also induced in a group of rabbits fed normolipidemic diet (standard chow) (n = 5) during the whole study.
Drugs were freshly dissolved every day in drinking water. To ensure complete drug intake, only water containing drugs was provided for the first 6 h and once it was finished, normal water was provided ad libitum for the remaining ∼18 h of each day. The weight of the animals was measured weekly to adjust the dosage of the drugs. After 6 weeks of treatment, rabbits were bled from the ear vein, and then anaesthetized and both femoral arteries from each animal were removed. To minimize any influence of the anatomical tapering of the femoral artery, each artery was cross-sectioned into two segments: proximal for histology, and distal for molecular biology studies. At the same time, four animals fed standard chow without any experimental intervention were killed and used as healthy controls.
Blood lipid measurements
Plasma total cholesterol and triglycerides were measured by standard enzymic methods (Thermo Electron Corporation). Plasma lipoproteins were isolated by sequential flotation-ultracentrifugation at the density interval of 1.006–1.019 g·mL−1 for very low-density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL), 1.019–1.063 g·mL−1 for LDL and 1.063–1.21 g·mL−1 for HDL. LDL-c and HDL-c were directly determined using commercial enzymatic assays.
Vascular histopathological studies
For light microscopy, proximal femoral artery segments were fixed in 4% buffered paraformaldehyde, dehydrated and embedded in paraffin. Sections (4 µm) were stained with orcein and Masson's trichrome. Morphometry was performed in digitized orcein-stained artery sections using computerised software (Image-Pro Plus, Media Cybernetics). We measured the lumen area, the areas encroached by the external elastic lamina (EEL) and the internal elastic lamina (IEL), the vessel diameter and the media thickness, and the following parameters were calculated: media area = EEL area − IEL area; neointimal lesion area = IEL area − lumen area. Femoral arteries were not perfused and fixed under a defined pressure. Therefore, to minimize this limitation, changes in neointimal lesion have been estimated by calculating the intima to media area ratio considered as a highly reproducible morphological parameter because it is independent of the dimensions of the vessels (Srámek et al., 2000; Gómez-Garre et al., 2006a). The vessel diameter and the media thickness were measured at the widest part of the shortest diameter in every given vessel section, which represents a point of a perfect (circular) arterial cross section. Sections with the maximal lesion were chosen for quantification.
The presence of lipid-rich areas and fibrosis was quantified in femoral artery sections stained with Masson's trichrome. After processing, lipids appear as predominately solvent-treated empty spaces, and collagen fibres are stained green. Lipid-rich areas, defined as spaces composed of clear, needle-shaped cholesterol clefts (representing ghost outlines of dissolved crystals) and/or clear, bubbly, granular, mostly anucleate necrotic debris of foam cells, and fibrosis were evaluated using computerized software (LeicaQwin software, Leica Corporation, St Galen, Switzerland) (Moreno et al., 2002; Gómez-Garre et al., 2006b).
The histological assessment was carried out by a pathologist, unaware of the treatments given to each sample.
Immunohistochemistry
Briefly, artery sections (5 µm thick) were dewaxed and rehydrated, and the endogenous peroxidase activity was quenched. The slides were washed and incubated in trypsin (0.1% trypsin/0.1% CaCl2 wt v−1) to expose antigenic sites. After blocking non-specific binding sites, sections were incubated with a mouse monoclonal antibody for rabbit macrophages (RAM-11, 1:50) or with a goat polyclonal anti-human monocyte chemoattractant protein-1 (MCP-1) antibody (40 µg·mL−1) in a humid atmosphere. Negative control slides were treated with diluted normal rabbit serum. Immunostained sites were revealed with the avidin-biotin immunoperoxidase method (ABComplex) with 0.05% 3,3′-diamino-benzidine as the chromogen. Sections were counterstained with Mayer's haematoxylin.
The intensity of RAM-11 and MCP-1 staining was evaluated using computerized software (LeicaQwin software) (Gómez-Garre et al., 2006a). The results are expressed as lesion area positively brown stained. For each artery, results of two to three sections were averaged to obtain the final results. The immunohistochemical assessments were made by a pathologist unaware of the treatments given to each sample.
RNA extraction
Pieces of distal femoral artery segments were homogenized and total RNA was obtained by the Trizol® method. The concentration and purity of the RNA was determined by measuring optical density at 260 and 280 nm with a NanoDrop® spectrophotometer.
TaqMan reverse transcription and real-time polymerase chain reaction
Relative mRNA abundance of MCP-1 was quantified by real-time TaqMan polymerase chain reaction (PCR). The PCR primers and TaqMan probe for MCP-1 (sense primer: 5′-GCTCATAGCAGTCGCCTTCAG-3′; antisense primer: 5′-GTGAATGTATAGCAGCAGGTGACT-3′; sense probe: 5′-TCCCATGTGCTTGCCC-3′) were designed using PrimerExpress software (Applied Biosystems) according to the rabbit MCP-1 sequence (GenBank accession number M57440). The expression of eucaryotic 18S rRNA (VIC/TAMRA Probe) was used as the endogenous control.
To obtain cDNA, 1 µg of isolated RNA from each individual sample was transcribed using a reverse transcription (RT) kit (High-Capacity cDNA Archive kit). Real-time PCR was performed with RT products (2 µL for MCP-1 and 1 µL for 18S) with a TaqMan Universal Master Mix on the ABI PRISM 7900 Detection System (Applied Biosystems) in a 384-well format.
Relative quantification of MCP-1 gene expression was performed with the comparative Ct method (ΔΔCt). The obtained Ct values were normalized to 18S rRNA expression and relative to a pooled sample of RNA from healthy rabbits.
Isolation of PBLs and NF-κB activity measurement
Three millilitres of blood was diluted with phosphate-buffered saline (1:2), poured over 3 mL of Ficoll-Paque and centrifuged at 400× g for 30 min at room temperature. The mononuclear cell layer was collected, washed twice with cold phosphate-buffered saline and resuspended in a cold protein extraction buffer (Gómez-Garre et al., 2006b).
Nuclear protein extracts from PBLs were obtained as previously described (Hernández-Presa et al., 1997) and the activity of NF-κB was evaluated by electrophoretic mobility shift assay (EMSA). Protein concentration was quantified by the bicinchoninic acid method and EMSA was performed with a commercial kit. HeLa cell nuclear extracts were used as positive control. To test the specificity of the assay, a 100-fold excess of unlabeled probe was added to the binding reaction.
Measurement of plasma CRP levels
Quantitative determination of CRP in rabbit plasma was performed using a specific and sensitive ELISA kit according to the manufacturer's instructions. Sensitivity level was ∼4 ng·mL−1.
Cell culture and migration experiments
For these studies, the human monocytic cell line (THP-1) was obtained from the American Type Culture Collection (Rockville, MD). THP-1 cells were maintained in suspension culture in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 U·mL−1 penicillin, 50 µg·mL−1 streptomycin and 2 mmol·L−1 glutamine at 37°C in an atmosphere of 5% CO2.
Chemotactic responses of THP-1 cells were assessed by Transwell® cell culture chambers with polycarbonate filters with 5 µm pores. A total of 3 × 105 cells were suspended in 100 µL RPMI 1640 containing 0.5% FCS and added to the upper compartment of the insert. Then, ezetimibe and/or simvastatin (converted to open acid form; Fenton et al., 1992) was added to THP-1 cells at different concentrations. After incubation at 37°C for 2 h, the lower chamber was filled with 100 ng·mL−1 recombinant human MCP-1 diluted in RPMI 1640 containing 0.5% FCS and THP-1 cells were allowed to migrate for 1 h at 37°C. The contents of the lower chamber were collected, and the number of migrated cells counted using an EPICS XL-MCL Coulter by passing each sample in the same predetermined time and flow conditions. Specific chemotaxis was expressed as the percentage of THP-1 cells that migrated in response to MCP-1 alone.
Cell viability
To test the effect of ezetimibe on cell survival, a total of 3 × 104 THP-1 cells per well in triplicate were incubated with or without different concentrations of ezetimibe. After 1 or 2 days of incubation, digital images were captured by light microscopy at 100× magnification, and cell viability was determined by the Trypan blue exclusion assay, in which cell survival was calculated as (the living cell number of treated group/the living cell number of untreated control group) × 100.
Statistical analysis
All data are expressed as mean ± SEM. Variables not normally distributed were log-transformed to improve their distribution for statistical testing and results transformed back for presentation in figures and tables. Individual arterial segments for each rabbit were evaluated separately. We used Student's t-test for comparisons between two groups and one-way anova for comparisons between more than two groups. Differences were considered statistically significant at P < 0.05. All calculations were performed using the software spss 12.0 for windows.
Materials
Xylazine (Rompun) was from Bayer, ketamine (Ketolar) from Parke-Davis and atropine from B. Braum Medicals SA. LDL-c and HDL-c was directly determined using commercial enzymic assays from Roche Diagnostics SL. For immunohistochemistry studies, RAM-11 antibody, ABComplex and 3,3′-diamino-benzidine were obtained from DAKO A/S, anti-human monocyte chemoattractant protein 1 (MCP-1) antibody from R&D Systems and Mayer's haematoxylin from Sigma-Aldrich. For RNA isolation, Trizol® method from Invitrogen was employed. All the reagents for real-time Taq-Man PCR experiments were purchased from Applied Biosystems. PBLs were isolated by Ficoll-Paque from Amersham. Nuclear proteins were quantified by the bicinchoninic acid method from Pierce Biotechnology and EMSA was performed with a commercial kit from Promega. Plasma CRP ELISA kit was from Alpha Diagnostic International. Culture medium, FCS and antibiotics were from Cambrex; Transwell® cell culture chambers were from Costar, and recombinant human MCP-1 was purchased from R&D systems.
Results
At the end of the study, there were no significant differences in food intake (P = 0.728), drinking pattern (P = 0.217) or body weight (P = 0.685) between animals from each experimental group (Table 1).
Table 1.
Feeding behaviour and body weight at the end of the study
| Healthy controls (n = 4) | ND (n = 5) | Untreated (n = 7) | Ezetimibe (n = 7) | Simvastatin (n = 10) | Eze + Simva (n = 10) | |
|---|---|---|---|---|---|---|
| Food intake (g·day−1) | Free access | 137 ± 43 | 118 ± 9 | 125 ± 5 | 122 ± 4 | 120 ± 3 |
| Water intake (mL·day−1) | Free access | 212 ± 12 | 182 ± 19 | 231 ± 21 | 184 ± 11 | 181 ± 17 |
| Body weight (kg) | 3.5 ± 0.1 | 2.9 ± 0.3 | 3.6 ± 0.2 | 3.3 ± 0.2 | 3.2 ± 0.1 | 3.3 ± 0.1 |
Data are mean ± SEM. Eze + Simva, ezetimibe + simvastatin; ND, normolipidemic diet.
Effect of ezetimibe on lipid levels
The administration of a hyperlipidemic diet to rabbits induced an increment in all plasma lipid levels (except in HDL-c), which was already significant after 1 week (total cholesterol: 1.6 ± 0.7 vs. 21.1 ± 5.4; VLDL: 0.4 ± 0.3 vs. 0.9 ± 0.3; LDL: 0.4 ± 0.0 vs. 19.6 ± 5.2; triglycerides: 0.6 ± 0.2 vs. 1.3 ± 0.3 mmol·L−1, P < 0.05). At this time, the femoral atherosclerosis procedure was carried out and animals were then randomized to different groups without significant differences in lipid levels among groups.
At the end of the study, rabbits with vascular damage and on an atherogenic diet were severely hyperlipidemic (except for HDL-c) compared with those fed normolipidemic diet and healthy controls (Table 2). The administration of ezetimibe had no effect on this raised plasma lipid profile and lipoprotein fractions (Table 2). Ezetimibe plus simvastatin and simvastatin alone tended to show lower levels of plasma total cholesterol, VLDL and LDL than untreated and ezetimibe-treated rabbits, although without reaching statistical significance in a multivariate analysis (Table 2). However, in univariate analysis, the combination of ezetimibe plus simvastatin induced a significant reduction in triglyceride levels with respect to untreated rabbits.
Table 2.
Plasma lipid levels and morphological parameters of femoral arteries of experimental animals at the end of the study
| Healthy controls | ND | Untreated | Ezetimibe | Simvastatin | Eze + Simva | |
|---|---|---|---|---|---|---|
| Plasma lipids | ||||||
| Total cholesterol (mmol·L−1) | 1.1 ± 0.2 | 1.2 ± 0.3 | 85.3 ± 6.3*† | 78.3 ± 19.2*† | 62.9 ± 3.1*† | 53.7 ± 10.4*† |
| VLDL (mmol·L−1) | 0.2 ± 0.0 | 0.2 ± 0.1 | 19.6 ± 3.8*† | 22.0 ± 8.3*† | 8.5 ± 3.3*† | 12.3 ± 6.8*† |
| LDL (mmol·L−1) | 0.4 ± 0.1 | 0.9 ± 0.3 | 64.9 ± 6.1*† | 55.7 ± 12.2*† | 55.4 ± 4.0*† | 42.3 ± 11.6*† |
| HDL (mmol·L−1) | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 |
| Triglycerides (mmol·L−1) | 1.1 ± 0.4 | 0.7 ± 0.3 | 3.3 ± 0.3*† | 2.8 ± 0.9*† | 2.8 ± 0.4*† | 1.8 ± 0.4*†# |
| Morphological data | ||||||
| External diameter (µm) | 808 ± 102 | 816 ± 153 | 932 ± 85 | 976 ± 55 | 865 ± 63 | 1038 ± 108 |
| Lumen area (µm2 103) | 222 ± 58 | 43 ± 4 | 17 ± 9 | 145 ± 37† | 93 ± 41† | 210 ± 79†# |
| Lesion area (µm2 103) | – | 374 ± 60 | 464 ± 77 | 290 ± 51† | 300 ± 64† | 268 ± 51†# |
| Media width (µm) | 115 ± 15 | 85 ± 21 | 90 ± 7 | 114 ± 22 | 98 ± 9 | 95 ± 7 |
Data are expressed as mean ± SEM, n = 6–18 arteries in each group.
P < 0.05 versus healthy control rabbits.
P < 0.05 versus normolipemic diet (ND) group and
P < 0.05 versus untreated rabbits. Eze + Simva, ezetimibe + simvastatin; ND, normolipidemic diet.
Effect of ezetimibe on atherosclerotic plaques
All rabbits with vascular injury developed neointimal hyperplasia in femoral arteries, although rabbits on hyperlipidemic diet without treatment showed the greatest intima/media ratio (Figure 1 and Table 2). Treatment with ezetimibe, simvastatin or the combination reduced the intima/media ratio by 13%, 27% and 28%, and increased lumen area by 8.5-, 5.5-and 12.3-fold, respectively, with respect to untreated rabbits. No changes were found in the media width (Table 2).
Figure 1.
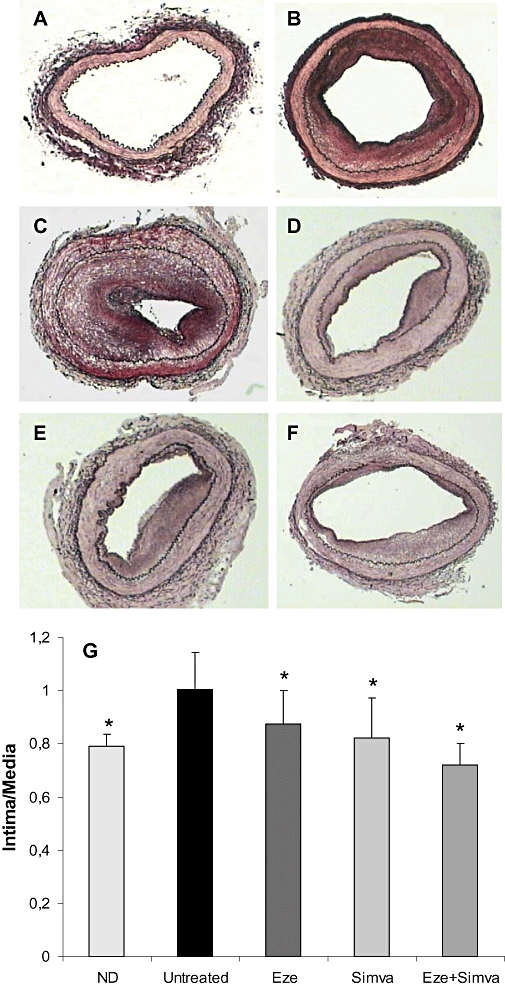
Morphological lesions of femoral arteries of healthy control rabbits (A), normolipidemic diet (ND, B), untreated (C), ezetimibe-treated (Eze, D), simvastatin-treated (Simva, E) and ezetimibe + simvastatin-treated (Eze + Simva, F) rabbits. Photographs show a representative orcein-stained femoral section from each group. No lesions can be observed in arteries from healthy control rabbits (A). Magnification 4×. (G) Quantitative analysis of neointimal lesion size. Data are mean ± SEM, n = 6–18 arteries in each group from 4 to 10 animals per group. *P < 0.05 versus untreated rabbits.
Femoral arteries from rabbits with vascular injury on hyperlipidemic diet showed neointimal lesions characterized by accumulation of lipid-loaded foam cells and a low collagen content, independent of the treatment group (Figure 2). Quantitative analysis of lipid-rich areas and fibrosis demonstrated that treatment with both ezetimibe and simvastatin alone significantly reduced lipid-rich areas within femoral lesions compared with untreated rabbits, despite maintaining similar plasma lipid concentrations (Figure 2F). Further reduction in plaque lipid content was observed after combined treatment. No significant differences were observed in the amount of plaque collagen between groups (Figure 2G).
Figure 2.
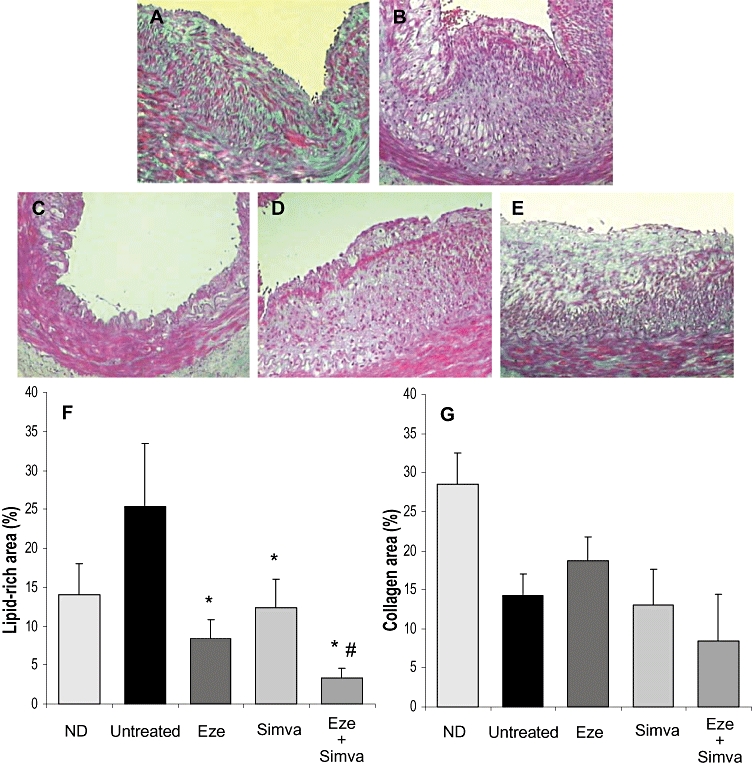
Representative photographs of Masson trichrome-stained sections of femoral arteries of normolipidemic diet (ND, A), untreated (B), ezetimibe-treated (Eze, C), simvastatin-treated (Simva, D) and ezetimibe + simvastatin-treated (Eze + Simva, E) rabbits. Magnification 12.5×. Quantitative analysis of lipid-rich areas (F) and fibrosis (G) within femoral lesions. Data are mean ± SEM, n = 6–18 arteries in each group from 4 to 10 animals per group. *P < 0.05 versus untreated rabbits. #P < 0.05 versus monotherapies.
Effect of ezetimibe on vascular inflammation
As lipid-rich areas are associated with monocyte/macrophage infiltration, their presence in the lesions was investigated by the anti-RAM-11 and an anti-MCP-1 antibody. Quantification of immunostained RAM-11-positive areas demonstrated that all treatments significantly reduced the area staining for number of monocytes/macrophages in the atherosclerotic lesions, in comparison with untreated hyperlipidemic rabbits (untreated: 228 ± 40; ezetimibe: 72 ± 16; simvastatin: 127 ± 37; ezetimibe + simvastatin: 30 ± 6 µm2 103, P < 0.05). The combination of ezetimibe plus simvastatin reduced monocyte/macrophage content more markedly than each drug alone (P < 0.01).
Inmmunohistochemical staining for MCP-1 protein is shown in Figure 3. Untreated rabbits showed the highest levels in both MCP-1 protein (Figure 3F) and mRNA (by real-time PCR) expression (Figure 3G) in atherosclerotic lesions. The administration of ezetimibe, simvastatin or the combination to hyperlipidemic rabbits significantly lowered both MCP-1 protein and mRNA without differences between groups (Figure 3F,G). This reduction was similar to that found in rabbits on the normolipidemic diet, even though plasma lipid levels were significantly higher.
Figure 3.
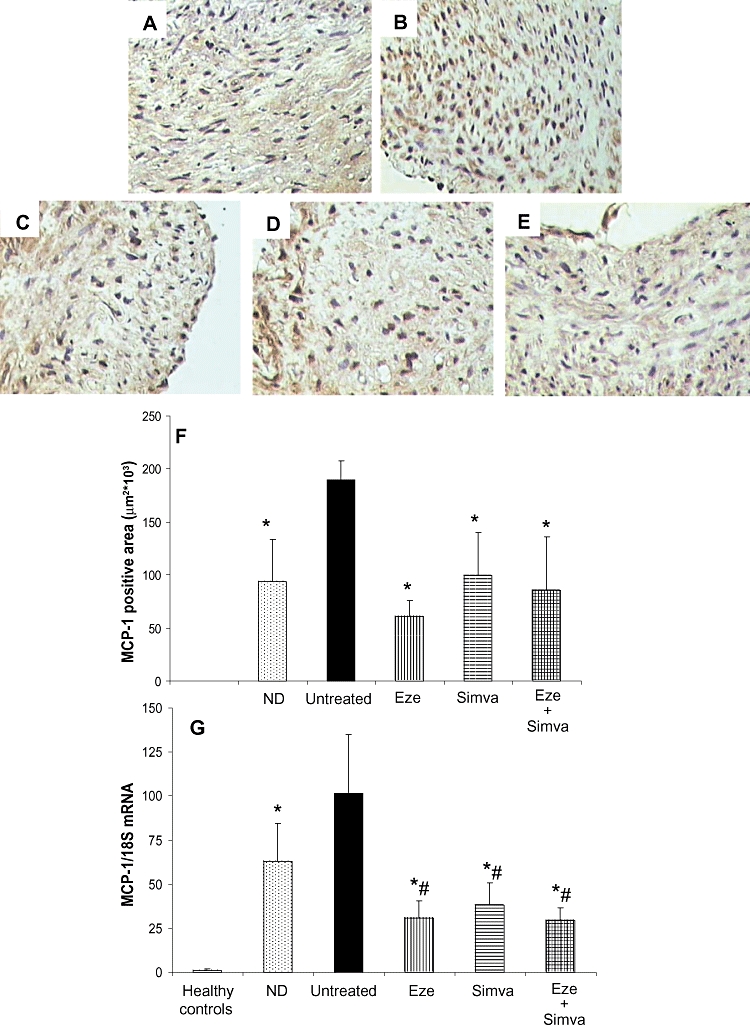
Immunolocalization of monocyte chemoattractant protein-1 (MCP-1) on femoral arteries from normolipidemic diet (ND, A), untreated (B), ezetimibe-treated (Eze, C), simvastatin-treated (Simva, D) and ezetimibe + simvastatin-treated (Eze + Simva, E) rabbits. Magnification 30×. Quantification of MCP-1 protein (F) and mRNA (G) expression in femoral lesions. Data are mean ± SEM, n = 6–18 arteries in each group from 4 to 10 animals per group. *P < 0.05 versus untreated rabbits. #P < 0.05 versus rabbits fed normolipidemic diet.
Effect of ezetimibe on systemic inflammation
In order to evaluate the activation state of PBLs, NF-κB activity was assayed by EMSA. Untreated hyperlipidemic rabbits showed an increased activity of NF-κB in PBLs, compared with those in healthy controls (Figure 4A,B). Treatment with ezetimibe, simvastatin or the combination reduced this high level of NF-κB activation by 61%, 64% and 64%, respectively, bringing it down to a level comparable to that shown in PBLs from the rabbits on the normolipidemic diet (Figure 4A,B).
Figure 4.
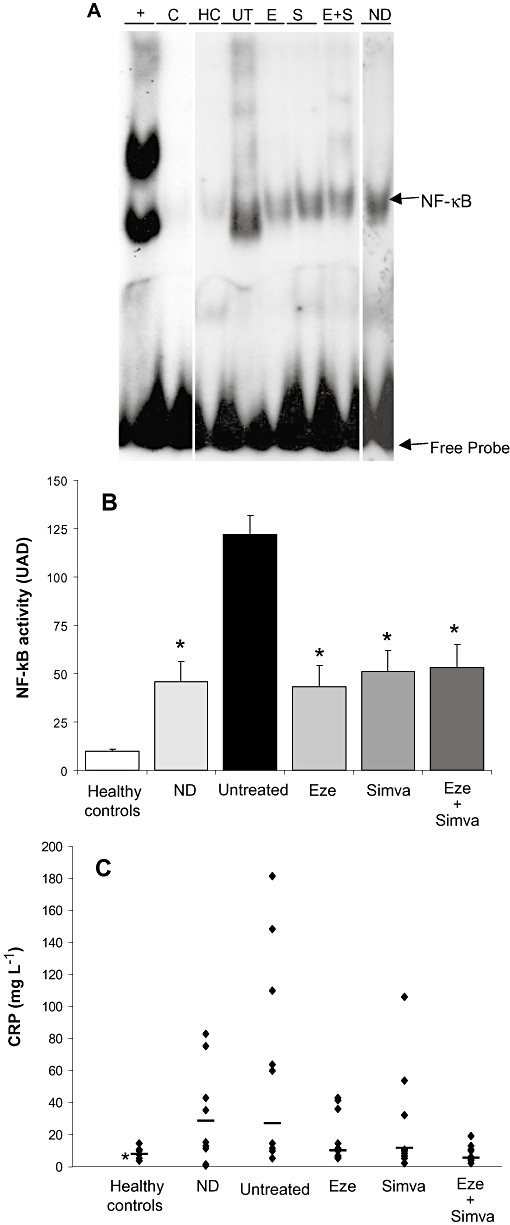
Effect of ezetimibe on systemic inflammation. (A) NF-κB activation of PBLs of healthy control (HC), normolipidemic diet (ND), untreated (UT), ezetimibe-treated (E), simvastatin-treated (S) and ezetimibe + simvastatin-treated (Eze + Simva) rabbits. Autoradiography shows a representative animal from each group. Lane +: HeLa cell nuclear extracts. Lane C: competitive assay showing the specificity of the assay. (B) Densitometric analysis of NF-κB bands. The individual bar values are the mean ± SEM of each group, n = 4–10 animals per group. *P < 0.05 versus untreated rabbits. (C) Plasma CRP levels in healthy control, normolipemic diet (ND), untreated, ezetimibe-treated (Eze), simvastatin-treated (Simva) and ezetimibe + simvastatin-treated (Eze + Simva) rabbits. Individual data are shown. Line represents average level. *P < 0.05 versus untreated rabbits. CRP, C-reactive protein; NF-κB, nuclear factor κB; PBL, peripheral blood leucocyte.
Plasma CRP levels were significantly higher in untreated rabbits than in healthy controls (Figure 4C). However, treatment with ezetimibe, simvastatin and the combination significantly lowered plasma CRP levels in comparison with untreated rabbits (Figure 4C). Only rabbits treated with ezetimibe plus simvastatin normalized CRP plasma levels.
Effect of ezetimibe on monocyte transmigration
On the basis of the above results, we decided to examine the effect of ezetimibe, alone and in combination, on MCP-1-directed monocyte cell migration. Our data demonstrate that pre-treatment of THP-1 monocytes with both ezetimibe and simvastatin for 2 h inhibited the migratory response in a dose-dependent manner (Figure 5). This inhibitory effect was higher when ezetimibe and simvastatin were added in combination.
Figure 5.
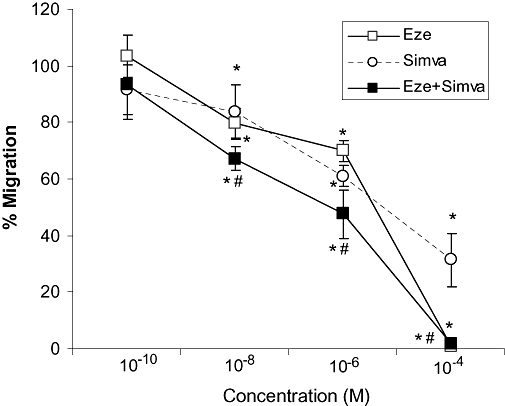
Effect of ezetimibe on monocyte migration. Cells were pre-treated with serial concentrations of ezetimibe (Eze), simvastatin (Simva) or the combination (Eze + Simva) for 2 h and then chemotaxis was induced by monocyte chemoattractant protein-1 (MCP-1) for 1 h. When combined, the same dose of ezetimibe and simvastatin was used. Results are mean ± SEM from a representative experiment of five independent assays performed in duplicate. *P < 0.05 versus the percentage of migrated cells in response to MCP-1 alone. #P < 0.05 versus monotherapies.
As cell migration was completely abolished when 10−4 mol·L−1 ezetimibe was added, control experiments were performed to assess cell integrity. Viability of THP-1 cells exposed to ezetimibe was >90% and remained unchanged up to the highest concentration (10−6 mol·L−1: 94 ± 8; 10−5 mol·L−1: 96 ± 6; 10−4 mol·L−1: 93 ± 3%).
Discussion
In this study, we have demonstrated that the administration of ezetimibe reduced the atherosclerotic lesion size, and decreased the plaque content in macrophage and in the chemoattractant protein MCP-1 in cholesterol-fed rabbits. In vitro, ezetimibe inhibited MCP-1-induced chemotaxis in THP-1 cells, providing a potential mechanism of beneficial action in addition to its lipid effects. Importantly, an additive anti-inflammatory effect was observed when ezetimibe was administered in combination with simvastatin.
It is now well established that inflammation plays a key role in the formation and progression of the atherosclerotic plaque, but also in its thrombotic complications (Ross, 1999; Libby and Aikawa, 2002). Thus, chronically elevated levels of circulating inflammatory proteins can be independent predictor values for cardiovascular events, even among apparently healthy subjects (Ross, 1999; Ridker et al., 2001). Our data demonstrated that ezetimibe exerts direct anti-inflammatory effects in femoral arteries and circulation. In comparison with the plaques from untreated rabbits, those from ezetimibe-treated rabbits were of smaller size, and of lower lipid and macrophage content. We also found a significant reduction in MCP-1 expression (both mRNA and protein) in atherosclerotic lesions from ezetimibe-treated rabbits in comparison with those from untreated animals, suggesting that this decrease could be, at least in part, responsible for the decrease in macrophage and subsequently lipid content within the plaques from ezetimibe-treated rabbits. The effect of ezetimibe seems not to be related solely to a decrease in plaque size, as the plaque area occupied by lipids, macrophages and MCP-1 was decreased by 64%, 68% and 68%, respectively, whereas the intima/media ratio was only reduced by 13%. The molecular mechanism underlying these anti-inflammatory vascular effects may well be the capacity of ezetimibe to suppress NF-κB activity, a transcriptional regulator of inflammatory proteins, such as MCP-1. In this context, we have shown that the administration of ezetimibe lowered PBL NF-κB activity with respect to untreated rabbits, to a level similar to that displayed by simvastatin-treated and normolipidemic diet groups. It had been previously reported that animals treated with simvastatin showed a significantly greater reduction of NF-κB activity than those in normolipidemic diet (Hernández-Presa et al., 2003). It is possible that this discrepancy could be due to the different diet protocol, as our control animals were never fed an atherogenic diet. Additionally, CRP plasma levels, found increased in untreated rabbits, were also reduced by ezetimibe. CRP, a classical plasma protein marker, is related to cardiovascular risk, and its reduction after cholesterol lowering is associated with improved clinical outcomes (Ridker et al., 2001; Libby and Aikawa, 2002; Ridker et al., 2005). There is also evidence indicating that high levels of CRP may be potentially atherogenic (Jialal et al., 2006). One important finding in our work is that there was not an association between CRP levels and cholesterol. In fact, ezetimibe decreased plasma CRP levels without changing plasma lipid profile.
Our results also showed that ezetimibe provided additional anti-inflammatory benefits when was administered in combination with simvastatin. Administration of ezetimibe plus simvastatin was associated with a more significant reduction in lipid and macrophage content within the plaque, as well as plasma CRP concentration, than each drug alone. It is well known that lesion size is not predictive of plaque stability in humans, and that inflammation is related to plaque instability (Libby and Aikawa, 2002). Although our data have been obtained in an experimental model of early accelerated atherosclerosis, which is not entirely representative of what occurs in humans, it is possible that the benefits of ezetimibe in the clinical practice could be due, at least in part, to the depletion of plaque lipids and macrophages, contributing to the plaque stabilization.
To elucidate the mechanism(s) by which ezetimibe could reduce plaque inflammation, we investigated whether ezetimibe could alter monocyte migration, measured as chemotaxis mediated by MCP-1, which is not only a marker of the development of atherosclerosis, but also of destabilization of atherosclerotic plaques. Our results showed that ezetimibe inhibited MCP-1-induced monocyte migration and that the inhibitory effect of the combination of ezetimibe and simvastatin was higher than those displayed by either drug given alone. Support for our results has come from the recent report of Kuhlencordt et al. (2009) who found that ezetimibe normalized vascular inflammation as it potently reduced vascular VCAM-1 protein expression and mononuclear cell infiltration in ApoE-deficient mice and in both ApoE-and eNOS-deficient mice. Interestingly, although this effect of ezetimibe was independent of eNOS function, previous studies have demonstrated that statins reduce the expression of several adhesion molecules most likely via increased eNOS expression and activity (Sasaki et al., 2003; Nachtigal et al., 2005), suggesting that combined therapy with both drugs could exert additive effects.
The anti-inflammatory signalling pathway of ezetimibe is unknown at present. However, APN/CD13, recently identified as a molecular target for ezetimibe (Kramer et al., 2005), induces chemotactic migration of leucocytes and can hydrolyse type IV collagen (Saiki et al., 1993; Riemann et al., 1997; Tani et al., 2000), supporting an important role for this membrane protein in leucocyte migration across blood vessels. In monocytes, it has been reported that APN/CD13 leads to the rapid activation of tyrosine kinases (Santos et al., 2000). Although ezetimibe decreased the surface expression of APN/CD13 in monocyte/macrophages, at present, we can only speculate about its role in ezetimibe-induced anti-inflammatory actions. Current experiments are being performed in our laboratory to study in detail the signal transmission pathway induced by ezetimibe in both monocytes and vascular cells.
The lack of synergism of the combined therapy on vascular lesions might be due, at least partially, to the chosen treatment duration or even the drug dose. Therefore, the effects of ezetimibe, alone or in combination, with other dose and/or a more prolonged schedule of drug administration should be further studied.
During the preparation of this manuscript, results from the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression trial have been reported (Kastelein et al., 2008). This study did not demonstrate significant differences in intima-media thickness between ezetimibe/simvastatin versus simvastatin alone over a 2 year period in patients with heterozygous familial hypercholesterolemia, a rare form of inherited high cholesterol levels that affects less than 0.2% of the population. To test if potential clinical benefits of ezetimibe are related to plaque stability, specifically designed clinical outcome studies are needed. Thus, conclusions regarding ezetimibe/statin combinations should not be drawn until the three large ongoing clinical outcome trials are completed within the next 2–3 years.
In conclusion, our data support beneficial effects of ezetimibe both on atherosclerosis progression and plaque stabilization and show additional anti-inflammatory benefits of its combination with simvastatin. Effects of ezetimibe on monocyte migration provide a potential mechanism of action in addition to its effects on lipids. Confirmation of this early evidence awaits the results of ongoing and future studies.
Acknowledgments
The authors thank Dr M Fuentes-Ferrer (Research Unit, Preventive Medicine Department, Hospital Clínico San Carlos) for his help with the statistical analysis and interpretation of the results. This work has been supported partially by research grants from Fondo de Investigación Sanitaria (07/0882), Spanish Network Research on Heart Failure (REDINSCOR, RD06/0003/0011) and Merck Sharp Dohme Spain. D.G.-G. is co-funded by Community of Madrid and the Spanish Ministry of Health-Human Resources Stabilization Program. M.L.G.-R. and P.M.-P. are fellows from Fundación Fernández-Cruz and Fundación Investigación Biomédica Hospital Clínico San Carlos respectively.
Glossary
Abbreviations:
- APN
aminopeptidase N
- CRP
C-reactive protein
- EEL
external elastic lamina
- EMSA
electrophoretic mobility shift assay
- FCS
fetal calf serum
- HDL-c
high-density lipoprotein cholesterol
- IEL
internal elastic lamina
- LDL-c
low-density lipoprotein cholesterol
- MCP-1
monocyte chemoattractant protein-1
- NF-κB
nuclear factor κB
- PBL
peripheral blood leucocyte
- PCR
polymerase chain reaction
- RT
reverse transcription
Conflict of interest
Professor Fernández-Cruz has received grants from Merck Sharp Dohme, AstraZeneca, Sanofi-Aventis and Pfizer, and served on the advisory board for AstraZeneca. Drs Gómez-Garre, Aragoncillo and Granados, and Ms González-Rubio and Muñoz-Pacheco have no additional relationships to report.
References
- Al-Shaer MH, Choueiri NE, Suleiman ES. The pivotal role of cholesterol absorption inhibitors in the management of dyslipidemia. Lipids Health Dis. 2004;3:22. doi: 10.1186/1476-511X-3-22. Available at: http://www.lipidworld.com/content/3/1/22 (DOI: 10.1186/1476-511X-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B, Dauzonne D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: chemistry, biological evaluations, and therapeutic prospects. Med Res Rev. 2006;26:88–130. doi: 10.1002/med.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–2038. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- Fenton RG, Kung HF, Longo DL, Smith MR. Regulation of intracellular actin polymerization by prenylated cellular proteins. J Cell Biol. 1992;117:347–356. doi: 10.1083/jcb.117.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firla B, Arndt M, Frank K, Thiel U, Ansorge S, Täger M, et al. Extracellular cysteines define ectopeptidase (APN, CD13) expression and function. Free Radic Biol Med. 2002;32:584–595. doi: 10.1016/S0891-5849(01)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Garre D, Martín-Ventura JL, Granados R, Sancho T, Torres R, Ruano M, et al. Losartan improves resistance artery lesions and prevents CTGF and TGF-beta production in mild hypertensive patients. Kidney Int. 2006a;69:1237–1244. doi: 10.1038/sj.ki.5000034. [DOI] [PubMed] [Google Scholar]
- Gómez-Garre D, Herraíz M, González-Rubio ML, Bernal R, Aragoncillo P, Carbonell A, et al. Activation of peroxisome proliferator-activated receptor-alpha and-gamma in auricular tissue from heart failure patients. Eur J Heart Fail. 2006b;8:154–161. doi: 10.1016/j.ejheart.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hernández-Presa MA, Bustos C, Ortego M, Tuñón J, Renedo G, Ruiz-Ortega M, et al. Angiotensin-converting enzyme inhibition prevents arterial NF-κB activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- Hernández-Presa MA, Ortego M, Tuñón J, Martín-Ventura JL, Mas S, Blanco-Colio LM, et al. Simvastatin reduces NF-kappaB activity in peripheral mononuclear and in plaque cells of rabbit atheroma more markedly than lipid lowering diet. Cardiovasc Res. 2003;57:168–177. doi: 10.1016/s0008-6363(02)00619-3. [DOI] [PubMed] [Google Scholar]
- Jialal I, Devaraj S, Singh U. C-reactive protein and the vascular endothelium: implications for plaque instability. J Am Coll Cardiol. 2006;47:1379–1381. doi: 10.1016/j.jacc.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, Jähne G, et al. Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. J Biol Chem. 2005;280:1306–1320. doi: 10.1074/jbc.M406309200. [DOI] [PubMed] [Google Scholar]
- Kuhlencordt PJ, Padmapriya P, Rützel S, Schödel J, Hu K, Schäfer A, et al. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis. 2009;202:48–57. doi: 10.1016/j.atherosclerosis.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- Mäki-Petäjä KM, Booth AD, Hall FC, Wallace SM, Brown J, McEniery CM, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852–858. doi: 10.1016/j.jacc.2007.04.076. [DOI] [PubMed] [Google Scholar]
- Moreno PR, Lodder RA, Purushothaman KR, Charash WE, O'Connor WN, Muller JE. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation. 2002;105:923–927. doi: 10.1161/hc0802.104291. [DOI] [PubMed] [Google Scholar]
- Nachtigal P, Kopecky M, Solichova D, Zdansky P, Semecky V. The changes in the endothelial expression of cell adhesion molecules and iNOS in the vessel wall after the short-term administration of simvastatin in rabbit model of atherosclerosis. J Pharm Pharmacol. 2005;57:197–203. doi: 10.1211/0022357055353. [DOI] [PubMed] [Google Scholar]
- Orsó E, Werner T, Wolf Z, Bandulik S, Kramer W, Schmitz G. Ezetimib influences the expression of raft-associated antigens in human monocytes. Cytometry A. 2006;69:206–208. doi: 10.1002/cyto.a.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. Pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 (PROVE IT-TIMI 22) investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Riemann D, Kehlen A, Thiele K, Lohn M, Langner J. Induction of aminopeptidase N/CD13 on human lymphocytes after adhesion to fibroblast-like synoviocytes, endothelial cells, epithelial cells, and monocytes/macrophages. J Immunol. 1997;158:3425–3432. [PubMed] [Google Scholar]
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sager PT, Capece R, Lipka L, Strony J, Yang B, Suresh R, et al. Effects of ezetimibe coadministered with simvastatin on C-reactive protein in a large cohort of hypercholesterolemic patients. Atherosclerosis. 2005;179:361–367. doi: 10.1016/j.atherosclerosis.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Saiki I, Fujii H, Yoneda J, Abe F, Nakajima M, Tsuruo T, et al. Role of aminopeptidase N (CD13) in tumor cell invasion and extracellular matrix degradation. Int J Cancer. 1993;54:137–143. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AN, Langner J, Herrmann M, Riemann D. Aminopeptidase N/CD13 is directly linked to signal transduction pathways in monocytes. Cell Immunol. 2000;201:22–32. doi: 10.1006/cimm.2000.1629. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Bharwani S, Jordan P, Joh T, Manas K, Warren A, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor pravastatin reduces disease activity and inflammation in dextran-sulfate induced colitis. J Pharmacol Exp Ther. 2003;305:78–85. doi: 10.1124/jpet.102.044099. [DOI] [PubMed] [Google Scholar]
- Srámek A, Bosch JG, Reiber JH, Van Oostayen JA, Rosendaal FR. Ultrasound assessment of atherosclerotic vessel wall changes: reproducibility of intima-media thickness measurements in carotid and femoral arteries. Invest Radiol. 2000;35:699–706. doi: 10.1097/00004424-200012000-00001. [DOI] [PubMed] [Google Scholar]
- Sudhop T, Lütjohann D, Kodal A, Ige IM, Tribble DL, Shah S, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- Tani K, Ogushi F, Huang L, Kawano T, Tada H, Hariguchi N, et al. CD13/aminopeptidase N, a novel chemoattractant for T lymphocytes in pulmonary sarcoidosis. Am J Respir Crit Care Med. 2000;161:1636–1642. doi: 10.1164/ajrccm.161.5.9902008. [DOI] [PubMed] [Google Scholar]
- Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283:157–163. [PubMed] [Google Scholar]


