Abstract
Background and purpose:
N-arachidonoyl dopamine (NADA) has complex effects on nociception mediated via cannabinoid CB1 receptors and the transient receptor potential vanilloid receptor 1 (TRPV1). Anandamide, the prototypic CB1/TRPV1 agonist, also inhibits T-type voltage-gated calcium channel currents (ICa). These channels are expressed by many excitable cells, including neurons involved in pain detection and processing. We sought to determine whether NADA and the prototypic arachidonoyl amino acid, N-arachidonoyl glycine (NAGly) modulate T-type ICa
Experimental approach:
Human recombinant T-type ICa (CaV3 channels) expressed in HEK 293 cells and native mouse T-type ICa were examined using standard whole-cell voltage clamp electrophysiology techniques.
Key results:
N-arachidonoyl dopamine completely inhibited CaV3 channels with a rank order of potency (pEC50) of CaV3.3 (6.45) ≥ CaV3.1 (6.29) > CaV3.2 (5.95). NAGly (10 µmol·L−1) inhibited CaV3 ICa by approximately 50% or less. The effects of NADA and NAGly were voltage- but not use-dependent, and both compounds produced significant hyperpolarizing shifts in CaV3 channel steady-state inactivation relationships. By contrast with anandamide, NADA and NAGly had modest effects on CaV3 channel kinetics. Both NAGly and NADA inhibited native T-type ICa in mouse sensory neurons.
Conclusions and implications:
N-arachidonoyl dopamine and NAGly increase the steady-state inactivation of CaV3 channels, reducing the number of channels available to open during depolarization. These effects occur at NADA concentrations at or below to those affecting CB1 and TRPV1 receptors. Together with anandamide, the arachidonoyl neurotransmitter amides, NADA and NAGly, represent a new family of endogenous T-type ICa modulators.
Keywords: T-type calcium channel, NADA, anandamide, N-arachidonoyl glycine, nociception, endocannabinoid, endovanilloid, acyl amino acid, arachidonoyl amino acid
Introduction
Endogenous compounds consisting of arachidonic acid conjugated with amino acids or neurotransmitter amines are a large class of molecules found in many tissues including brain and spinal cord (Huang et al., 2001; 2002; Milman et al., 2006; Saghatelian et al., 2006; Rimmerman et al., 2008). While details of their synthesis and metabolism are only beginning to emerge, several of these compounds have been shown to have profound effects on nociception (Burstein et al., 2000; Huang etal., 2001; 2002), inflammation (Burstein et al., 2007) and vascular function (O'Sullivan et al., 2004; Milman et al., 2006) following systemic administration or incubation with tissue in vitro. The molecular targets of these compounds are also beginning to be defined, and in some respects they resemble those of the endogenous cannabinoid agonist, anandamide (AEA) (N-arachidonoyl ethanolamide). Thus, N-arachidonoyl dopamine (NADA) is an agonist at cannabinoid CB1 receptors (Bisogno et al., 2000), transient receptor potential vanilloid receptor 1 (TRPV1) receptors (Huang et al., 2002) and a weak inhibitor of a major AEA hydrolysing enzyme, fatty acid amide hydrolase (FAAH, Bisogno et al., 2000). Intriguingly, the N-arachidonoyl amide most closely related to AEA, N-arachidonoyl glycine (NAGly), has very low affinity for CB1 receptors (Sheskin et al., 1997), modest inhibitory potency at FAAH (Huang et al., 2001) and negligible activity at TRPV1 receptors (De Petrocellis et al., 2000). It is, however, the prototypic agonist for the orphan G protein coupled receptor, GPR 18 (Kohno et al., 2006), and an agonist of another orphan receptor, GPR 92 (Oh et al., 2008).
The mechanisms through which NADA and NAGly modulate nociception remain incompletely understood. NADA has pro- and anti-nociceptive effects, depending on the route and site of administration (Bisogno et al., 2000; Huang et al., 2002). In different experiments, both pro- and anti-nociceptive effects have been explained on the basis of TRPV1 activation (Sagar et al., 2004; Huang and Walker, 2006), while some anti-nociceptive actions involve CB1 receptors (Sagar et al., 2004). There is also evidence for CB1/TRPV1-independent actions of NADA in sensory neurons (Price et al., 2004). The antinociceptive effects of NAGly are not sensitive to CB1 antagonists (Succar et al., 2007; Vuong et al., 2008), indicating that its main mechanism of action is unlikely to be elevation of endocannabinoids following inhibition of FAAH. Recent studies have described selective NAGly inhibition of the glycine transporter GlyT2A and complex effects on glycine receptors (Wiles et al., 2006; Yang et al., 2008), both of which could conceivably contribute to anti-nociception. As part of a search for possible sites of action relevant to the anti-nociceptive effects of NADA and NAGly, we examined the effects of these compounds on T-type calcium channels, low-voltage-activated channels that have an important role in sensory processing and are an emerging target for analgesics (Shin et al., 2008). We report that NADA and NAGly robustly inhibit recombinant human and native mouse T-type calcium channels. The effects are broadly similar to those previously reported for AEA (Chemin et al., 2001; 2007), but quite distinct from the effects of NADA and NAGly on high-voltage-activated, N-type calcium channels (Guo et al., 2008).
Methods
Cell culture
HEK 293 cells stably transfected with plasmids containing cDNA for the human CaV3.1, CaV3.2 or CaV3.3 (Cribbs etal., 1998; 2000; Gomora et al., 2002, Ross et al., 2008) were cultivated in Dulbecco's modified Eagle's Medium supplemented with 100 U penicillin, 100 µg streptomycin, 10% fetal bovine serum or donor bovine serum and 1 mg·mL−1 G418 (Invitrogen, Mt. Waverly, Australia).
Isolation of sensory neurons
All animal procedures were approved by the Royal North Hospital Animal Care and Ethics Committee. Male C57Bl6 mice at least 8 weeks old were anaesthetized with isofluorane, decapitated and the trigeminal ganglia removed. Adult mouse trigeminal ganglion neurons were isolated as described in Ross et al. (2008). Briefly, ganglia were placed in a modified HEPES-buffered saline (mHBS) containing (in mM): 130 NaCl, 2.5 KCl, 1.8 CaCl2, 10 MgCl2. 10 HEPES, 10 glucose (pH to 7.3 with NaOH, osmolarity 330 ± 5 mosmol). The ganglia were cut into pieces with iridectomy scissors and incubated in mHBS containing 20 U·mL−1 papain for 25 min at 37°C. The reaction was stopped with mHBS containing 1 mg·mL−1 BSA and 1 mg·mL−1 trypsin inhibitor (Type II-O). The tissue was then washed with mHBS and cells released by gentle trituration through fire-polished Pasteur pipettes. Cells were plated onto tissue culture dished and used within 8 h of isolation.
Electrophysiology
Voltage-gated calcium channel currents (ICa) in HEK 293 cells were recorded in the whole-cell configuration of the patch-clamp method (Hamill et al., 1981) at room temperature, unless otherwise noted (Ross et al., 2008). Dishes were perfused with HBS containing (in mmol·L−1): 140 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose (pH to 7.3, osmolarity = 330 ± 5 mosmol). For recording CaV3.1 and 3.2 currents, cells were bathed in an external solution containing (in mM): 140 tetraethylammonium chloride, 2.5 CsCl, 10 HEPES, 10 glucose, 1 MgCl2, 5 CaCl2 (pH to 7.3, osmolarity = 330 ± 5 mosmol). For recording CaV3.3 currents, 5 mmol·L−1 CaCl2 was replaced by 5 mmol·L−1 BaCl2 in the external solution (see Ross et al., 2008). Recordings were made with fire-polished borosilicate glass pipettes with resistance ranging from 2 to 3 MΩ. For recording CaV3.1 and 3.2 currents, the internal solution contained (in mmol·L−1): 130 CsCl, 10 HEPES, 2 CaCl2, 10 EGTA, 5 MgATP (pH to 7.3, osmolarity = 285 ± 5 mosmol). For recording of CaV3.3 currents, 10 mmol·L−1 EGTA was replaced by 10 mmol·L−1 BAPTA, and the concentration of MgATP was reduced to 1 mmol·L−1. Recordings were made with a HEKA EPC 10 amplifier with Patchmaster acquisition software (HEKA Elektronik, Lambrecht/Pfalz, Germany), an Axopatch 1D amplifier using pClamp 5 software (Molecular Devices, Sunnyvale, CA, USA) and an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) using AxoGraph X software (http://axographx.com/). Data were sampled at 5–20 kHz, filtered at 2 kHz and recorded on a hard disk for later analysis. Series resistance ranged from three to 10 MΩ, and was compensated by at least 80% in all experiments. Leak subtraction using a P over 4 protocol (with 10 mV test steps) was used for some experiments where cells were being stepped to a single potential, but it was not employed for experiments where more complex waveforms were applied to the cells (e.g. inactivation). Uncompensated leak in these latter experiments did not exceed −30 pA at −106 mV, and cells with a leak current of greater than −30 pA were not used for any experiment. Cells were exposed to drugs via flow pipes positioned approximately 200 µm from the cell.
Recordings from trigeminal ganglion sensory neurons were made as outlined in Borgland et al. (2001). The solutions were the same as those outlined above, except that T-type ICa were recorded in an external TEA solution containing 2.5 mmol·L−1 Ca2+ and 1 mmol·L−1 Mg2+. Other membrane currents were recorded using HBS as the external solution. Recordings were made from small to medium sized cells (<25 µm diameter) which were identified as Type 1 or Type 2 cells from their ICa signatures derived from a current–voltage protocol, as outlined in Borgland et al. (2001). Type 1 neurons express little or no T-type ICa (measured at a test potential of −40 mV) and typically express TRPV1 and µ-opioid receptors. Type 2 neurons have significant ICa at a test potential of −40 mV, do not express TRPV1 and are not sensitive to µ-opioid agonists.
Effects of drug vehicle (ethanol)
Drugs were kept in concentrated stock solutions in ethanol and stored at −20°C. Daily dilutions from these stocks were made; the final ethanol concentration in all solutions was 0.1%. Ethanol at this concentration did not significantly affect the properties of the CaV3 channels. After 10 min recording in control conditions or ethanol (0.1%), the V50 for activation for the CaV3 channels was not different: CaV3.1: 43 ± 1 mV, 43 ± 1 mV; CaV3.2: 40 ± 2 mV, 39 ± 2 mV; CaV3.3: −37 ± 1 mV, −35 ± 2 mV (n = 5 to 9 cells for each condition). The amplitudes of the currents elicited by stepping from −106 mV to −26 mV were also similar after 10 min in control conditions and 10 min in ethanol (0.1%): CaV3.1: −642 ± 115 pA, −742 ± 150 pA; CaV3.2: −331 ± 74 pA, −258 ± 77 pA; CaV3.3: −1.82 ± 0.4 nA, −2.02 ± 0.4 nA (n = 5 to 9 cells for each condition). Acute application of ethanol (0.1%) inhibited CaV3.1 channels activated by stepping from −86 mV to −26 mV by 1 ± 1% (n = 12).
Data analysis
Concentration–response (ICa) curves were generated by fitting data to a sigmoidal dose–response function in GraphPad Prism 4. Steady-state activation curves were generated from current–voltage relationships, while steady-state inactivation curves were generated by measuring the peak current from a 50 ms step to −26 mV following a series of 5 s steps ranging from potentials of −126 mV to −46 mV. Reported potentials are corrected for a junction potential of −6 mV. Activation curves were generated by fitting data to a Boltzmann sigmoidal function Y = 1/(1 + e((V0.5−Vm)/Slope)). Inactivation curves were generated by fitting data to a Boltzmann sigmoidal function Y = 1 − 1/(1 + e((V0.5−Vm)/Slope)).
Statistical significance for comparing the V0.5 values of activation and inactivation was determined using unpaired t-tests comparing values of V0.5 calculated for individual experiments. In order to compare the changes in the time constant of inactivation and deactivation, two-way anova was used with a Bonferroni post-test to compare values at different potentials.
Materials
N-arachidonoyl dopamine, NAGly and AEA were obtained from either Alexis Biochemicals (Lausen, Switzerland), Biomol (Plymouth Meeting, PA, USA) or Cayman Chemical (Ann Arbor, MI, USA). Where possible, the same drug was purchased from several sources. In all cases, results were similar with drugs purchased from different suppliers. Papain was from Worthington, and all other drugs and chemicals were from Sigma Australia.
Drug and molecular target nomenclature conforms to the BJP Guide to Receptors and Channels (Alexander et al., 2008).
Results
N-arachidonoyl glycine is structurally very similar to the endocannabinoid AEA, differing only by having an additional oxygen molecule, that is, having a terminal carboxylic acid instead of alcohol. AEA is a reasonably potent inhibitor of T-type calcium channels (Chemin et al., 2001; 2007), so we examined the effects NAGly on T-type calcium channels in mouse trigeminal sensory neurons (Borgland et al., 2001). Superfusion of NAGly onto Type 2 trigeminal ganglion neurons inhibited the ICa evoked by a step from −86 mV to −46 mV (Figure 1). The highest concentration of NAGly testable (30 µmol·L−1) inhibited the currents at −46 mV by 80 ± 8% (n = 6), 10 µmol·L−1 NAGly inhibited the currents by 52 ± 7%. As the low-voltage-activated calcium currents in native sensory neurons are likely to represent a mixed population of channels, we examined the effects of NAGly and the CB1/TRPV1 agonist NADA on recombinant human CaV3 channels stably expressed in HEK293 cells.
Figure 1.
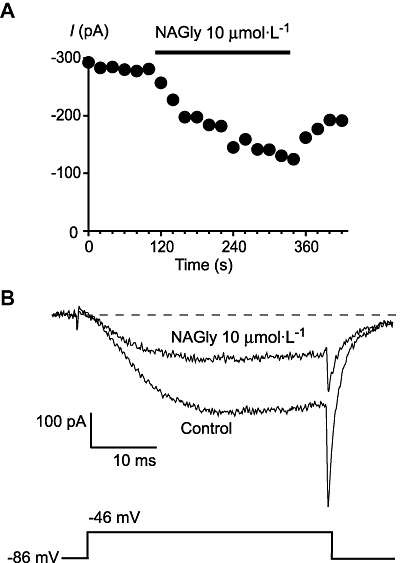
N-arachidonoyl glycine (NAGly) inhibits native T-type calcium channels. Whole-cell patch clamp recordings were made from acutely isolated mouse trigeminal ganglion neurons. Currents were evoked by stepping from −86 mV to −46 mV. (A) Time plot of the peak amplitude of the ICa at −46 mV, illustrating the effects of 10 µmol·L−1 NAGly (perfused for the duration of the bars). (B) Example traces from the above cell in the absence and presence of NAGly. The dotted line represents zero current. ICa, voltage-gated calcium channel current.
N-arachidonoyl glycine and NADA inhibited each of the human CaV3 subtypes (Figures 2 and 3). The inhibitory effects of lower concentrations of drug on CaV3 channels could be at least partially reversed by washing. At a concentration of 10 µmol·L−1 or 30 µmol·L−1, NADA completely inhibited each of the CaV3 channels, while NAGly was considerably less effective, with about 50% or less inhibition of each channel at a concentration of 10 µmol·L−1 (Figure 4). The potency of NADA and NAGly inhibition of CaV3 channels was determined by superfusing single concentrations of drug onto cells repetitively stepped from −86 mV to −26 mV. NADA inhibited CaV3.1 with pEC50 of 6.29 ± 0.03, CaV3.2 with a pEC50 of 5.95 ± 0.02 and CaV3.3 with a pEC50 of 6.45 ± 0.02. The endogenous compound N-oleoyl dopamine (18 : 1ω9, 10 µmol·L−1, Chu et al., 2003) inhibited CaV3 channels to similar degree as NADA (10 µmol·L−1), but the unsaturated N-palmitoyl dopamine (C16, 10 µmol·L−1) was much less effective (Figure 3). NAGly inhibited CaV3.1 channels with a notional EC50 of 16 µmol·L−1, but the highest concentration tested (30 µmol·L−1) did not completely inhibit the channels (Figure 4).
Figure 2.
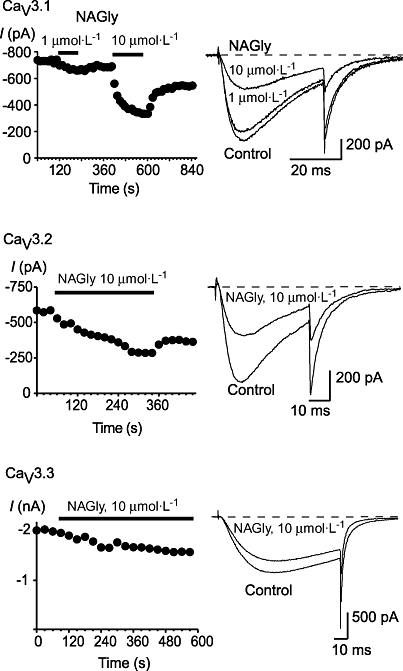
N-arachidonoyl glycine (NAGly) inhibits recombinant human T-type calcium channels. Whole-cell patch clamp recordings were made from human CaV3.1, CaV3.2 and CaV3.3 channels stably expressed in HEK 293 cells. Currents were evoked by stepping from −86 mV to −26 mV. The effect of NAGly on each of CaV3.1, CaV3.2 and CaV3.3. are illustrated, with a representative time plot and example traces. Each trace is an example of at least six similar experiments. The dotted line represents zero current.
Figure 3.
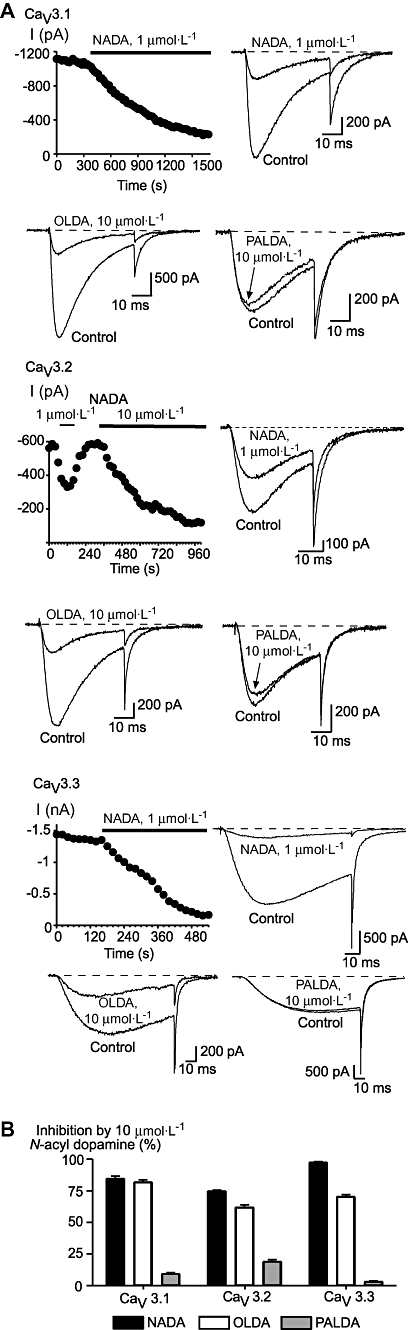
N-acyl dopamine compounds inhibit recombinant human T-type calcium channels. Whole-cell patch clamp recordings were made from human CaV3.1, CaV3.2 and CaV3.3 channels stably expressed in HEK 293 cells. Currents were evoked by stepping from −86 mV to −26 mV. (A) The effect of N-arachidonoyl dopamine (NADA) N-oleoyl dopamine (OLDA) and N-palmitoyl dopamine (PALDA) on each of CaV3.1, CaV3.2 and CaV3.3. are illustrated, with a time plot for the effects of NADA. (B) A summary of the effects of 10 µmol·L−1 of each of the N-acyl dopamines on each of the recombinant human CaV3 channels. The bars are the mean ± SEM of 6–9 cells for each drug on each channel. The dotted line represents zero current.
Figure 4.
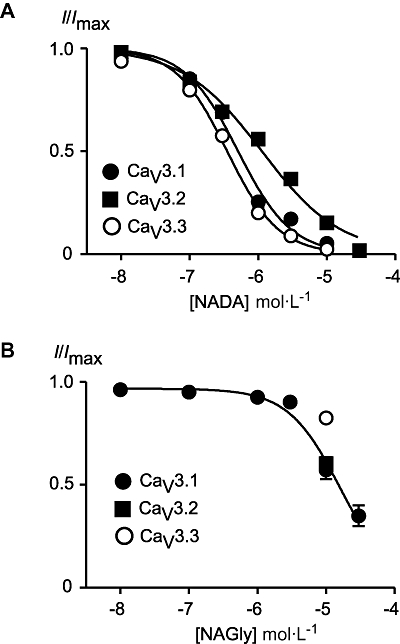
Concentration–response plots for N-arachidonoyl dopamine (NADA) and N-arachidonoyl glycine (NAGly) inhibition of CaV3 channels. Whole-cell patch clamp recordings were made from human CaV3.1, CaV3.2 and CaV3.3 channels stably expressed in HEK 293 cells. Currents were evoked by stepping from −86 mV to −26 mV. A single concentration of drug was superfused over each cell, each point represents the mean ± SEM of at least six cells. (A) NADA inhibits CaV3 channels with a rank order of CaV3.3 (350 nmol·L−1) ≥ CaV3.1 (500 nmol·L−1) > CaV3.2 (1.13 µmol·L−1). (B) Concentration-response plot for NAGly inhibition of CaV3.1. The highest concentration of NAGly tested was 30 µmol·L−1, assuming complete inhibition the notional EC50 was 16 µmol·L−1. The inhibition of CaV3.2 and CaV3.3 by 10 µmol·L−1 NAGly is plotted for comparison.
We further examined the interactions of NADA and NAGly on CaV3.1 by examining whether inhibition of the channel was use-dependent or influenced by the membrane potential at which the cell was voltage clamped during the experiment. The amount and macroscopic time-course of inhibition of CaV3.1 were the same for NADA (300 nmol·L−1) and NAGly (10 µmol·L−1) whether the currents were evoked at 1 Hz or 0.05 Hz (Figure 5). However, the amount of inhibition by NADA (300 nmol·L−1) and NAGly (10 µmol·L−1) was strongly influenced by the holding potential of the cell, with significantly greater inhibition at −86 mV than −126 mV (Figire 5). Inclusion of the competitive inhibitor of G protein activation, GDPβS (1.2 mmol·L−1), failed to affect the inhibition of CaV3.1 by NADA (300 nmol·L−1; 50 ± 5% in GTP vs. 49 ± 6% in GDPβS, n = 6 each) or NAGly (10 µmol·L−1; 52 ± 2% in GTP vs. 53 ± 8% in GDPβS, n = 6 each) applied 10 min after breaking into the cells.
Figure 5.
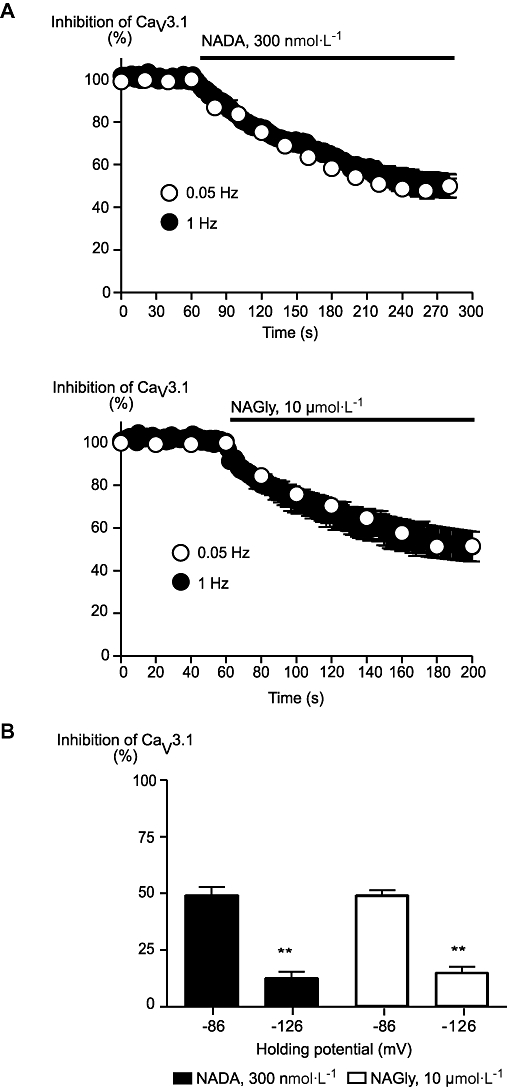
Inhibition of CaV3.1 by N-arachidonoyl dopamine (NADA) and N-arachidonoyl glycine (NAGly) was voltage- but not use-dependent. Whole-cell patch clamp recordings were made from human CaV3.1 channels stably expressed in HEK 293 cells. (B) The time course of inhibition of CaV3.1 currents by NADA (300 nmol·L−1) and NAGly (10 µmol·L−1) were similar when currents were evoked by stepping from −86 mV to −26 mV at 1 Hz or 0.05 Hz. (B) The inhibition of CaV3.1 by NADA (300 nmol·L−1) and NAGly (10 µmol·L−1) was significantly less when cells were voltage clamped at −126 mV and stepped to −26 mV than when cells were stepped to −26 mV from a holding potential of −86 mV. **P < 0.01 versus −86 mV, Student's t-test.
We compared the effects of NADA (500 nmol·L−1), NAGly (10 µmol·L−1) and AEA (300 nmol·L−1) on CaV3.1 channel kinetics by comparing the effects of 5 min applications of compound with time-matched controls. These concentrations of drug inhibited the peak current currents by about 50% (Figure 6). Currents were elicited from a holding potential of −106 mV and we measured the time to peak and time constant of channel inactivation from an open state. Data from test potentials of −46 mV (approximately 50% of channels active) and −26 mV (all channels open) are illustrated in (Figure 6). NAGly did not affect time to peak or inactivation from an open state at either potential, while NADA modestly accelerated inactivation from an open state at −26 mV (P < 0.05, Figure 6). AEA significantly accelerated open-state inactivation kinetics of CaV3.1 channels at both potentials (P < 0.01), and also decreased the time to peak at −46 mV, consistent with the previously reported effects of AEA on CaV3.1 (Chemin et al., 2001). NADA did not affect time to peak of CaV3.2 or CaV3.3 currents evoked from −106 mV. NADA or NAGly did not affect the time to peak or deactivation time constant of currents evoked from a holding potential of −86 to a test potential of −26 mV in any CaV3 channel (Table 1).
Figure 6.
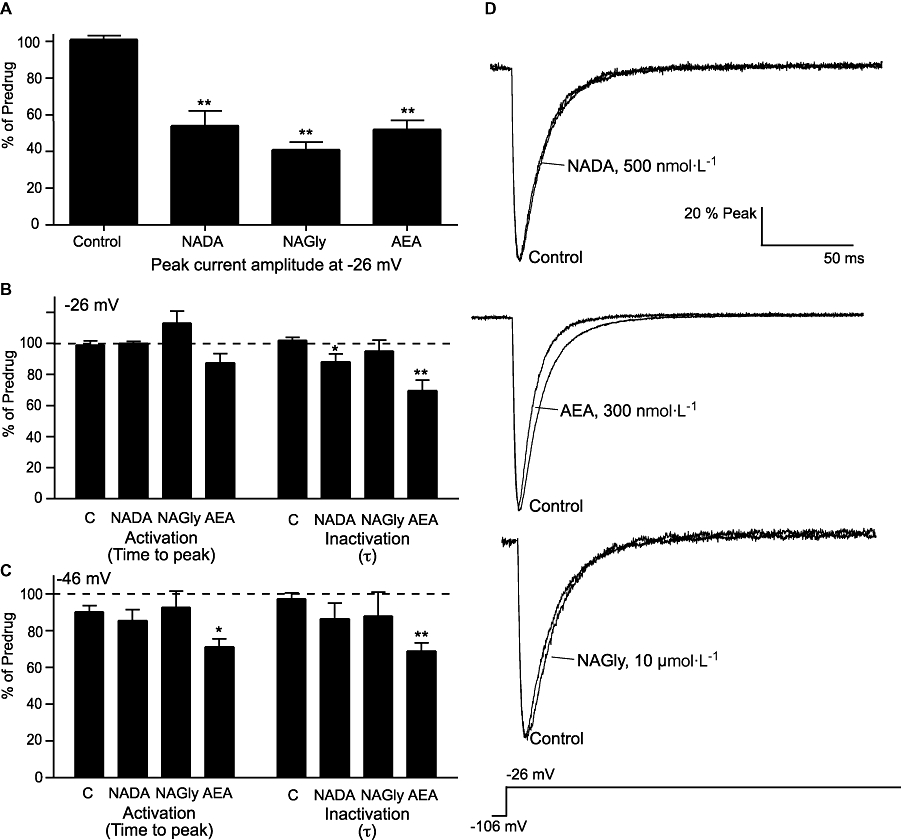
N-arachidonoyl dopamine (NADA) and N-arachidonoyl glycine (NAGly) do not strongly affect CaV3.1 channel kinetics. Whole-cell patch clamp recordings were made from human CaV3 channels stably expressed in HEK 293 cells. Channel activation from a holding potential of −106 mV was measured 5 min after breaking into the cell and then again after 5 min in NADA (500 nmol·L−1), NAGly (10 µmol·L−1) or anandamide (AEA, 300 nmol·L−1). The graphs illustrate: (A) current amplitude at −26 mV, the time to peak and time constant of inactivation at (B) −26 mV and (C) −46 mV. The values in drug are expressed as a percentage of the values at 5 min, control cells were continuously superfused with vehicle alone. Each bar represents the mean ± SEM of at least six cells. Statistical comparisons were made with control cells recorded on the same day (Student's t-test, *P < 0.05, **P < 0.01). Panel (D) illustrates typical currents elicited by a step from −106 mV to −26 mV recorded in control conditions and after 5 min in NADA, AEA and NAGly. Currents have been normalized to the peak inward current to allow ready comparison of inactivation kinetics.
Table 1.
The effects of NADA and NAGly on the kinetics of CaV3 channels
| CaV channel |
Time to peak (ms) |
Deactivation τ (ms) |
||||
|---|---|---|---|---|---|---|
| Control | NADA | NAGly | Control | NADA | NAGly | |
| 3.1 | 7.2 ± 0.3 | 6.2 ± 0.3 | 7.3 ± 0.3 | 3.2 ± 0.1 | 3.1 ± 0.1 | 3.2 ± 0.2 |
| 3.2 | 11.2 ± 0.4 | 11.5 ± 0.6 | 11.6 ± 0.4 | 3.1 ± 0.2 | 3.1 ± 0.2 | 3.1 ± 0.2 |
| 3.3 | 43 ± 1 | 44 ± 2 | 43 ± 2 | 2.4 ± 0.1 | 2.4 ± 0.2 | 2.3 ± 0.1 |
Cells expressing recombinant CaV3 channels were voltage clamped at −86 mV and then stepped to −26 mV. For examples of these experiments see Figures 2 and 3. The time to peak was measured directly and the decay of the current following repolarization of the membrane to −86 mV fit with a single exponential function. The concentration of NADA was 300 nmol·L−1 for CaV3.1 and CaV3.3 and 1 µmol·L−1 for CaV3.2. 10 µmol·L−1 NAGly was used for each channel. There were no differences in time to peak or deactivation for any current with either drug (paired t-test vs. predrug values for each cell). n = 6–8 for each condition.
NADA, N-arachidonoyl dopamine; NAGly, N-arachidonoyl glycine.
Effects of NADA and NAGly on channel activation and inactivation
AEA inhibits CaV3 channels in part by increasing steady-state inactivation and thus reducing the numbers of channels available to open during a depolarization. We examined whether the inhibition of CaV3 channels by NADA or NAGly could be due to effects on channel availability or activation. Activation curves were constructed by stepping cells from −106 mV to potentials between −86 and +59 mV, and then were repeated after 5 min perfusion of approximately EC50 concentrations of NADA (Figure 7), NAGly (Figure 8) and AEA (not shown). In the presence of the compounds there were small (2–3 mV) shifts in the potential at which half the channels were activated, these shifts were not different from those seen with time-matched vehicle controls (Table 2).
Figure 7.
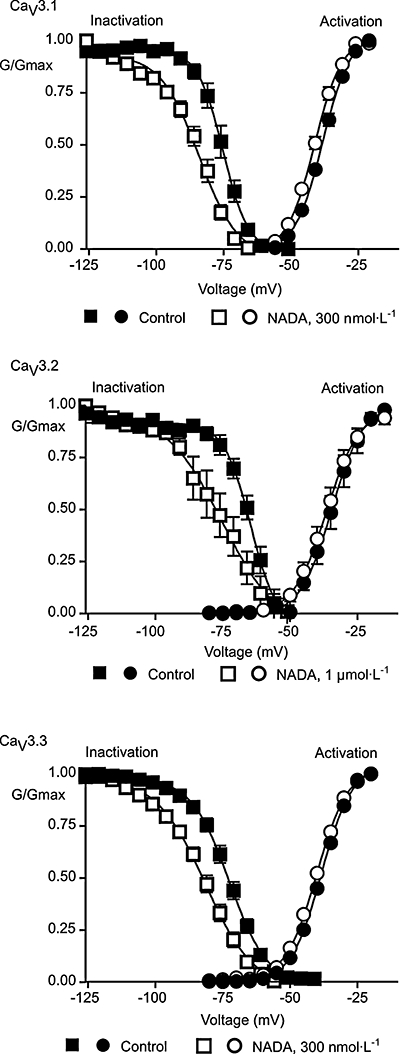
N-arachidonoyl dopamine (NADA) affects steady-state inactivation but not activation of CaV3 channels. Whole-cell patch clamp recordings were made from human CaV3 channels stably expressed in HEK 293 cells, 5 min after breaking into the cell and then again after 5 min in NADA. To measure channel activation, cells were voltage clamped at −106 mV and stepped to potentials above −86 mV in 5 mV increments. To measure steady-state inactivation, cells were voltage clamped for 5 s at potentials between −126 mV and −46 mV, and then stepped to a test potential of 26 mV. The peak current at each test potential is plotted for activation curves, the current at −26 mV following 5 s at the indicated holding potential is plotted for inactivation. Curves are a Boltzmann fit of the data (see Methods). NADA did not affect activation, but produced a significant hyperpolarizing shift in the membrane at which 50% of the channels are inactivated for each CaV3 subtype (Table 2).
Figure 8.

N-arachidonoyl glycine (NAGly) affects steady-state inactivation but not activation of CaV3.1. Whole-cell patch clamp recordings were made from human CaV3.1 channels stably expressed in HEK 293 cells, 5 min after breaking into the cell and then again after 5 min in NAGly (10 µmol·L−1). To measure channel activation, cells were voltage clamped at −106 mV and stepped to potentials above −86 mV in 5 mV increments. To measure steady-state inactivation, cells were voltage clamped for 5 s at potentials between −126 mV and −46 mV, and then stepped to a test potential of −26 mV. The peak current at each test potential is plotted for the activation curve, the current at −26 mV following 5 s at the indicated holding potential is plotted for inactivation. Curves are a Boltzmann fit of the data (see Methods). NAGly did not affect activation, but produced a significant hyperpolarizing shift in the membrane at which 50% of the CaV3.1 channels are inactivated (Table 2).
Table 2.
The effects of NADA, NAGly and anandamide (AEA) on the parameters of steady-state activation and inactivation of CaV3 channels
| Drug | CaV channel |
Channel V0.5 (mV) |
|
|---|---|---|---|
| Activation | Inactivation | ||
| 300 nmol·L−1 NADA | 3.1 | −3 ± 0.3 | −11 ± 1** |
| 1 µmol·L−1 NADA | 3.2 | −2.5 ± 2 | −10 ± 3** |
| 300 nmol·L−1 NADA | 3.3 | −1 ± 0.3 | −10 ± 1** |
| 10 µmol·L−1 NAGly | 3.1 | 1 ± 1 | −8.5 ± 1** |
| 300 nmol·L−1 AEA | 3.1 | −2.7 ± 0.5 | ND |
| No drug | 3.1 | −2 ± 1 | −2 ± 3 |
| No drug | 3.2 | 1 ± 2 | −2 ± 2 |
| No drug | 3.3 | −2 ± 2 | −2 ± 2 |
Cells expressing recombinant CaV3 channels were voltage clamped at −106 mV and then stepped to potentials above −86 mV (activation) or stepped for 5 s to potentials between −126 and −36 mV before stepping to the test potential of −26 mV. The resulting peak currents were fitted to a Boltzmann equation. Changes in the voltage for half activation/inactivation (V0.5) of the curves are reported. The “No drug” values represent time-dependent changes under our recording conditions. Curves for NADA and NAGly are illustrated in Figures 7 and 8. NADA, N-arachidonoyl dopamine; NAGly, N-arachidonoyl glycine; ND, not determined.
*P < 0.05,
P < 0.01 from control.
Steady-state inactivation was determined by holding cells at −106 mV and then stepping them for 5 s to test potentials between −126 mV and −51 mV before measuring the current following a step to −26 mV. This was repeated after 5 min in approximately EC50 concentrations of drug. NADA produced a significant hyperpolarizing shift in the membrane potential at which 50% of the channels were available for activation for all channels examined (Figure 7, Table 2). A similar effect was observed for NAGly (10 µmol·L−1) on CaV3.1 (Figure 8, Table 2). The shifts in steady-state inactivation in cells exposed to vehicle alone for 5 min were less than 2 mV (Table 2). The increase in steady-state inactivation is likely to make a major contribution to the inhibition of CaV3 channel currents by NADA and NAGly.
In small to medium sized mouse trigeminal ganglion neurons, T-type ICa and capsaicin responses are largely segregated into two different populations of neurons (Borgland et al., 2001; Roberts et al., 2002). Type 1 cells are usually sensitive to capsaicin but do not express T-type ICa while Type 2 cells express prominent T-type ICa but are insensitive to capsaicin. Superfusion of NADA (300 nmol·L−1) inhibited the ICa evoked by stepping Type 2 neurons from −86 mV to −46 mV by 30 ± 7% (Figure 9). NADA alone did not produce an inward current in Type 2 cells. NADA is also an agonist at TRPV1 receptors, and superfusion of NADA (300 nmol·L−1) produced modest inward currents in capsaicin-sensitive Type 1 cells, but not Type 1 cells insensitive to capsaicin (n = 6) (Figure 9). The NADA currents were 10 ± 6% of the size of the current produced by a subsequent application of high concentration of capsaicin (10 µmol·L−1, mean current 1.5 ± 0.5 nA, n = 7). NAGly (10 µmol·L−1) did not activate currents in capsaicin-sensitive Type 1 cells at room temperature (n = 6) and only produced a small inward current at 33°C in capsaicin-sensitive Type 1 cells (16 ± 8 pA, n = 6).
Figure 9.
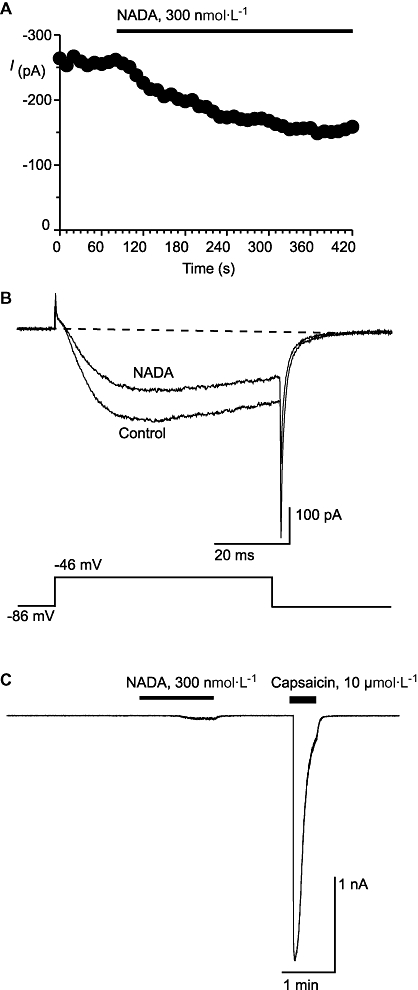
N-arachidonoyl dopamine (NADA) inhibits native T-type calcium channels and activates TRPV1. Whole-cell patch clamp recordings were made from acutely isolated mouse trigeminal ganglion neurons. Currents were evoked by stepping from −86 mV to −46 mV. (A) Time plot of the peak amplitude of the ICa at −46 mV, illustrating the effect of NADA (perfused for the duration of the bar). (B) Typical traces from the same cell as above in (A), in the absence and presence of NADA. (C) Example trace from a Type 1 sensory neuron voltage clamped at −60 mV and superfused with NADA and a maximally effective concentration of the TRPV1 agonist capsaicin. NADA (300 nmol·L−1) produced an inward current of, on average, 10 ± 6% of the capsaicin (10 µmol·L−1) current. The dotted line represents zero current. These traces are not leak subtracted. ICa, voltage-gated calcium channel current; TRPV1, transient receptor potential vanilloid receptor 1.
Discussion
The major finding of this study is that NADA inhibited T-type calcium channels with a similar potency to its agonist actions at native CB1 or TRPV1 receptors and with a similar or greater potency to the inhibitory effects of the prototypic endocannabinoid AEA at CaV3 channels (Chemin et al., 2001). At concentrations of 300 nmol·L−1 to 1 µmol·L−1, NADA strongly inhibited native and recombinant T-type calcium channels, while other studies have reported EC50 values of approximately 1 µmol·L−1 for NADA activation of native TRPV1 (Huang et al., 2002, Price et al., 2004) and CB1 receptors (Bisogno, et al., 2000). By contrast, NAGly, the prototypic arachidonoyl amino acid and close structural analogue of AEA, was a much weaker inhibitor of T-type calcium channels than AEA or NADA. Interestingly, unlike AEA or NADA, NAGly is also not a CB1 receptor ligand (Sheskin et al., 1997), has little activity at TRPV1 (De Petrocellis et al., 2000) and does not activate a recently described AEA-stimulated current in mouse trigeminal neurons (Roberts et al., 2008).
T-type calcium channels are involved in a wide range of physiological processes (Perez-Reyes, 2003, Shin et al., 2008), including many that are also modulated by activation of CB or TRPV1 receptors. For several reasons, it is difficult to assess the contribution of NADA modulation of T-type channels to the known effects of NADA administered to animals. First, the role of T-type channels cannot be readily dissected pharmacologically because of the lack of inhibitors selective for T-type channels over other voltage-gated calcium channels (Heady et al., 2001). Conversely, while there are neurobehavioural assays of nociception, sleep and seizure activity sensitive to known T-type channel inhibitors (Perez-Reyes, 2003; Shin et al., 2008), none of these assays are specific assays for T-type channel blockers. Second, many of the drugs used to antagonize the effects of NADA at CB1 receptors or TRPV1 also inhibit T-channels at pharmacologically relevant concentrations. These include the CB1 antagonists SR 141716A (Chemin et al., 2001) and AM251 (Ross et al., 2008) and the TRPV1 antagonist capsazepine, (Docherty et al., 1997; Connor, unpublished observations). Thus, while the reversal of a NADA effect by any of these agents provides good evidence for the involvement of the cognate protein, pre-treatment with these ligands will occlude any effects of subsequently administered NADA mediated by actions on T-type channels.
The effects of NADA and NAGly on CaV3 channel activity shared some but not all of the hallmarks of AEA modulation of the channel. All three ligands produce strong hyperpolarizing shifts in the membrane potential at which CaV3 channels inactivate, and this would have the effect of reducing the number of channels available to open from all but the most negative membrane potentials. This is a common mechanism for modulation of CaV3 channels, and is seen with arachidonic acid (Zhang et al., 2000; Talavera et al., 2004; Chemin et al., 2007) and cannabinoid ligands such as Δ9-tetrahydrocannabinol and cannabidiol (Ross et al., 2008). However, AEA (and arachidonic acid) both have effects on the kinetics of CaV3 channels, manifested as an acceleration of channel opening and open-state inactivation, with this latter effect further limiting calcium entry through CaV3 channels. Neither NADA nor NAGly strongly affected channel opening or open-state inactivation, which is similar to the effect of cannabidiol on CaV3 channels (Ross et al., 2008). NADA inhibition of CaV3 channels, particularly CaV3.3, was more potent than cannabidiol (EC50 approximately 800 nmol·L−1 for CaV3.1 and CaV3.2, 4 µmol·L−1 for CaV3.3) and Δ9-tetrahydrocannabinol (EC50 approximately 1 µmol·L−1 for CaV3.1 and CaV3.2, 4 µmol·L−1 for CaV3.3) recorded in identical conditions, (Ross et al., 2008) while that of NAGly was similar to that of arachidonic acid (Chemin et al., 2007). The rank order of potency for NADA effects on CaV3 channels (3.3 ≥ 3.1 > 3.2) is also distinct from that reported for AEA (3.2 > 3.3 > 3.1) (Chemin et al., 2001) and cannabidiol/Δ9-tetrahydrocannabinol (3.1 = 3.2 > 3.3) (Ross et al., 2008).
It is likely, but not proven, that NADA and NAGly were acting directly on the CaV3 channels. NADA has not been reported to be a ligand for any G protein coupled receptor other than the CB1 receptor and, while HEK 293 cells express mRNA for the NAGly-activated GPR 18 (Kohno et al., 2006, Johnson and Connor, unpublished observations), the inhibition of CaV3.1 by either compound was not sensitive to the non-specific inhibitor of G protein activation, GDPβS. Receptor- or second messenger-dependent modulation of T-type calcium channels is complex (Perez-Reyes, 2003) but we are not aware of any mechanisms described that produce inhibitory effects on all three isoforms of CaV3 channels that are similar to those observed in this study (Hildebrand et al., 2007; Iftinca et al., 2007; Tao et al., 2008).
The site(s) of action for fatty acids and their derivatives modulating CaV3 channels is not known and there is limited information about where drugs that modulate CaV3 channels could bind to affect channel function. Unsaturated fatty acids seem to be relatively non-selective inhibitors of CaV3 channels, and a hyperpolarizing shift in channel inactivation potential is commonly observed with lipid-soluble modulators of CaV3 (Heady et al., 2001; Ross et al., 2008) and other voltage-dependent channels (Lundbaek 2008). Amphiphiles which act in the plasma membrane to increase bilayer elasticity produce hyperpolarizing shifts in the inactivation of N-type calcium channels and voltage-gated sodium channels (Lundbaek et al., 1996; 2004), reminiscent of the effects of AEA, NADA and NAGly. The partial reversibility of NADA, NAGly and AEA (Chemin et al., 2001) are also consistent with an interaction mediated through the plasma membrane. However, the effects of AEA on bilayer lipid dynamics have been suggested to be minimal (Tian et al., 2005) and those of NADA and NAGly are unknown. Further evidence inconsistent with a major effect of NADA and NAGly on lipid elasticity, at the concentrations that inhibit CaV3 channels, comes from a study showing that NADA (10 µmol·L−1) had no effect on native N-type calcium channels while NAGly (10 µmol·L−1) enhanced N-channel activation (Guo et al., 2008). The differences in the absolute potency (up to 30-fold), differences in rank order of potency at CaV3 subtypes and the distinct effects on CaV3.1 kinetics of iso-inhibitory concentrations of AEA, NADA and NAGly suggest that a specific site mediates the effects of these compounds. This site seems to have a sufficiently well defined structure to be sensitive to the nature of the head group of the compounds and we suggest is likely to be part of the CaV3 channel itself.
In this study, we have shown that endogenous acyl amides are powerful inhibitors of T-type calcium channels, with potencies similar to that previously reported for their major sites of action, CB1 receptors and TRPV1 ion channels. T-type calcium channels, CB1 receptors and TRPV1 are often co-expressed, and thus at concentrations previously shown to modulate peripheral nociception (Chu et al., 2003; Sagar et al., 2004; Huang and Walker, 2006) and synaptic transmission (Marinelli et al., 2007), NADA is also likely to be strongly affecting T-type calcium channels in these tissues. However, confirmation that T-type calcium channel modulation contributes to the physiological or pharmacological effects of NADA and NAGly awaits the development of more selective T-type calcium channel modulators, or studies in CaV3 knockout animals.
Acknowledgments
This work was supported by National Health and Medical Research Council of Australia Project 402564 to MC. HR was supported by a University of Sydney postgraduate award and Kolling Institute award, AG was supported by a University of Sydney postgraduate award and Kolling Institute award. HR, AG and MC performed experiments, HR and MC wrote the paper. Thanks to Catherine Morris and Ann Rittenhouse for their helpful discussion of the membrane effects of arachidonic acid and related compounds.
Glossary
Abbreviations:
- FAAH
fatty acid amide hydrolase
- ICa
voltage-gated calcium channel current
- NADA
N-arachidonoyl dopamine
- NAGly
N-arachidonoyl glycine
- TRPV1
transient receptor potential vanilloid receptor 1
Conflict of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Melck D, Bobrov MY, Gretskaya NM, Bezuglov VV, De Petrocellis L, et al. N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351:817–824. [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Christie MJ. Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse. J Physiol. 2001;536:35–47. doi: 10.1111/j.1469-7793.2001.t01-1-00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Burstein SH, Adams JK, Bradshaw HB, Fraioli C, Rossetti RG, Salmonsen RA, et al. Potential anti-inflammatory actions of the elmiric (lipoamino) acids. Bioorg Med Chem. 2007;15:3345–3355. doi: 10.1016/j.bmc.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Perez-Reyes E, Nargeot J, Lory P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 2001;20:7033–7040. doi: 10.1093/emboj/20.24.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Nargeot J, Lory P. Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. J Biol Chem. 2007;282:2314–2323. doi: 10.1074/jbc.M610033200. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, et al. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- Cribbs LL, Gomora JC, Daud AN, Lee JH, Perez-Reyes E. Molecular cloning and functional expression of CaV3.1c, a T-type calcium channel from human brain. FEBS Lett. 2000;466:54–58. doi: 10.1016/s0014-5793(99)01756-1. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JD, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Piper AC. Capsazepine block of voltage-activated calcium channels in adult dorsal root ganglion neurones in culture. Br J Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomora JC, Murbartian J, Arias JM, Lee J-H, Perez-Reyes E. Cloning and expression of the human T-type channel CaV3.3: insights into prepulse facilitation. Biophys J. 2002;83:229–241. doi: 10.1016/s0006-3495(02)75164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Williams DJ, Ikeda SR. N-arachidonyl serine, a putative endocannabinoid, alters the activation of N-type calcium channels in sympathetic neurons. J Neurophysiol. 2008;100:1147–1151. doi: 10.1152/jn.01204.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heady TN, Gomora JC, MacDonald TL, Perez-Reyes E. Molecular pharmacology of T-type calcium channels. Jpn J Pharmacol. 2001;85:339–350. doi: 10.1254/jjp.85.339. [DOI] [PubMed] [Google Scholar]
- Hildebrand ME, David LS, Hamid J, Mulatz K, Garcia E, Zamponi GW, et al. Selective inhibition of CaV3.3 T-type calcium channels by Gαq/11-coupled muscarinic acetylcholine receptors. J Biol Chem. 2007;282:21043–21055. doi: 10.1074/jbc.M611809200. [DOI] [PubMed] [Google Scholar]
- Huang SM, Walker JM. Enhancement of spontaneous and heat-evoked activity in spinal nociceptive neurons by the endovanilloid/endocannabinoid N-arachidonyldopamine (NADA) J Neurophysiol. 2006;95:1207–1212. doi: 10.1152/jn.00395.2005. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Petros TJ, Chang S-Y, Zavitsanos PA, Zipkin RE, et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276:42639–42634. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, de Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftinca M, Hamid J, Chen L, Varela D, Tadayonnejad R, Altier C, et al. Regulation of T-type calcium channels by Rho-associated kinase. Nat Neurosci. 2007;10:854–860. doi: 10.1038/nn1921. [DOI] [PubMed] [Google Scholar]
- Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, et al. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347:827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- Lundbaek JA. Lipid bilayer-mediated regulation of ion channel function by amphiphilic drugs. J Gen Physiol. 2008;131:421–429. doi: 10.1085/jgp.200709948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek JA, Birn P, Girshman J, Hansen AJ, Andersen OS. Membrane stiffness and channel function. Biochemistry. 1996;35:3825–3830. doi: 10.1021/bi952250b. [DOI] [PubMed] [Google Scholar]
- Lundbaek JA, Birn P, Hansen AJ, Sogaard R, Nielsen C, Girshman J, et al. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of micelle-forming amphiphiles and cholesterol. J Gen Physiol. 2004;123:599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Florenzano F, Fezza F, Viscomi MT, van der Stelt M, et al. N-arachidonoyl-dopamine tune synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology. 2007;32:298–308. doi: 10.1038/sj.npp.1301118. [DOI] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Baktai S, et al. N-arachidonyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci USA. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA) Br J Pharmacol. 2004;141:803–812. doi: 10.1038/sj.bjp.0705643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Yoon JM, Moon MJ, Hwang J-I, Choe H, Lee JE, et al. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem. 2008;283:21054–21064. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neurons activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol. 2004;141:1118–1130. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmerman N, Bradshaw HB, Hughes HV, Chen JS-C, Hu SS-J, McHugh D, et al. N-Palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol Pharmacol. 2008;74:213–224. doi: 10.1124/mol.108.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LA, Christie MJ, Connor M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br J Pharmacol. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LA, Ross HR, Connor M. Methanandamide activation of a novel current in mouse trigeminal ganglion sensory neurons in vitro. Neuropharmacology. 2008;54:172–180. doi: 10.1016/j.neuropharm.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Ross HR, Napier I, Connor M. Inhibition of recombinant human T-type calcium channels by Δ9-Tetrahydrocannabinol and cannabidiol. J Biol Chem. 2008;283:16124–16134. doi: 10.1074/jbc.M707104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Smith PA, Millns PJ, Smart D, Kendall DA, Chapman V. TRPV1 and CB1 receptor-mediated effects of the endovanilloid/endocannabinoid N-arachidonoyl-dopamine on primary afferent fibre and spinal cord neuronal responses in the rat. Eur J Neurosci. 2004;20:175–184. doi: 10.1111/j.1460-9568.2004.03481.x. [DOI] [PubMed] [Google Scholar]
- Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45:9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the rat brain cannabinoid receptor. J Med Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- Shin H-S, Cheong E-J, Choi S, Lee J, Na HS. T-type calcium channels as therapeutic targets in the nervous system. Curr Opin Pharmacol. 2008;8:33–41. doi: 10.1016/j.coph.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Succar R, Mitchell VA, Vaughan CW. Actions of N-arachidonyl-glycine in a rat inflammatory pain model. Mol Pain. 2007;3:24. doi: 10.1186/1744-8069-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Staes M, Janssens A, Droogmans G, Nilius B. Mechanism of arachidonic acid modulation of the T-type calcium channel α1g. J Gen Physiol. 2004;124:225–238. doi: 10.1085/jgp.200409050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Hildebrand ME, Liao P, Liang MC, Tan G, Li S, et al. Activation of corticotrophin-releasing factor receptor 1 selectively inhibits CaV3.2 T-type calcium channels. Mol Pharmacol. 2008;73:1596–1609. doi: 10.1124/mol.107.043612. [DOI] [PubMed] [Google Scholar]
- Tian X, Guo J, Yao F, Yang D-P, Makriyannis A. The conformation, location and dynamic properties of the endocannabinoid ligand anandamide in a membrane bilayer. J Biol Chem. 2005;280:29788–29795. doi: 10.1074/jbc.M502925200. [DOI] [PubMed] [Google Scholar]
- Vuong LAQ, Mitchell VA, Vaughan CW. Actions of N-arachidonyl-glycine in a rat neuropathic pain model. Neuropharmacology. 2008;54:189–193. doi: 10.1016/j.neuropharm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Wiles AL, Pearlman RJ, Rosvall M, Aubrey K, Vandenberg RJ. N-arachidonyl glycine inhibits the glycine transporter, GLYT2a. J Neurochem. 2006;99:781–786. doi: 10.1111/j.1471-4159.2006.04107.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Aubrey KR, Alroy I, Harvey RJ, Vandenberg RJ, Lynch JW. Subunit-specific modulation of glycine receptors by cannabinoids and N-arachidonyl-glycine. Biochem Pharmacol. 2008;76:1014–1023. doi: 10.1016/j.bcp.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cribbs L, Satin J. Arachidonic acid modulation of α1H, a cloned human T-type calcium channel. Am J Physiol Heart Circ Physiol. 2000;278:H184–H193. doi: 10.1152/ajpheart.2000.278.1.H184. [DOI] [PubMed] [Google Scholar]


