Abstract
Background and purpose:
We investigated the effect of nitric oxide synthase (NOS) inhibition on polymorphonuclear cell (PMN) influx in zymosan or lipopolysaccharide (LPS)-induced arthritis and peritonitis.
Experimental approach:
Wistar rats received intra-articular (i.art.) zymosan (30–1000 µg) or LPS (1–10 µg). Swiss C57/Bl6 mice genetically deficient in intercellular adhesion molecule-1 (ICAM-1−/−) or in β2-integrin (β2-integrin−/−) received zymosan either i.art. or i.p. PMN counts, leukotriene B4 (LTB4), tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) levels were measured in joint and peritoneal exudates. Groups received the NOS inhibitors NG-nitro-L-arginine methyl ester (LN), nitro-L-arginine, N-[3-(aminomemethyl)benzyl] acetamide or aminoguanidine, prior to zymosan or LPS, given i.p. or s.c. in the arthritis and peritonitis experiments respectively. A group of rats received LN locally (i.art. or i.p.), 30 min prior to 1 mg zymosan i.art.
Key results:
Systemic or local NOS inhibition significantly prevented PMN migration in arthritis while increasing it in peritonitis, regardless of stimuli, concentration of NOS inhibitors and species. NOS inhibition did not alter TNF-α and IL-10 but decreased LTB4 in zymosan-induced arthritis. LN administration significantly inhibited PMN influx into the joints of ICAM-1−/− and β2-integrin−/− mice with zymosan-arthritis, while not altering PMN influx into the peritoneum of mice with zymosan-peritonitis.
Conclusions and implications:
Nitric oxide has a dual modulatory role on PMN influx into joint and peritoneal cavities that is stimulus- and species-independent. Differences in local release of LTB4 and in expression of ICAM-1 and β2-integrin account for this dual role of NO on PMN migration.
Keywords: Neutrophils, zymosan, arthritis, leukotrienes, cytokines, adhesion molecules
Introduction
Neutrophil migration into inflammatory sites is a crucial step in host defence, whereas inflammatory tissue damage is also associated with neutrophil accumulation. The release of proteolytic lysosomal enzymes, metalloproteinases and reactive oxygen/nitrogen-derived species mediate neutrophil damage to tissues. Therefore, strategies to limit neutrophil trafficking are positively associated with the clinical benefit obtained in inflammatory diseases, such as rheumatoid arthritis (O'Dell, 2004). The recruitment of neutrophils in arthritis is a multi-mediator process with the participation of leukotriene B4 (LTB4), the complement fragment C5a, cytokines and chemokines. We have previously shown that antigen-induced neutrophil migration requires the chemokines macrophage inflammatory protein (MIP)-1α and MIP-2 and CD4+ T-cell-derived tumour necrosis factor-α (TNF-α) acting through an LTB4-dependent mechanism (Ramos et al., 2006).
The events involved in neutrophil migration require interaction of reciprocal adhesion molecules present on neutrophils and endothelial cells. Rolling is mediated by E- and P-selectins (on endothelial cells) and L-selectin (on leukocytes) interacting with their respective carbohydrate ligands. Thereafter, adhesion and transmigration are mediated by the leukocyte β2-integrins, CD11a/CD18, CD11b/CD18 and CD11c/CD18, which interact with immunoglobulins, intercellular adhesion molecule-1 (ICAM-1), ICAM-2 and ICAM-3, while other integrins, very late antigen-4 (VLA-4) and α5β3 interact with vascular cell adhesion molecule-1 (VCAM-1) and platelet-endothelial cell adhesion molecule-1, present mainly on endothelial cells (Muller, 2003). These processes are regulated by a variety of mediators, including nitric oxide (NO) (Mulligan et al., 1998).
There are several reports showing that NO modulates neutrophil migration. Selective inhibition of both the inducible NOS (iNOS) and endothelial NOS isoforms increases neutrophil adhesion to endothelium, whereas NO donors decrease both adhesion and transmigration of leukocytes (Hickey and Kubes, 1997; Dal Secco et al., 2003). Moreover, NO was shown to down-regulate the expression of the adhesion molecules, MAC-1 (CD11b/CD18), selectins (P, L), ICAM-1 (CD54) and VCAM-1 (Lefer and Lefer, 1996; Spiecker et al., 1998; Sato et al., 1999; Dal Secco et al., 2006). In the peritoneum, we showed that NO released by both constitutive and iNOS depressed neutrophil recruitment that was partially due to a reduction in the apoptosis of emigrated neutrophils into the peritoneal cavity (Secco et al., 2003). Recently, we demonstrated that this effect is mediated via a soluble guanylate cyclase-dependent mechanism (Dal Secco et al., 2006).
There are controversies regarding the role of NO in the neutrophil migration into joints and peritoneal cavities. In the zymosan-induced peritonitis in mice genetically deficient for the iNOS gene (iNOS−/−), a slight increase in neutrophil recruitment was observed 1 h after injection of zymosan, as compared with wild-type mice. However, between 2 and 4 h, neutrophil migration was significantly decreased, which was coupled to a reduction of MIP-2 and interleukin-10 (IL-10) levels in the peritoneal exudate (Ajuebor et al., 1998). On the other hand, the accumulation of neutrophils in the synovium of mice subjected to zymosan-arthritis was similar in iNOS−/− and wild-type animals (van de Loo et al., 1998). In streptococcal cell wall-induced arthritis in rats, NOS inhibitors reduced neutrophil influx (McCartney-Francis et al., 2001). However, in ovalbumin-induced arthritis in rats, the NOS inhibitor, nitro-L-arginine, did not alter neutrophil influx (Bombini et al., 2004).
Tissue specificities, concentration of the mediator, animal species and inflammatory stimuli may all account for these apparent discrepancies regarding the role of these molecules in neutrophil recruitment. Herein, we demonstrate that NOS inhibition either decreases or increases neutrophil migration in acute arthritis or peritonitis, respectively, regardless of the concentration of NOS inhibitors, animal species and inflammatory stimuli. This was not influenced by the local release of TNF-α and IL-10. On the other hand, NOS inhibition significantly decreased LTB4 release into the joints in zymosan-arthritis, while not altering LTB4 levels in zymosan-peritonitis. In addition, differences in the local expression of the adhesion molecules β2-integrin and ICAM-1 also seem to be responsible for the phenomenon.
Methods
Animals
All animal procedures and these experimental protocols were approved by the local ethics committees on animal experimentation at the Faculty of Medicine both of the Federal University of Ceará and of the University of São Paulo, Ribeirão Preto-SP, Brazil. All efforts were made to minimize animal suffering and the number of animals used associated with valid statistical evaluation. Male Wistar rats (180–200 g) or male Swiss mice (25–30 g) (n = 6 per group) were provided by the central animal house of the Federal University of Ceará, Fortaleza-CE, Brazil. Experiments with C57/Bl6, mice genetically deficient for the β2-integrin (β2-integrin−/−) or for ICAM-1 (ICAM-1−/−) (18–20 g) (n = 6 per group) were carried out at the Department of Pharmacology of the Faculty of Medicine, University of São Paulo, Ribeirão Preto-SP, Brazil. Breeding pairs of mice with targeted disruption of the ICAM-1 and β2-integrin genes were obtained from Jackson Laboratories (Bar Harbor, ME, USA). They were housed in cages in temperature-controlled rooms with 12 h light/dark cycles and free access to food and water.
Induction of arthritis and peritonitis – assessment of cell counts and determination of LTB4, TNF-α and IL-10 levels
Rats received an intra-articular (i.art.) injection of either zymosan (30–1000 µg 50 µL−1 total volume) or lipopolysaccharide (LPS) from E. coli O111:B4 (1–10 µg in 50 µL total volume), dissolved in sterile saline, or saline (50 µL) into their right knee joints. Mice received i.art. injection of zymosan (30–100 µg in 25 µL total volume) or saline (25 µL) into their right knee joints. Other groups of rats received either 1000 µg zymosan or 10 µg LPS i.p. or saline and the mice groups received either 30–100 µg zymosan or saline i.p. The animals were terminally anesthetized (chloral hydrate 400 mg·kg−1 i.p.), killed by cervical dislocation and ex-sanguinated, either 4 or 6 h after injection of the stimuli, for the peritonitis or arthritis experiments respectively.
The articular cavities were then washed twice with 200 µL (rats) or 50 µL (mice) whereas the peritoneal cavities were washed with 7 mL (rats) or 2 mL (mice) of PBS containing 10 mmol·L−1 EDTA. The exudates were collected by aspiration for determination of total cell counts using a Neubauer chamber. After centrifuging (500× g for 10 min), the supernatants were stored for determination of LTB4, TNF-α and IL-10, using ELISA. Briefly, 96-well microtiter plates (Nunc Immunoplates) were coated overnight at 4°C with immunoaffinity-purified polyclonal antibodies against the respective cytokines. These antibodies were provided by Dr S Poole (National Institute for Biological Standards and Control, United Kingdom). After blocking the plates (1% albumin for 1 h), concentrations of cytokines and samples were loaded in duplicate for 2 h (22°C). A secondary rabbit biotinylated immunoaffinity-purified antibody was added, followed by incubation for 1 h (22°C). Finally, 100 µL of avidin-horseradish peroxidase (1:5000 dilution; DAKO A/S, Denmark) was added to each well; after 30 min, the plates were washed and the colour reagent o-phenylenediamine (40 µg·well−1) was added. After 15 min, the reaction was stopped with 1 mol·L−1 H2SO4 and the optical density was measured at 490 nm. Cytokine concentration was expressed as pg·mL−1.
Drug treatments
Evaluation of the dose-range, stimuli and various NOS inhibitors on the polymorphonuclear cell (PMN) influx into the joints or peritoneum
In an attempt to test the effect of systemic NOS inhibition on cell influx, the animals subjected to arthritis received the test compounds intra-peritoneally (i.p.) whereas those subjected to peritonitis received test compounds subcutaneously (s.c.). Groups of rats received the non-selective NOS inhibitors, NG-nitro-L-arginine methyl ester (LN 10–30 mg·kg−1) given either i.p. or s.c. for arthritis and peritonitis experiments, respectively, 30 min prior to injection of zymosan. Other groups received LN 1 mg·kg−1 i.art. or LN 10 mg·kg−1 i.p. prior to 1 mg zymosan, to evaluate the effect of local NOS inhibition.
Other NOS inhibitors tested included the non-selective NOS inhibitor NG-nitro-L-arginine (NA 50 mg·kg−1) or the selective iNOS inhibitors, aminoguanidine (AG 50 mg·kg−1) or N-[3-(aminomethyl)benzyl] acetamide (1400W: 1 mg·kg−1) given 30 min prior to the zymosan, either i.p. or s.c. for arthritis or peritonitis experiments respectively.
In an attempt to test the effect in another species, groups of mice received LN (30 mg·kg−1) i.p. or s.c. 30 min before injection of zymosan into the joints or the peritoneum respectively. The doses were chosen on the basis of previous experiments (Secco et al., 2003).
Statistical analysis
Results are expressed as the mean ± SEM. To compare the differences between means, we used one-way anova followed by Tukey's test. P < 0.05 was considered significant.
Chemicals and reagents
Most agents were purchased from Sigma (St. Louis, MO, USA). 1400W and the LTB4 ELISA kit were purchased from Cayman Chemical Co. (Ann Arbor, MI, USA).
Results
Systemic and local effects of NOS inhibition in the neutrophil migration in zymosan-induced arthritis and peritonitis in rats
In previous reports (Rocha et al., 1999; Benjamim et al., 2000), we have demonstrated that the cells obtained either in the peritoneal or joint exudates, 4 or 6 h after the injection of zymosan, respectively, are mostly neutrophils (≥90%).
The results of NOS inhibition, whether non-selective (LN or NA) or selective for iNOS (AG or 1400W) in zymosan-induced arthritis and peritonitis in rats are shown in Figure 1. NOS inhibition significantly decreased neutrophil migration into the joints of rats subjected to zymosan-induced arthritis (Figure 1A), regardless of the zymosan concentration. Also, local (i.art.) administration of LN significantly reduced the neutrophil influx in the zymosan-induced-arthritis (Figure 1B). On the other hand, NOS inhibition significantly increased cell migration into the peritoneal cavity of rats after injection of 1 mg zymosan, whether LN was given systemically (Figure 1C) or locally (Figure 1D).
Figure 1.
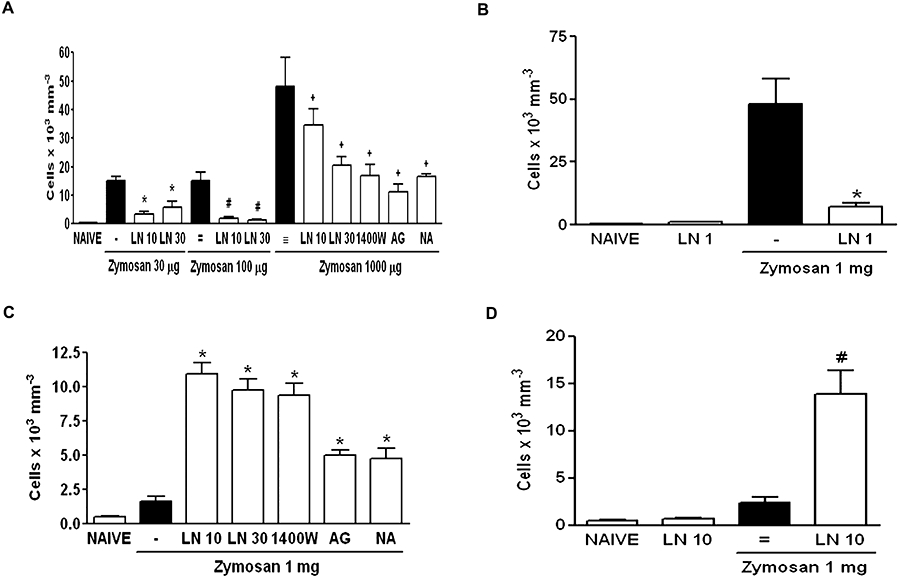
Systemic and local effect of NOS inhibition in the neutrophil migration in zymosan (Zy)-induced arthritis and peritonitis in rats. For systemic experiments, rats received either (30–1000 µg) zymosan intra-articular (i.art.), 1 mg zymosan i.p. or saline. LN (10–30 mg·kg−1), 1400W (1 mg·kg−1), aminoguanidine (AG − 50 mg·kg−1) or nitro-L-arginine (NA − 50 mg·kg−1) were injected i.p. or s.c. in arthritis and peritonitis, respectively, 30 min before the zymosan. For local experiments, rats received 1 mg LN i.art. or 10 mg LN i.p. 30 min prior to the injection of 1 mg zymosan i.art. or i.p. respectively; (A,B) and (C,D) represent systemic and local effect of NOS inhibition in arthritis and peritonitis respectively. Non-treated (bars marked -, =, ≡) rats were given saline (i.p. or s.c. for arthritis and peritonitis respectively) 30 min prior to zymosan. For local experiments, non-treated (-, =) animals were given i.art. or i.p. saline prior to zymosan. Naïve animals received only saline i.art or i.p. Results are expressed as the mean ± SEM of number of cells for each group of six animals. *P < 0.05 compared with non-treated (-); #P < 0.05 compared with non-treated (=); +P < 0.01 compared with non-treated (≡). 1400W, N-[3-(aminomemethyl)benzyl] acetamide; AG, aminoguanidine; i.art., intra-articular; LN, NG-nitro-L-arginine methyl ester; NA, NG-nitro-L-arginine; NOS, nitric oxide synthase.
Systemic effect of NOS inhibition in the neutrophil influx in endotoxin (LPS)-induced arthritis and peritonitis in rats
Inasmuch as the effect of LN was similar over a large dose-range, we evaluated the results using another stimulus. LN administration dose-dependently and significantly decreased neutrophil influx into the joint exudates of rats subjected to LPS-induced arthritis, regardless of the dose of LPS used (Figure 2A). Additionally, LN significantly increased neutrophil migration into the peritoneal cavity of rats subjected to LPS-induced peritonitis (Figure 2B).
Figure 2.
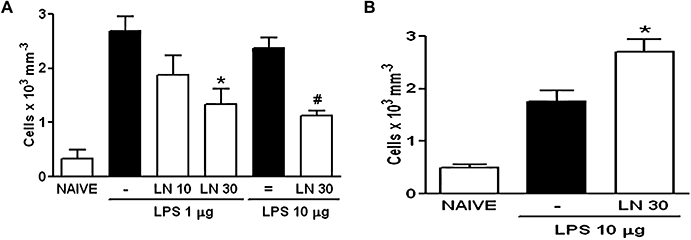
Systemic effect of NOS inhibition in the neutrophil influx in lipopolysaccharide (LPS)-induced arthritis and peritonitis in rats. Rats received i.p. or s.c. LN (10–30 mg·kg−1) 30 min prior to i.art. or i.p. LPS (1–10 µg) respectively. Non-treated (-, =) animals received i.p. or s.c. saline prior to LPS. Naïve animals received only saline; (A) and (B) represent data from arthritis and peritonitis models respectively. Results are expressed as the mean ± SEM of number of cells for each group of six animals. *P < 0.05 compared with non-treated (-); #P < 0.001 compared with non-treated (=). LN, NG-nitro-L-arginine methyl ester; NOS, nitric oxide synthase.
Analysis of LTB4, TNF-α and IL-10 levels in the exudates of zymosan-induced arthritis and peritonitis in rats
The exudates collected from the animals used for cell influx studies, shown in Figure 1, were used for assessment of LTB4, TNF-α and IL-10 levels. Figure 3A shows that the LTB4 levels in the joint exudates were significantly reduced when the animals with zymosan-arthritis were pretreated with the non-selective NOS inhibitor, LN. However, LN pretreatment did not alter LTB4 levels in the peritoneal cavity of rats subjected to zymosan-peritonitis. The levels in naïve animals were below detection limits. Neither TNF-α nor IL-10 levels in both joint and peritoneal cavities of rats with zymosan-arthritis or peritonitis were significantly altered by the administration of LN 30 min prior to the zymosan (Figure 3B,C respectively).
Figure 3.
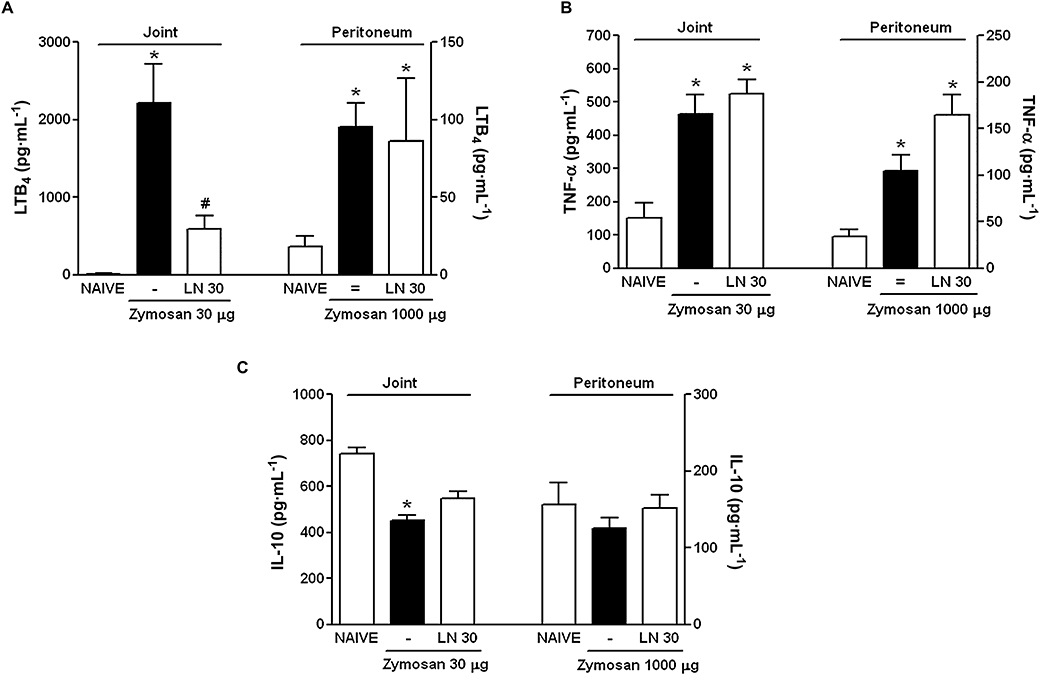
Analysis of LTB4, TNF-α and IL-10 levels in the exudates of zymosan-induced arthritis and peritonitis. Rats received 30 mg·kg−1 LN i.p. or s.c. 30 min prior to the injection of 30 µg zymosan i.art. or 1000 µg i.p. respectively. Non-treated (-, =) animals were given i.p. or s.c. saline prior to zymosan. Naïve animals received only saline. Results are expressed as the mean ± SEM of LTB4 (A), TNF-α (B) and IL-10 (C) for each group of six animals, using ELISA. *P < 0.05 compared with Naïve; #P < 0.05 compared with non-treated (-). IL-10, interleukin-10; LN, NG-nitro-L-arginine methyl ester; LTB4, leukotriene B4; TNF-α, tumor necrosis factor-α.
Systemic effect of NOS inhibition in the neutrophil influx in zymosan-induced arthritis and peritonitis in wild-type, β2-integrin−/− and ICAM-1−/− mice
Similar to what was seen in rats, the systemic administration of LN significantly decreased the neutrophil influx in mice with zymosan arthritis (Figure 4A), while significantly increasing the neutrophil influx in mice with zymosan-induced peritonitis (Figure 4B).
Figure 4.
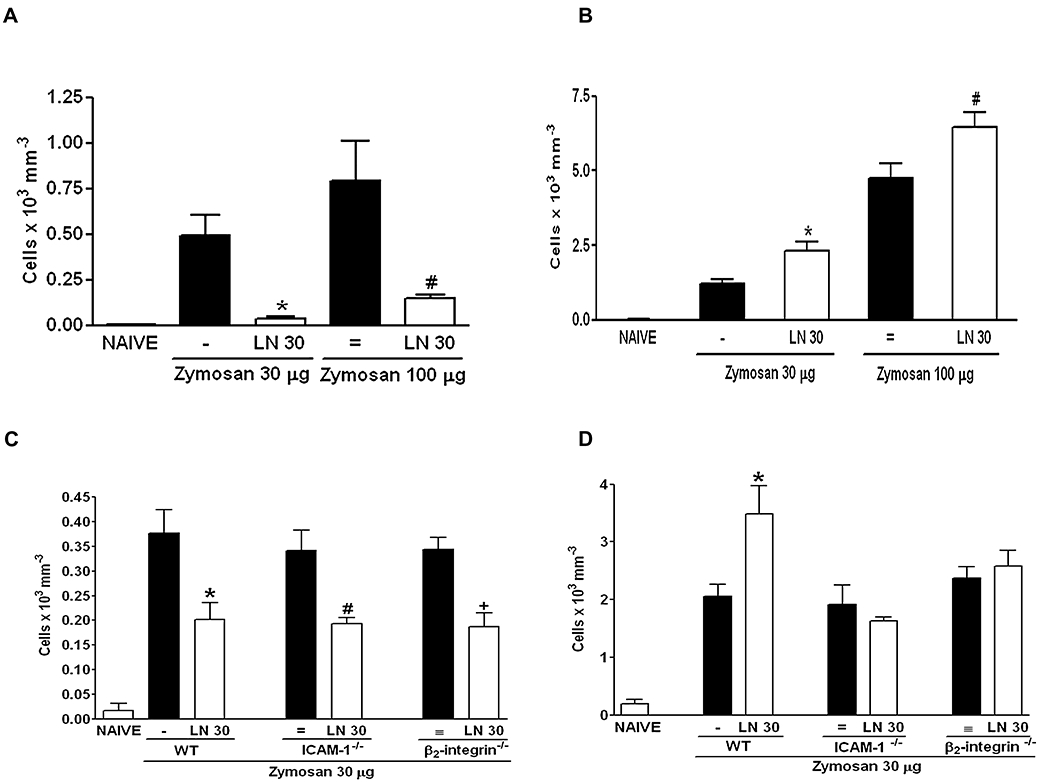
Systemic effect of NOS inhibition in the neutrophil influx in zymosan-induced arthritis and peritonitis in β2-integrin (β2−/−) and ICAM-1 (ICAM-1−/−) genetically deficient mice. Wild-type (WT) mice received 30 mg·kg−1 LN i.p. or s.c. 30 min prior to i.art. or i.p. zymosan (30–100 µg) respectively. Non-treated (-, =) animals were given only saline i.p. or s.c. prior to zymosan. Naïve animals received only saline; (A,C) and (B,D) represent data from arthritis and peritonitis respectively. β2−/− and ICAM-1−/− mice received 30 mg·kg−1 LN i.p. or s.c. 30 min prior to i.art. or i.p. zymosan (30µg) respectively. Results are expressed as the mean ± SEM of number of cells for each group of six animals. *P < 0.05 compared with non-treated (-); #P < 0.05 compared with non-treated (=).i.art., intra-articular; ICAM-1, intercellular adhesion molecule-1; ICAM-1−/−, mice genetically deficient for ICAM-1; LN, NG-nitro-L-arginine methyl ester; NOS, nitric oxide synthase.
Figure 4C and D show that the number of neutrophils in the articular or peritoneal exudates of animals stimulated by zymosan did not differ in wild-type, β2-integrin−/− or ICAM-1−/− mice. Moreover, Figure 4C illustrates that LN significantly reduced the neutrophil influx into the joints of mice with zymosan-induced arthritis, regardless of being administered to wild-type, β2-integrin−/− or ICAM-1−/− mice. On the other hand, LN administration did not alter the neutrophil influx into the peritoneal cavity of β2-integrin−/− and ICAM-1−/− mice with zymosan-peritonitis, compared with the increase in the neutrophil influx observed in wild-type animals (Figure 4D).
Discussion
The present study provides evidence that NO has a dual effect on neutrophil influx. NOS inhibition, whether non-selective or selective for the iNOS isoenzyme, significantly decreased neutrophil migration into inflamed joints while increasing neutrophil influx when similar stimuli were applied to the peritoneal cavity.
We had previously demonstrated a direct association of neutrophil infiltration and increase in the local release of nitrite (used as an index of NO production) in zymosan-arthritis (Rocha et al., 2002). In this report, the effect of NOS inhibition in the zymosan-arthritis occurred over a full range of zymosan concentrations. Therefore, the possibility that high concentrations of zymosan produced such a pronounced cell migration into the joints that a potentiating effect caused by NOS inhibition, as demonstrated in the peritoneum, could not be detected seems unlikely (Ajuebor et al., 1998). Among other possibilities to explain the apparent discrepancy in neutrophil influx between arthritis and peritonitis models, we included stimulus specificities, animal species and tissue characteristics.
We found that treatment of mice with zymosan arthritis with LN produced the same results observed in rats. Similar data were reported in guinea-pigs, where the combined intradermal administration of zymosan-activated plasma and LN significantly inhibited neutrophil and eosinophil accumulation (Teixeira et al., 1993). In the antigen-induced arthritis model in rabbits, the administration of LN also inhibited neutrophil migration (Mello et al., 1997), which was similar to the results achieved in rats subjected to stretococcal cell wall-induced arthritis treated with NG-monomethyl-L-arginine (L-NMMA) (McCartney-Francis et al., 1993). Thus, species differences were ruled out as a cause for the dual effect of NO in cell migration.
Local vasoconstriction secondary to constitutive NOS inhibition could explain our data (Paul-Clark et al., 2001). The rich vascular supply to the synovium, coupled with the vasodilatation and increased vascular permeability during inflammation, makes this possibility also unlikely (Rocha et al., 1999). In addition, we have demonstrated that AG reduces neutrophil influx in zymosan arthritis, without altering systemic blood pressure (Rocha et al., 2002).
We reproduced previous data showing that NOS inhibition increased neutrophil migration in both zymosan and LPS peritonitis, in both rats and mice (Benjamim et al., 2000; Tavares-Murta et al., 2001; Crosara-Alberto et al., 2002). In a more recent study, we confirmed those data and we have also shown that NOS inhibition led to an increase in the expression of ICAM-1 in mesenteric vessels, thus providing a possible mechanism to explain the increase in neutrophil influx caused by NOS inhibition in peritonitis (Dal Secco et al., 2006). The iNOS−/− mice, subjected to different inflammatory models, showed increased leukocyte recruitment into the peritoneal cavity, compared with wild-type mice (Benjamim et al., 2000; Secco et al., 2003). However, one report has shown that iNOS−/− mice subjected to zymosan-peritonitis had decreased cell influx (Ajuebor et al., 1998). Those authors associated this inhibitory effect to low levels of MIP-2 and IL-10 in the peritoneal exudate. The suppressive activity of IL-10 in PMN migration (Cassatella et al., 1993; Haskóet al., 1998) does not depend on iNOS modulation. Differences in the models studied may account for these discrepancies. In vitro studies have shown that L-NMMA decreases formyl-Met-Leu-Phe-induced chemotaxis (Kaplan et al., 1989). It has also been shown that low NO levels could promote neutrophil recruitment whereas high levels would inhibit (van Uffelen et al., 1996; Wanikiat et al., 1997). As the NOS inhibitor LN was effective over a full range of concentrations in the present in vivo study, we excluded this possibility.
We and others have shown an increase in LTB4 release in arthritis models, as well as a suppression of neutrophil migration provided by LTB4 blockade (Crooks and Stockley, 1998; Rocha et al., 2004). In vitro, using peripheral leukocytes of rheumatoid arthritis patients, the administration of an LTB4 antagonist inhibited the expression of the CD11b/CD18 (Mac-1) cell adhesion molecules (Alten et al., 2004). The fact that NOS inhibition decreased LTB4 levels in the joint but not in the peritoneal cavity suggests that NO has a different modulatory on LTB4 levels, depending on the tissue. However, the very low levels of LTB4 in the peritoneum made it difficult to demonstrate significant differences between the groups treated with the vehicle and the NOS inhibitor. Stimulation with an NO donor reduced LTB4 synthesis by cultured rat peritoneal macrophages (Brock et al., 2003). However, to our knowledge, there are no in vivo reports showing that NOS inhibition differentially modulates LTB4 release into joint and peritoneal cavities.
Modulation by cytokines could also be involved in the effects of NO. Considering the prominent role played by TNF-α (Sedgwick et al., 2000) and IL-10 (Alten et al., 2004) in neutrophil migration, we analysed the effect of NOS inhibition on their release into the inflamed cavities. Surprisingly, though, neither TNF-α nor IL-10 levels were significantly affected by the administration of LN, thus ruling out this possibility.
We have previously shown that ICAM-1−/− mice had similar neutrophil influx into the peritoneal cavity, as compared with wild-type animals (Dal Secco et al., 2006). Similar data were reported in other studies, using thioglycollate or glycogen-induced peritonitis (Steeber et al., 1999; Crockett et al., 2004). This result led us to conclude that ICAM-1is not essential for neutrophil recruitment. In the present study, we not only reproduced those data, but also showed that this is equally true for the β2-integrin molecule, as neutrophil numbers did not differ in β2-integrin−/− animals, compared with wild-type controls. These results apply for both peritoneal and joint cavities. Due to the multi-mediator characteristic of cell migration, we speculate that in the absence of either ICAM-1 or β2-integrin, other molecules (e.g. CD11a/CD18, CD11b/CD18, CD11c/CD18, VLA-4, αVβ3, ICAM-2, ICAM-3) may become more relevant in promoting neutrophil influx.
The data on the genetically modified animals suggest that tissue specificities account for the different effect of NO in cell migration. Indeed, the blockade of neutrophil influx by NOS inhibition persisted in those animals with zymosan-arthritis, whereas NOS inhibition was no longer effective in increasing neutrophil influx, either in ICAM-1−/− or β2-integrin−/− mice, when peritonitis was induced with zymosan.
It is tempting to speculate on the characteristics of the peritoneal and joint cavities. Synoviocytes are immersed in an extracellular matrix with no apparent contact. Type A synoviocytes resemble macrophages whereas type B are fibroblast-like cells. Dendritic cells, as well as mastocytes, are scattered throughout the synovium, comprising a minor though significantly functional cell component that work as antigen-presenting cells (Tran et al., 2005). A rich vascular supply is provided by a fenestrated capillary network. This unique arrangement renders the synovium a basement membrane-free tissue, where nothing but the endothelium separates blood constituents from the surrounding tissue. The peritoneum is a typical mesothelium, with a structured basement membrane. The present results show an interesting functional difference regarding the modulation of adhesion molecules by NO between the joint and peritoneal cavities. Pharmacological strategies to halt or at least control cell infiltration, aiming to achieve therapeutic benefits, should take into account that options may vary, depending on the tissue targeted.
In summary, we provide evidence that neither ICAM-1 nor β2-integrin expression are necessary for neutrophil recruitment into joints and peritoneum. The dual modulatory role of NO in the acute neutrophil migration in these tissues is at least partially due to a local differential effect on LTB4 release as well as on the expression of these cell adhesion molecules.
Acknowledgments
This work was supported by grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo). We thank Ana Karine R. M. Leite (student of Doctorate in Medical Sciences – Federal University of Ceará) and Maria Marcela Fernandes Monteiro and Ana Kátia dos Santos for collaboration in the experiments and Giuliana Bertozi Francisco for assistance with the ELISA analysis.
Glossary
Abbreviations:
- 1400W
N-[3-(aminomemethyl)benzyl] acetamide
- AG
aminoguanidine
- β2-integrin−/−
mice genetically deficient for the β2-integrin
- i.art.
intra-articular
- ICAM-1
intercellular adhesion molecule-1
- ICAM-1−/−
mice genetically deficient for ICAM-1; IL-10, interleukin-10
- iNOS
inducible NOS
- iNOS−/−
mice genetically deficient for the iNOS gene
- LN
NG-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- LPS
lipopolysaccharide
- LTB4
leukotriene B4
- MIP
macrophage inflammatory protein
- NA
NG-nitro-L-arginine
- PMN
polymorphonuclear cell
- TNF-α
tumour necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
- VLA-4
very late antigen-4
Conflicts of Interest
None.
References
- Ajuebor MN, Virág L, Flower RJ, Perretti M, Szabó C. Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology. 1998;95:625–630. doi: 10.1046/j.1365-2567.1998.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alten R, Gromnica-Ihle E, Pohl C, Emmerich J, Steffgen J, Roscher R, et al. Inhibition of leukotriene B4-induced CD11B/CD18 (Mac-1) expression by BIIL 284, a new long acting LTB4 receptor antagonist, in patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:170–176. doi: 10.1136/ard.2002.004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamim CF, Ferreira SH, Cunha FQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis. 2000;182:214–223. doi: 10.1086/315682. [DOI] [PubMed] [Google Scholar]
- Bombini G, Canetti C, Rocha FA, Cunha FQ. Tumour necrosis factor-alpha mediates neutrophil migration to the knee synovial cavity during immune inflammation. Eur J Pharmcol. 2004;496:197–204. doi: 10.1016/j.ejphar.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Brock TG, McNish RW, Mancuso P, Coffey MJ, Peters-Golden M. Prolonged lipopolysaccharide inhibits leukotriene synthesis in peritoneal macrophages: mediation by nitric oxide and prostaglandins. Prostaglandins Other Lipid Mediat. 2003;71:131–145. doi: 10.1016/s1098-8823(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin-10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett ET, Remelius C, Hess K, Al-Ghawi H. Gene deletion of P-Selectin and ICAM-1 does not inhibit neutrophil infiltration into peritoneal cavity following cecal ligation-puncture. BMC Clin Pathol. 2004;4:1–13. doi: 10.1186/1472-6890-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–178. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- Crosara-Alberto DP, Darini ALC, Inoue RY, Silva JS, Ferreira SH, Cunha FQ. Involvement of NO in the failure of neutrophil migration in sepsis induced by Staphylococcus aureus. Br J Pharmacol. 2002;136:645–658. doi: 10.1038/sj.bjp.0704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Secco D, Moreira AP, Freitas A, Silva JS, Rossi MA, Ferreira SH, et al. Nitric oxide inhibits neutrophil migration by a mechanism dependent on ICAM-1: role of soluble guanylate cyclase. Nitric Oxide. 2006;15:77–86. doi: 10.1016/j.niox.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Haskó G, Virág L, Egnaczyk G, Salzman AL, Szabó C. The crucial role of IL-10 in the suppression of the immunological response in mice exposed to staphylococcal enterotoxin B. Eur J Immunol. 1998;28:1417–1425. doi: 10.1002/(SICI)1521-4141(199804)28:04<1417::AID-IMMU1417>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hickey MJ, Kubes P. Role of nitric oxide in regulation of leukocyte-endothelial cell interactions. Exp Physiol. 1997;82:339–348. doi: 10.1113/expphysiol.1997.sp004029. [DOI] [PubMed] [Google Scholar]
- Kaplan SS, Billiar T, Curran RD, Zdziarski UE, Simmons RL, Basford RE. Inhibition of chemotaxis with NG-monomethyl-L-arginine: a role for cyclic GMP. Blood. 1989;74:1885–1887. [PubMed] [Google Scholar]
- Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc Res. 1996;32:743–751. [PubMed] [Google Scholar]
- McCartney-Francis N, Allen JB, Mizel DE, Albina JE, Xie QW, Nathan CF, et al. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993;178:749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney-Francis NL, Song X, Mizel DE, Wahl SM. Selective inhibition of inducible nitric oxide synthase exacerbates erosive joint disease. J Immunol. 2001;166:2734–2740. doi: 10.4049/jimmunol.166.4.2734. [DOI] [PubMed] [Google Scholar]
- Mello SB, Novaes GS, Laurindo IM, Muscara MN, Maciel FM, Cossermelli W. Nitric oxide synthase inhibitor influences prostaglandin and interleukin-1 production in experimental arthritic joints. Inflamm Res. 1997;46:72–77. doi: 10.1007/s000110050086. [DOI] [PubMed] [Google Scholar]
- Muller WA. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:326–333. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, Lentsch AB, Ward PA. In vivo recruitment of neutrophils: consistent requirements for L-arginine and variable requirements for complement and adhesion molecules. Inflammation. 1998;22:327–339. doi: 10.1023/a:1022356301181. [DOI] [PubMed] [Google Scholar]
- O'Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–2602. doi: 10.1056/NEJMra040226. [DOI] [PubMed] [Google Scholar]
- Paul-Clark MJ, Gilroy DW, Willis D, Willoughby DA, Tomlinson A. Nitric oxide synthase inhibitors have opposite effects on acute inflammation depending on their route of administration. J Immunol. 2001;166:1169–1177. doi: 10.4049/jimmunol.166.2.1169. [DOI] [PubMed] [Google Scholar]
- Ramos CDL, Fernandes KSS, Canetti C, Teixeira MM, Silva JS, Cunha FQ. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1α, TNF-α and LTB4. Eur J Immunol. 2006;36:2025–2034. doi: 10.1002/eji.200636057. [DOI] [PubMed] [Google Scholar]
- Rocha FAC, Aragão AGM, Jr, RC Oliveira, Pompeu MML, Vale MR, Ribeiro RA. Periarthritis promotes gait disturbance in zymosan-induced arthritis in rats. Inflamm Res. 1999;48:485–490. doi: 10.1007/s000110050491. [DOI] [PubMed] [Google Scholar]
- Rocha FAC, Teixeira MM, Rocha JCS, Girão VCC, Bezerra MM, Ribeiro RA, et al. Blockade of leukotriene B4 prevents articular incapacitation in rat zymosan-induced arthritis. Eur J Pharmacol. 2004;497:81–86. doi: 10.1016/j.ejphar.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rocha JCS, Peixoto MEB, Jancar S, Cunha FQ, Ribeiro RA, Rocha FAC. Dual effect of nitric oxide in articular inflammatory pain in zymosan-induced arthritis in rats. Br J Pharmacol. 2002;136:588–596. doi: 10.1038/sj.bjp.0704755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Walley KR, Klut ME, English D, Dyachkova Y, Hogg JC, et al. Nitric oxide reduces the sequestration of polymorphonuclear leukocytes in lung by changing deformability and CD 18 expression. Am J Respir Crit Care Med. 1999;159:1469–1476. doi: 10.1164/ajrccm.159.5.9808063. [DOI] [PubMed] [Google Scholar]
- Secco DD, Paron JA, De Oliveira SHP, Ferreira SH, Silva JS, Cunha FQ. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide. 2003;9:153–164. doi: 10.1016/j.niox.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Riminton DS, Cyster JG, Körner H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–113. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- Spiecker M, Darius H, Kaboth K, Hübner F, Liao JK. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol. 1998;63:732–739. [PubMed] [Google Scholar]
- Steeber DA, Tang ML, Green NE, Zhang XQ, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and ICAM-1 pathways. J Immunol. 1999;163:2176–2186. [PubMed] [Google Scholar]
- Tavares-Murta BM, Cunha FQ, Ferreira SH. Nitric oxide mediates the inhibition of neutrophil migration induced by systemic administration of LPS. Inflammation. 2001;25:247–253. doi: 10.1023/a:1010927921018. [DOI] [PubMed] [Google Scholar]
- Teixeira MM, Williams TJ, Hellewell PG. E-type prostaglandins enhance local oedema formation and neutrophil accumulation but suppress eosinophil accumulation in guinea pig skin. Br J Pharmacol. 1993;110:416–422. doi: 10.1111/j.1476-5381.1993.tb13826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CN, Lundy SK, Fox DA. Synovial biology and T cells in rheumatoid arthritis. Pathophysiology. 2005;12:183–189. doi: 10.1016/j.pathophys.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo FA, Arntz OJ, van Enckevort FH, van Lent PL, van den Berg WB. Reduced cartilage proteoglycan loss during zymosan-induced gonarthritis in NOS2-deficient mice and in anti-interleukin-1-treated wild-type mice with unabated joint inflammation. Arthritis Rheum. 1998;41:634–646. doi: 10.1002/1529-0131(199804)41:4<634::AID-ART10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- van Uffelen BE, de Koster BM, van den Broek PJ, Van Steveninck J, Elferink JG. Modulation of neutrophil migration by exogenous gaseous nitric oxide. J Leukoc Biol. 1996;60:94–100. doi: 10.1002/jlb.60.1.94. [DOI] [PubMed] [Google Scholar]
- Wanikiat P, Woodward DF, Armstrong RA. Investigation of the role of nitric oxide and cyclic GMP in both the activation and inhibition of human neutrophils. Br J Pharmacol. 1997;122:1135–1145. doi: 10.1038/sj.bjp.0701477. [DOI] [PMC free article] [PubMed] [Google Scholar]


