Abstract
Background and purpose:
During the development of atherosclerotic plaques, vascular smooth muscle cells (VSMCs) migrate from the media to the intima through the basement membrane and interstitial collagenous matrix, and proliferate to form neointima. Here, we investigate the mechanism of VSMC migration and proliferation caused by aggretin, a snake venom integrin α2β1 agonist.
Experimental approach:
Cultures of rat and human VSMCs were treated with aggretin and the signal transduction pathways induced by this agonist were examined by Western blotting, immunoprecipitation and electrophoretic mobility shift assay techniques.
Key results:
Aggretin-induced VSMC proliferation was blocked by a monoclonal antibody (mAb) against integrin α2 (AII2E10) or against the platelet-derived growth factor receptor (PDGFR)-β. Proliferation was also blocked by inhibition of the tyrosine kinase Src with PP2, phospholipase C (PLC) with U73122, extracellular signal-regulated kinase (ERK) with PD98059 or nuclear factor-kappa B (NF-kB) activation with pyrrolidine dithiocarbamate (PDTC). VSMC migration towards immobilized aggretin was increased in a modified Boyden chamber and this effect was blocked by α2β1-Src-PLC-MAPK axis inhibitors, but not by PDTC, PDGFR-β mAb, or a phosphoinositide-3 kinase inhibitor, LY294002. Aggretin stimulated the phosphorylation of PDGFR-β, Src and ERK in a time-dependent manner. NF-kB translocation and platelet-derived growth factor (PDGF)-BB production were also observed. The ERK activation, NF-kB translocation and PDGF-BB production were blocked by PP2, U73122 and PD98059.
Conclusions and implications:
Aggretin induces VSMC proliferation and migration mainly through binding to integrin α2β1, and subsequently activates Src, PLC and ERK pathways, inducing NF-kB activation and PDGF production.
Keywords: aggretin, integrin α2β1, smooth muscle cell, NF-kB, PDGF
Introduction
Vascular smooth muscle cells (VSMCs) contribute to the pathogenesis of atherosclerosis and restenosis by proliferation, migration from the media to the intima, and deposition of abundant extracellular matrix (ECM) in the neointima. In fact, several matrix proteins produced after vascular injury, including fibronectin, tenascin and type I collagen, stimulate VSMC proliferation and/or migration in vitro (Gotwals et al., 1996). The nature of the local environment, particularly the components of the ECM, dramatically regulate cell behaviour (Skinner et al., 1994). A majority of cell-matrix interactions is mediated by specific membrane receptors of the integrin family. Occupancy and clustering of integrins can activate intracellular signalling pathways and induce the formation of transcription factors and the subsequent gene expression (Hynes, 1992).
Collagen is known to stimulate the migration and growth of numerous cell types, including VSMCs (Xiang et al., 2000). These actions of collagen are mediated through cell surface receptors, including integrins (Hynes, 1992). The ligation of integrin is known to activate signalling pathways including the activation of the c-Src and Ras/extracellular signal-regulated kinase (ERK) pathways (Giancotti and Ruoslahti, 1999). Different ECM proteins interact with distinct integrins on cells (Stupack, 2005). For example, VSMCs adhere to collagen through the integrins α2β1 and α1β1, while they adhere to fibronectin through the integrin α2β1 (Glukhova et al., 1994).
Snake venoms contain many unique components that affect cell-matrix interaction. Aggretin, a potent platelet-aggregating protein purified from Calloselasma rhodostoma venom, consists of α and β subunits which share sequences homologous to those of C-type lectins (Chung et al., 1999). In previous studies, we showed that aggretin induced platelet aggregation, most likely via integrin α2β1 and glycoprotein Ib (GPIb) receptors (Chung et al., 2001). In addition, aggretin exhibits pro-angiogenic activities, including promotion of human umbilical vein endothelial cell (HUVEC) proliferation, migration and Matrigel-induced capillary tube formation in vitro, and induction of neovascularization in a chick chorioallantoic membrane angiogenesis model in vivo. The major target site of aggretin on HUVECs is integrin α2β1 (Chung et al., 2004). Thus, we investigated if aggretin exerted any effect on VSMCs through α2β1 ligation.
Nuclear factor-kappa B (NF-kB) is a dimeric transcription factor involved in inflammatory and immune responses. Cellular stimulation by proinflammatory cytokines and other agents activates an IkB kinase complex to phosphorylate IkB proteins. Subsequent polyubiquitination and proteasomal degradation of IkB leads to the translocation of NF-kB into the nucleus (Karin and Ben-Neriah, 2000). Activated nuclear NF-kB has been detected in smooth muscle cells after balloon injury to rat carotid arteries and in the smooth muscle cells of human atherosclerotic lesions. It was further identified in situ in macrophages, endothelial cells, and VSMCs in the intima and media of atherosclerotic vessel sections (Landry et al., 1997). These data strongly suggest a causative role for NF-kB in the development and maintenance of atherosclerosis.
In this study, we investigated the effect of aggretin on VSMC proliferation and migration, and the signal transduction pathways involved. To evaluate contribution of aggretin in modulating the integrin α2β1-mediated signalling pathway in VSMCs, we studied the Src, phospholipase C (PLC), and ERK cascades and their cross-regulation. Our analysis revealed that aggretin induced the activation of Src, platelet-derived growth factor receptor (PDGFR)-β, ERK phosphorylation and NF-kB translocation, leading to production of platelet-derived growth factor (PDGF)-BB.
Methods
Cell culture
Human aortic smooth muscle cells (HASMCs) purchased from Clonetics were cultured in smooth muscle cell growth medium-2 (SM-GM2) containing 2 ng·mL−1 human basic fibroblast growth factor, 0.5 ng·mL−1 human epidermal growth factor, 50 ng·mL−1 amphotericin-B, 5% fetal bovine serum (FBS); 50 µg·mL−1 gentamicin and 5 µg·mL−1 bovine insulin (all purchased from Clonetics). For all experiments, early-passage (passages 5 to 7) HASMCs were grown to 80% confluence, and made quiescent by serum starvation (0.4% FBS) for 24 h.
Rat A10 VSMCs were obtained from American Type Culture Collection (A10 CRL 14776). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM), containing 10% fetal calf serum (Gibco) supplemented with glutamax I (Gibco), 100 IU·mL−1 penicillin G (sodium salt), 100 mg·mL−1 streptomycin and 0.25 mg·mL−1 amphotericin B (antibiotic-antimitotic solution, Gibco).
Cell proliferation
Human aortic smooth muscle cells and VSMCs (5 × 103 cells per well) were seeded in 96-well plates (Costar) for attachment, and cells were grown in SM-GM2 or DMEM, or in the presence of aggretin or various inhibitors or antibodies for 48 h before assay. For the assay using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), cells were incubated with MTT at a final concentration of 0.5 mg·mL−1 for 4 h. After incubation, the medium was aspirated, and the cells were dissolved in dimethyl sulphoxide and then measured for developed colour absorbance at 550 nm. For control, HASMCs and VSMCs were grown in SM-GM2 or DMEM with or without 10% FBS for 48 h and then assayed for cell proliferation
Migration assay (haptotaxis)
HASMC and VSMC migration assays were performed using the modified Boyden chamber model (Transwell apparatus, 8.0 µmol·L−1 pore size, Costar), as previously described, with modification (Leavesley et al., 1993). Polycarbonate filters (Transwell inserts) were coated with aggretin (0.6 µg), collagen (0.2 µg) or bovine serum albumin (BSA) (20 µg) overnight, respectively, and the lower chamber was filled with 0.6 mL DMEM. VSMCs (1 × 104 cells·mL−1, 200 µL) were placed in the upper chamber of the Transwell in the absence or presence of A2IIE10 for 30 min. After 16 h incubation, all non-migrant cells were removed from the upper face of the Transwell membrane with a cotton swab, and the migrant cells were fixed and stained with 0.5% toluidine blue in 4% paraformaldehyde. Migration was quantified by counting the number of stained cells per 100× field with an inverted contrast phase microscope (Nikon, Japan), and then photographed.
Binding assays of aggretin towards VSMCs
Flow cytometric studies were performed to assay the binding and target receptor for aggretin in VSMCs. VSMCs were suspended in phosphate-buffered saline (PBS)/1% BSA and fixed with 1% paraglutaldehyde for at 4°C 30 min. Following washing with PBS/1% BSA, the cells were pretreated with A2IIE10, Agkistin or 7E3 at 4°C for 1 h. Treated cells were washed twice and then incubated with fluorescein-5-isothiocyanate (FITC)-conjugated aggretin for 30 min at 4°C with a continuous shaking. After incubation, cells were washed twice, resuspended in PBS and analysed immediately using a FACalibur (Becton Dickinson, CA, USA) at excitation and emission wavelengths of 488 and 525 nm respectively. Fluorescence signals from 10 000 cells were collected to calculate the mean fluorescence intensity of a single cell and the percentage of positively stained cells.
Immunoprecipitation and Western blotting
Vascular smooth muscle cells were pretreated with various concentrations of aggretin for different time intervals. Reactions were terminated with lysis buffer, and after sonication of the cells, the supernatant was isolated by centrifugation. PDGFR was immunoprecipitated from the supernatant using anti-PDGFR antibody for 1 h, and then protein A/G plus Sepharose was added for 1 h. The pelleted protein A/G plus Sepharose was washed three times with 1 mL of Tris-saline buffer, and eluted with Laemmli sodium dodecyl sulphate (SDS) reducing buffer. The immunoprecipitates or VSMC lysates were separated by SDS-PAGE using 8% gels, and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% (w/v) non-fat milk dissolved in Tris/phosphate/saline/Tween (TBS-T) and incubated with primary and secondary antibodies, which were diluted in TBS-T containing 1% non-fat milk. Blots were washed for at least 1 h in TBS-T after each incubation with antibodies, and developed using an enhanced chemiluminescence (ECL) detection system. Primary antibodies and horseradish peroxidase-conjugated secondary antibodies were used at a concentration of 1 µg·mL−1.
NF-kB translocation
For study of NF-kB p65 translocation, cells were rinsed with PBS and suspended in hypotonic buffer A (10 mmol·L−1 HEPES, pH 7.6, 10 mmol L−1 KCl, 1 mmol L−1 DTT, 0.1 mmol·L−1 EDTA and 0.5 mmol·L−1 phenylmethylsulphonyl fluoride) for 10 min on ice and vortexed for 10 s. The lysates were separated into cytosolic and nuclear fractions by centrifugation at 12 000 g for 2 min. The supernatants containing cytosolic proteins were collected. A pellet containing nuclei was suspended in buffer C (20 mmol·L−1 HEPES, pH 7.6, 1 mmol·L−1 EDTA, 1 mmol·L−1 DTT, 0.5 mmol·L−1 phenylmethylsulphonyl fluoride, 25% glycerol and 0.4 mol·L−1 NaCl) for 30 min on ice. The supernatants containing nuclei proteins were collected by centrifugation at 12 000 g for 20 min and stored at −70°C. All protein concentrations were determined by colorimetric assay using a Bio-Rad assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts (40 µg) of each protein from cytosolic or nuclei fractions were separated by 10% polyacrylamide-SDS gel and then electrotransferred to polyvinylidene difluoride membranes.
Electrophoretic mobility shift assay (EMSA)
Electrophoretic mobility shift assay was performed by using LightShift® Chemiluminescent EMSA kit (Pierce Inc.) according to the manufacturer's protocol. Binding reaction was initiated by adding 5 µg nuclear extract to binding buffer, 1 µg poly(deoxyinosinic-deoxycytidylic acid) and 100 nmol·L−1 biotin-labelled target double-stranded oligonucleotide incubated for 30 minutes at 37°C. The reaction was terminated by adding 5 µL of 5X DNA loading dye and then placing samples on ice before electrophoresis on a 5% native polyacrylamide gel. The samples on gel were then transferred onto Hybone™-N Nylon membrane (Amersham, Buckinghamshire, UK). The membrane was cross-linked at 120 mJ·cm−1 for 1 min, and then developed by adding the blocking buffer and streptavidin-HRP conjugate. NF-kB consensus site probe sequence: 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (Liu et al., 2006).
Transfection with small interfering RNA (siRNA)
Vascular smooth muscle cells (1 × 106 cells) were transfected with integrin α2 siRNA or non-targeting siRNA (negative siRNA) in 100 µL serum-free media with RNAi-Mate. A mixture of three siRNA sequences targeting the integrin α2 was used at total concentration of 1.5 µg per sequence. After 48 h, flow cytometry analysis for the integrin α2 was performed to confirm RNA suppression.
The integrin α2 sequences were as follows: sequence 1, sense UGAAUUGUCUGGCGUAUAATT, antisense UUAUACGCCAGACAAUUCATT; sequence 2, sense CAACUGGGAUCUGUUCUGATT, antisense UCAGAACAGAUCCCAGUUGTT; sequence 3, sense GCCAAUGAGCCGAGAAUUATT, antisense UAAUUCUCGGCUCAUUGGCTT (Jafri et al., 2008).
PDGF-BB production
Platelet-derived growth factor-BB concentrations of cell lysates were determined, following the manufacturer's instructions, using a quantitative enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA). All analyses and calibrations were carried out in triplicate. PDGF-BB immunoreactivity was determined as compared with recombinant PDGF-BB standards. Optical densities were determined at 450 nm using a microtiter plate spectrophotometer.
Statistical analysis
All values are presented as mean ± standard error. Differences between groups were assessed by one-way anova and Newman-Keuls multiple comparison test where appropriate. P values less than 0.05 (P < 0.05) were considered significant difference.
Materials
Aggretin and agkistin were purified from Calloselasma rhodostoma and Formosan Agkistrodon acutus venom, as described previously (Huang et al., 1995; Yeh et al., 2000). Protein A/G plus-Sepharose, peroxidase-conjugated anti-mouse antibody, anti-PDGFR, anti-NF-kB p65, peroxidase-conjugated anti-rabbit antibody, anti-phospho-c-Src α polyclonal antibody (Tyr 216), anti-α-tubulin antibody (TU-02), anti-nucleolin antibody (sc-8031), anti-phospho-ERK1/2 (E-4) and an ECL detection system were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); anti-α2 monoclonal antibody (mAb) (A2IIE10), anti-α1 mAb (5E8D9) and anti-phosphotyrosine mAb (4G10) were obtained from Upstate Biotechnology Inc. (Lake Placid, NY, USA); FITC was from Molecular Probes (USA); Collagen type I, MTT, pyrrolidine dithiocarbamate (PDTC), LY294002, PD98059 and BSA were from Sigma Co., (USA); PP2 was obtained from Calbiochem (San Diego, CA, USA); U73122 was obtained from Calbiochem. (La Jolla, CA, USA); DMEM, FBS and all culture reagents were purchased from Gibco BRL (USA); RNAi-Mate transfection reagent, integrin α2 siRNA and non-targeting siRNA were obtained from MDBio Inc. (Taipei, Taiwan). Our drug/molecular target nomenclature conforms to the BJP Guide to Receptors and Channels (Alexander et al., 2008).
Results
Effect of aggretin on VSMC and HASMC proliferation and migration
Our working hypothesis is that integrin α2β1 plays an important role in SMC proliferation. Thus, we used this natural, specific integrin α2β1 agonist, aggretin to examine its effect in stimulating VSMCs and HASMCs. The SMC proliferation assay was performed by determining cell metabolic activity with MTT. As shown in Figure 1A,B, aggretin significantly increased cell proliferation in a concentration-dependent manner, compared with serum-free controls (10∼80% increase). VSMC proliferation were partially inhibited by an integrin α2 mAb, A2IIE10 (50 µg·mL−1, about 60% inhibition) and almost completely blocked by the Src inhibitor, PP2 (10 µmol·L−1) (Salazar and Rozengurt, 1999), a PLC inhibitor, U73122 (10 µmol·L−1) (Tatrai et al., 1994), an ERK inhibitor, PD98059 (30 µmol·L−1) (Dudley et al., 1995) and the NF-kB activation inhibitor, PDTC (20 µmol·L−1) (Sherman et al., 1993) as compared with those of the control (Figure 1A). HASMC proliferation was also partially inhibited by an integrin α2 mAb, A2IIE10 (50 µg·mL−1, about 62% inhibition) and almost completely blocked by the ERK inhibitor, PD98059 (30 µmol·L−1, about 94% inhibition), compared with that in the control cultures (Figure 1B). On the other hand, the proliferation effect induced by aggretin was not affected by LY294002, a phosphoinositide-3 kinase (PI3K) inhibitor (10 µmol·L−1) (Baumann and West, 1998). PP2, U73122, PD98059 and LY294002 use dimethyl sulfoxide (DMSO) as their solvent. Vehicle controls showed no significant effects in this assay. These results suggest that aggretin-SMC interaction promotes cell proliferation and this effect is mainly mediated by integrin α2β1, and that the activation of Src, PLC, ERK and NF-kB are involved in the proliferation cascades. However, the activation of the PI3K/Akt cascade was not involved in VSMC and HASMC proliferation. To evaluate if growth factor was involved in VSMC proliferation, PDGFR-β mAb was used. PDGFR-β mAb concentration-dependently inhibited VSMC proliferation (25 and 50 µg·mL−1, 45% and 75% inhibition).
Figure 1.
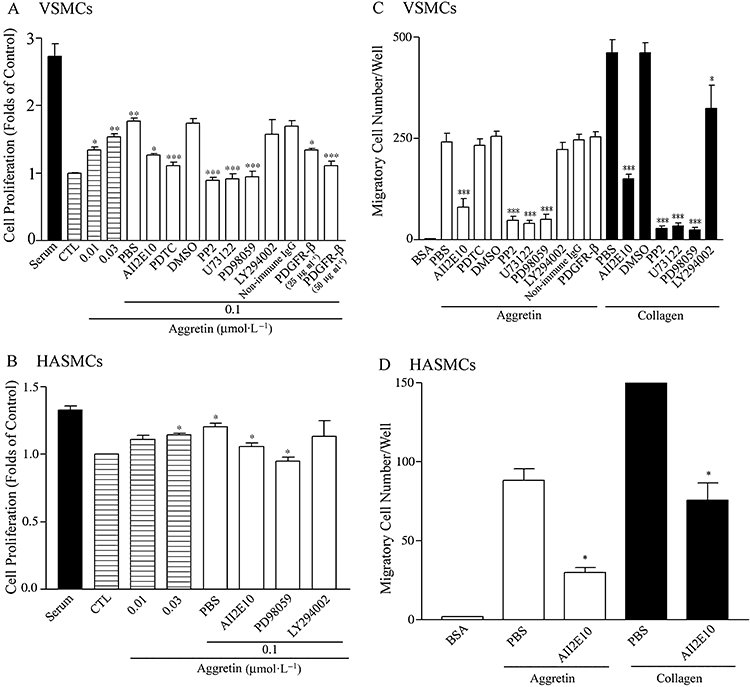
Effects of aggretin on proliferation (A,B) and migration (C,D) of rat VSMCs and HASMCs. (A, B) VSMCs or HASMCs (1 × 104 cell·mL−1) were seeded on 24-well plates. After attachment, cells were treated with an indicated concentration of aggretin for 48 h in the absence or presence of various inhibitors, and followed by MTT assay. Results are expressed as cell viability relative to the control cells (CTL, as 1.0) in the absence of serum (DMEM). The inhibitor studies with 0.1 µmol·L−1 aggretin were compared with the PBS (0.1 µmol·L−1 aggretin alone) group. (C, D) Migration of VSMCs and HASMCs (5 × 104 cells·mL−1) incubated in the absence or presence of various inhibitors, was followed by placing them in the upper chamber of a Transwell containing a gelatin-coated filter membrane, using collagen (0.2 µg), aggretin (0.6 µg) or BSA (20 µg) as the coating of the lower filter. Haptotaxis was allowed to proceed for 16 h. After fixation and removing non-migrated cells, cells that migrated to the underside of the filter membrane were quantified by a phase-contrast light microscope under a high power field (HPF, magnification × 100). All experiments were conducted in triplicate and similar results were repeated at least four times. Data are presented as mean ± SEM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 as compared with control. BSA, bovine serum albumin; DMEM, Dulbecco's modified Eagle's medium; HASMCs, human aortic smooth muscle cells; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; PBS, phosphate-buffered saline; VSMCs, vascular smooth muscle cells.
As shown in Figure 1C,D, VSMCs and HASMCs migrated through aggretin or collagen-coating inserts, and both of these cell migrations were similarly blocked by A2IIE10 (50 µg·mL−1), indicating that α2 integrin is essential for cell migration. VSMC migration was also abolished by PP2, U73122, PD98059, but not by LY294002, indicating that Src, PLC, ERK and PI3K/Akt may exert their effects in diverse signalling pathways. As the activation of integrin α2β1 in VSMCs may increase PDGF production (Figure 6), the involvement of PDGF-BB in VSMC migration was investigated. In the presence of PDGFR-β mAb, however, VSMC migration was not affected (Figure 1C). Thus, VSMC migration caused by aggretin is primarily modulated through integrin α2β1 activation, but is not correlated with activation of the PDGFR-β.
Figure 6.

Aggretin induced PDGF-BB production and its regulation. VSMCs (1 × 107 cells) were cultured in the presence of aggretin (0.1 µmol·L−1) for different times or in the presence of various inhibitors for 24 h. The cell lysates were collected, and PDGF-BB concentrations of cell lysates were determined by quantitative enzyme-linked immunosorbent assay. Aggretin did not induce PDGF-BB production until 24 h of incubation. This late phase output was inhibited by all the compounds tested except LY294002, a PI3K inhibitor. The data are representative of at least three experiments. Data are presented as mean ± SEM (n = 4). *P < 0.05 as compared with control. PDGF-BB, platelet-derived growth factor-BB; VSMCs, vascular smooth muscle cells.
Binding assay of aggretin towards VSMC
To study the target site of aggretin on VSMCs, the cells were incubated with FITC-conjugated aggretin to examine the binding reaction. The increment of relative fluorescence intensity of the bound FITC-aggretin was concentration-dependent, reaching saturation at a concentration of 10 µmol·L−1 (Figure 2A). 5E8D9, a mAb against α1 integrin, agkistin, a snake venom GPIb antagonist and antibody 7E3 raised against integrin αvβ3 showed little effect on FITC-aggretin binding towards VSMCs (Figure 2B). In contrast, A2IIE10 (25 and 50 µg·mL−1) inhibited aggretin binding (39 and 69%) to VSMCs in a concentration-dependent manner (Figure 2B).
Figure 2.
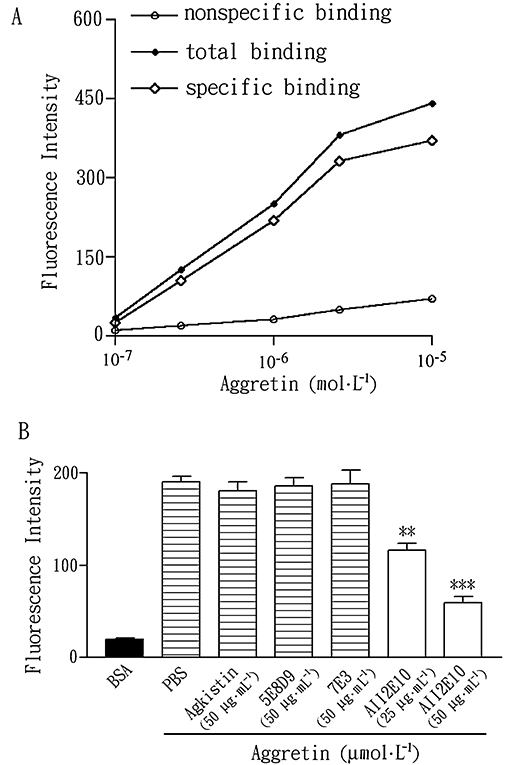
Effects of anti-α2 integrin mAb, anti-α1 integrin mAb and GPIb antagonist on the binding of FITC-conjugated aggretin to VSMCs. (A) VSMCs (1 × 106 cells) were incubated with various concentrations of FITC-conjugated aggretin or FITC-conjugated BSA for 30 min and then analysed by flow cytometry. This is representative of three similar results. (B) Quantitative analyses of FITC-aggretin and FITC-BSA with the various mAb and inhibitors, are presented as mean fluorescence intensity. Data are presented as mean ± SEM (n = 3). **P < 0.01, ***P < 0.001 as compared with that of control. BSA, bovine serum albumin; FITC, fluorescein-5-isothiocyanate; GPIb, glycoprotein Ib; mAb, monoclonal antibody; VSMCs, vascular smooth muscle cells.
Effect of aggretin on PDGFR-β and Src phosphorylation in VSMCs
There is Src-dependent crosstalk between α2β1 integrin and PDGFR-β (Hollenbeck et al., 2004). PDGF is a well-known SMC agonist and the phosphorylated receptor transduces its signal by binding to intracellular signalling proteins, including the Src family of non-receptor tyrosine kinases, which in turn activates the downstream pathway implicated in cellular proliferation, including ERK (Bornfeldt et al., 1995). We evaluated the effect of aggretin on PDGFR-β and Src phosphorylation. After incubation of VSMCs with aggretin for various time intervals, the PDGFR-β of cell lysates was immunoprecipitated and immunoblotted. Figure 3A shows the tyrosine phosphorylation of immunoprecipitated aggretin-treated VSMC lysates. Phosphorylation of PDGFR-β induced by aggretin (0.1 µmol·L−1) was significantly elevated, as compared with that of the control after 1/12 h of aggretin pretreatment, and this effect persisted for 3 h; the sequential phosphorylation of PDGFR-β was observed after 24 h of aggretin treatment. This long-term effect may be related to PDGF-BB production. As Src is also important in integrin α2β1 signalling (Inoue et al., 2003), the activation of Src was evaluated in aggretin-stimulated VSMCs. After aggretin pretreatment, aggretin induced Src activation of VSMCs in a time-dependent manner. This effect was evident at time points ranging from 5 min to 1 h (P < 0.05) after aggretin stimulation (Figure 3B).
Figure 3.

Effect of aggretin on VSMC PDGFR-β and Src phosphorylation. (A) VSMCs (1 × 106 cells) were cultured in the presence of aggretin (0.1 µmol·L−1) for various times and the reactions were stopped with lysis buffer. Immunoprecipitates (IP) of PDGFR-β were formed as described in the methods. VSMC lysates were applied to SDS-PAGE. Tyrosine-phosphorylated proteins were detected with Western blot (WB) using anti-phosphotyrosine mAb (4G10) coupled with ECL, and then reprobed with PDGFR-β mAb. (B) VSMCs (1 × 106 cells) were cultured in the presence of aggretin (0.1 µmol·L−1) for different times, and the reactions were stopped with lysis buffer. Src activation was detected with WB using anti-phospho-Src pAb (Tyr 216) coupled with ECL, whereas α-tubulin was taken as an internal control. Summary data of PDGFR-β and Src phosphorylation were presented (on the right) as mean density, as determined by a densitometer. Densitometric band intensities were normalized to static controls, and fold increases were calculated. The data are representative of at least three experiments. Data are presented as mean ± SEM (n = 4). *P < 0.05 as compared with control. ECL, enhanced chemiluminescence; PDGFR-β, platelet-derived growth factor receptor-β; SDS, sodium dodecyl sulphate; VSMC, vascular smooth muscle cell.
ERK activation and its regulation
It is documented that matrix proteins and growth factors are able to stimulate ERK phosphorylation in migratory cells (Robinson and Cobb, 1997), and the ERK inhibitor, PD98059, can also affect VSMC proliferation and migration, so we were interested in identifying ERK involvement in integrin-ligand interaction. Utilization of the phosphorylation-specific antibody against ERK1/2 for immunoblotting revealed activation of the ERK1/2 pathway in aggretin-stimulated VSMCs. After VSMC exposure to aggretin (0.1 µmol·L−1) for various time intervals, ERK1/2 phosphorylation was markedly increased in a time-dependent manner (Figure 4A), and the ERK of VSMCs lysates were phosphorylated within 5 min and partially inactivated at 1 h. Moreover, the activation of ERK1/2 was abolished by the Src inhibitor, PP2 (10 µmol·L−1) and the PLC inhibitor, U73122 (10 µmol·L−1) (Figure 4B).
Figure 4.

Aggretin causes ERK activation and its regulation. VSMCs (1 × 106 cells) were cultured in the presence of aggretin (0.1 µmol·L−1) for different times (A) or in the presence of various inhibitors for 30 min (B). The reactions were stopped with lysis buffer. ERK activation was detected with Westen blots (WB) using anti-phospho-ERK1/2 mAb coupled with ECL, with α-tubulin was taken as an internal control. Summary data of ERK phosphorylation are presented (on the right) as mean density, determined by densitometer. Densitometric band intensities were normalized to static controls, and fold increases were calculated. The data are representative of at least three experiments. Data are presented as mean ± SEM (n = 4). *P < 0.05 as compared with control. ECL, enhanced chemiluminescence; ERK, signal-regulated kinase; VSMCs, vascular smooth muscle cells.
NF-kB activation is involved in integrin α2β1 ligation
An important role for NF-kB in controlling the proliferation of various cell types has been demonstrated (Bargou et al., 1997). It is possible that integrin ligation may also involve NF-kB activation. To monitor NF-kB activation in VSMCs after aggretin stimulation, we studied the translocation of NF-kB from cytosol to nucleus. Figure 5A displays a decrease of cytosolic NF-kB and an increase in the nucleus in a time-dependent manner. This translocation effect was more pronounced at 15 and 30 min (increases of 1.98 ± 0.56- and 2.30 ± 0.81-fold respectively). We also investigated the influence of pharmacological inhibitors on NF-kB activation in VSMCs. In parallel studies (Figure 5B), preincubation of VSMCs with Src, PLC and ERK inhibitors eliminated the NF-kB translocation effect induced by aggretin. NF-kB DNA binding and transcriptional activity were also confirmed by EMSA analysis (Figure 5C). Results were similar to NF-kB translocation studies (Figure 5A,B).
Figure 5.

Integrin α2β1 ligation triggers NF-kB activation. VSMCs (1 × 107 cells) were cultured in the presence of aggretin (0.1 µmol·L−1) for different times (A) or in the presence of various inhibitors for 30 min (B). The cytosolic and nuclear fractions were obtained as described in the methods. The reactions were stopped with lysis buffer. NF-kB activation was detected with Western blots using anti-NF-kB p65 pAb coupled with ECL. Summary data of NF-kB translocation are presented below the blots, as mean density, determined by densitometer. Densitometric band intensities were normalized to static controls, and fold increases were calculated. (C) EMSA was performed in the absence or presence of NF-kB binding sequence to confirm the NF-kB activation condition in A and B. VSMCs were also pretreated with aggretin (0.1 µmol·L−1) for different time intervals or in the presence of various inhibitors for 30 min. Nuclear extracts were preincubated with biotin-labelled double-stranded oligonucleotide NF-kB, and reaction products were analysed on 5% nondenaturing polyacrylamide gels. (D) FACS analysis for integrin α2β1 expression after siRNA interference. (E) EMSA was performed in the absence or presence of integrin α2 siRNA in aggretin-induced NF-kB activation. The data are representative of at least three experiments. Data are presented as mean ± SEM (n = 4). *P < 0.05 as compared with control. ECL, enhanced chemiluminescence; EMSA, electrophoretic mobility shift assay; NF-kB, nuclear factor-kappa B; siRNA, small interfering RNA; VSMCs, vascular smooth muscle cells.
To precisely evaluate the role of integrin α2β1, the relevant siRNA was used to suppress the expression of α2-subunit and was confirmed by flow cytometry. The expression of integrin α2β1 was dramatically decreased, compared with non-transfected cells (Figure 5D). Aggretin-induced activation of NF-kB was significantly inhibited in A10 cells transfected with integrin α2 siRNA (Figure 5E). Cells transfected with non-targeting siRNA showed no difference in integrin α2β1 expression (data not show) and NF-kB activation (Figure 5E), compared with non-transfected cell.
Regulation of PDGF-BB production by aggretin
We next explored the contribution of PDGF-BB production to aggretin-stimulated VSMCs. As shown in Figure 6, no increment in PDGF-BB production was observed within the first 6 h, but a dramatic increase detected after 24 h exposure to aggretin. As compared with the PDGFR-β phosphorylation pattern, PDGF-BB production 24 h after aggretin treatment may result in sequential phosphorylation of PDGFR-β (Figure 3A, 24 h). To investigate the signalling proteins required for PDGF-BB production in aggretin-stimulated VSMCs, we measured the level of PDGF-BB in cells pretreated with various kinase inhibitors. PP2, U73122 and PD98059 almost completely eliminated the enhancing effect of aggretin on PDGF-BB production, but LY294002 did not affect PDGF production (Figure 6). PDTC (20 µmol·L−1) also effectively suppressed PDGF-BB production. These data suggest that in VSMCs, the Src/PLC/ERK cascade and NF-kB play crucial roles in promoting PDGF-BB production triggered by aggretin.
Discussion
Many matrix molecules that promote cell migration, such as collagen, act as attachment factors because it is necessary for a cell to adhere to a substrate to gain traction for migration (Elliott et al., 2005). It is interesting to connect these behaviours of VSMCs with integrin signalling and so activation of kinase cascades coupled with integrin ligation has been investigated. In this study, ligand-binding demonstrated that the major binding site of aggretin is integrin α2β1, but not α1β1, GPIb or integrin αvβ3 expressed on VSMCs (Figure 2B). Skinner et al. (1994) showed that cultured SMCs expressed α2, α3, α5 and α v subunits with little α1 or β3 (Skinner et al., 1994). Given that the rate of matrix turnover in normal blood vessels is slow, it is most likely that expression of the α2β1 collagen receptor, which can mediate migration, collagen turnover and gel contraction, and α1β1 may be expressed only during periods of active remodeling, such as in development and wound repair (Franco et al., 2002). In other study, Katsue et al. identified a novel aggretin (i.e. rhodocytin)-binding 32-kDa surface megakaryocyte/platelet-specific protein, the C-type lectin receptor, CLEC-2 (Suzuki-Inoue et al., 2006). However, α2β1 integrin is expressed primarily on cultured VSMCs and there is no literature suggesting that CLEC-2 is expressed in VSMCs, thus excluding the involvement of CLEC-2 as a target for aggretin in VSMCs.
The VSMC response to aggretin is mainly mediated by the α2 integrin receptor, because both the enhancement of migration and proliferation effects induced by aggretin were blocked by A2IIE10, a mAb against α2 integrin. Ligation of integrins induces a cascade of intracellular signals, regulates gene expression and contributes to proliferation, migration and differentiation (Stromblad et al., 1996). Therefore, we investigated the integrin α2-mediated signalling pathways of VSMCs induced by aggretin. Some studies have demonstrated that integrin α2β1 enhances PDGF-BB-dependent proliferation and the synergistic effect of collagen on PDGF-BB-induced VSMC proliferation appears to reflect transactivated PDGFR-β receptor (Hollenbeck et al., 2004). In this report, when smooth muscles were treated with aggretin, the tyrosine phosphorylation of PDGFR-β and Src were markedly increased within 5 min in parallel, compared with the serum-free control (Figure 3). Src is necessary and sufficient for smooth muscle cell proliferation and migration (Cho et al., 2005). The Src kinase activity is also required for integrin-mediated NF-kB activation (Courter et al., 2005). We showed that Src activation was involved in integrin ligation and may be related to PDGFR-β phosphorylation. Accordingly, some authors have described the role of the Src family of kinases in αvβ3/EGF and α2β1/PDGFR crosstalk (Moro et al., 2002; Hollenbeck et al., 2004); however, the pathways linking integrin ligation to activation of Src, and the downstream signalling proteins involved, are not yet fully defined.
The proliferation of several phenotypes of cells is mediated by growth factor or cytokine-induced mitogen-activated protein kinases (MAPKs), a family of serine-threonine proteins. MAPKs consist of ERK, p38 MAPK (p38) and c-Jun NH2-terminal kinase (JNK) (Cowan and Storey, 2003). The activation of ERK induced by various substances, such as PDGF, increased proliferation of human airway smooth muscle cells (Karpova et al., 1997). Studies also have revealed that loss of the α7β1 integrin results in VSMC hyperplasia through ERK activation (Welser et al., 2007). The mechanism of ERK activation in aggretin-stimulated VSMCs was elucidated in this study (Figure 4A). The phosphorylation of other MAPK pathways, p38 and JNK, were not observed with aggretin-stimulated VSMCs (data not shown). These findings suggest that aggretin-induced VSMC proliferation is related to the activation of the ERK pathway, and Src and PLC may be the upstream signalling molecules.
The PI3K/Akt/PKB cascade is another integrin-mediated cell signalling pathway. PI3K is known to be activated by integrin engagement and is important in cell spreading in normal fibroblastd (Berrier et al., 2000). Although PI3K/Akt was also activated after aggretin treatment of VSMCs (data not shown), pretreatment of VSMCs with a PI3K inhibitor did not affect the proliferation and migration induced by aggretin (Figure 1A,C). These results indicate that ERK and PI3K/Akt may contribute to the diverse signalling pathway modulating the different function of VSMCs. As the phosphorylation of p38 and JNK were not observed in aggretin-stimulated VSMCs (data not shown), they are not involved in aggretin-mediated cell proliferation and migration.
Various reports suggest a role for the ubiquitous transcription factor NF-kB in the mitogenic growth control of a variety of cell types. The activated NF-kB binds to specific promoter regions of target genes in the nucleus. Previous studies demonstrated that NF-kB activation is enhanced in intimal lesions (Wilson et al., 2000). Furthermore, evidence indicates that NF-kB activation was increased in endothelial cells of regions pre-disposed to lesion formation and, additionally, that NF-kB can bind to the promoter regions of PDGF-BB (Hajra et al., 2000). Li et al. also reported that angiotensin II and oxidized-LDL promoted NF-kB activation, which mediated, at least in part, PDGF-BB expression in cultured endothelial cells (Zhou et al., 2003). According to our experiments, incubation with aggretin stimulates VSMCs, resulting in NF-kB activation in 15–30 min (Figure 5A). These findings also indicate that the aggretin-induced NF-kB translocation in VSMCs was dependent on Src, PLC and ERK, thus implicating integrin α2β1 ligation for triggering NF-kB activation (Figure 5B,C). In vitro, siRNA transfection also supported an important role for integrin α2β1 in aggretin-induced signal transduction in VSMCs. Aggretin-induced NF-kB activation was almost completely inhibited in A 10 cells silenced with siRNA for integrin α2 (Figure 5D,E). These results indicate that the integrin α2β1 is an important receptor in aggretin-mediated VSMC signalling.
As PDGF-BB production was blocked by the NF-kB inhibitor, PDTC, the consequence of NF-kB activation is the promotion of PDGF-BB expression (Figure 6), suggesting a close relationship between the induction of NF-kB activation and the expression of PDGF-BB in vivo. Although aggretin induced PDGFR-β trans-activation in the early phase, after 24 h the activation of PDGFR-β following aggretin stimulation may be through PDGF-BB release, as no significant PDGF-BB release was detected during the first 6 h of incubation.
In conclusion, we have shown that aggretin exhibited a significant promoting effect on VSMC proliferation and migration. The binding of FITC-conjugated aggretin to VSMCs was specifically inhibited by a mAb A2IIE10 raised against α2 integrin. Aggretin stimulated Src and ERK phosphorylation and, in turn, induced NF-kB activation. Aggretin-induced NF-kB activation was abolished by transfection with siRNA for integrin α2, indicating that the NF-kB activation may primarily through α2β1 ligation. Additionally, an increase in NF-kB translocation enhances PDGF-BB expression in aggretin-stimulated VSMCs. Taken together, we demonstrate that an increased proliferation and migration of VSMCs is initially triggered by α2β1 ligation of aggretin, and eventually a delayed elevation of PDGF-BB production, linking α2β1 engagement, the activation of PDGFR-β, Src-PLC-ERK, NF-kB translocation, PDGF production and feedback activation of PDGFR in mediating cell proliferation.
Acknowledgments
We appreciate the financial support of National Science Council, Taiwan.
Glossary
Abbreviations:
- ECM
extracellular matrix
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- GPIb
glycoprotein Ib
- HASMC
human aortic smooth muscle cell
- HUVEC
human umbilical vein endothelial cell
- MAPK
mitogen-activated protein kinase
- NF-kB
nuclear factor-kappa B
- PDGF
platelet-derived growth factor
- PDTC
pyrrolidine dithiocarbamate
- PI3K
phosphoinositide-3 kinase
- PLC
phospholipase C
- VSMC
vascular smooth muscle cell
Conflict of interest
None.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd) 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, West SC. DNA end-joining catalyzed by human cell-free extracts. Proc Natl Acad Sci USA. 1998;95:14066–14070. doi: 10.1073/pnas.95.24.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier AL, Mastrangelo AM, Downward J, Ginsberg M, LaFlamme SE. Activated R-ras Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains. J Cell Biol. 2000;151:1549–1560. doi: 10.1083/jcb.151.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt KE, Raines EW, Graves LM, Skinner MP, Krebs EG, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann N Y Acad Sci. 1995;766:416–430. doi: 10.1111/j.1749-6632.1995.tb26691.x. [DOI] [PubMed] [Google Scholar]
- Cho HM, Choi SH, Hwang KC, Oh SY, Kim HG, Yoon DH, et al. The Src/PLC/PKC/MEK/ERK signaling pathway is involved in aortic smooth muscle cell proliferation induced by glycated LDL. Mol Cells. 2005;19:60–66. [PubMed] [Google Scholar]
- Chung CH, Au LC, Huang TF. Molecular cloning and sequence analysis of aggretin, a collagen-like platelet aggregation inducer. Biochem Biophys Res Commun. 1999;263:723–727. doi: 10.1006/bbrc.1999.1457. [DOI] [PubMed] [Google Scholar]
- Chung CH, Peng HC, Huang TF. Aggretin, a C-type lectin protein, induces platelet aggregation via integrin alpha(2)beta(1) and GPIb in a phosphatidylinositol 3-kinase independent pathway. Biochem Biophys Res Commun. 2001;285:689–695. doi: 10.1006/bbrc.2001.5228. [DOI] [PubMed] [Google Scholar]
- Chung CH, Wu WB, Huang TF. Aggretin, a snake venom-derived endothelial integrin alpha 2 beta 1 agonist, induces angiogenesis via expression of vascular endothelial growth factor. Blood. 2004;103:2105–2113. doi: 10.1182/blood-2003-07-2483. [DOI] [PubMed] [Google Scholar]
- Courter DL, Lomas L, Scatena M, Giachelli CM. Src kinase activity is required for integrin alphaVbeta3-mediated activation of nuclear factor-kappaB. J Biol Chem. 2005;280:12145–12151. doi: 10.1074/jbc.M412555200. [DOI] [PubMed] [Google Scholar]
- Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206:1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JT, Woodward JT, Langenbach KJ, Tona A, Jones PL, Plant AL. Vascular smooth muscle cell response on thin films of collagen. Matrix Biol. 2005;24:489–502. doi: 10.1016/j.matbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Franco CD, Hou G, Bendeck MP. Collagens, integrins, and the discoidin domain receptors in arterial occlusive disease. Trends Cardiovasc Med. 2002;12:143–148. doi: 10.1016/s1050-1738(01)00165-7. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Glukhova MA, Frid MG, Koteliansky VE. Phenotypic changes of human aortic smooth muscle cells during development and in adult. J Atheroscler Thromb. 1994;1(Suppl.)(1):S47–S49. doi: 10.5551/jat1994.1.supplemment1_s47. [DOI] [PubMed] [Google Scholar]
- Gotwals PJ, Chi-Rosso G, Lindner V, Yang J, Ling L, Fawell SE, et al. The alpha1beta1 integrin is expressed during neointima formation in rat arteries and mediates collagen matrix reorganization. J Clin Invest. 1996;97:2469–2477. doi: 10.1172/JCI118693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck ST, Itoh H, Louie O, Faries PL, Liu B, Kent KC. Type I collagen synergistically enhances PDGF-induced smooth muscle cell proliferation through pp60src-dependent crosstalk between the alpha2beta1 integrin and PDGFbeta receptor. Biochem Biophys Res Commun. 2004;325:328–337. doi: 10.1016/j.bbrc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Huang TF, Liu CZ, Yang SH. Aggretin, a novel platelet-aggregation inducer from snake (Calloselasma rhodostoma) venom, activates phospholipase C by acting as a glycoprotein Ia/IIa agonist. Biochem J. 1995;309:1021–1027. doi: 10.1042/bj3091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol. 2003;160:769–780. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M, Donnelly B, Allen S, Bondoc A, McNeal M, Rennert PD, et al. Cholangiocyte expression of alpha2beta1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G16–G26. doi: 10.1152/ajpgi.00442.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Karpova AY, Abe MK, Li J, Liu PT, Rhee JM, Kuo WL, et al. MEK1 is required for PDGF-induced ERK activation and DNA synthesis in tracheal myocytes. Am J Physiol. 1997;272:L558–L565. doi: 10.1152/ajplung.1997.272.3.L558. [DOI] [PubMed] [Google Scholar]
- Landry DB, Couper LL, Bryant SR, Lindner V. Activation of the NF-kappa B and I kappa B system in smooth muscle cells after rat arterial injury. Induction of vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1. Am J Pathol. 1997;151:1085–1095. [PMC free article] [PubMed] [Google Scholar]
- Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993;121:163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhan M, Hannay JA, Das P, Bolshakov SV, Kotilingam D, et al. Wild-type p53 inhibits nuclear factor-kappaB-induced matrix metalloproteinase-9 promoter activation: implications for soft tissue sarcoma growth and metastasis. Mol Cancer Res. 2006;4:803–810. doi: 10.1158/1541-7786.MCR-06-0201. [DOI] [PubMed] [Google Scholar]
- Moro L, Dolce L, Cabodi S, Bergatto E, Erba EB, Smeriglio M, et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Salazar EP, Rozengurt E. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J Biol Chem. 1999;274:28371–28378. doi: 10.1074/jbc.274.40.28371. [DOI] [PubMed] [Google Scholar]
- Sherman MP, Aeberhard EE, Wong VZ, Griscavage JM, Ignarro LJ. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochem Biophys Res Commun. 1993;191:1301–1308. doi: 10.1006/bbrc.1993.1359. [DOI] [PubMed] [Google Scholar]
- Skinner MP, Raines EW, Ross R. Dynamic expression of alpha 1 beta 1 and alpha 2 beta 1 integrin receptors by human vascular smooth muscle cells. Alpha 2 beta 1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- Stromblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG. Integrins as a distinct subtype of dependence receptors. Cell Death Differ. 2005;12:1021–1030. doi: 10.1038/sj.cdd.4401658. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- Tatrai A, Lee SK, Stern PH. U-73122, a phospholipase C antagonist, inhibits effects of endothelin-1 and parathyroid hormone on signal transduction in UMR-106 osteoblastic cells. Biochim Biophys Acta. 1994;1224:575–582. doi: 10.1016/0167-4889(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Welser JV, Lange N, Singer CA, Elorza M, Scowen P, Keef KD, et al. Loss of the alpha7 integrin promotes extracellular signal-regulated kinase activation and altered vascular remodeling. Circ Res. 2007;101:672–681. doi: 10.1161/CIRCRESAHA.107.151415. [DOI] [PubMed] [Google Scholar]
- Wilson SH, Caplice NM, Simari RD, Holmes DR, Jr, Carlson PJ, Lerman A. Activated nuclear factor-kappaB is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis. 2000;148:23–30. doi: 10.1016/s0021-9150(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Xiang D, Huang D, Gai L, Liu H. Relationship between expression of type III collagen and phenotype of vascular smooth muscle cells in neointimal of stented coronary artery. Chin Med J (Engl) 2000;113:324–327. [PubMed] [Google Scholar]
- Yeh CH, Wang WC, Hsieh TT, Huang TF. Agkistin, a snake venom-derived glycoprotein Ib antagonist, disrupts von Willebrand factor-endothelial cell interaction and inhibits angiogenesis. J Biol Chem. 2000;275:18615–18618. doi: 10.1074/jbc.C000234200. [DOI] [PubMed] [Google Scholar]
- Zhou L, Dong J, Yu M, Yin H, She M. Age-dependent increase of NF-kappaB translocation and PDGF-B expression in aortic endothelial cells of hypercholesterolemic rats. Exp Gerontol. 2003;38:1161–1168. doi: 10.1016/s0531-5565(03)00170-0. [DOI] [PubMed] [Google Scholar]


