Abstract
Background and purpose
Cannabidiol (CBD) is a non-psychotomimetic compound from Cannabis sativa which induces anxiolytic- and antipsychotic-like effects in rodents. These effects could be mediated by facilitation of the endocannabinoid system or by the activation of 5-HT1A receptors. As either of these mechanisms could promote adaptation to inescapable stress, the aim of the present work was to test the hypothesis that CBD would attenuate the autonomic and behavioural consequences of restraint stress (RS). We also investigated if the responses to CBD depended on activation of 5-HT1A receptors.
Experimental approach
Male Wistar rats received i.p. injections of vehicle or CBD (1, 10 or 20 mg kg−1) and 30 min later were submitted to 60 min of restraint where their cardiovascular responses were recorded. The protocol of the second experiment was similar to the first one except that animals received i.p. injections of the 5-HT1A receptor antagonist WAY100635 (0.1 mg kg−1) before CBD treatment and exposure to restraint. 24 h later they were also tested in the elevated plus-maze (EPM), an animal model of anxiety.
Key results
Exposure to RS increased blood pressure and heart rate and induced an anxiogenic response in the EPM 24 h later. These effects were attenuated by CBD. WAY100635 by itself did not change the cardiovascular and anxiogenic response to RS, but blocked the effects of CBD.
Conclusion and implications
The results suggest that CBD can attenuate acute autonomic responses to stress and its delayed emotional consequences by facilitating 5-HT1A receptor-mediated neurotransmission.
Keywords: cannabinoids, cardiovascular system, elevated plus-maze, 5-HT1A receptor
Introduction
Marijuana (from Cannabis sativa) is one of the most widely abused drugs in the world. In humans, it elicits subjective changes that include euphoria, heightened sensitivity to external stimuli and relaxation (Martin et al., 1991; Compton et al., 1992; Johns, 2001). The major constituent of cannabis is Δ9-tetrahydrocannabinol (THC) and this is thought to be the main ingredient responsible for its psychoactive properties (Mechoulam, 1970; Mechoulam et al., 1970; Ilan et al., 2005). The discovery of specific binding sites for THC led to the discovery of the cannabinoid receptors (Devane et al., 1988; Matsuda et al., 1990; Munro et al., 1993) and, so far, two sub-types of the cannabinoid receptor have been identified, CB1 and CB2 (Pertwee, 2005; nomenclature follows Alexander et al., 2008). The activation of CB1 receptors by THC is thought to account for most of the central effects of cannabis (Huestis et al., 2001). Anandamide and 2-arachidonoyl glycerol, referred to as endocannabinoids, are the major endogenous agonists of the CB1 receptor (Di Marzo et al., 1998; Piomelli, 2003).
Cannabidiol (CBD), another cannabinoid generally found in relatively high concentrations in cannabis, exhibits a somewhat different pharmacology compared with THC (Mechoulam et al., 2002). CBD attenuates the psychotomimetic and anxiogenic effects of THC in humans (Karniol et al., 1974; Zuardi et al., 1982). Moreover, systemic administration of CBD induced antipsychotic (Zuardi et al., 1991; Zuardi et al., 2006) and anxiolytic-like effects (Guimaraes et al., 1990; Resstel et al., 2006).
The mechanism of action of CBD is not fully understood. It has a low affinity for cannabinoid receptors (Petitet et al., 1998; Thomas et al., 1998) but can block the reuptake of anandamide (Bisogno et al., 2001) and its metabolism by the enzyme, fatty acid amide hydrolase (FAAH), (Watanabe et al., 1998; Di Marzo et al., 1999; Mechoulam and Hanus, 2002; Mechoulam et al., 2002). Moreover, CBD may possess agonistic properties at 5-HT1A receptors (Russo et al., 2005). Although there are contradictory results, several studies indicate that activation of these receptors can induce anxiolytic-like effects and mediate adaptation to stress (Blier and de Montigny, 1994; Blier and Ward, 2003; Joca et al., 2003; Joca et al., 2007).
Acute restraint is an uncontrollable stress situation that produces endocrine and autonomic responses characterized by increases in glucocorticoids levels, blood pressure and heart rate (HR) (Tavares and Correa, 2006; Hsu et al., 2007). These responses are accompanied by activation of several brain structures (Pacak and Palkovits, 2001). In addition to physiological responses, animals submitted to restraint also develop behavioural changes reflected, for example, in reduced exploratory activity in an open field 24 h after stress (Kennett et al., 1985a; 1987; Mechiel Korte and De Boer, 2003), increased immobility in a forced swimming test (Sevgi et al., 2006) and reduced exploration of the open arms of an elevated plus-maze (EPM) (Guimaraes et al., 1993; Padovan and Guimaraes, 2000). These stress-induced behavioural changes can be attenuated by systemic or intracerebral administration of anxiolytic and antidepressant drugs (Kennett et al., 1985a; 1987; Guimaraes et al., 1993; Padovan and Guimaraes, 2000; Mechiel Korte and De Boer, 2003). A possible effect of CBD on these changes, however, has not yet been investigated. Therefore, in the present work we tested the hypothesis that systemic administration of CBD would attenuate the acute physiological changes and the behavioural consequences of restraint stress. We also evaluated the involvement of 5-HT1A receptors in the effects of CBD in this model.
Methods
Animals
The Institution's Animal Ethics Committee approved housing conditions and experimental procedures for animals. Male Wistar rats weighing 230–250 g were used. The animals were provided by our local Animal farm facility. After arriving at the Animal Care Unit of the Department of Pharmacology, School of Medicine of Ribeirão Preto, University of Sao Paulo, the animals were kept in groups of four per cage for 48 h. After this adaptation period they are housed individually in plastic cages with free access to food and water under a 12 h light/dark cycle (lights on at 06:30 h) for a further 24 h period before being subjected to the surgical procedure (see below).
Surgical preparation
Twenty-four hours before being submitted to restraint stress, animals had a polyethylene catheter implanted into the femoral artery under anaesthesia (tribromoethanol, 250 mg kg−1 i.p.), for the recording of arterial blood pressure and HR. The catheter was exposed on the dorsum of the animals and attached to the skin, allowing arterial pressure recordings from conscious animals.
Acute restraint
Experiment 1
In the morning period (between 7 a.m. and 12 a.m.), the animals were transferred to the experimental room in their home box. Mean arterial pressure (MAP) and HR were recorded using an HP-7754A amplifier (Hewlett Packard, Palo Alto, CA, USA) connected to a signal acquisition board (Biopac M-100, Goleta, CA, USA) and computer processed. After a few minutes of baseline recording, rats received a single i.p. injection of one of the following drugs: vehicle or CBD (1, 10 or 20 mg kg−1). Thirty minutes later they were submitted to a 60 min restraint period in a small plastic cylindrical restraining tube (diameter = 6.5 cm and length = 15 cm). After the restraint period the animals returned to their cages. Each animal was submitted to only one session of restraint to prevent the development of stress tolerance (Guimaraes et al., 1993).
Experiment 2
Based on the results obtained in the first experiment, we chose the dose of 10 mg kg−1 of CBD to use in this second study. The protocol was similar to the first experiment except that before the restraint period the animals received, first, an i.p. injection of vehicle or WAY (0.1 mg kg−1) followed, 30 min later, by a second injection of vehicle or CBD (10 mg kg−1). As in experiment 1, 30 min after the last injection the animals were restrained for 60 min. One day later they were tested in the EPM. We also had a general control group of unrestrained animals treated with the saline+CBD vehicle that were tested 24 h later in the EPM.
The EPM test
The EPM test was conducted as described before (Padovan and Guimaraes, 2000). Briefly, the apparatus consisted of two opposite open arms (50 × 10 cm) crossed at a right angle by two arms of the same dimensions enclosed by 40 cm high walls with no roof. The maze was located 50 cm above the floor. Rodents naturally avoid the open arms of the EPM and anxiolytic compounds typically increase the exploration of these arms without changing the number of enclosed-arm entries (Pellow et al., 1985; Carobrez and Bertoglio, 2005). The EPM was cleaned and dried before each session and the Ethovision software (Version 1.9, Noldus, Netherlands) was employed for behavioural analysis.
Data analysis
Mean arterial pressure and HR values were continuously recorded for 10 min before the 60 min restraint stress period. Data were expressed as means ± SEM of MAP or HR changes (respectively Δ MAP and Δ HR) sampled at 5 min intervals. Points sampled during the 10 min before restraint were used as control baseline value. MAP and HR changes were analysed using two-way anova with treatment as independent factor and time as repeated measurement factor. When interaction between the factors was observed, groups were compared using one-way anova followed by Bonferroni's post hoc test.
The per cent of entries (100 × open/total entries) and time spent in the open arms (100 × open/open + enclosed) of the EPM were calculated for each rat. These data, together with the number of enclosed arm entries, were analysed by one-way anova followed by Bonferroni's post hoc test. Values of P < 0.05 were taken as showing statistically significant differences between means.
Drugs
The following drugs were used: CBD (THC Pharma, Frankfurt, Germany): 1, 10 or 20 mg kg−1, suspended in polyoxyethylenesorbitan monooleate (Tween 80) 2%-saline (Resstel et al., 2006), WAY100635 (WAY, Sigma, St. Louis, MO, USA): 0.1 mg kg−1 dissolved in saline (Kaster et al., 2005) and tribromoethanol (Aldrich, St. Louis, MO, USA). The solutions were prepared immediately before use and injected intraperitoneally in a volume of 1 mL kg−1. The appropriate vehicles were used in each experiment.
Results
Experiment 1
Effects of CBD on cardiovascular responses to acute restraint
Systemic injection of CBD (1, 10 and 20 mg kg−1) did not affect baseline blood pressure (F4,25 = 1.2, P > 0.05) or HR (F4,25 = 0.9, P > 0.05) values when compared with vehicle control (n = 6, Table 1). As represented in Figure 1, acute restraint induced a marked and sustained increase of HR and MAP during the 60 min test. There were significant effects of treatment (MAP: F3,240 = 93.5, P < 0.001; HR: F3,240 = 123, P < 0.001), time (MAP: F14,240 = 83.6, P < 0.001; HR: F14,240 = 27.9, P < 0.001) and treatment versus time interaction (MAP: F42,240 = 5.1, P < 0.01; HR: F42,240 = 7.9, P < 0.01).
Table 1.
Basal values of the MAP and HR in vehicle (control), CBD and WAY100635 treated rats
| Group | MAP (mmHg) | HR (bpm) | |
|---|---|---|---|
| Control | n = 6 | 105 ± 2 | 338 ± 13 |
| CBD 1 mg | n = 5 | 103 ± 3 | 363 ± 14 |
| CBD 10 mg | n = 5 | 100 ± 3 | 349 ± 11 |
| CBD 20 mg | n = 5 | 97 ± 3 | 369 ± 9 |
| WAY100635 | n = 5 | 99 ± 2 | 371 ± 14 |
| F4,25 = 1.5, P > 0.05 | F4,25 = 1.3, P > 0.05 |
The values in the table represent the means ± SE. One-way anova.
CBD, cannabidiol; HR, heart rate; MAP, mean arterial pressure.
Figure 1.
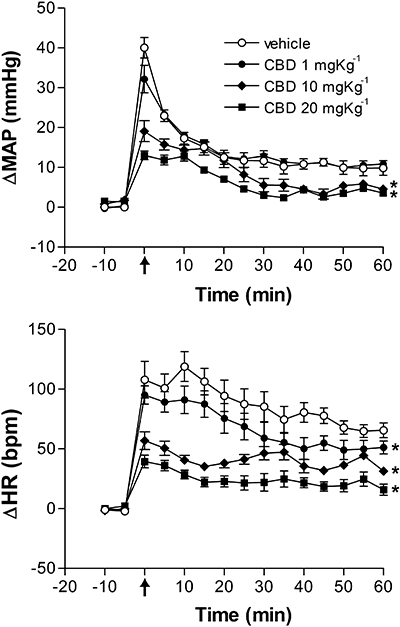
Effects of different doses of cannabidiol (CBD, 1, 10 or 20 mg kg−1, n = 5 per group) or vehicle (Tween 80 2%-saline, 1 mL kg−1, n = 6) on changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) of animals submitted to 60 min of restraint stress. The arrow indicates the beginning of the restraint period. Data shown represent the means ± SEM. *P < 0.05, compared with vehicle group; anova followed by Bonferroni's post hoc test.
Cannabidiol decreased the stress-induced cardiovascular responses at doses of 10 and 20 mg kg−1 (HR, F3,16 = 19.9, P < 0.001, MAP, F3,16 = 14.6, P < 0.001). The dose of 1 mg kg−1 was also able to attenuate HR responses (P < 0.05). A dose-dependency was demonstrated by nonlinear regression analysis and showed a significant correlation between doses and attenuation of the increased MAP (r2 = 0.82, df = 13, P < 0.01) and HR (r2 = 0.73, df = 13, P < 0.01) (Fig. 2).
Figure 2.
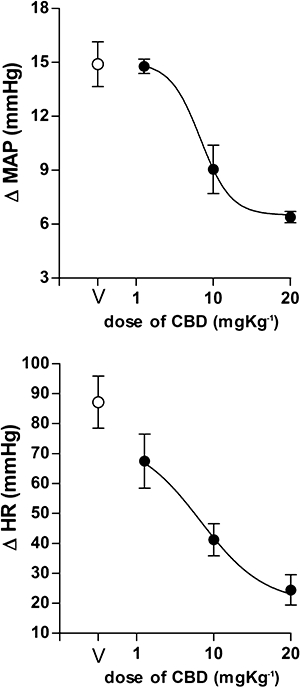
Mean arterial pressure (ΔMAP) and heart rate (ΔHR) changes in response to the injection of increasing doses of cannabidiol (CBD, 1, 10, 20 mg kg−1, n = 5/group) in rats. V: vehicle (Tween 80 2%-saline, 1 mL kg−1, n = 6). Dose- effect curves were generated by nonlinear regression analysis. Data shown represent the means ± SEM of the variation of MAP and HR during the 60 min of acute restraint.
Experiment 2a
Effects of WAY100635 on CBD effects on acute cardiovascular responses to restraint
WAY (n = 5) did not affect baseline values of blood pressure (F4,25 = 1.2, P > 0.05) or HR (F4,25 = 0.9, P > 0.05) compared with CBD (10 mg kg−1, n = 6) and vehicle (n = 6, Table 1).
There were significant effects of restraint (MAP: F3,270 = 35.95, P < 0.001; HR: F3,270 = 104.3, P < 0.001), time (MAP: F14,270 = 59.66, P < 0.001; HR: F14,270 = 66.4, P < 0.001) and treatment versus time interaction (MAP: F42,270 = 2.1, P < 0.01; HR: F42,270 = 1.9, P < 0.01) (Fig. 3).
Figure 3.

Effects of pre-treatment with of saline (Sal) or WAY100635 (WAY, 0.1 mg kg−1) followed by second injection of vehicle (Veh, Tween 80 2%-saline) or cannabidiol (CBD, 10 mg kg−1) immediately before a 60 min restraint period on increase in the mean arterial pressure (ΔMAP) and heart rate (ΔHR) induced by restraint stress. The arrow indicates the beginning of the restraint period. Data shown represent the mean ± SEM of five to six animals. *P < 0.05, compared with vehicle group; anova followed by Bonferroni's post hoc test.
The decrease of the cardiovascular responses by systemic administration of CBD (10 mg kg−1) was prevented by WAY (MAP: F1,135 = 139.5, P < 0.001; HR: F1,135 = 290.3, P < 0.001) (Fig. 3). The latter drug, by itself, had no effect on cardiovascular responses to restraint (MAP: F1,135 = 1.3, P > 0.05; HR: F1,135 = 1.1, P > 0.05) (Fig. 3).
Typical experimental recordings showing the effects of CBD and WAY on cardiovascular responses observed during acute restraint can be seen in Figure 4.
Figure 4.

Mean arterial pressure (MAP), pulsatile arterial pressure (PAP) and heart rate (HR) individual recordings showing the cardiovascular changes evoked by acute restraint in animals treated with vehicle (control, Tween 80 2%-saline), cannabidiol (CBD) or cannabidiol after WAY100635 (WAY+CBD). The restraint period started at time 0.
Experiment 2b
Effects of CBD and WAY100635 on delayed behavioural consequences in the EPM induced by restraint
Acute restraint induced a significant decrease in the percentage of open arm entries (F3,25 = 7.72, P < 0.001) compared with unrestrained controls (n = 6), when tested 24 h after stress (Fig. 5). CBD administration in restrained rats (n = 6) increased the percentage of open arm entries (P < 0.001, Fig. 5) compared with controls (vehicle-treated restrained animals, n = 6). This effect was prevented by pre-administration of WAY (P < 0.001, n = 6). No effect was found in animals treated only with WAY (P > 0.05, n = 6) (Fig. 5). There was also no effect on the percentage time spent in the open arms and in the number of enclosed arm entries (P > 0.05).
Figure 5.
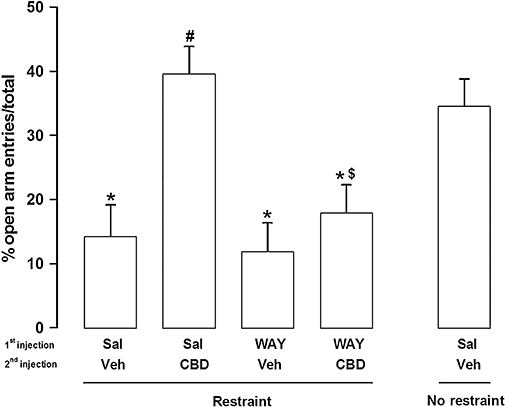
Effects in the elevated plus-maze (EPM) of a first systemic injection of saline (Sal) or WAY100635 (WAY, 0.1 mg kg−1) followed by a second injection of vehicle (Veh, Tween 80 2%-saline) or cannabidiol (CBD, 10 mg kg−1) immediately before a 60 min restraint period (n = 6 per group). A non-stressed group (no-restraint) that received i.p. injections of saline followed by vehicle was used as general control. The EPM test was performed 24 h after the restraint period. Data represent the mean (±SEM) per cent of open arm entries. *P < 0.05, compared with control group; #P < 0.05, compared with restraint-vehicle group; $P < 0.05, compared with restraint-CBD group; anova followed by Bonferroni's post hoc test.
Discussion
The present results showed that (i) CBD reduced the pressor and tachycardic responses to restraint stress, in a dose-dependent manner; (ii) CBD attenuated the increased anxiety behaviour caused by a previous exposure to restraint; and (iii) effects of CBD on cardiovascular and behavioural responses to restraint can be blocked by WAY100635, a 5-HT1A receptor antagonist.
Acute exposure to restraint stress has been shown to evoke several physiological and behavioural changes (Tavares and Correa, 2006; Hsu et al., 2007) and, as expected, in the present study animals exhibited significant increases in MAP and HR during restraint. Moreover, they showed decreased exploration of the open arms of the EPM 24 h after restraint stress. These delayed behavioural consequences of acute restraint stress have been described in several models, including the open field and EPM (Kennett et al., 1985a; Guimaraes et al., 1993; Padovan and Guimaraes, 2000; Mechiel Korte and De Boer, 2003), and are sensitive to systemic and intra-cerebral injection of anxiolytic and antidepressant drugs (Kennett et al., 1985a; Kennett et al., 1987; Guimaraes et al., 1993; Padovan and Guimaraes, 2000; Mechiel Korte and De Boer, 2003).
Cannabidiol did not induce any significant change in basal MAP and HR which agrees with the reported lack of significant cardiovascular effects of this drug (McQueen et al., 2004; Resstel et al., 2006). At low doses, it also does not interfere with memory and learning processes (Lichtman et al., 1995; Fadda et al., 2004; 2006). It is unlikely, therefore, that the attenuation of the cardiovascular and delayed behavioural responses to restraint depend on direct cardiovascular effects or memory impairment induced by the drug, but rather on an attenuation of the emotional response to stress. In agreement with this proposal, acute administration of CBD has been shown to induce anxiolytic-like effects in several animal models, including the EPM, Vogel conflict test and contextual fear conditioning (Guimaraes et al., 1990; Onaivi et al., 1990; Moreira et al., 2006; Resstel et al., 2006). The effective doses of CBD in these previous studies were, in general, similar to ours. In the study by Guimaraes et al. (1990), however, CBD produced an inverted U-shaped dose-response curve, with the highest dose (20 mg kg−1) being ineffective. The reasons for this difference are unknown, but may depend on the model used (Calabrese, 2008). As the EPM test is based on exploratory activity, it could be more prone to interference of non-specific drug effects that affects this parameter (Calabrese, 2008). In addition, at least for classical anxiolytics such as diazepam, the EPM is more sensitive than other models such as the Vogel conflict test (see Calabrese, 2008).
The mechanisms of the anxiolytic and anti-stress effects of CBD are not clear. It has a low affinity for cannabinoid receptors (Petitet et al., 1998; Thomas et al., 1998) although, under certain circumstances, it may act as an antagonist of CB1- and CB2-receptor agonists (Pertwee et al., 2002). CBD could also block the reuptake and metabolism of anandamide, facilitating endocannabinoid-mediated neurotransmission (Watanabe et al., 1998; Di Marzo et al., 1999; Bisogno et al., 2001; Mechoulam et al., 2002). However, several effects of CBD have been shown to be independent of the endocannabinoid system (Hayakawa et al., 2007). More recently, Russo et al. (2005) reported that CBD can displace the 5-HT agonist [3H]8-OH-DPAT from cloned human 5-HT1A receptors expressed in Chinese hamster ovary cultured cells. Moreover, using signal transduction studies, this work also showed that CBD can act as an agonist at these receptors. The observation that CBD has agonistic properties at 5-HT1A receptors has been supported by in vivo studies where the neuroprotective and anti-oxidative effects induced by CBD were blocked by pre-treatment with the 5-HT1A antagonist WAY100135 (Mishima et al., 2005; Hayakawa et al., 2007). Our results corroborate these findings, by showing that the stress-attenuating effects induced by CBD were prevented by systemic pretreatment with WAY100635, a selective antagonist at these receptors. In agreement with our results, a recent study from our laboratory showed that CBD interacts with 5-HT1A receptors in the dorsolateral periaqueductal gray to produce anxiolytic-like effects in the EPM (Campos and Guimaraes, 2008).
5-HT1A receptors are widely distributed in the brain, especially in structures traditionally related to stress and anxiety, such as the raphé nuclei, hippocampus, prefrontal cortex, amygdala and hypothalamus (Chalmers and Watson, 1991). Although the role of 5-HT in anxiety is still a matter of intense debate (Millan, 2003), several pieces of evidence suggest that activation of 5-HT1A receptors facilitates adaptation to stress (Kostowski et al., 1992; Dekeyne et al., 2000; Tsuji et al., 2000; Blier and Ward, 2003; Joca et al., 2003; Kagamiishi et al., 2003; Rioja et al., 2004; Joca et al., 2007). For example, pre-administration of a 5-HT1A agonist before an acute immobilization period blocked the stress-induced anxiogenic effect observed in an elevated T-maze test performed 24 h later (Rioja et al., 2004). Similar results were reported by Tsuji et al. (2000), who observed that administration of 5-HT1A receptor agonists before restraint stress attenuated, in a dose-dependent manner, the development of stress-induced anxiogenic effect observed 24 h later in the hole-board test of anxiety. Data from 5-HT1A receptor knockout mice give further support to this hypothesis, as these animals display anxiogenic-like behaviour in different paradigms (Zhuang et al., 1999; Tsetsenis et al., 2007). It is proposed that the lack of this receptor would promote a bias in the processing of threatening cues which could render the animal more susceptible to the development of behavioural consequences of stress (Tsetsenis et al., 2007). On the other hand, facilitation of this neurotransmission would mediate adaptation to stress (Graeff et al., 1996). This suggestion is supported by results showing that behavioural adaptation to stress is accompanied by sensitization of 5-HT1A-mediated neurotransmission (Kennett et al., 1985b; 1987; Samad and Haleem, 2007) and that an increased expression of this receptor in the brain is associated with reduced anxiety-like behaviour (Kusserow et al., 2004). Therefore, these studies support the possibility that activation of 5-HT1A receptors protects animals against various emotional changes caused by stressful stimuli, perhaps by facilitating mechanisms involved in the ability to cope with the stressful situation.
The exact mechanism through which 5-HT1A agonists induce their anxiolytic activity remains unclear. 5-HT1A receptors are located presynaptically (somatodendritic autoreceptors) in 5-hydroxytryptaminergic cell bodies in the raphé nuclei of the brain stem and postsynaptically, predominantly in limbic structures such as the hippocampus and the hypothalamus (Verge et al., 1985; 1986; Chalmers and Watson, 1991). It is still controversial whether the anxiolytic-like effects induced by acute systemic administration of 5-HT1A agonists are due to the activation of the pre- or the post-synaptic receptors (File et al., 1996; Lopez-Rubalcava, 1996). Moreover, depending on the structure where post-synaptic 5-HT1A receptors are located, their activation may lead to anxiolytic or anxiogenic-like effects (Graeff et al., 1996; Zangrossi et al., 2001). Therefore, the exact mechanism of action of 5-HT1A agonists as anxiolytic compounds is complex and still warrants further investigation.
In conclusion, the present findings indicate that CBD, by activating 5-HT1A receptors, can attenuate physiological and behavioural responses to restraint stress. This finding raises the possibility that CBD could be useful for treating psychiatric disorders thought to involve impairment of stress-coping mechanisms, such as depression and post-traumatic stress disorder.
Acknowledgments
The authors wish to thank Ivanilda A.C. Fortunato and José Carlos A. for technical help. The present research was supported by grants from CNPq and FAPESP.
Glossary
Abbreviations
- CBD
cannabidiol
- EPM
elevated plus-maze
- HR
heart rate
- MAP
mean arterial pressure
- THC
Δ9-tetrahydrocannabinol
Conflicts of interest
The authors state no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd) 2008;153(Suppl.2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. An assessment of anxiolytic drug screening tests: hormetic dose responses predominate. Crit Rev Toxicol. 2008;38:489–542. doi: 10.1080/10408440802014238. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimaraes FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain – a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201–209. [PubMed] [Google Scholar]
- Dekeyne A, Brocco M, Adhumeau A, Gobert A, Millan MJ. The selective serotonin (5-HT)1A receptor ligand, S15535, displays anxiolytic-like effects in the social interaction and Vogel models and suppresses dialysate levels of 5-HT in the dorsal hippocampus of freely-moving rats. A comparison with other anxiolytic agents. Psychopharmacology (Berl) 2000;152:55–66. doi: 10.1007/s002130000449. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T, Melck D. Metabolism of anandamide and 2-arachidonoylglycerol: an historical overview and some recent developments. Lipids. 1999;34(Suppl.):S319–S325. doi: 10.1007/BF02562332. [DOI] [PubMed] [Google Scholar]
- Fadda P, Robinson L, Fratta W, Pertwee RG, Riedel G. Differential effects of THC- or CBD-rich cannabis extracts on working memory in rats. Neuropharmacology. 2004;47:1170–1179. doi: 10.1016/j.neuropharm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Fadda P, Robinson L, Fratta W, Pertwee RG, Riedel G. Scopolamine and MK801-induced working memory deficits in rats are not reversed by CBD-rich cannabis extracts. Behav Brain Res. 2006;168:307–311. doi: 10.1016/j.bbr.2005.11.022. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Chiaretti TM, Graeff FG, Zuardi AW. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 1990;100:558–559. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Del Bel EA, Padovan CM, Netto SM, de Almeida RT. Hippocampal 5-HT receptors and consolidation of stressful memories. Behav Brain Res. 1993;58:133–139. doi: 10.1016/0166-4328(93)90098-b. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Irie K, Fujioka M, et al. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J Neurochem. 2007;102:1488–1496. doi: 10.1111/j.1471-4159.2007.04565.x. [DOI] [PubMed] [Google Scholar]
- Hsu HR, Chen TY, Chan MH, Chen HH. Acute effects of nicotine on restraint stress-induced anxiety-like behavior, c-Fos expression, and corticosterone release in mice. Eur J Pharmacol. 2007;566:124–131. doi: 10.1016/j.ejphar.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005;16:487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- Joca SR, Padovan CM, Guimaraes FS. Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res. 2003;978:177–184. doi: 10.1016/s0006-8993(03)02943-3. [DOI] [PubMed] [Google Scholar]
- Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry. 2001;178:116–122. doi: 10.1192/bjp.178.2.116. [DOI] [PubMed] [Google Scholar]
- Kagamiishi Y, Yamamoto T, Watanabe S. Hippocampal serotonergic system is involved in anxiety-like behavior induced by corticotropin-releasing factor. Brain Res. 2003;991:212–221. doi: 10.1016/j.brainres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9 – tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Santos AR, Rodrigues AL. Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test. Brain Res Bull. 2005;67:53–61. doi: 10.1016/j.brainresbull.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Dickinson SL, Curzon G. Central serotonergic responses and behavioural adaptation to repeated immobilisation: the effect of the corticosterone synthesis inhibitor metyrapone. Eur J Pharmacol. 1985a;119:143–152. doi: 10.1016/0014-2999(85)90290-0. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Dickinson SL, Curzon G. Enhancement of some 5-HT-dependent behavioural responses following repeated immobilization in rats. Brain Res. 1985b;330:253–263. doi: 10.1016/0006-8993(85)90684-5. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Dourish CT, Curzon G. Antidepressant-like action of 5-HT1A agonists and conventional antidepressants in an animal model of depression. Eur J Pharmacol. 1987;134:265–274. doi: 10.1016/0014-2999(87)90357-8. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Dyr W, Krzascik P, Jarbe T, Archer T. 5-Hydroxytryptamine1A receptor agonists in animal models of depression and anxiety. Pharmacol Toxicol. 1992;71:24–30. doi: 10.1111/j.1600-0773.1992.tb00515.x. [DOI] [PubMed] [Google Scholar]
- Kusserow H, Davies B, Hortnagl H, Voigt I, Stroh T, Bert B, et al. Reduced anxiety-related behaviour in transgenic mice overexpressing serotonin 1A receptors. Brain Res Mol Brain Res. 2004;129:104–116. doi: 10.1016/j.molbrainres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Lopez-Rubalcava C. Pre- or postsynaptic activity of 5-HT1A compounds in mice depends on the anxiety paradigm. Pharmacol Biochem Behav. 1996;54:677–686. doi: 10.1016/0091-3057(96)00018-4. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Bond SM, Smith PJ, Balali-Mood K, Smart D. Cannabidiol lacks the vanilloid VR1-mediated vasorespiratory effects of capsaicin and anandamide in anaesthetised rats. Eur J Pharmacol. 2004;491:181–189. doi: 10.1016/j.ejphar.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechiel Korte S, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Marihuana chemistry. Science. 1970;168:1159–1166. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Hanus L. Cannabidiol: an overview of some chemical and pharmacological aspects. Part I: chemical aspects. Chem Phys Lipids. 2002;121:35–43. doi: 10.1016/s0009-3084(02)00144-5. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Shani A, Edery H, Grunfeld Y. Chemical basis of hashish activity. Science. 1970;169:611–612. doi: 10.1126/science.169.3945.611. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36:1077–1082. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Guimaraes FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1466–1471. doi: 10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253:1002–1009. [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Padovan CM, Guimaraes FS. Restraint-induced hypoactivity in an elevated plus-maze. Braz J Med Biol Res. 2000;33:79–83. doi: 10.1590/s0100-879x2000000100011. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA, Craib SJ, Thomas A. )-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur. J Pharmacol. 2002;456:99–106. doi: 10.1016/s0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of delta9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63:PL1–PL6. doi: 10.1016/s0024-3205(98)00238-0. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Moreira FA, Correa FM, Guimaraes FS. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res. 2006;172:294–298. doi: 10.1016/j.bbr.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Rioja J, Santin LJ, Garcia M, Dona A, De Pablos L, Cuadrado MI, et al. 5-HT1A receptor activation before acute stress counteracted the induced long-term behavioral effects. Ann N Y Acad Sci. 2004;1018:333–338. doi: 10.1196/annals.1296.041. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Samad N, Haleem DJ. Serotonin-1A receptor responsiveness in stress and following adaptation to stress. Pak J Pharm Sci. 2007;20:115–119. [PubMed] [Google Scholar]
- Sevgi S, Ozek M, Eroglu L. L-NAME prevents anxiety-like and depression-like behavior in rats exposed to restraint stress. Methods Find Exp Clin Pharmacol. 2006;28:95–99. doi: 10.1358/mf.2006.28.2.977840. [DOI] [PubMed] [Google Scholar]
- Tavares RF, Correa FM. Role of the medial prefrontal cortex in cardiovascular responses to acute restraint in rats. Neuroscience. 2006;143:231–240. doi: 10.1016/j.neuroscience.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–292. [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Takeda H, Matsumiya T. Different effects of 5-HT1A receptor agonists and benzodiazepine anxiolytics on the emotional state of naive and stressed mice: a study using the hole-board test. Psychopharmacology (Berl) 2000;152:157–166. doi: 10.1007/s002130000514. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Patey A, Gozlan H, el Mestikawy S, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol. 1985;113:463–464. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, et al. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ogi H, Nakamura S, Kayano Y, Matsunaga T, Yoshimura H, et al. Distribution and characterization of anandamide amidohydrolase in mouse brain and liver. Life Sci. 1998;62:1223–1229. doi: 10.1016/s0024-3205(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Jr, Viana MB, Zanoveli J, Bueno C, Nogueira RL, Graeff FG, et al. Serotonergic regulation of inhibitory avoidance and one-way escape in the rat elevated T-maze. Neurosci Biobehav Rev. 2001;25:637–645. doi: 10.1016/s0149-7634(01)00047-1. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21:52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Rodrigues JA, Cunha JM. Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology (Berl) 1991;104:260–264. doi: 10.1007/BF02244189. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimaraes FS. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39:421–429. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]


