Abstract
Background and purpose
Transient lower oesophageal sphincter relaxations (TLESRs) are the main mechanism underlying gastro-oesophageal reflux and are a potential pharmacological treatment target. We evaluated the effect of the CB1/CB2 receptor agonist Δ9-tetrahydrocannabinol (Δ9-THC) on TLESRs in dogs. Based on these findings, the effect of Δ9-THC was studied in healthy volunteers.
Experimental approach
In dogs, manometry was used to evaluate the effect of Δ9-THC in the presence and absence of the CB1 receptor antagonist SR141716A on TLESRs induced by gastric distension. Secondly, the effect of 10 and 20 mg Δ9-THC was studied in 18 healthy volunteers in a placebo-controlled study. Manometry was performed before and for 3 h after meal ingestion on three occasions.
Key results
In dogs, Δ9-THC dose-dependently inhibited TLESRs and reduced acid reflux rate. SR141716A significantly reversed the effects of Δ9-THC on TLESRs. Similarly, in healthy volunteers, Δ9-THC significantly reduced the number of TLESRs and caused a non-significant reduction of acid reflux episodes in the first postprandial hour. In addition, lower oesophageal sphincter pressure and swallowing were significantly reduced by Δ9-THC. After intake of 20 mg, half of the subjects experienced nausea and vomiting leading to premature termination of the study. Other side-effects were hypotension, tachycardia and central effects.
Conclusions and implications
Δ9-THC significantly inhibited the increase in meal-induced TLESRs and reduced spontaneous swallowing in both dogs and humans. In humans, Δ9-THC significantly reduced basal lower oesophageal sphincter pressure. These findings confirm previous observations in dogs and indicate that cannabinoid receptors are also involved in the triggering of TLESRs in humans.
Keywords: gastro-oesophageal reflux disease, cannabinoid, transient lower oesophageal sphincter relaxation, Δ9-tetrahydrocannabinol
Introduction
Gastro-oesophageal reflux disease (GERD) is a common disorder with symptoms like heartburn, regurgitation and retrosternal pain (Heading, 1999), resulting from prolonged acid exposure of the oesophageal mucosa. The lower oesophageal sphincter (LES) plays a central role in regulating flow across the gastro-oesophageal junction by generating a tonic pressure to prevent reflux of gastric contents into the oesophagus. Transient lower oesophageal sphincter relaxations (TLESRs), occurring in the absence of a swallow, are the main mechanism underlying reflux in healthy volunteers and in patients with GERD (Dent et al., 1980; Dodds et al., 1982; Holloway, 2000). Therefore, pharmacological inhibition of TLESRs is a potential target to treat GERD.
TLESRs are triggered by gastric distension resulting from an increased activation of gastric mechanoreceptors in the subcardiac region of the stomach. This leads to activation of a reflex pathway involving gastric vagal afferents, brainstem integrative circuits and efferent inhibitory pathways to the LES and crural diaphragm (Mittal et al., 1995). Vagal cooling and cervical vagotomy abolish the triggering of TLESRs, indicating that TLESRs are mediated by a vago-vagal pathway (Martin et al., 1986; Abrahams et al., 2002). The insight in the neural pathway and the neurotransmitters involved in this reflex has increased enormously (Hirsch et al., 2002). To date the most potent pharmacological agents to reduce the rate of TLESRs are GABAB receptor agonists. The GABAB receptor agonist baclofen reduces the rate of TLESRs and the number of acid reflux episodes in normal subjects (Lidums et al., 2000) and in GERD patients (Zhang et al., 2002; Koek et al., 2003; Vela et al., 2003). This effect most likely depends on inhibition of mechanosensitive gastric vagal afferents and their central synaptic connections with brain stem neurons, leading to a raised threshold for action potential firing and reduction of transmitter release respectively (Partosoedarso et al., 2001).
Cannabinoid (CB) receptors, like GABAB receptors, belong to the superfamily of G protein-coupled receptors (Pertwee, 1997; 1999). There are two types of CB receptors, the CB1 and CB2 receptors. CB1 receptors are mainly localized in the central nervous system whereas CB2 receptors are particularly associated with the immune system. Interestingly, CB1 receptors have been located in brain areas involved in the triggering of TLESRs as well as in the nodose ganglion from which vagal afferents emanate (Van Sickle et al., 2001; Partosoedarso et al., 2003). Moreover a study in dogs showed that the CB receptor agonist WIN 55,212-2 reduced the occurrence of TLESRs in response to gastric distension by 80% (Lehmann et al., 2002). A study in ferrets confirmed the involvement of CB1 receptors in the central regulation of LES relaxation and showed the presence of CB1 receptors in the brain centres involved in the triggering of TLESRs (Partosoedarso et al., 2003). These data indicate that CB1 agonists may be clinically useful to reduce TLESRs in humans and may have the potential to be used as reflux inhibitors.
To date, no specific CB1 agonists are available for human use. However, marijuana and its main psychoactive component Δ9-tetrahydrocannabinol (Δ9-THC) are frequently used as anti-emetics (Tortorice and O'Connell, 1990; Tramer et al., 2001) or as an appetite stimulant for example in patients who fail to respond adequately to conventional therapies, in particular cancer patients receiving chemotherapy or radiotherapy, or patients undergoing HIV therapy (Porter and Felder, 2001; Tramer et al., 2001; Massa and Monory, 2006). Δ9-THC has approximately equal affinity for the CB1 and CB2 receptors, but its efficacy is less at the CB2 receptor (Grotenhermen, 2003). In the present study, therefore, we first evaluated whether Δ9-THC had an inhibitory effect in dogs on the triggering of TLESRs, as previously shown for the specific CB1 agonist WIN 55,212-2. The pharmacodynamic/kinetic relationship in dogs was then used to design a study in healthy human volunteers evaluating its effect on the occurrence of meal-induced TLESRs.
Methods
Dog study
Animals
Ten adult male and female Labrador retrievers were used in the experiments. Cervical oesophagostomies were made, and after recovery from surgery the dogs were accustomed to rest in a Pavlov stand. Before each experiment, the dogs were fasted overnight with free access to water. A washout of at least 3 days was allowed between experiments. All procedures were approved by the Ethical Committee for Animal Experiments of the Göteborg region.
Protocol
The dogs were intubated with a water-perfused Dentsleeve multilumen catheter to record gastric, LES and oesophageal pressures. An antimony pH electrode was placed 3 cm above the LES to measure acid reflux episodes and a water-perfused catheter was placed in the hypopharynx to measure swallows.
TLESRs were stimulated by infusion into the stomach through the central lumen of the assembly of an acidified liquid nutrient (30 ml kg−1; 100 ml min−1) followed by air insufflation (500 ml min−1) to maintain gastric pressure between 10 ± 1 mm Hg.
The number of TLESRs was measured for a 45 min period starting from the infusion of the liquid. TLESRs were defined as a rapid decrease of LES pressure (>1 mm Hg s−1) to a pressure <2 mm Hg above gastric pressure and a duration of >1 s, without any pharyngeal signal <2 s before onset (Lehmann et al., 1999).
Δ9-THC was administered intragastrically (2 ml kg−1), 30 min before the start of the experiment (T = 0). In experiments with the CB1 antagonist SR141716A, the compound was administered intragastrically (2 ml kg−1) 45 min before start of measurements, followed by Δ9-THC 15 min later. Except for TLESRs and acid reflux episodes, basal LES pressure, latency time from start to first TLESR and swallowing rate were determined. Basal LES pressure was defined as the average pressure during the 45 min experimental period. Reflux episodes were defined as a drop in pH > 1 u within 5 s. The average pH in the 15 s following nadir pH should be <4.
Plasma sampling and analysis
Blood samples were taken from a foreleg vein from two dogs at each dose. After separation of the plasma the samples were stored at −18°C until analysis. The analysis of the plasma was performed by using MS/MS detection after liquid extraction and chromatographic separation; 100 µl plasma was extracted by shaking with 700 µl methyl-tert-buthyl ether/hexane 50/50 v/v (volume in volume) for 30 min. A 450 µl sample of the organic phase was evaporated to dryness and reconstituted in 150 µl 30% acetonitrile, 0.2% formic acid. A 10 µl aliquot was injected onto a reversed phase C18 column, eluted by a fast gradient and detected by electrospray tandem mass spectrometry in positive ion mode.
Drugs
Δ9-THC was purchased from Sigma Aldrich (Stockholm, Sweden) and was diluted in 5% ethanol, 80% polyethylene glycol and 15% physiological saline (0.9% NaCl). The intragastrically used doses of Δ9-THC were 0.25 µmol kg−1 (0.079 mg kg−1), 0.40 µmol kg−1 (0.126 mg kg−1) and 0.60 µmol kg−1 (0.189 mg kg−1). SR141716A [rimonabant; N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride] was dissolved in 5% ethanol, 60% polyethylene glycol and 35% physiological saline (0.9% NaCl). The intragastrically used dose of SR141716A was 0.22 µmol kg−1 (0.1 mg kg−1).
Data analysis and statistics
Each dog served as its own control. All parameters were calculated with regard to the mean of five preceding control experiments for each dog. Data are presented as means ± s.e.mean. Statistical analysis was performed by using Student's paired t-tests. A P value <0.05 was regarded as statistically significant.
Human study
Subjects
Studies were performed in 18 healthy volunteers (male), ranging in age from 19 to 51 years (median 21.5). Subjects were free of any gastrointestinal symptoms, had no history of gastrointestinal surgery and did not take any medication known to influence gastrointestinal motility. They had no history of cannabis use or any other drug of abuse for at least 2 months prior to the study, and frequent cannabis users were excluded. Urine samples were taken to check for any recent drug abuse. Each volunteer gave written informed consent, and the study was approved by the Medical Ethical Committee of the Academic Medical Centre.
Study design
The effect of Δ9-THC (10 and 20 mg, p.o.) was evaluated in a randomized double-blind placebo-controlled study. On three different study days, all with a minimum interval of 1 week, subjects underwent combined oesophageal manometry and pH measurements. Intake of alcohol and nicotine was not allowed 24 h prior to and during the study. The studies were performed after an overnight fast. A cannula was inserted into a forearm vein for blood sampling. The manometric and pH catheter were introduced through an anaesthetized nostril and positioned so that the sleeve straddled the LES. The pH electrode was located 5 cm above the proximal margin of the LES. All studies were performed in the sitting position. Recording started just after dosing (T = 0). After 45 min of basal recording, the subjects received a meal (550 kcal), consisting of 2 pancakes (2 × 100 g) with jam (2 × 15 g) and orange juice (200 ml) to trigger the occurrence of TLESRs. After the meal (T = 60), patients remained upright, and manometric/pH recordings were obtained for three more hours. Blood pressure and heart rate were recorded before dosing, every 15 min for the first 2 h of the study, and every 30 min for the next 2 h. Blood samples were taken before dosing, at T = 30, 60, 120, 240 and 480 min. After the recording period, subjects had to stay for four more hours in the hospital for further monitoring of blood pressure and pulse once every hour. In order to screen for drug side-effects, symptoms of dizziness, nausea and sleepiness were assessed during the total study period.
Recording methods
Oesophageal manometry was performed by using a 10 lumen assembly with a sleeve sensor incorporated at its distal end to monitor LES pressure. Side holes monitored pressure in the stomach (1 cm below the distal margin of the sleeve) and at 2, 5, 8, 11, 14, 17 and 20 cm above the LES. A side hole in the pharynx monitored swallows. The side holes and the sleeve were perfused with degassed distilled water at 0.3 ml min−1 and 0.6 ml min−1 respectively, by using a pneumohydraulic capillary perfusion pump. The pharyngeal side hole was perfused with air at 0.6 ml min−1 to reduce pharyngeal triggering of TLESRs. Pressures were sensed by external transducers connected to a polygraph. To measure acid reflux, pH was recorded with an antimony electrode with built-in reference, positioned 5 cm above the proximal margin of the LES. Before and after the study the pH electrode was calibrated at 37°C by using pH 1.0 and 7.0 buffer solutions. Signals were digitalized, computer-processed, stored and analysed by using commercially available software (Polygram, Synectics Medical, Stockholm, Sweden).
Materials
The Δ9-THC used was dronabinol (Marinol); the 10 lumen assembly was obtained from Dentsleeve (Adelaide, Australia); the pneumohydraulic capillary perfusion pump from Dentsleeve Pty (Belair, South Australia); the polygraph was from Synectics Medical (Stockholm, Sweden) and the pH 1.0 and 7.0 buffer solutions were from Medtronic (Skovlunde, Denmark).
Data analysis
Basal LES pressure was measured at end-expiration relative to intragastric pressure and was determined as visual means of 1 min periods every 15 min.
TLESRs were evaluated according to previously published criteria (Holloway et al., 1995): (i) absence of swallowing for 4 s before to 2 s after the onset of LES relaxation; (ii) relaxation rate of ≥1 mm Hg s−1; (iii) time from onset to complete relaxation of ≤10 s; and (iv) nadir pressure of ≤2 mm Hg. LES relaxations associated with a swallow that fulfilled the above mentioned criteria ii, iii and iv, and that lasted more than 10 s were included as TLESR. TLESRs were counted for each subject during the preprandial period and the three postprandial hours. In addition, the percentage of TLESRs accompanied by an acid reflux episode was determined, as well as the proportion of acid reflux episodes associated with a TLESR and the total amount of acid reflux episodes and total acid exposure time.
Acid reflux episodes were defined as a decrease in oesophageal pH below 4 for more than 5 s with the pH drop > 1 pH u or, if basal oesophageal pH was already below 4, as a further decrease in pH of at least 1 pH u. Slow drifts of pH below 4 were included in the analysis of the total duration of oesophageal acid exposure but were not scored as acid reflux episodes. The rate of spontaneous swallowing was determined by counting the pharyngeal pressure waves.
Plasma sampling and analysis
Blood samples were taken from a forearm vein at six time points during each study day. After separation of plasma, samples were stored at −20°C until analysis. Metabolism of Δ9-THC occurs mainly in the liver by hydroxylation and oxidation. Nearly 100 metabolites have been identified for Δ9-THC (Grotenhermen, 2003). The two major metabolites are 11-hydroxy-THC and 11-nor-9-carboxy-THC. Analysis of Δ9-THC, 11-hydroxy-THC and 11-nor-9-carboxy-THC was done by Pharma Bio Research (Assen, The Netherlands) through high performance liquid chromatography with tandem mass spectrometric detection, in compliance with the principles of Good Laboratory Practice. Only the results of Δ9-THC plasma concentration are discussed in this study.
Statistical analysis
Data are presented as the means ± s.e.mean. For statistical analysis, Student's paired t-test and a mixed model using pairwise comparisons were used. P value < 0.05 was considered to be significant. Based on previous studies and on the assumption that a reduction in TLESRs of 40% would occur between placebo and active drug, a sample size of 18 subjects was estimated to be required for detection of this difference at the 5% significance level with 80% power.
Results
Dog study
Plasma THC levels
Δ9-THC was introduced intragastrically at three different doses: 0.25 µmol kg−1 (0.079 mg kg−1), 0.40 µmol kg−1 (0.126 mg kg−1) and 0.60 µmol kg−1 (0.189 mg kg−1). Maximum Δ9-THC plasma levels were reached after 30–40 min at all doses of Δ9-THC (Fig. 1A). No apparent differences in plasma exposure were seen between 0.4 and 0.6 µmol kg−1.
Figure 1.
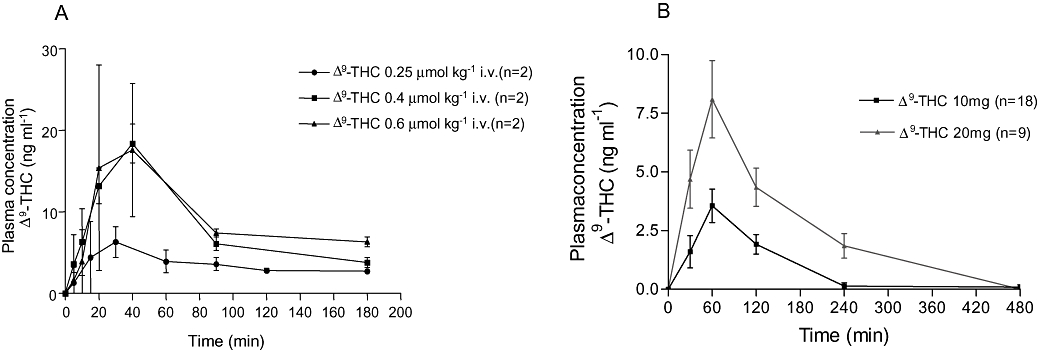
Mean Δ9-tetrahydrocannabinol (Δ9-THC) plasma concentration in dogs (A) and humans (B). Peak plasma concentrations occurred 40 and 60 min after drug administration in dogs and humans respectively, and declined rapidly with time. Note the difference in scaling of the axes.
No obvious side-effects were observed at the present doses. A higher dose, 0.8 µmol kg−1, induced emesis in one out of two dogs, and no further experiments were done at this dose.
TLESRs and reflux episodes
The average number of TLESRs in all control experiments was 9.5 ± 0.4 (10 dogs, n = 68). Δ9-THC produced a dose-dependent inhibition of TLESRs (Fig. 2). At the highest dose, the rate of TLESRs was significantly reduced during Δ9-THC compared with that in the control experiments (Fig. 3A). Similarly, the number of reflux episodes was significantly reduced by the two highest doses of Δ9-THC (Fig. 3B). The latency time to the occurrence of the first TLESR in the experiments was significantly increased at 0.4 µmol kg−1 (Fig. 4C).
Figure 2.
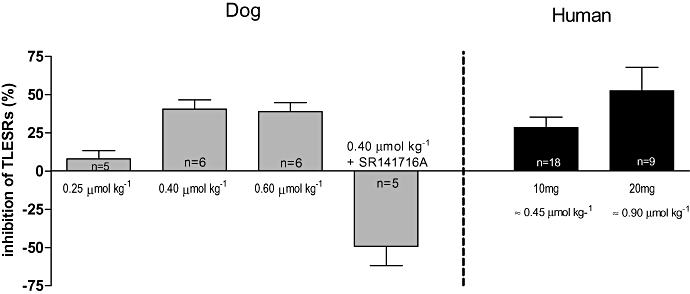
Percentage of inhibition by different doses of Δ9-tetrahydrocannabinol in dog and human studies. Maximal inhibition of transient lower oesophageal sphincter relaxations (TLESRs) occurred up to 40% and 53% in dogs and humans respectively, with the selective CB1 receptor antagonist SR141716A reversing the inhibitory effect of Δ9-tetrahydrocannabinol.
Figure 3.
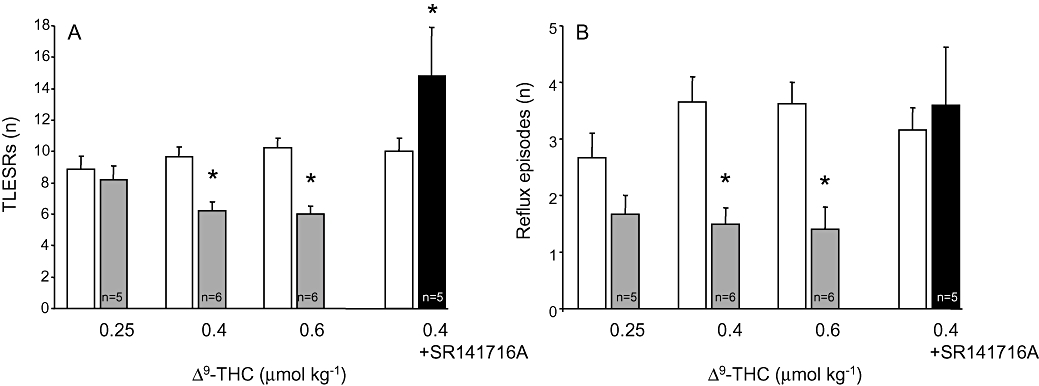
(A) transient lower oesophageal sphincter relaxations (TLESRs) in dogs. Δ9-tetrahydrocannabinol (Δ9-THC) produced a dose-dependent inhibition of TLESRs. At the highest dose, the rate of TLESRs was significantly reduced from 10.2 ± 0.6 in control experiments to 6.0 ± 0.6 during Δ9-THC. SR141716A reversed the inhibitory effect of Δ9-THC. (B) The number of reflux episodes was significantly reduced by Δ9-THC at the two highest doses. *P < 0.05 compared with controls (Student's paired t-test).
Figure 4.
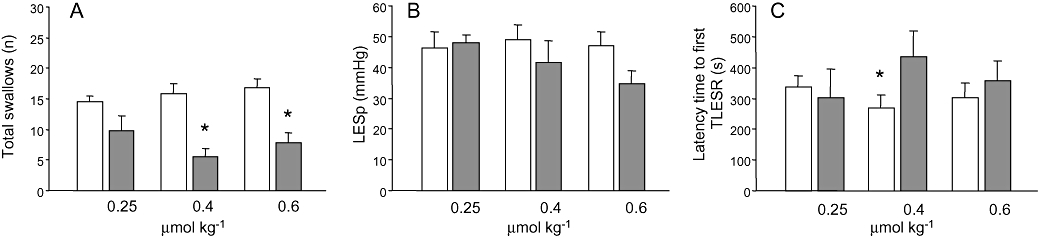
(A) Swallowing rate in dogs was significantly reduced by Δ9-THC at 0.4 and 0.6 µmol kg−1. (B) No significant effect was seen on basal LES pressure in dogs. (C) Latency time to the first transient lower oesophageal sphincter relaxation (TLESR) was significantly increased at the dose of 0.4 µmol kg−1. *P < 0.05 compared with controls (Student's paired t-test). LESp, lower oesophageal sphincter pressure.
Administration of the selective CB1 antagonist SR141716A (0.22 µmol kg−1) prevented the inhibitory response on TLESRs produced by Δ9-THC, 0.4 µmol kg−1 (n = 5). The overall response was a significant increase in the number of TLESRs (Fig. 2).
Basal LES pressure, swallowing rate
Basal LES pressure in control conditions was 45.4 ± 1.6 mm Hg. As shown in Figure 4B, Δ9-THC slightly lowered basal LES pressure, but this effect did not reach statistical significance. In contrast, the rate of swallowing was significantly reduced by Δ9-THC at 0.4 and 0.6 µmol kg−1 (Fig. 4A). SR141716A blocked the reduction of reflux episodes and swallows induced by Δ9-THC 0.4 µmol kg−1.
Human study
Plasma THC levels
Maximum Δ9-THC plasma levels were reached at T = 60 min during both doses of Δ9-THC and declined rapidly with time (Fig. 1B). Compared with Δ9-THC 10 mg, Δ9-THC 20 mg reached higher plasma levels and was detected in the plasma for a longer period of time (Fig. 1B). There was a large inter-individual variation in plasma concentrations, with a range in peak concentrations of 11.8 ng ml−1 during Δ9-THC 10 mg and 13.6 ng ml−1 during Δ9-THC 20 mg.
Side-effects
All 18 subjects tolerated the lowest Δ9-THC dose. However, due to nausea and vomiting in the first hour after meal intake, only nine subjects completed the Δ9-THC 20 mg session. Other reported side-effects were dizziness, loss of concentration, sleepiness and confusion. Less frequently reported were a dry mouth and headache. Manifestation of side-effects started at approximately T = 60 min, that is at the start of the first postprandial hour and coincided with peak plasma levels. The side-effects were maximal around T = 120 min and lasted for 3–4 h.
TLESRs and reflux
Before meal intake, the basal rate of TLESRs was comparable in the three different groups (Fig. 5C). During placebo, meal intake resulted in an increase of TLESRs. This increase was most pronounced in the first postprandial hour, whereas in the second and third hours, the number of TLESRs was only marginally increased (Fig. 5A). Treatment with Δ9-THC dose-dependently reduced meal-induced increase of TLESRs during the first postprandial hour (Figs. 5B and 5C). This corresponds to an inhibition by Δ9-THC 10 and 20 mg of 28.5 ± 6.7% and 52.5 ± 15.3% respectively (Fig. 2). In line with this, latency time to the first TLESR was significantly increased by Δ9-THC 20 mg (Fig. 6A). During the second and third postprandial hours, however, TLESR rate almost returned to preprandial level and did not differ between the three groups (Fig. 5C).
Figure 5.
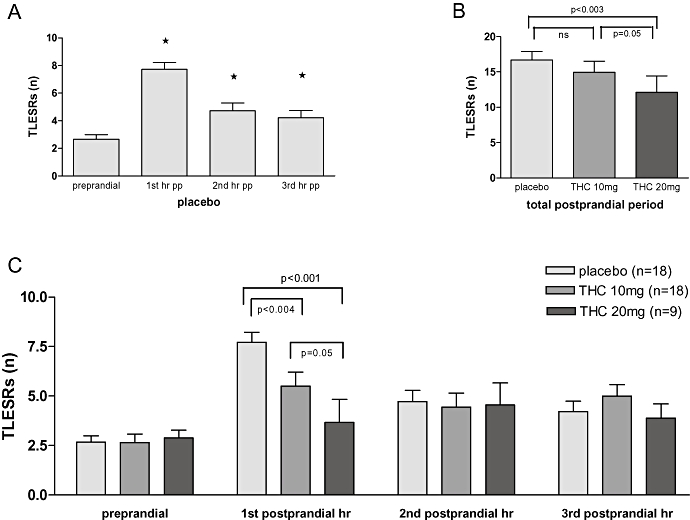
TLESRs in humans. (A) Meal induced increase in transient lower oesophageal sphincter relaxations (TLESRs) was most pronounced during the first postprandial hour. (B) Δ9-tetrahydrocannabinol (Δ9-THC) 20 mg, but not Δ9-THC 10 mg, significantly reduced the rate of TLESRs during the total three postprandial hours (mixed model using pairwise comparisons). (C) Δ9-THC dose-dependently decreased the rate of TLESRs during the first postprandial hour (placebo 7.7 ± 0.5; Δ9-THC 10 mg 5.5 ± 0.7; Δ9-THC 20 mg 3.7 ± 1.2) (mixed model using pairwise comparisons). No reduction in TLESRs was seen during the second and third postprandial hours *P < 0.05 compared with the preprandial period during placebo (Student's paired t-test).
Figure 6.
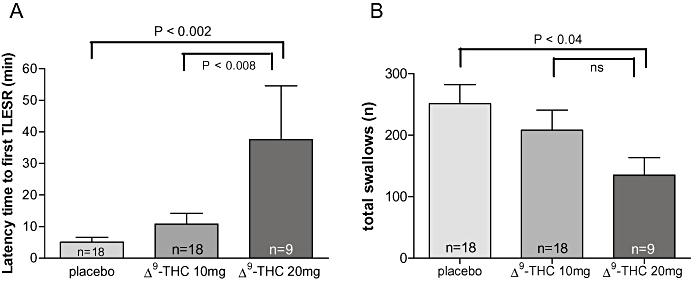
(A) Latency time in humans was increased significantly from 5.1 ± 1.5 min during placebo to 37.7 ± 17.0 min during Δ9-THC 20 mg (P < 0.002). No significant effect was seen with Δ9-THC 10 mg compared with placebo. (B) Mean swallowing rate in humans. Δ9-THC 20 mg induced a significant inhibition of swallowing rate [placebo 251 ± 31.1; Δ9-THC 10 mg 208 ± 32.6 (ns); Δ9-THC 20 mg 135 ± 28.4 (P < 0.04)] (mixed model using pairwise comparisons). Δ9-THC, Δ9-tetrahydrocannabinol; TLESRs, transient lower oesophageal sphincter relaxations.
During basal recordings, acid reflux episodes did not occur in either of the study groups. Similar to the number of TLESRs, meal ingestion resulted in an increase in acid reflux episodes in the 3 h postprandial period and in acid exposure time (2.9 ± 1.2%) during placebo. Δ9-THC did not reduce either the total number of postprandial acid reflux episodes (Fig. 7), or the total acid exposure time [Δ9-THC 10 mg 3.2 ± 0.8% (ns); Δ9-THC 20 mg 4.4 ± 1.5% (ns)]. During the first postprandial hour, however, the reflux episode rate was decreased with Δ9-THC 20 mg compared with placebo (Fig. 7) but, due to the very low number of episodes involved, this did not reach statistical significance.
Figure 7.
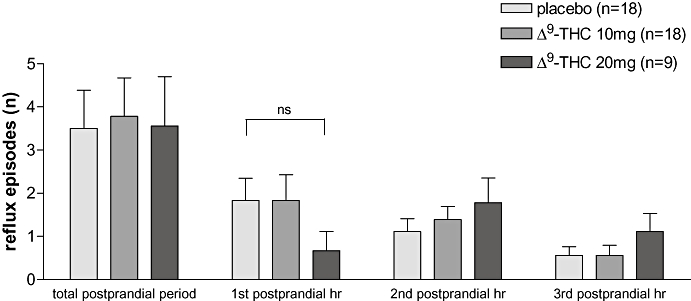
Acid reflux episodes in humans. If the total postprandial period is taken into account there is no difference in reflux episode rate. During the first postprandial hour the amount of reflux episodes was decreased from 1.83 ± 0.5 (placebo) to 0.67 ± 0.4 [Δ9-tetrahydrocannabinol (Δ9-THC) 20 mg]. This difference, however, was not significant, most likely due to the very few episodes detected.
The percentage of TLESRs accompanied by gastro-oesophageal reflux did not significantly differ between the groups (placebo 17%; Δ9-THC 10 mg 20%; Δ9-THC 20 mg 23%). Acid reflux occurred during a TLESR in 84% of all reflux episodes in the placebo group, compared with 79% in the Δ9-THC 10 mg group (ns) and 87% in the Δ9-THC 20 mg (ns).
Basal LES pressure
Basal LES pressure was comparable in all three groups. Meal ingestion resulted in a reduction of LES pressure. This effect was maximal directly after the meal at T = 60 min, and returned to baseline around T = 150 min. Δ9-THC induced a further decrease in LES pressure after meal intake compared with placebo. This further decrease started at T = 45 min, was maximal around T = 100 min, and recovered slowly with time (Fig. 8). No difference in LES pressure between Δ9-THC 10 mg and 20 mg was observed.
Figure 8.
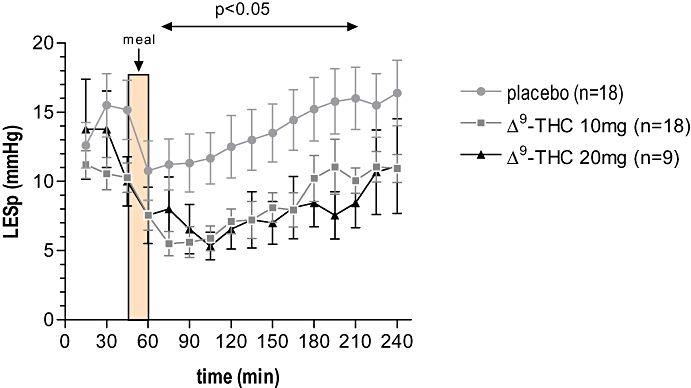
Effect of Δ9-tetrahydrocannabinol (Δ9-THC) on basal LES pressure in humans. Both Δ9-THC 10 mg and 20 mg significantly decreased basal LES pressure starting around T = 60 min and this recovered slowly with time (P < 0.05) (mixed model using pairwise comparisons). LESp, lower oesophageal sphincter pressure.
Swallowing rate
Total swallowing rate was significantly decreased by Δ9-THC 20 mg compared with placebo (Fig. 6B). This decrease was most pronounced during the second and third postprandial hours.
Blood pressure and pulse
Diastolic and systolic blood pressure were decreased significantly only at T = 45 min and T = 60 min (T = 60; placebo:130 (111–143)/79 (63–94) mm Hg; Δ9-THC 10 mg: 124 (112–140)/73 (59–89) mm Hg; Δ9-THC 20 mg: 125 (114–140)/71 (66–77) mm Hg (P = 0.003).
Tachycardia occurred in both Δ9-THC groups, but was more pronounced after Δ9-THC 20 mg (Fig. 9). Tachycardia started at T = 45 min and reached its maximum around T = 90 min. After Δ9-THC 10 mg the tachycardia lasted till 210 min, whereas after Δ9-THC 20 mg, tachycardia lasted until the end of the experiment (Fig. 9).
Figure 9.
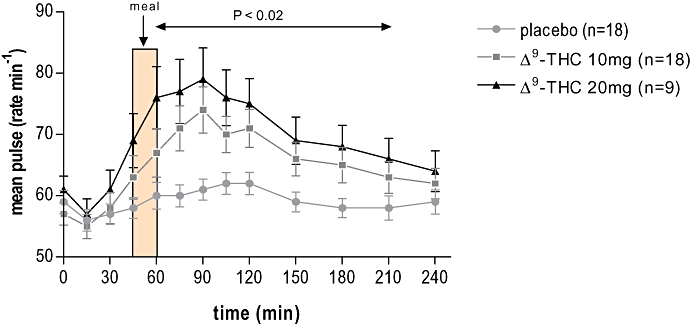
The effect of the various treatments on mean heart rate in humans. During Δ9-tetrahydrocannabinol (Δ9-THC) there was a significant tachycardia starting at T = 45 min, with its maximum effect at T = 90 min (placebo 61 beats min−1, Δ9-THC 10 mg 74 beats min−1 and THC 20 mg 79 beats min−1); heart rate recovered slowly with time after administration of Δ9-THC. The tachycardia was most marked with Δ9-THC 20 mg (mixed model using pairwise comparisons).
Discussion
TLESRs are the main mechanism underlying gastro-oesophageal reflux, and as such may represent an important potential target for pharmacological treatment of GERD (Dent et al., 1980; Dodds et al., 1982). Insight into the neurotransmitters mediating TLESRs is therefore of crucial importance. Recently, Lehmann et al. (2002) showed that exogenous and endogenous activation of CB1 receptors inhibits TLESRs in dogs; the CB1 agonist WIN 55,212-2 reduced the occurrence of TLESRs by 80%. In line with this, our study showed that a single dose of the mixed CB1/CB2 agonist Δ9-THC caused a 40% reduction in TLESRs in the same dog model, increased the latency time to the first TLESR and reduced the number of acid reflux episodes. Whether there is a true difference in maximal effect between WIN 55,212-2 and Δ9-THC cannot be ascertained from the present study. Based on these findings, the active dosage was estimated for a human study in which we studied the effect of two different doses of Δ9-THC on the occurrence of meal-induced TLESRs in healthy subjects. Although the peak plasma levels obtained after a single oral dose of 10 and 20 mg Δ9-THC in healthy subjects was only half of that reached in dogs, TLESRs were significantly inhibited by Δ9-THC. Up to 52% of the TLESRs were inhibited by 20 mg Δ9-THC, but this effect was only observed during the first postprandial hour. It should be emphasized although that in our study, meal intake failed to increase the number of TLESRs during the second and third postprandial hours making it difficult to demonstrate a reduction. Alternatively, the lack of effect on TLESRs in the second and third postprandial hours could result from insufficient serum levels of Δ9-THC. The peak Δ9-THC plasma concentration indeed occurred at the start of the first postprandial hour and declined rapidly with time. However, basal LES pressure was significantly reduced during the entire study, arguing against this possibility. Alternatively, basal LES pressure is more sensitive to Δ9-THC than TLESR.
In addition to its effect on TLESRs, Δ9-THC 20 mg also tended to decrease the amount of reflux episodes by 37% during the first postprandial hour. This effect, however, did not reach statistical significance, most likely due to the very low number of reflux episodes recorded, even during placebo. As the percentage of reflux episodes during TLESRs was not affected by Δ9-THC, the reduction in reflux episodes observed in the first postprandial hour most likely results from the inhibition of TLESRs. On the other hand, the total acid exposure time was not affected by Δ9-THC, suggesting a prolonged acid exposure per acid reflux episode. As clearance of refluxate relies on sufficient swallowing, this finding could be explained by the decrease in swallowing rate observed after Δ9-THC. Suppression of spontaneous swallowing is also observed after baclofen (Blackshaw et al., 1999; Lehmann et al., 1999; Lidums et al., 2000; Zhang et al., 2002) and WIN 55,212-2 (Lehmann et al., 2002).
It should be noted although that Δ9-THC is a non-selective CB receptor agonist binding to other receptors such as the recently described CB receptor GPR55 (Pertwee, 2007; Ryberg et al., 2007). Moreover Δ9-THC has 5-HT3 receptor blocking properties (Fan, 1995), which may be relevant to our current findings as these receptors have been shown to play a role in the occurrence of TLESRs (Ruth et al., 1998; Holloway, 2001). However, the highly selective CB1 receptor antagonist SR141716A (Pertwee, 1997; 1999) effectively blocked the inhibitory effect of Δ9-THC in dogs and even stimulated the occurrence of TLESRs. The stimulating effect induced by the combination of Δ9-THC and SR141716A in the present study (40%) was higher compared with the combination of WIN 55,212-2 and SR141716A (10%) but comparable with previous observations observed with a single administration of SR141716A (Lehmann et al., 2002). Although we cannot exclude the possibility that the effects are due to an interaction with other receptors, the data obtained with the selective CB1 receptor antagonist SR141716A in dogs suggest that, at least in dogs, Δ9-THC interacts with CB1 receptors. Ideally, these experiments should have also been tested in humans. However, experiments in healthy subjects evaluating the combination of Δ9-THC and a selective CB1 antagonist were not performed for ethical reasons, that is, in view of the observed side-effects at the highest dose of THC tested.
Although the exact mechanism of action of Δ9-THC cannot be determined from our experiments, we speculate that it acts peripherally on the afferent limb of the neural pathway and/or within the brainstem nuclei controlling TLESRs. In the human brain, CB1 receptors are present in the dorsal motor nucleus of the vagus, providing the preganglionic parasympathetic motor innervation of the foregut, and in the nucleus tractus solitarius (NTS), which receives sensory afferent fibres from the autonomic nervous system (Glass et al., 1997). In addition, CB1 receptor expression has been observed in cell bodies of the nodose ganglion (Partosoedarso et al., 2003). No expression, however, has been shown on vagal efferent neurons (Van Sickle et al., 2001). Therefore, Δ9-THC most probably inhibits TLESRs via the CB1 receptor on central terminals of vagal primary afferents or on interneurons in the NTS synapsing with vagal motor neurons. The latter site of action may be more likely because vagotomy does not affect CB1-like immunoreactivity in the ferret NTS (Partosoedarso et al., 2003).
Although the observed inhibitory effect of Δ9-THC on spontaneous swallowing rate might well be a manifestation of diminished salivation, it could also provide further evidence for a central mechanism of action. Clearly, future studies are required to prove this hypothesis.
A drawback of our study is the occurrence of emesis, and subsequent vomiting in half of the subjects at the highest dose tested leading to premature termination. This was rather unexpected as Δ9-THC, in the same dose range, is clinically used as an anti-emetic in patients undergoing chemotherapy (Tramer et al., 2001). Possibly the combination of meal ingestion and oesophageal intubation may have led to these effects. On the other hand, vomiting is a well documented side-effect of Δ9-THC (Grotenhermen, 2003). Other frequent side-effects were tachycardia, hypotension and psychotropic effects, starting approximately 60 min after drug administration and lasting till the end of the study.
Blockade of TLESRs may be a more physiological approach to prevent both acid and non-acid reflux than currently available therapies, a feature especially important in patients who fail to respond or insufficiently respond to proton pump inhibitors. Previous studies with baclofen indeed showed a significant reduction in reflux episodes and improved symptoms in GERD patients. Although our study shows that Δ9-THC reduces the occurrence of TLESRs by more than 50%, there are several reasons why the use of a mixed CB1/CB2 agonist is not an attractive approach to treat GERD. First of all, administration of Δ9-THC resulted in significant central and cardiovascular side-effects. Secondly, the LES pressure and spontaneous swallowing were both significantly reduced by Δ9-THC, facilitating reflux and impairing the clearance of refluxate respectively. To what extent the same applies to a selective CB1 agonist cannot be concluded from this study, but clearly potential new CB agonists should be devoid of these properties before they can be considered as interesting new drugs to treat reflux.
In conclusion, the present study demonstrates that Δ9-THC significantly inhibits the increase in TLESRs evoked by meal ingestion and reduces spontaneous swallowing in both dogs and humans. Furthermore, Δ9-THC reduces basal LES pressure in humans. These findings confirm previous findings in dogs and indicate that CB receptors are also involved in the triggering of TLESRs in humans.
Acknowledgments
This study was supported by AstraZeneca, Mölndal, Sweden and was part of a collaboration between AstraZeneca and the Department of Gastroenterology of the Academic Medical Centre, Amsterdam, the Netherlands.
Glossary
Abbreviations
- GERD
gastro-oesophageal reflux disease
- LES
lower oesophageal sphincter
- TLESRs
transient lower oesophageal sphincter relaxations
- CB
cannabinoid
- Δ9-THC
Δ9-tetrahydrocannabinol
- DMN
dorsal motor nucleus of the vagus
- NTS
nucleus tractus solitarius
- PPIs
proton pump inhibitors
Conflicts of interest
JJ, AC, MR and AL are all full-time employees of AstraZeneca. GB has participated as an investigator in clinical studies sponsored by AstraZeneca.
References
- Abrahams TP, Partosoedarso ER, Hornby PJ. Lower oesophageal sphincter relaxation evoked by stimulation of the dorsal motor nucleus of the vagus in ferrets. Neurogastroenterol Motil. 2002;14:295–304. doi: 10.1046/j.1365-2982.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Staunton E, Lehmann A, Dent J. Inhibition of transient LES relaxations and reflux in ferrets by GABA receptor agonists. Am J Physiol. 1999;277:G867–G874. doi: 10.1152/ajpgi.1999.277.4.G867. [DOI] [PubMed] [Google Scholar]
- Dent J, Dodds WJ, Friedman RH, Sekiguchi T, Hogan WJ, Armdorfer RC, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256–267. doi: 10.1172/JCI109667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307:1547–1552. doi: 10.1056/NEJM198212163072503. [DOI] [PubMed] [Google Scholar]
- Fan P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Heading RC. Prevalence of upper gastrointestinal symptoms in the general population: a systematic review. Scand J Gastroenterol. 1999;231(Suppl.):3–8. [PubMed] [Google Scholar]
- Hirsch DP, Tytgat GN, Boeckxstaens GE. Transient lower oesophageal sphincter relaxations – a pharmacological target for gastro-oesophageal reflux disease? Aliment Pharmacol Ther. 2002;16:17–26. doi: 10.1046/j.1365-2036.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- Holloway RH. The anti-reflux barrier and mechanisms of gastro-oesophageal reflux. Baillieres Best. Pract Res Clin Gastroenterol. 2000;14:681–699. doi: 10.1053/bega.2000.0118. [DOI] [PubMed] [Google Scholar]
- Holloway RH. Systemic pharmacomodulation of transient lower esophageal sphincter relaxations. Am J Med. 2001;111(Suppl. 8A):178S–185S. doi: 10.1016/s0002-9343(01)00853-1. [DOI] [PubMed] [Google Scholar]
- Holloway RH, Penagini R, Ireland AC. Criteria for objective definition of transient lower esophageal sphincter relaxation. Am J Physiol. 1995;268:G128–G133. doi: 10.1152/ajpgi.1995.268.1.G128. [DOI] [PubMed] [Google Scholar]
- Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut. 2003;52:1397–1402. doi: 10.1136/gut.52.10.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A, Antonsson M, Bremner-Danielsen M, Flardh M, Hansson-Branden L, Karrberg L. Activation of the GABA(B) receptor inhibits transient lower esophageal sphincter relaxations in dogs. Gastroenterology. 1999;117:1147–1154. doi: 10.1016/s0016-5085(99)70400-2. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Blackshaw LA, Branden L, Carlsson A, Jensen J, Nygren E, et al. Cannabinoid receptor agonism inhibits transient lower esophageal sphincter relaxations and reflux in dogs. Gastroenterology. 2002;123:1129–1134. doi: 10.1053/gast.2002.36025. [DOI] [PubMed] [Google Scholar]
- Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH. Control of transient lower esophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in normal subjects. Gastroenterology. 2000;118:7–13. doi: 10.1016/s0016-5085(00)70408-2. [DOI] [PubMed] [Google Scholar]
- Martin CJ, Patrikios J, Dent J. Abolition of gas reflux and transient lower esophageal sphincter relaxation by vagal blockade in the dog. Gastroenterology. 1986;91:890–896. doi: 10.1016/0016-5085(86)90691-8. [DOI] [PubMed] [Google Scholar]
- Massa F, Monory K. Endocannabinoids and the gastrointestinal tract. J Endocrinol Invest. 2006;29:47–57. [PubMed] [Google Scholar]
- Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–610. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Partosoedarso ER, Young RL, Blackshaw LA. GABA(B) receptors on vagal afferent pathways: peripheral and central inhibition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G658–G668. doi: 10.1152/ajpgi.2001.280.4.G658. [DOI] [PubMed] [Google Scholar]
- Partosoedarso ER, Abrahams TP, Scullion RT, Moerschbaecher JM, Hornby PJ. Cannabinoid1 receptor in the dorsal vagal complex modulates lower oesophageal sphincter relaxation in ferrets. J Physiol. 2003;550:149–158. doi: 10.1113/jphysiol.2003.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Pertwee RG. GPR55: a new member of the cannabinoid receptor clan? Br J Pharmacol. 2007;152:984–986. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Ruth M, Hamelin B, Rohss K, Lundell L. The effect of mosapride, a novel prokinetic, on acid reflux variables in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1998;12:35–40. doi: 10.1046/j.1365-2036.1998.00268.x. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorice PV, O'Connell MB. Management of chemotherapy-induced nausea and vomiting. Pharmacotherapy. 1990;10:129–145. [PubMed] [Google Scholar]
- Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323:16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard LJ, MacKie K, Davison JS, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- Vela MF, Tutuian R, Katz PO, Castell DO. Baclofen decreases acid and non-acid post-prandial gastro-oesophageal reflux measured by combined multichannel intraluminal impedance and pH. Aliment Pharmacol Ther. 2003;17:243–251. doi: 10.1046/j.1365-2036.2003.01394.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lehmann A, Rigda R, Dent J, Holloway RH. Control of transient lower oesophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in patients with gastro-oesophageal reflux disease. Gut. 2002;50:19–24. doi: 10.1136/gut.50.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]


