Abstract
Background and purpose
Galantamine, a weak acetylcholine esterase (AChE) inhibitor and allosteric potentiator of nicotinic ACh receptors (nAChRs), improves apomorphine-induced deficits in prepulse inhibition (PPI), sensory information-processing deficits, via a nAChR-independent mechanism. The present study examined the role of muscarinic ACh receptors (mAChRs) in the effect of galantamine, and studied the mechanism of galantamine-induced increases in prefrontal ACh levels in mice.
Experimental approach
Apomorphine (1 mg kg−1) was administered to male ddY mice (9–10 weeks old) to create a PPI deficit model. Extracellular ACh concentrations in the prefrontal cortex were measured by in vivo microdialysis.
Key results
Galantamine- and donepezil-mediated improvements in apomorphine-induced PPI deficits were blocked by the preferential M1 mAChR antagonist telenzepine. The mAChR agonist oxotremorine also improved apomorphine-induced PPI deficits. Galantamine, like donepezil, increased extracellular ACh concentrations in the prefrontal cortex. Galantamine-induced increases in prefrontal ACh levels were partially blocked by the dopamine D1 receptor antagonist SCH23390, but not by antagonists of mAChRs (telenzepine) and nAChRs (mecamylamine). Galantamine increased dopamine, but not 5-HT, release in the prefrontal cortex.
Conclusions and implications
Galantamine improves apomorphine-induced PPI deficits by stimulating mAChRs through increasing brain ACh levels via a dopamine D1 receptor-dependent mechanism and AChE inhibition.
Keywords: galantamine, donepezil, prepulse inhibition, apomorphine, AChE inhibitor, muscarinic ACh receptor (mAChR), ACh release, dopamine release
Introduction
Central cholinergic dysfunction causes the cognitive symptoms of various neurological diseases (Friedman, 2004). Schizophrenic patients do not show decreased cell density in the nucleus basalis of Meynert, a typical pathological change in Alzheimer's disease, but do show decreased levels of nicotinic ACh receptors (nAChRs) and M1/M4 muscarinic ACh receptors (mAChRs) (Friedman, 2004). In addition, single-photon emission computed tomography in living, unmedicated schizophrenic patients showed fewer mAChRs in several brain regions including the frontal cortex (Raedler et al., 2003), and neuropharmacological studies showed that atypical antipsychotic drugs preferentially increase ACh concentrations in the prefrontal cortex (Ichikawa et al., 2002; Shirazi-Southall et al., 2002). These observations indicate that cholinergic alterations may be involved in the sensory gating abnormalities seen in schizophrenia, providing a rationale for pharmacological approaches directed at cholinergic targets to enhance the cognitive abilities of schizophrenic patients.
Prepulse inhibition (PPI) refers to the normal inhibition of the startle response when a weak stimulus (the prepulse) immediately precedes an intense startling stimulus (the pulse) (Graham, 1975). The PPI of startle is an operational measure of the pre-attentive filtering process known as sensorimotor gating, and abnormalities in pre-attentive information processing may be predictive of, or lead to, complex cognitive deficits in schizophrenia (Braff et al., 1999; Geyer et al., 2001; Swerdlow et al., 2006). In addition, PPI performance may be related to cognitive processes in healthy males (Bitsios et al., 2006). The acetylcholine esterase (AChE) inhibitors, galantamine and donepezil, improve apomorphine-induced PPI deficits in rats (Hohnadel et al., 2007) and mice (Koda et al., 2008), but the mechanism of this improvement is unclear. Galantamine is a weaker AChE inhibitor than donepezil (Woodruff-Pak et al., 2002), but has additional allosteric potentiating effects at nAChRs (Maelicke et al., 2001; Santos et al., 2002; Dajas-Bailador et al., 2003; Samochocki et al., 2003). In addition, nicotine administration enhances PPI in both human and animals (Acri et al., 1994; Kumari et al., 1996; 1997; Adler et al., 1998) and reverses apomorphine-induced PPI deficits in rats via nicotinic α7 receptors (Suemaru et al., 2004). However, the beneficial effects of AChE inhibitors are not blocked by the nAChR antagonists, mecamylamine and methyllycaconitine (Koda et al., 2008), and mAChR agonists reverse apomorphine-induced PPI deficits in rats (Stanhope et al., 2001; Jones et al., 2005). Therefore, we examined the role of mAChRs in the improvement of apomorphine-induced PPI deficits by AChE inhibitors in mice using the preferential M1 mAChR antagonist, telenzepine (Eltze et al., 1985; Doods et al., 1987; Bymaster et al., 1993). In this relation, we compared the effects of galantamine and donepezil on extracellular ACh levels in the prefrontal cortex, since the prefrontal cortex plays a key role in the regulation of PPI of acoustic startle in animal models (Bubser and Koch, 1994; Swerdlow et al., 1995; de Jong and van den Buuse, 2006). In addition, we examined the effect of galantamine on prefrontal dopamine and 5-HT levels to study the mechanism(s) underlying the effect of galantamine on extracellular ACh levels, since prefrontal ACh release is regulated by dopamine (Imperato et al., 1993; Acquas et al., 1994; Hersi et al., 1995; Di Cara et al., 2007), and 5-HT (Consolo et al., 1996; Somboonthum et al., 1997).
Methods
Animals
Procedures involving animals and their care were conducted according to Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society. Male ddY mice (8 weeks old) were housed in groups of 5–6 per cage (24 × 17 × 12 cm3) under controlled environmental conditions (22 ± 1°C; 12–12 h light–dark cycle, lights on at 08:00 h; food and water ad libitum) for at least 1 week before being used in the experiment. We used a total of 336 mice in all the experiments; different mice were used in each experiment.
Measurement of startle response and PPI
All PPI testing took place within startle chambers acquired from San Diego Instruments (San Diego, CA, USA) as previously reported (Sakaue et al., 2003; Koda et al., 2008). Each startle chamber consists of a 5.1 cm (outside diameter) Plexiglas cylinder mounted on a platform (20.4 cm length × 12.7 cm width × 0.4 cm thick) with a piezoelectric accelerometer unit attached below the Plexiglas cylinder. The piezoelectric unit transduces vibrations into signals that are rectified and stored by a microcomputer interface. The Plexiglas cylinder and platform are located in a sound-attenuated chamber (San Diego Instruments) with a loudspeaker (28 cm above the cylinder), and house light. Calibration procedures using a vibrating standardization unit (San Diego Instruments) were performed between experiments to ensure equivalent sensitivities across the chambers. The sound levels for background noise and various stimuli in each chamber were calibrated with a digital sound-level meter.
Each test session began by placing a mouse in the Plexiglas cylinder where it was left undisturbed. After a background noise of 65 dB had been presented for the 5 min acclimation period, each mouse was exposed to four consecutive blocks with a total of 100 trials over the approximately 30 min test session. One block consisted of 25 trials including five different trial types: pulse-alone trials in which a 40 ms broadband 120 dB burst was presented; three different prepulse-pulse trials in which the onset of a 20 ms broadband noise preceded the onset of the 120 dB startle pulse by 100 ms (prepulse intensities 3, 6 and 9 dB above the 65 dB background noise were used); and no-stimulation trials in which only the background noise was presented. Trials were presented in a pseudo-random order separated by an average of 15 s (range 7–23 s). The startle response was recorded for 100 ms (measuring the response every 1 ms) starting at the onset of each startle stimulus. The maximum startle amplitude recorded during the 100 ms sampling window was used as the dependent variable.
Surgery and microdialysis procedures
Mice were anesthetized with sodium pentobarbital (40 mg kg−1, i.p.) and stereotaxically implanted with a guide cannula (one site per animal) for a dialysis probe (Eicom, Kyoto, Japan) in the prefrontal cortex (A + 1.9 mm, L − 0.5 mm, V − 3.8 mm, from the bregma and skull) (Franklin and Paxinos, 1997). The cannula was cemented in place with dental acrylic, and the animal was kept warm and allowed to recover from anaesthesia. Postoperative analgesia was provided with a single injection of buprenorphine (0.1 mg kg−1, i.p.) (Ago et al., 2006a; 2007). The active probe membranes were 3 mm long in the prefrontal cortex of mice. On the day after surgery, the probe was perfused with Ringer's solution (147.2 mmol·L−1 NaCl, 4.0 mmol·L−1 KCl and 2.2 mmol·L−1 CaCl2; Fuso Pharmaceutical Industries, Ltd., Osaka, Japan) at a constant flow rate of 2 µL min−1 for the dopamine and 5-HT simultaneous assay or 1 µL min−1 for the ACh assay. A stabilization period of 3 h was established before the onset of the experiments. Microdialysis samples (20 µL) were collected every 10 min for the dopamine and 5-HT simultaneous assay or 20 min for the ACh assay, and injected immediately onto a high-performance liquid chromatography column for detection of dopamine and 5-HT (Ago et al., 2002; 2003; 2006a; 2007) and ACh (Ago et al., 2006b; Sato et al., 2007) as previously reported. After the experiments, Evans Blue dye was microinjected through the cannula to histologically verify the position of the probe.
Data analysis
All data are expressed as the mean ± SEM. For the acoustic startle response profile, the amount of PPI was calculated as a percentage score for each prepulse trial type. The following formula was used: %PPI = 100 − {[(startle response to prepulse-pulse trial)/(startle response to pulse-alone trial)] × 100}. Startle amplitude was calculated as the average response to all of the pulse-alone trials and analysed using one-way analysis of variance (anova) followed by the Tukey–Kramer test. Data for PPI were analysed using two-way ANOVA for treatment as the intersubject factor and repeated measures with prepulse intensity as the intrasubject factor. The post hoc individual comparisons were performed with the Tukey–Kramer test. Data from the ‘no stim’ trials are not included in the results because the values were negligible, relative to values on trials containing startle stimuli. For in vivo microdialysis studies, all data were calculated as per cent change from the dialysate basal concentrations, with 100% defined as the average of three fractions before administration. Analyses were made using two-way ANOVA for treatment as the intersubject factor and repeated measures with time as the intrasubject factor. Statistical analyses were made using a software package Statview 5.0 J for Apple Macintosh computer (SAS Institute Inc., Cary, NC, USA). A value of P < 0.05 was considered statistically significant.
Drugs
The following drugs were used: galantamine (Janssen Pharmaceutical K.K., Tokyo, Japan); donepezil (Mitsubishi Tanabe Pharma Co., Yokohama, Japan); apomorphine, SCH23390, oxotremorine, mecamylamine and telenzepine (Sigma, St Louis, MO, USA). All other commercially available chemicals used in the experiments were of superfine quality. Galantamine, donepezil, SCH23390, oxotremorine, mecamylamine and telenzepine were dissolved in saline (0.9% solution of NaCl). Apomorphine was dissolved in saline containing 0.1% w/v ascorbic acid. Drugs were administered at 10 mL kg−1 intraperitoneally (galantamine, donepezil, SCH23390, oxotremorine, mecamylamine) or subcutaneously (apomorphine, telenzepine).
Results
Effect of telenzepine, a preferential M1 mAChR antagonist, on galantamine- and donepezil-induced reversal of PPI deficits in apomorphine-treated mice
Apomorphine (1 mg kg−1, s.c.) caused a marked reduction of PPI of the acoustic startle response in mice. Both galantamine (3 mg kg−1, i.p.) and donepezil (3 mg kg−1, i.p.) reversed apomorphine-induced PPI deficits, as previously reported (Koda et al., 2008). These improvements were significantly antagonized by telenzepine (3 or 10 mg kg−1, s.c.) (Fig. 1), whereas telenzepine alone did not affect PPI or the startle response of naïve mice (data not shown). Galantamine, donepezil and telenzepine did not affect the startle response of apomorphine-treated mice (Table 1).
Figure 1.
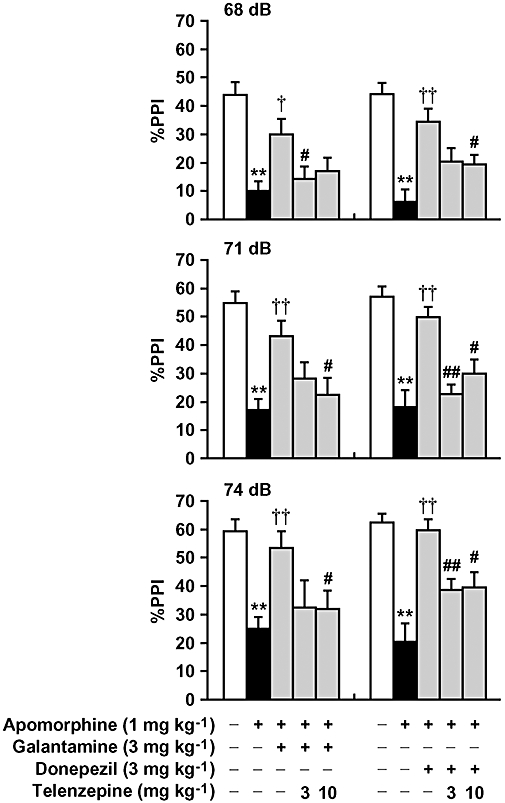
Effect of telenzepine on galantamine- and donepezil-induced reversal of PPI deficits in apomorphine-treated mice. Apomorphine (1 mg kg−1, s.c.) was injected 10 min before the experiments. Galantamine (3 mg kg−1, i.p.) or donepezil (3 mg kg−1, i.p.) was injected 30 min before the experiments. Telenzepine (3 and 10 mg kg−1, s.c.) was administered 30 min before galantamine or donepezil treatment. Data are expressed as the mean ± SEM from 8–18 mice. **P < 0.01, compared with vehicle/saline-treated mice; †P < 0.05, ††P < 0.01, compared with vehicle treatment group in apomorphine-treated mice; #P < 0.05, ##P < 0.01, compared with galantamine or donepezil treatment group in apomorphine-treated mice using Tukey–Kramer's post hoc test, following repeated measures two-way anova (main effects of prepulse intensity (F2,156 = 60.768, P < 0.0001 and F2,132 = 52.956, P < 0.0001 for galantamine and donepezil respectively) and treatment (F4,78 = 10.032, P < 0.0001 and F4,66 = 16.369, P < 0.0001 for galantamine and donepezil respectively); no significant interaction between treatment and prepulse intensity (F8,156 = 0.906, P > 0.05 and F8,132 = 1.194, P > 0.05 for galantamine and donepezil respectively).
Table 1.
Effects of galantamine, donepezil, oxotremorine and telenzepine on the startle response of apomorphine-treated mice
| Treatment |
Average startle amplitude |
||
|---|---|---|---|
| Galantamine | Donepezil | Oxotremorine | |
| Normal mice | |||
| Vehicle | 361 ± 45 | 327 ± 37 | 342 ± 56 |
| Apomorphine-treated mice | |||
| Vehicle | 291 ± 27 | 286 ± 38 | 283 ± 58 |
| 0.01 mg kg−1 | 206 ± 18 | ||
| 0.03 mg kg−1 | 234 ± 40 | ||
| 0.1 mg kg−1 | 196 ± 36 | ||
| AChE inhibitor | |||
| +Vehicle | 281 ± 34 | 305 ± 33 | |
| +Telenzepine 3 mg kg−1 | 333 ± 51 | 483 ± 87 | |
| +Telenzepine 10 mg kg−1 | 406 ± 56 | 302 ± 44 | |
Apomorphine (1 mg kg−1, s.c.) was administered 10 min before the experiments. Galantamine (3 mg kg−1, i.p.), donepezil (3 mg kg−1, i.p.) or oxotremorine (0.01–0.1 mg kg−1, i.p.) was injected 30 min before the experiments. Telenzepine (3 and 10 mg kg−1, s.c.) was administered 30 min before galantamine or donepezil treatment. Data are expressed as the mean ± SEM from 8–18 mice. One-way anova indicated that galantamine/telenzepine (F4,78 = 1.393, P > 0.05), donepezil/telenzepine (F4,66 = 2.208, P > 0.05) and oxotremorine (F4,64 = 1.695, P > 0.05) did not affect the startle response of apomorphine-treated mice.
Effect of oxotremorine, a non-selective mAChR agonist, on apomorphine-induced deficits in PPI of the acoustic startle response
Apomorphine (1 mg kg−1, s.c.) induced deficits in PPI of the acoustic startle response in mice. The mAChR agonist, oxotremorine (0.01–0.1 mg kg−1, i.p.), dose-dependently reversed apomorphine-induced PPI deficits in mice (Fig. 2), whereas oxotremorine alone did not affect PPI or the startle response of naïve mice (data not shown). Oxotremorine did not affect the startle response of apomorphine-treated mice (Table 1).
Figure 2.
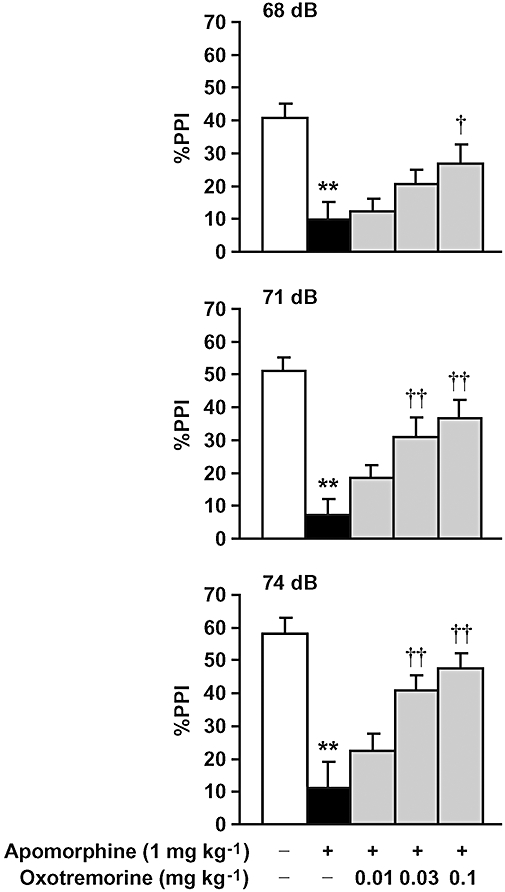
Effect of oxotremorine on apomorphine-induced deficits in PPI of the acoustic startle response in mice. Oxotremorine (0.01–0.1 mg kg−1, i.p.) was injected 30 min before the experiments. Apomorphine (1 mg kg−1, s.c.) was administered 10 min before the experiments. Data are expressed as the mean ± SEM from 10–16 mice. **P < 0.01, compared with vehicle/saline-treated mice; †P < 0.05, ††P < 0.01, compared with vehicle treatment group in apomorphine-treated mice using Tukey–Kramer's post hoc test, following repeated measures two-way ANOVA (main effects of prepulse intensity (F2,128 = 25.160, P < 0.0001) and treatment (F4,64 = 12.448, P < 0.0001); significant interaction between treatment and prepulse intensity (F8,128 = 2.025, P = 0.0484)).
Effects of galantamine and donepezil on extracellular ACh concentrations in the prefrontal cortex
Basal extracellular levels (means ± SEM) of ACh (not corrected for in vitro probe recovery) in the absence (n = 64) and presence (n = 25) of neostigmine in the perfusion solution were 30 ± 2 and 249 ± 23 fmol per 20 µL respectively (data are obtained from Figs 3 and 4). Galantamine (1 and 3 mg kg−1, i.p.) and donepezil (1 and 3 mg kg−1, i.p.) produced a robust increase in extracellular ACh concentrations in the prefrontal cortex in the absence and presence of neostigmine in the perfusion solution (Fig. 3). Inhibition of increase in ACh levels by neostigmine was greater in the effect of donepezil than in that of galantamine.
Figure 3.
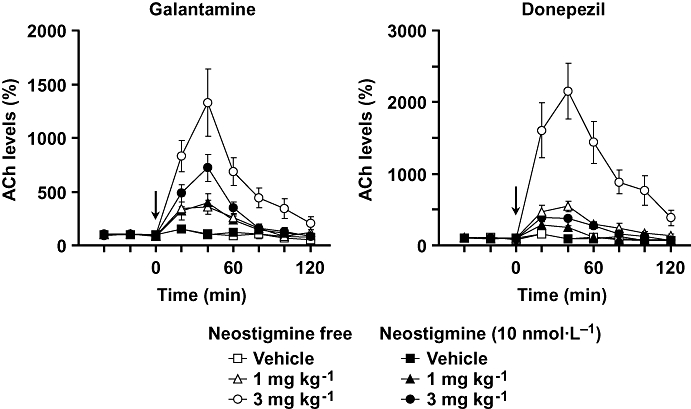
Effects of galantamine and donepezil on extracellular ACh levels in the prefrontal cortex of mice. Galantamine or donepezil at doses of 1 and 3 mg kg−1 were injected i.p. at 0 min (arrow). Ringer's solution was perfused with or without neostigmine at 10 nmol·L−1 in the probe. Data are expressed as the mean ± SEM from 3–7 mice. Repeated measures two-way anova indicated that galantamine significantly increased prefrontal ACh levels [interaction (treatment × time): F16,96 = 6.245, P < 0.0001 and F16,72 = 8.092, P < 0.0001 for absence and presence of neostigmine respectively]. Donepezil also increased prefrontal ACh levels (F16,88 = 13.444, P < 0.0001 and F16,80 = 9.023, P < 0.0001 for absence and presence of neostigmine respectively).
Figure 4.
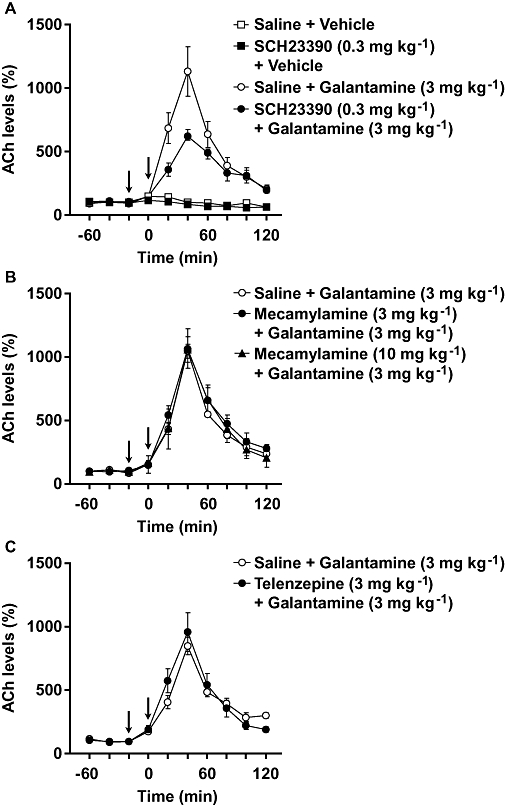
Effects of antagonists of dopamine D1 receptors (SCH23390), nAChR (mecamylamine) and mAChR (telenzepine) on galantamine-induced increase in prefrontal ACh levels in mice. Ringer's solution was perfused without neostigmine in the probe. Galantamine (3 mg kg−1) was injected i.p. at 0 min (right arrow). SCH23390 (0.3 mg kg−1, i.p.) (A), mecamylamine (3 and 10 mg kg−1, i.p.) (B) and telenzepine (3 mg kg−1, s.c.) (C) were injected 20 min before galantamine treatment (left arrow). Data are expressed as the mean ± SEM from 3–4 mice. Repeated measures two-way anova indicated that SCH23390 attenuated galantamine-induced increase in ACh levels, although SCH23390 itself did not affect basal extracellular ACh levels [interaction (treatment × time): F9,54 = 1.214, P > 0.05 and F9,54 = 5.465, P < 0.0001 for basal and galantamine-induced increase respectively]. On the other hand, neither mecamylamine [interaction (treatment × time): F18,72 = 0.261, P > 0.05] nor telenzepine (F9,54 = 0.864, P > 0.05) affected galantamine-induced increase in ACh levels.
Effects of SCH23390, a dopamine-D1 receptor antagonist, mecamylamine, a non-selective nAChR antagonist, and telenzepine on galantamine-induced increase in prefrontal ACh levels
Galantamine (3 mg kg−1, i.p.) caused a robust increase in ACh levels, which could be attenuated by SCH23390 (0.3 mg kg−1, i.p.), but not by mecamylamine (3 and 10 mg kg−1, i.p.) or telenzepine (3 mg kg−1, s.c.) (Fig. 4). SCH23390 alone did not affect basal extracellular ACh levels.
Effect of galantamine on extracellular levels of dopamine and 5-HT in the prefrontal cortex
Basal extracellular levels (means ± SEM) of dopamine and 5-HT (not corrected for in vitro probe recovery) were 3.30 ± 0.54 fmol per 20 µL (n = 24) and 6.71 ± 1.40 fmol per 20 µL (n = 12) respectively (data are obtained from Figs 5 and 6). Galantamine (1 and 3 mg kg−1) significantly increased extracellular dopamine, but not 5-HT, levels (Fig. 5).
Figure 5.
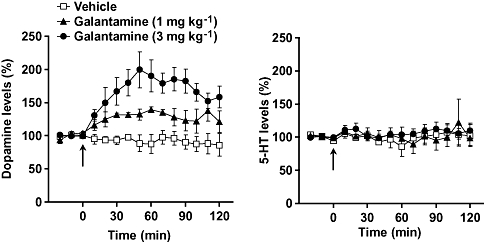
Effects of galantamine on extracellular levels of dopamine and 5-HT in the prefrontal cortex of mice. Galantamine at doses of 1 and 3 mg kg−1 were injected i.p. at 0 min (arrow). Data are expressed as the mean ± SEM from four mice. Repeated measures two-way anova indicated that galantamine produced a significant increase in levels of dopamine [interaction (treatment × time): F28,126 = 4.878, P < 0.0001], but not 5-HT (F28,126 = 0.460, P > 0.05).
Figure 6.
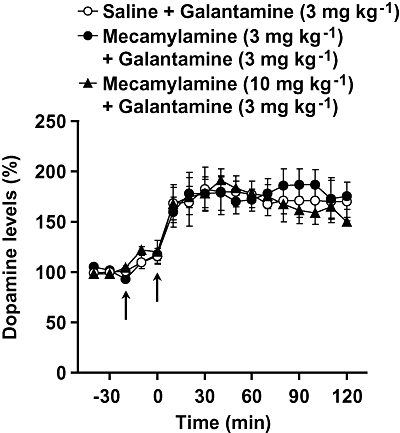
Effect of mecamylamine on galantamine-induced increase in prefrontal dopamine levels. Galantamine (3 mg kg−1) was injected i.p. at 0 min (right arrow). Mecamylamine (3 and 10 mg kg−1, i.p.) was injected 20 min before galantamine treatment (left arrow). Data are expressed as the mean ± SEM from 3–5 mice. Repeated measures two-way anova indicated that mecamylamine did not affect galantamine-induced increase in dopamine levels [interaction (treatment × time): F32,144 = 0.332, P > 0.05].
Effect of mecamylamine on galantamine-induced increase in prefrontal dopamine levels
Galantamine (3 mg kg−1, i.p.) caused a robust increase in dopamine levels, but this effect was not inhibited by mecamylamine (3 and 10 mg kg−1, i.p.) (Fig. 6).
Discussion
A disruption of cerebral cholinergic pathways may contribute to the cognitive deficits of schizophrenia, and AChE inhibitors, like ACh receptor agonists, have therapeutic potential for these deficits (Friedman, 2004). We have recently reported in mice that the AChE inhibitors, galantamine and donepezil, improved apomorphine-induced PPI disruption (Koda et al., 2008), as occurs in rats (Hohnadel et al., 2007). Galantamine is a weak AChE inhibitor and potentiates nAChR activity (Dajas-Bailador et al., 2003; Samochocki et al., 2003), although nAChR antagonists (mecamylamine and methyllycaconitine) do not block its ability to modulate PPI deficits (Koda et al., 2008). The present study demonstrated that the preferential M1 mAChR antagonist, telenzepine (Eltze et al., 1985; Doods et al., 1987; Bymaster et al., 1993), blocked galantamine and donepezil-mediated improvements in apomorphine-induced PPI deficits. This suggests that endogenous ACh preferentially interacts with mAChRs, although both mAChRs and nAChRs are responsible for the improvement of apomorphine-induced PPI deficits (Stanhope et al., 2001; Suemaru et al., 2004; Jones et al., 2005). In addition, the non-selective mAChR agonist, oxotremorine, improved apomorphine-induced PPI deficits in mice. Galantamine, like donepezil, then increased brain ACh levels to improve PPI deficits via mAChRs.
Galantamine at low doses (0.01–0.63 mg kg−1 s.c. for the prefrontal cortex and 0.16–0.63 mg kg−1 s.c. for the hippocampus) increases brain ACh levels in rats (Di Cara et al., 2007), and donepezil increases extracellular ACh concentrations in the cortex (Rogers et al., 1991; Giacobini et al., 1996), hippocampus (Kawashima et al., 1994; Kosasa et al., 1999; Hatip-Al-Khatib et al., 2004) and striatum (Isomae et al., 2002) in rats. Ours is the first study to show that galantamine and donepezil increase extracellular ACh concentrations in the prefrontal cortex of mice. Galantamine is much more potent in rats than in mice: the effective doses of galantamine in the prefrontal cortex of rats (Di Cara et al., 2007) and mice (in this study) were 0.01–0.63 and 1–3 mg kg−1 respectively. Doses of galantamine that increased prefrontal ACh levels (3 mg kg−1) also improved apomorphine-induced PPI deficits in mice. The same dose also improved performance of mice with a nucleus basalis magnocellularis lesion in two different tasks (passive avoidance and swim maze) (Sweeney et al., 1990).
We found that galantamine and donepezil similarly increase brain ACh levels, although another report (Geerts et al., 2005) indicated that donepezil was 3–15 times more potent than galantamine at inhibiting brain AChE. In addition, the present study showed that inhibition of increase in ACh levels by neostigmine was much greater in the effect of donepezil than in that of galantamine. These observations suggest that mechanisms other than AChE inhibition were involved in the galantamine-induced increases in prefrontal ACh levels. Dopamine D1 receptors facilitate ACh release in the prefrontal cortex and hippocampus of rats (Imperato et al., 1993; Acquas et al., 1994; Hersi et al., 1995; Di Cara et al., 2007). Galantamine, but not donepezil, enhances dopamine release in the prefrontal cortex in rats, although only at a low dose (0.1 mg kg−1) (Schilström et al., 2007). We found that galantamine increased extracellular dopamine, but not 5-HT, concentrations in the prefrontal cortex, and the effect of galantamine on ACh levels was partially blocked by the dopamine D1 receptor antagonist, SCH23390. A dopamine D1 receptor-mediated mechanism may thus contribute to the ability of galantamine to increase prefrontal ACh levels. nAChR-mediated ACh release also occurs in rat brain (Tani et al., 1998; Reid et al., 1999), but nAChR and mAChR antagonists did not affect the ability of galantamine to modify prefrontal ACh levels. We have found in separate experiments that mecamylamine (3 mg kg−1) blocked nicotine-induced hypolocomotion and hypothermia in mice. This suggests that mecamylamine under these conditions does indeed block nAChRs, in agreement with the previous studies (Freeman et al., 1987; Damaj et al., 1995; Castañéet al., 2002). Galantamine may increase prefrontal ACh levels via two different mechanisms, AChE inhibition and through activation of dopamine D1 receptors by increasing dopamine release. Concerning the mechanism of galantamine-induced increase in dopaminergic neurotransmission, Schilström et al. (2007) reported that galantamine-induced increase in dopamine cell firing in the ventral tegmental area was prevented by mecamylamine, but not by the muscarinic receptor antagonist scopolamine in anaesthetized rats. However, the present study showed that galantamine-induced increase in dopamine release in the prefrontal cortex of awake mice was not affected by mecamylamine. Alternatively, Alés et al. (2006) have reported that galantamine can potentiate neurotransmitter release by blocking small conductance Ca2+-activated K+ channels. It remains to be established how galantamine might increase dopamine release in the prefrontal cortex.
In conclusion, we have shown that galantamine and donepezil improved PPI deficits in apomorphine-treated mice in an mAChR-dependent manner. We also showed that galantamine, like donepezil, increases prefrontal ACh levels. Galantamine-induced increases in prefrontal ACh levels required both AChE inhibition and activation of dopamine D1 receptors.
Acknowledgments
This study was supported in part by a grant from Janssen Pharmaceutical K.K.
Glossary
Abbreviations
- AChE
acetylcholine esterase
- anova
analysis of variance
- mAChR
muscarinic ACh receptor
- nAChR
nicotinic ACh receptor
- PPI
prepulse inhibition
Conflict of interest
The authors state no conflict of interest.
References
- Acquas E, Day JC, Fibiger HC. The potent and selective dopamine D1 receptor agonist A-77636 increases cortical and hippocampal acetylcholine release in the rat. Eur J Pharmacol. 1994;260:85–87. doi: 10.1016/0014-2999(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Acri JB, Morse DE, Popke EJ, Grunberg NE. Nicotine increases sensory gating measured as inhibition of the acoustic startle reflex in rats. Psychopharmacology (Berl) 1994;114:369–374. doi: 10.1007/BF02244861. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Ago Y, Sakaue M, Baba A, Matsuda T. Selective reduction by isolation rearing of 5-HT1A receptor-mediated dopamine release in vivo in the frontal cortex of mice. J Neurochem. 2002;83:353–359. doi: 10.1046/j.1471-4159.2002.01128.x. [DOI] [PubMed] [Google Scholar]
- Ago Y, Koyama Y, Baba A, Matsuda T. Regulation by 5-HT1A receptors of the in vivo release of 5-HT and DA in mouse frontal cortex. Neuropharmacology. 2003;45:1050–1056. doi: 10.1016/s0028-3908(03)00304-6. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Uda M, Kajii Y, Abe M, Baba A, et al. Attenuation by the 5-HT1A receptor agonist, osemozotan, of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacology. 2006a;51:914–922. doi: 10.1016/j.neuropharm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Ago Y, Sato M, Nakamura S, Baba A, Matsuda T. Lack of enhanced effect of antipsychotics combined with fluvoxamine on acetylcholine release in rat prefrontal cortex. J Pharmacol Sci. 2006b;102:419–422. doi: 10.1254/jphs.sc0060187. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Kajita N, Uda M, Hashimoto H, Baba A, et al. Ritanserin reverses repeated methamphetamine-induced behavioral and neurochemical sensitization in mice. Synapse. 2007;61:757–763. doi: 10.1002/syn.20421. [DOI] [PubMed] [Google Scholar]
- Alés E, Gullo F, Arias E, Olivares R, Carcía AG, Wanke E, et al. Blockade of Ca2+-activated K+ channels by galantamine can also contribute to the potentiation of catecholamine secretion from chromaffin cells. Eur J Pharmacol. 2006;548:45–52. doi: 10.1016/j.ejphar.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG, Theou K, Frangou S. Increased prepulse inhibition of the acoustic startle response is associated with better strategy formation and execution times in healthy males. Neuropsychologia. 2006;44:2494–2499. doi: 10.1016/j.neuropsychologia.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 1999;156:596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Bubser M, Koch M. Prepulse inhibition of the acoustic startle response of rats is reduced by 6 hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology (Berl) 1994;113:487–492. doi: 10.1007/BF02245228. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Heath I, Hendrix JC, Shannon HE. Comparative behavioral and neurochemical activities of cholinergic antagonists in rats. J Pharmacol Exp Ther. 1993;267:16–24. [PubMed] [Google Scholar]
- Castañé A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Consolo S, Arnaboldi S, Ramponi S, Nannini L, Ladinsky H, Baldi G. Endogenous serotonin facilitates in vivo acetylcholine release in rat frontal cortex through 5-HT1B receptors. J Pharmacol Exp Ther. 1996;277:823–830. [PubMed] [Google Scholar]
- Dajas-Bailador FA, Heimala K, Wonnacott S. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine is transduced into cellular responses in neurons: Ca2+ signals and neurotransmitter release. Mol Pharmacol. 2003;64:1217–1226. doi: 10.1124/mol.64.5.1217. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Welch SP, Martin BR. In vivo pharmacological effects of dihydro-β-erythroidine, a nicotinic antagonists, in mice. Psychopharmacology (Berl) 1995;117:67–73. doi: 10.1007/BF02245100. [DOI] [PubMed] [Google Scholar]
- Di Cara B, Panayi F, Gobert A, Dekeyne A, Sicard D, De Groote L, et al. Activation of dopamine D1 receptors enhances cholinergic transmission and social cognition: a parallel dialysis and behavioural study in rats. Int J Neuropsychopharmacol. 2007;10:383–399. doi: 10.1017/S1461145706007103. [DOI] [PubMed] [Google Scholar]
- Doods HN, Mathy MJ, Davidesko D, van Charldorp KJ, de Jonge A, van Zwieten PA. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987;242:257–262. [PubMed] [Google Scholar]
- Eltze M, Gonne S, Riedel R, Schlotke B, Schudt C, Simon WA. Pharmacological evidence for selective inhibition of gastric acid secretion by telenzepine, a new antimuscarinic drug. Eur J Pharmacol. 1985;112:211–224. doi: 10.1016/0014-2999(85)90498-4. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, Inc; 1997. [Google Scholar]
- Freeman GB, Sherman KA, Gibson GE. Locomotor activity as a predictor of times and dosages for studies of nicotine's neurochemical actions. Pharmacol Biochem Behav. 1987;26:305–312. doi: 10.1016/0091-3057(87)90123-7. [DOI] [PubMed] [Google Scholar]
- Friedman JI. Cholinergic targets for cognitive enhancement in schizophrenia: focus on cholinesterase inhibitors and muscarinic agonists. Psychopharmacology (Berl) 2004;174:45–53. doi: 10.1007/s00213-004-1794-x. [DOI] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005;1033:186–193. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NE. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Giacobini E, Zhu XD, Williams E, Scherman KA. The effect of the selective reversible acetylcholinesterase inhibitor, E2020, on extracellular acetylcholine and biogenic amine levels in rat cortex. Neuropharmacology. 1996;35:205–211. doi: 10.1016/0028-3908(95)00157-3. [DOI] [PubMed] [Google Scholar]
- Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Hatip-Al-Khatib I, Takashi A, Egashira N, Iwasaki K, Fujiwara M. Comparison of the effect of TAK-147 (zanapezil) and E-2020 (donepezil) on extracellular acetylcholine level and blood flow in the ventral hippocampus of freely moving rats. Brain Res. 2004;1012:169–176. doi: 10.1016/j.brainres.2004.03.067. [DOI] [PubMed] [Google Scholar]
- Hersi AI, Richard JW, Gaudreau P, Quirion R. Local modulation of hippocampal acetylcholine release by dopamine D1 receptors: a combined receptor autoradiography and in vivo dialysis study. J Neurosci. 1995;15:7150–7157. doi: 10.1523/JNEUROSCI.15-11-07150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnadel E, Bouchard K, Terry AV., Jr Galantamine and donepezil attenuate pharmacologically induced deficits in prepulse inhibition in rats. Neuropharmacology. 2007;52:542–551. doi: 10.1016/j.neuropharm.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J, Dai J, O'Laughlin IA, Fowler WL, Meltzer HY. Atypical, but not typical, antipsychotic drugs increase cortical acetylcholine release without an effect in the nucleus accumbens or striatum. Neuropsychopharmacology. 2002;26:325–339. doi: 10.1016/S0893-133X(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Imperato A, Obinu MC, Gessa GL. Stimulation of both dopamine D1 and D2 receptors facilitates in vivo acetylcholine release in the hippocampus. Brain Res. 1993;618:341–345. doi: 10.1016/0006-8993(93)91288-4. [DOI] [PubMed] [Google Scholar]
- Isomae K, Ishikawa M, Ohta M, Ogawa Y, Hasegawa H, Kohda T, et al. Effects of T-82, a new quinoline derivative, on cholinesterase activity and extracellular acetylcholine concentration in rat brain. Jpn J Pharmacol. 2002;88:206–212. doi: 10.1254/jjp.88.206. [DOI] [PubMed] [Google Scholar]
- Jones CK, Eberle EL, Shaw DB, McKinzie DL, Shannon HE. Pharmacologic interactions between the muscarinic cholinergic and dopaminergic systems in the modulation of prepulse inhibition in rats. J Pharmacol Exp Ther. 2005;312:1055–1063. doi: 10.1124/jpet.104.075887. [DOI] [PubMed] [Google Scholar]
- de Jong IE, van den Buuse M. SCH 23390 in the prefrontal cortex enhances the effect of apomorphine on prepulse inhibition of rats. Neuropharmacology. 2006;51:438–446. doi: 10.1016/j.neuropharm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Sato A, Yoshizawa M, Fujii T, Fujimoto K, Suzuki T. Effects of the centrally acting cholinesterase inhibitors, tetrahydroaminoacridine and E2020, on the basal concentration of extracellular acetylcholine in the hippocampus of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:523–528. doi: 10.1007/BF00173022. [DOI] [PubMed] [Google Scholar]
- Koda K, Ago Y, Kawasaki T, Hashimoto H, Baba A, Matsuda T. Galantamine and donepezil differently affect isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2008;196:293–301. doi: 10.1007/s00213-007-0962-1. [DOI] [PubMed] [Google Scholar]
- Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. Effect of donepezil hydrochloride (E2020) on basal concentration of extracellular acetylcholine in the hippocampus of rats. Eur J Pharmacol. 1999;380:101–107. doi: 10.1016/s0014-2999(99)00545-2. [DOI] [PubMed] [Google Scholar]
- Kumari V, Checkley SA, Gray JA. Effects of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology (Berl) 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter PA, Checkley SA, Gray JA. Effects of acute subcutaneous nicotine on prepulse inhibition of the acoustic startle reflex in healthy male non-smokers. Psychopharmacology (Berl) 1997;132:389–395. doi: 10.1007/s002130050360. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Samochocki M, Jostock R, Fehrenbacher A, Ludwig J, Albuquerque EX, et al. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer's disease. Biol Psychiatry. 2001;49:279–288. doi: 10.1016/s0006-3223(00)01109-4. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS, et al. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160:118–127. doi: 10.1176/appi.ajp.160.1.118. [DOI] [PubMed] [Google Scholar]
- Reid RT, Lloyd GK, Rao TS. Pharmacological characterization of nicotine-induced acetylcholine release in the rat hippocampus in vivo: evidence for a permissive dopamine synapse. Br J Pharmacol. 1999;127:1486–1494. doi: 10.1038/sj.bjp.0702683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Yamanishi Y, Yamatsu K. E2020: the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Cambridge: Birkhäuser Boston; 1991. pp. 314–320. [Google Scholar]
- Sakaue M, Ago Y, Baba A, Matsuda T. The 5-HT1A receptor agonist, MKC-242, reverse isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2003;170:73–79. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Höffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Santos MD, Alkondon M, Pereira EF, Aracava Y, Eisenberg HM, Maelicke A, et al. The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Mol Pharmacol. 2002;61:1222–1234. doi: 10.1124/mol.61.5.1222. [DOI] [PubMed] [Google Scholar]
- Sato M, Ago Y, Koda K, Nakamura S, Kawasaki T, Baba A, et al. Role of postsynaptic serotonin1A receptors in risperidone-induced increase in acetylcholine release in rat prefrontal cortex. Eur J Pharmacol. 2007;559:155–160. doi: 10.1016/j.ejphar.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Schilström B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32:43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- Somboonthum P, Matsuda T, Asano S, Sakaue M, Baba A. MKC-242, a novel 5-HT1A receptor agonist, facilitates cortical acetylcholine release by a mechanism different from that of 8-OH-DPAT in awake rats. Neuropharmacology. 1997;36:1733–1739. doi: 10.1016/s0028-3908(97)00174-3. [DOI] [PubMed] [Google Scholar]
- Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB, et al. The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J Pharmacol Exp Ther. 2001;299:782–792. [PubMed] [Google Scholar]
- Suemaru K, Yasuda K, Umeda K, Araki H, Shibata K, Choshi T, et al. Nicotine blocks apomorphine-induced disruption of prepulse inhibition of the acoustic startle in rats: possible involvement of central nicotinic α7 receptors. Br J Pharmacol. 2004;142:843–850. doi: 10.1038/sj.bjp.0705855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JE, Bachman ES, Coyle JT. Effects of different doses of galantamine, a long-acting acetylcholinesterase inhibitor, on memory in mice. Psychopharmacology (Berl) 1990;102:191–200. doi: 10.1007/BF02245921. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Lipska BK, Weinberger DR, Braff DL, Jaskiw GE, Geyer MA. Increased sensitivity to the sensorimotor gatingdisruptive effects of apomorphine after lesions of medial prefrontal cortex or ventral hippocampus in adult rats. Psychopharmacology (Berl) 1995;122:27–34. doi: 10.1007/BF02246438. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Tani Y, Saito K, Imoto M, Ohno T. Pharmacological characterization of nicotinic receptor-mediated acetylcholine release in rat brain – an in vivo microdialysis study. Eur J Pharmacol. 1998;351:181–188. doi: 10.1016/s0014-2999(98)00314-8. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lander C, Geerts H. Nicotinic cholinergic modulation: galantamine as a prototype. CNS Drug Rev. 2002;8:405–426. doi: 10.1111/j.1527-3458.2002.tb00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


