Abstract
Background and purpose
Arginase and nitric oxide (NO) synthase share the common substrate L-arginine, and arginase inhibition is proposed to increase NO production by increasing intracellular levels of L-arginine. Many different inhibitors are used, and here we have examined the effects of these inhibitors on vascular tissue.
Experimental approach
Each arginase inhibitor was assessed by its effects on isolated rings of aorta and mesenteric arteries from rats by: (i) their ability to preserve the tolerance to repeated applications of the endothelium-dependent agonist acetylcholine (ACh); and (ii) their direct vasorelaxant effect.
Key results
In both vessel types, tolerance (defined as a reduced response upon second application) to ACh was reversed with addition of L-arginine, (S)-(2-boronethyl)-L-cysteine HCl (BEC) or NG-Hydroxy-L-arginine (L-NOHA). On the other hand, Nω-hydroxy-nor-L-arginine (nor-NOHA) significantly augmented the response to ACh, an effect that was partially reversed with L-arginine. No effect on tolerance to ACh was observed with L-valine, nor-valine or D,L, α-difluoromethylornithine (DFMO). BEC, L-NOHA and nor-NOHA elicited endothelium-independent vasorelaxation in both endothelium intact and denuded aorta while L-valine, DFMO and nor-valine did not.
Conclusions and implications
BEC and L-NOHA, but not nor-NOHA, L-valine, DFMO or nor-valine, significantly reversed tolerance to ACh possibly conserving L-arginine levels and therefore increasing NO bioavailability. However, both BEC and L-NOHA caused endothelium-independent vasorelaxation in rat aorta, suggesting that these inhibitors have a role beyond arginase inhibition alone. Our data thus questions the interpretation of many studies using these antagonists as specific arginase inhibitors in the vasculature, without verification with other methods.
Keywords: arginase, L-arginine, aorta, mesenteric arteries, nitric oxide, vasorelaxation
Introduction
Nitric oxide (NO) bioavailability is compromised in many cardiovascular disease states. As L-arginine is rate limiting in NO production, this amino acid precursor has been postulated as a factor contributing to NO availability. Also, because the enzyme arginase catalyses the catabolism of L-arginine to form ornithine and urea, many argue that arginase can be manipulated to influence NO bioavailability. In this context, increased expression and/or activity of arginase has been demonstrated in many vascular pathologies such as pulmonary hypertension (associated with sickle cell disease) (Morris et al., 2003), primary pulmonary arterial hypertension (Xu et al., 2004), ischaemia-reperfusion (Hein et al., 2003), uraemia (Thuraisingham et al., 2002) as well as in animal models of arterial hypertension (Johnson et al., 2005), aging (Berkowitz et al., 2003; Santhanam et al., 2007), sexual arousal (Berkowitz et al., 2003; Cama et al., 2003a), diabetes (Romero et al., 2008) and atherosclerosis (Ming et al., 2004; Ryoo et al., 2006; 2008). Furthermore, inhibition of arginase has been shown to stimulate NO production (Chicoine et al., 2004), while over-expression of arginase I or II decreases intracellular L-arginine concentrations and suppresses NO synthesis (Li et al., 2001). However, because NG-hydroxy-L-arginine (L-NOHA), a reaction intermediate of NO synthase (NOS), is also a potent intracellular inhibitor of arginase (Boucher et al., 1994; Daghigh et al., 1994) some of the effects observed may not confined to substrate competition alone.
Both vascular endothelial and smooth muscle cells express arginase I and II, but their distribution appears to be vessel- and species-dependent (Buga et al., 1996; Zhang et al., 2001; Bachetti et al., 2004; Ming et al., 2004). In addition to the production of urea, arginase is also involved in the biosynthesis of polyamines and the amino acids, ornithine, proline and glutamate (Cederbaum et al., 2004). As such it is not surprising that amino acids such as ornithine, leucine, valine, lysine, isoleucine and nor-valine inhibit arginase (Hunter and Downs, 1945). Among them, ornithine is the most potent of the competitive amino acids, with nor-valine, a non-competitive inhibitor, demonstrating similar potency (Hunter and Downs, 1945). Another commonly used inhibitor is the indirectly acting, irreversible inhibitor of ornithine decarboxylase, D,L, α-difluoromethylornithine (DFMO), which increases ornithine levels endogenously (Selamnia et al., 1998). Despite the relatively high concentrations required to inhibit arginase, the use of nor-valine, L-valine and DFMO have recently been reported in the context of studying NO function (Ming et al., 2004; Santhanam et al., 2007; Lewis et al., 2008).
As L-NOHA also acts as a substrate for NO production, its utility as a specific arginase inhibitor is limited, although it is still used as a specific arginase inhibitor (Sakai et al., 2004; Holan et al., 2006). Due to the complications with the use of L-NOHA, Nω-hydroxy-nor-L-arginine (nor-NOHA) was synthesized and reported to be more potent than L-NOHA, but not a substrate for NO (Tenu et al., 1999). Aside from these inhibitors, several boron-based inhibitors have been designed. Among these are (S)-(2-boronethyl)-L-cysteine HCl (BEC) and 2(S)-amino-6-boronohexanoic acid (ABH) (Colleluori and Ash, 2001), both of which have been shown to effectively inhibit arginase (Khangulov et al., 1995).
Due to the potential for manipulating intracellular L-arginine stores, and possibly increasing NO bioavailability in disease states, the interest in this field of research has escalated, resulting in the recent use of many of these inhibitors (Santhanam et al., 2007; Bagnost et al., 2008; Lewis et al., 2008). To address which of the arginase inhibitors is most specific and appropriate for studying functional NO effects in the vasculature, a comparative assessment of these arginase inhibitors was performed.
Methods
Animals
All animal procedures and the study protocol was approved by the Alfred Medical Research and Education Precinct (AMREP) Animal Ethics Committee (applications E/0323/2003/B, E/0238/2004/2004B and E/0352/2004/B) that adheres to the National Health and Medical Research Council (NHMRC) of Australia Code of Practice for the Care and Use of Animals for Scientific Purposes. Male Sprague-Dawley rats were housed under standard laboratory conditions with access to food and water ad libitum. Animals were killed by decapitation and exsanguination following overexposure to 80% CO2 and 20% O2.
Vascular reactivity
The thoracic aorta and mesenteric arteries were excised and placed into ice-cold modified Krebs solution (composition in mmol·L−1: NaCl 119, KCl 4.7, MgSO4·7H20 1.17, NaHCO3 25, KH2PO4 1.18, CaCl2 2.5, glucose 11 and EDTA 0.03). The adipose and connective tissue were removed. Rat aorta was sectioned into eight rings of 3 mm length and mesenteric arteries into eight rings of 2 mm length with the aid of a dissecting microscope (Olympus, Tokyo). In some of the vessels, endothelium denudation of thoracic aortic rings was performed by gently rubbing the lumen of the aorta against a wire. For mesenteric arteries, this procedure was achieved by pulling a strand of human hair backwards and forwards through the lumen of the vessel.
Aortic rings and mesenteric arteries were mounted in organ baths and on a wire-myograph as previously described (Lewis et al., 1997; Kimura et al., 2002). Once the vessels were mounted and incubated for an equilibration period of 30 min, all vessels were subjected to an oxygenated and pre-warmed (37°C) high potassium physiological salt solution (KPSS in mmol·L−1; KCl 123, MgSO4·7H20 1.17, NaHCO3 25, KH2PO4 1.18, CaCl2 2.5, glucose 6.05 and EDTA 0.03) until a plateau contractile response was observed. The vessel was rinsed three times with oxygenated and pre-warmed (37°C) modified Krebs’ solution. Endothelium integrity or successful denudation was confirmed by pre-constricting the vessel with a constrictor agonist followed by addition of acetylcholine (ACh) (1 µmol·L−1). Rat aorta was pre-constricted with noradrenaline (10 nmol·L−1), while for mesenteric arteries, the modified Krebs solution was replaced with 40 mmol·L−1 potassium salt solution (40 mmol·L−1 KCl, composition in mmol·L−1; NaCl 84, KCl 40, MgSO4·7H20 1.17, NaHCO3 25, KH2PO4 1.18, CaCl2 2.5, glucose 11 and EDTA 0.03). Potassium was chosen as the constrictor for mesenteric arteries since at the concentration used, any effect of endothelial-derived hyperpolarizing factor (EDHF) would be negated, unmasking the contribution of NO to vasodilatation (Chen and Suzuki, 1990). Where applicable, for intact (i.e. non-denuded) vessels, a relaxation response of >80% in rat aorta and >60% in mesenteric arteries was set as the inclusion criteria upon addition of 1 µmol·L−1 ACh. Vessels were deemed denuded when relaxation was less than 10% in either aorta or mesenteric arteries.
Experimental design
Effect of L-arginine supplementation and arginase inhibitors on NO function
Cumulative full concentration–response curves in half log increments to the endothelium- and NO-dependent dilator, ACh, (1 nmol·L−1–10 µmol·L−1), were obtained and repeated 30 min apart. Between each curve, vessels were washed, and a total of three concentration–response curves to ACh were obtained successively, 30 min apart, in the absence of any treatment and used as the comparative time control experiment.
In separate baths and myograph chambers, vessels were incubated with L-arginine (1 µmol·L−1 or 10 µmol·L−1) or the arginase inhibitors: BEC (100 µmol·L−1), nor-NOHA (10 µmol·L−1), L-NOHA (10 µmol·L−1), DFMO (10 µmol·L−1), L-valine (10 µmol·L−1) or nor-valine (10 µmol·L−1) for 30 min before and during the repeat concentration–response curve to ACh. Therefore, matched controls were obtained for each compound, such that (−) and (+) denotes the ACh concentration–response curve performed before and after addition of L-arginine or the arginase inhibitor as indicated. The concentration of the arginase inhibitor used during the incubation period was determined from pilot studies. Vasorelaxant responses in rat aorta were performed on vessels pre-constricted with noradrenaline and in mesenteric arteries with 40 mmol·L−1 KCl.
The role of the endothelium on the direct vasodilatory effect of arginase inhibitors
Full cumulative concentration–response curves in half log increments to the arginase inhibitors: BEC (aorta and mesenteric arteries: 0.1 µmol·L−1–3 mmol·L−1), nor-NOHA (aorta and mesenteric arteries: 0.1 µmol·L−1–3 mmol·L−1), L-NOHA (aorta: 1 µmol·L−1–1 mmol·L−1, mesenteric arteries: 0.1 µmol·L−1–3 mmol·L−1), DFMO, L-valine or nor-valine (aorta: 1 µmol·L−1–3 mmol·L−1, mesenteric arteries: 10 µmol·L−1–3 mmol·L−1) were obtained in endothelium intact and denuded vessels. Experiments were performed in rat aortic rings pre-constricted with noradrenaline and in mesenteric arteries pre-constricted with 40 mmol·L−1 KCl. Concentration–response curves were also obtained to L-NOHA, nor-NOHA and BEC in the presence of the soluble guanlylyl cyclase (sGC) inhibitor, 1H-[1,2,4]-oxadiazolol[4,3-1]quinoxaline-1-one (ODQ, 10 µmol·L−1) or the NOS inhibitor, NG-nitro-L-arginine-methyl ester (L-NAME, 100 µmol·L−1).
Data analysis and statistics
All vasorelaxation responses were expressed as percentage relaxation from the pre-constriction response to noradrenaline in aortic rings, or to 40 mmol·L−1 KCl in mesenteric arteries. Rmax depicts the maximum relaxation response obtained. Variable slope sigmoidal concentration–response curves to each agonist were fitted and graphed, and the potency [−log EC50 (M) i.e. the concentration giving 50% of the maximum response] calculated for individual curves by using GraphPad Prism (V. 4.01, USA). Results were analysed by Student's t-test (paired or unpaired, as appropriate). Where three curves were compared, comparisons were made by using a one-way anova. Statistical analysis was performed by using GraphPad Prism where P < 0.05 was considered statistically significant. All data are presented as mean ± SEM.
Drugs and reagents
Arginase inhibitors, L-NOHA (NG-hydroxy-L-arginine monoacetate salt), nor-NOHA (Nω-hydroxy-nor-L-arginine diacetate salt) and BEC, were purchased from Calbiochem, USA. DFMO, L-NAME, L-valine, nor-valine, ODQ, noradrenaline bitartrate, ACh chloride, sodium nitroprusside dihydrate and L-arginine were purchased from Sigma-Aldrich, St. Louis, MO, USA. Drug stock solutions were made in milliQ water and stored at −20°C. All drugs were diluted in modified Krebs solution, with the exception of ODQ that was made up in 100% ethanol on the day of experiment and stored on ice until ready for use. Salts used in modified KPSS, modified Krebs solution and 40 mmol·L−1 KCl were all purchased from Merck P/L, Kilsyth, Victoria, Australia. Drug/molecular target nomenclature conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2008).
Results
L-arginine and tolerance to ACh in aorta
As shown in Figure 1A and consistent with previous findings (Hogan et al., 2005), a successive reduction in both potency (rightward shift in concentration–response curve) and maximal efficacy, Rmax, was observed with each successive construction of a full concentration–response curve to ACh. Upon addition of either 1 µmol·L−1 or 10 µmol·L−1 of L-arginine, 30 min prior to performing the second ACh concentration–response curve, the shift or ‘tolerance’ to ACh was no longer present (Fig. 1B), suggesting that depletion of intracellular L-arginine, over time plays a role in the observed tolerance.
Figure 1.
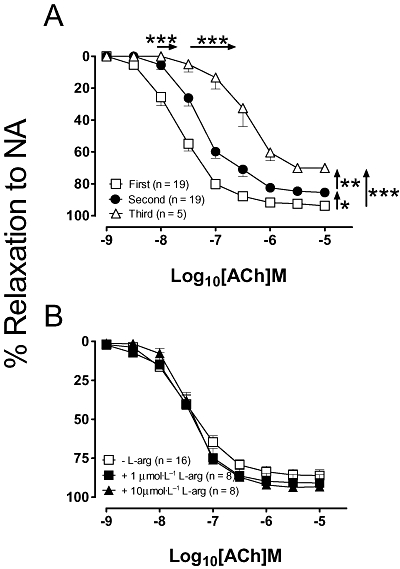
(A) Successive concentration–response curves to acetylcholine (ACh), repeated 30 min apart. In separate experiments, (B) the second application of ACh was performed in the presence of either 1 µmol·L−1 or 10 µmol·L−1 of L-arginine (L-arg). All data are presented as mean ± SEM. The horizontal and vertical arrows refer to changes in EC50 and Rmax respectively; *P < 0.05, **P < 0.01 and ***P < 0.001 by using a one-way anova with Tukey's post hoc analysis. NA, noradrenaline.
Arginase inhibitors and tolerance to ACh in aorta
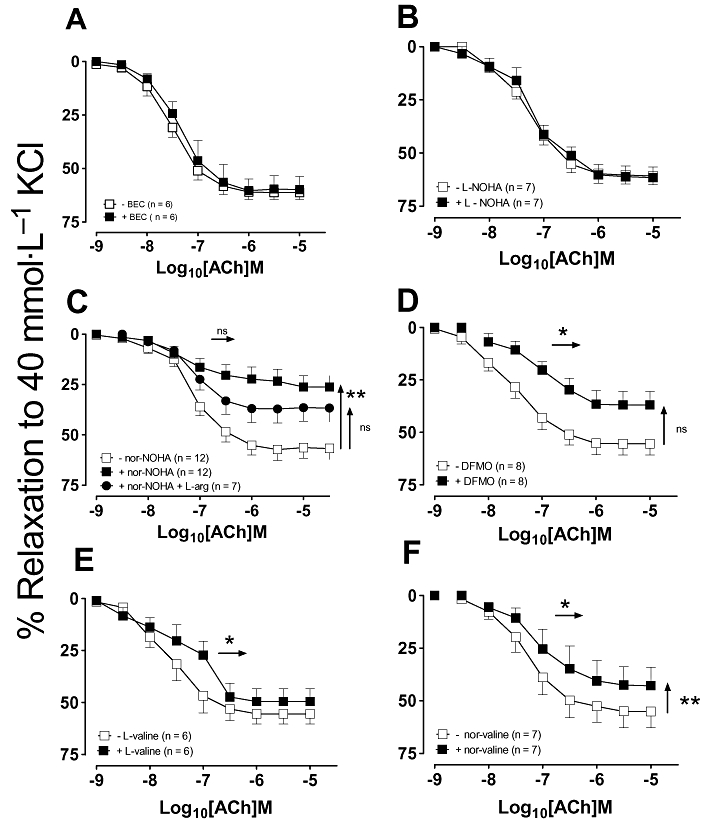
The ability of six different arginase inhibitors to reverse ACh tolerance was investigated in rat isolated aortic rings (Fig. 2). In the presence of BEC and L-NOHA, tolerance to ACh was not observed, that is, there was no significant difference in either the EC50 or Rmax values in ACh in the presence of the arginase inhibitor (P > 0.05; Fig. 2A,B). In contrast, nor-NOHA enhanced the shift to the right substantially and reduced the maximum of the second ACh concentration–response curve when compared with the second control ACh concentration–response curve (EC50, 0.3 ± 0.1 vs. 0.09 ± 0.02 µmol·L−1; Rmax, 34 ± 9 vs. 85 ± 2%; n = 15–19; P < 0.05; Fig. 2C), an effect that was partially restored by L-arginine. DFMO and the equipotent (to DFMO) competitive and non-competitive arginase inhibitors, L-valine and nor-valine had no significant effect on the EC50 of the ACh-induced tolerance, albeit in the presence of L-valine and nor-valine there was no longer a significant difference in the maximal response (Fig. 2D–F).
Figure 2.
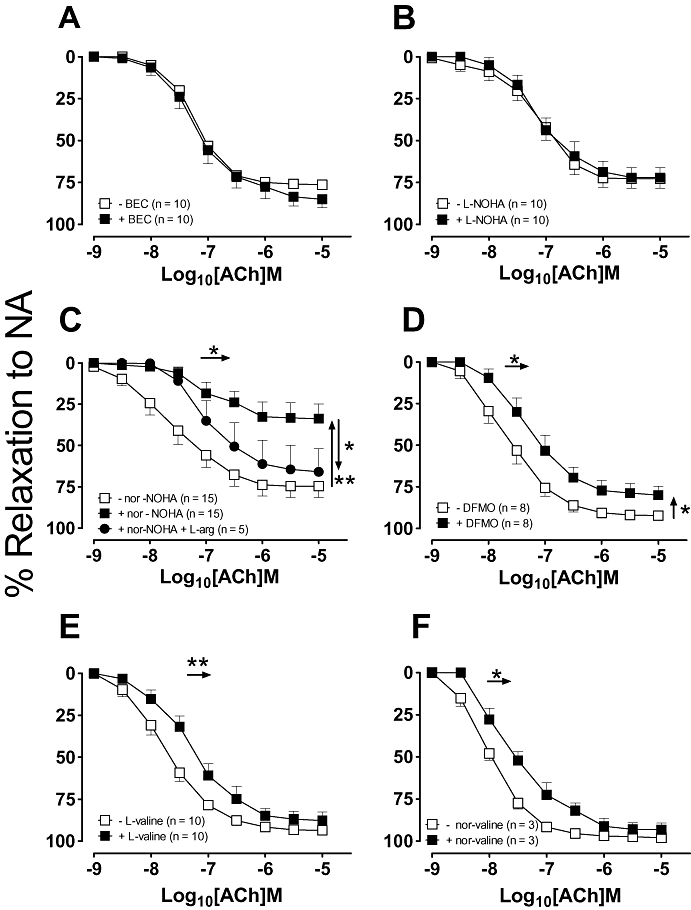
Concentration–response curves to ACh were repeated 30 min after the addition of either (A) 100 µmol·L−1 BEC, (B) 10 µmol·L−1 L-NOHA, (C) 10 µmol·L−1 nor-NOHA, (D) 10 µmol·L−1 DFMO, (E) 10 µmol·L−1 L-valine or (F) 10 µmol·L−1 nor-valine. All data are presented as mean ± SEM where *P < 0.05 and **P < 0.01 by using a paired Student's t-test comparison of the EC50 (horizontal arrows) or Rmax (vertical arrows) before and after the addition of an arginase inhibitor. ACh, acetylcholine; BEC, (S)-(2-boronethyl)-L-cysteine HCl; DFMO, D,L, α-difluoromethylornithine; L-NOHA, NG-hydroxy-L-arginine; NA, noradrenaline; nor-NOHA, Nω-hydroxy-nor-arginine.
Arginase inhibitors as vasodilators in aorta
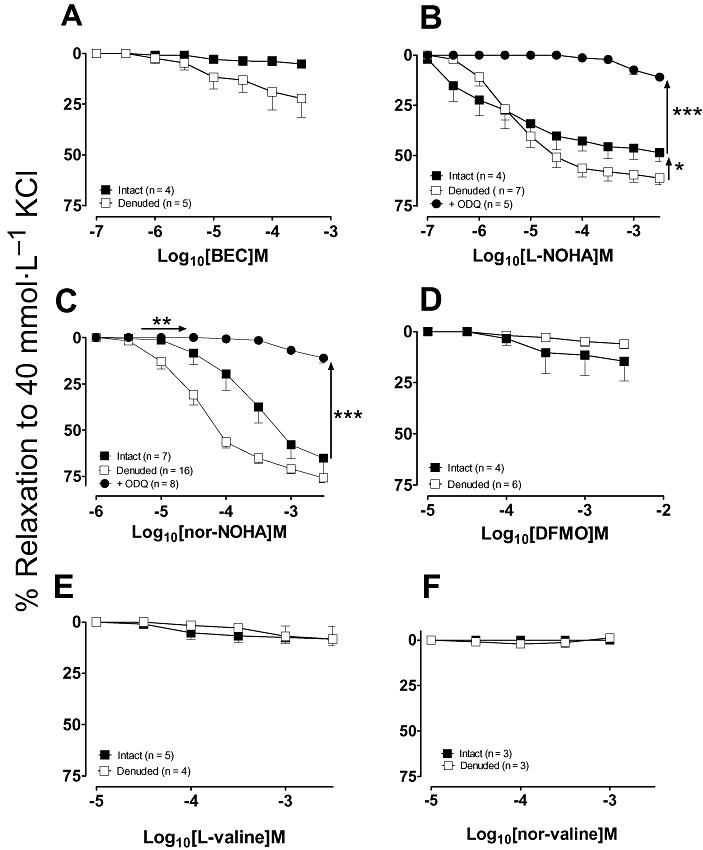
Since arginase expression has been reported in both endothelial and vascular smooth muscle cells (Berkowitz et al., 2003; Buchwalow et al., 2004; Johnson et al., 2005), the effects of the arginase inhibitors were examined in both endothelium-intact and denuded vessels. As shown in Figure 3A, BEC elicited concentration-dependent vasorelaxation which was non-endothelium-dependent (EC50 and Rmax, intact vs. denuded rings: 13 ± 4 µmol·L−1 and 80 ± 6% vs. 62 ± 26 µmol·L−1 and 67 ± 8%, n = 11–15; P > 0.05). Similarly, L-NOHA and nor-NOHA induced vasorelaxation in both intact and denuded vessels with comparable potencies (Fig. 3B,C). In endothelium-denuded aorta, vasorelaxation to BEC, L-NOHA and nor-NOHA, was significantly attenuated in the presence of the sGC inhibitor, ODQ (10 µmol·L−1) suggesting a cGMP-dependent mechanism. Responses to L-NOHA were attenuated by the NOS inhibitor L-NAME (100 µmol·L−1) in both intact and denuded aorta (P < 0.05) while those to BEC were unaffected. DFMO, L-valine and nor-valine did not induce significant vasorelaxation (see Fig. 3D–F) when compared with their time controls (data not shown), which coincided with their reduced ability to reverse tolerance to ACh.
Figure 3.
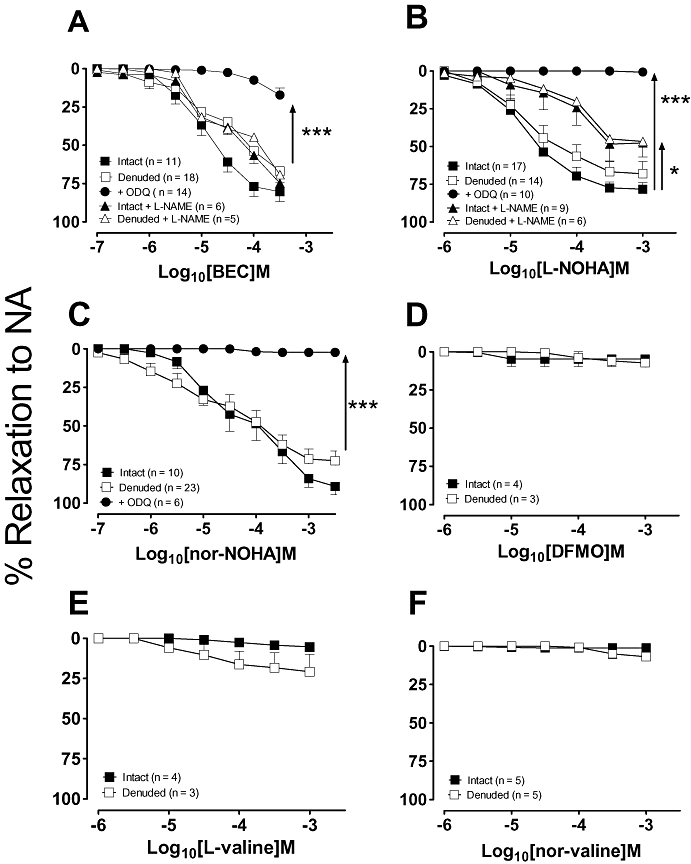
Concentration–response curves to the arginase inhibitors: (A) BEC, (B) L-NOHA, (C) nor-NOHA, (D) DFMO, (E) L-valine and (F) nor-valine were performed in endothelium-intact and denuded aortic rings pre-constricted with NA. Responses to L-NOHA, nor-NOHA and BEC were also performed in the presence of the cGMP inhibitor, ODQ (10 µmol·L−1) in endothelium-denuded vessels or the NOS inhibitor L-NAME (100 µmol·L−1) in endothelium-intact and denuded vessels. All responses are expressed as % relaxation to NA and as mean ± SEM where *P < 0.05 (−nor-NOHA vs. +nor-NOHA) and ***P < 0.001 by using an unpaired Student's t-test. BEC, (S)-(2-boronethyl)-L-cysteine HCl; DFMO, D,L, α-difluoromethylornithine; L-NAME, NG-nitro-L-arginine-methyl ester; L-NOHA, NG-hydroxy-L-arginine; NA, noradrenaline; nor-NOHA, Nω-hydroxy-nor-arginine; ODQ, 1H-[1,2,4]-oxadiazolol[4,3-1]quinoxaline-1-one.
L-arginine and tolerance to ACh in mesenteric arteries
While NO is thought to play a significant role in the vasodilatory profile in conduit vessels, it has been frequently reported to play only a minor role in resistance vessels such as mesenteric arteries, where a larger contribution by other vasodilators such as EDHF is reported (Wu et al., 1993; Shimokawa et al., 1996; Chataigneau et al., 1999). Therefore, to negate the effects of EDHF, which dilates via K+ channels, all experiments were performed by using a high K+ solution (40 mmol·L−1) as the vasoconstrictor. Hence, under these conditions, ACh-induced relaxation is NOS-dependent and is abolished in the presence of L-NAME (data not shown). When similar experiments to those described above, but this time in mesenteric arteries were performed, tolerance to ACh was also observed (EC50 values, first vs. second control concentration–response curves, 0.1 ± 0.01 vs. 0.4 ± 0.2 µmol·L−1; n = 13; P < 0.05; Fig. 4A) but without an effect on the maximal response to ACh (P > 0.05). As observed in aortic rings, supplementation with either 1 µmol·L−1 or 10 µmol·L−1 L-arginine abolished the rightward shift in the concentration–response curve to ACh (P > 0.05; Fig. 4B).
Figure 4.
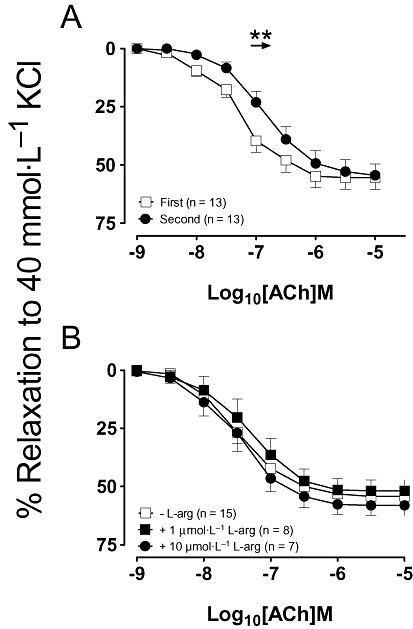
(A) Concentration–response curves to acetylcholine (ACh) were repeated 30 min apart in mesenteric artery rings pre-constricted with 40 mmol·L−1 KCl. The second application of ACh was also performed (B) in the presence of either 1 µmol·L−1 or 10 µmol·L−1 of L-arginine (L-arg). All data are presented as mean ± SEM where **P < 0.01 by using a paired Student's t-test.
Arginase inhibitors and tachyphylaxis to ACh in mesenteric arteries
As we had observed in aortic rings, there was no tolerance to ACh in rings of mesenteric arteries in the presence of BEC or L-NOHA (Fig. 5A,B). Again, nor-NOHA significantly enhanced the tolerance to ACh, a finding, which again was partially reversed with addition of 100 µmol·L−1 of L-arginine (Fig. 5C). DFMO, L-valine and nor-valine, likewise, did not reduce tolerance to Ach (Fig. 5D–F).
Figure 5.
The second concentration–response curve to ACh was repeated in mesenteric artery rings after a 30 min incubation with either (A) 100 µmol·L−1 BEC, (B) 10 µmol·L−1 L-NOHA, (C) 10 µmol·L−1 nor-NOHA, (D) 10 µmol·L−1 DFMO, (E) 10 µmol·L−1 L-valine or (F) 10 µmol·L−1 nor-valine. All data are presented as mean ± SEM where *P < 0.05 and **P < 0.001 by using a paired t-test comparison of the EC50 and Rmax before and after the addition of an arginase inhibitor. ACh, acetylcholine; BEC, (S)-(2-boronethyl)-L-cysteine HCl; DFMO, D,L, α-difluoromethylornithine; L-NOHA, NG-hydroxy-L-arginine; nor-NOHA, Nω-hydroxy-nor-arginine.
The endothelium and arginase inhibitors in mesenteric arteries
In contrast to the finding in aortic rings, BEC did not induce significant vasorelaxation in mesenteric artery rings (Fig. 6A). In mesenteric arteries, L-NOHA and nor-NOHA induced concentration-dependent vasorelaxation, similar to those observed in the aorta, and both were abolished in the presence of the sGC inhibitor, ODQ (see Fig. 6B,C). There were no vasorelaxant responses to L-valine, DFMO or nor-valine in intact or denuded mesenteric arteries (P > 0.05; two-way anova, when compared with time control; Fig. 6D–F).
Figure 6.
Concentration–response curves to the arginase inhibitors: (A) BEC, (B) L-NOHA, (C) nor-NOHA, (D) L-valine, (E) DFMO and (F) nor-valine were performed in endothelium-intact and denuded mesenteric arteries pre-constricted with 40 mmol·L−1 KCl. Concentration–response curves to L-NOHA and nor-NOHA were also performed in the presence of sGC inhibitor, ODQ (10 µmol·L−1), in endothelium-denuded vessels. All responses are presented as mean ± SEM, where *P < **P < 0.01 and ***P < 0.001 by using an unpaired Student's t-test. BEC, (S)-(2-boronethyl)-L-cysteine HCl; DFMO, D,L, α-difluoromethylornithine; L-NOHA, NG-hydroxy-L-arginine; nor-NOHA, Nω-hydroxy-nor-arginine; ODQ, 1H-[1,2,4]-oxadiazolol[4,3-1]quinoxaline-1-one; sGC, soluble guanlylyl cyclase.
Discussion
Several studies have reported that arginase inhibition restores endothelial function in various animal models of disease including hypertension (Rodriguez et al., 2004; Zhang et al., 2004; Demougeot et al., 2005; Johnson et al., 2005; Bagnost et al., 2008), diabetes (Romero et al., 2008), atherosclerosis (Ming et al., 2004; Ryoo et al., 2008) and aging (Berkowitz et al., 2003; Santhanam et al., 2007). This data are consistent with the hypothesis that arginase competes with NOS for the catabolism of L-arginine and that the inhibition of arginase allows increased production of NO. Many of these papers use the commonly available arginase inhibitors with the assumption of arginase and endothelial specificity. The current paper, however, shows that these arginase inhibitors each have differing effects in the vasculature and that some caution should be exercised with their use and in the subsequent interpretation of the data obtained particularly in diseased models.
An in vitro method of inducing decreased NO bioavailability by repeated applications of ACh so that a significant shift to the right in the concentration–response curve to ACh indicated decreased potency and a reduction in the response to 10 µmol·L−1 ACh indicated decreased maximal effect, in the rat aorta. In the mesenteric arteries, a modest decrease in potency was observed without changes to the maximal effect. ACh is a well-known endothelium-dependent dilator in conduit vessels, where it releases NO via a NOS-dependent mechanism (Furchgott and Zawadzki, 1980). However, in resistance vessels, such as the mesenteric arteries utilized in this study, the majority of the relaxation to ACh is mediated via EDHF, rather than NO, as we and others (Hogan et al., 2005) have shown relaxation is only partially reduced by NOS inhibitors. In both the current study and in bovine intrapulmonary arterial vessels (Gold et al., 1989), reduced responses to ACh suggest that depletion of intracellular L-arginine over time plays a role in tolerance, as L-arginine supplementation prevented this loss of response. To examine functionally whether arginase inhibition increased intracellular L-arginine and could therefore prevent tolerance to ACh, several arginase inhibitors were examined by using a protocol, similar to that used for L-arginine in both the aorta and the mesenteric arteries. Interestingly, only two of the arginase inhibitors examined, BEC and L-NOHA, effectively prevented ACh tolerance in aortic and mesenteric artery preparations. This suggests that indeed some but not all arginase inhibitors can conserve intracellular L-arginine stores to the level required to allow the preservation of ACh responses, mediated by NO.
Based on the published inhibitory constants of the six commercially available arginase inhibitors utilized in this study, it was anticipated that BEC would be the most potent, followed by nor-NOHA, L-NOHA and collectively least potent: DFMO, L-valine and nor-valine (Hunter and Downs, 1945; Daghigh et al., 1994; Custot et al., 1997; Cama et al., 2003b). While BEC and L-NOHA were able to prevent tolerance to ACh, DFMO and the valine amino acids failed to inhibit the ACh-induced shift to the right in the concentration–response curves or reductions in the maximum response. These findings fit with the notion that these compounds are comparatively poorer inhibitors of arginase and as such are not as effective in the vasculature, in relation to improving L-arginine levels to a level where a functional advantage is observed. It has been reported that the synthetic derivative of the intermediate L-NOHA, nor-NOHA, is not a substrate for or inhibitor of NOS unlike L-NOHA itself (Daghigh et al., 1994) and as such it has been proposed to be a more specific inhibitor of arginase than L-NOHA (Tenu et al., 1999). However, in both aorta and mesenteric arteries, nor-NOHA caused further inhibition of responses to ACh, an effect that was partially restored by L-arginine supplementation. This result was unexpected because nor-NOHA is 40 times more potent than L-NOHA as an arginase inhibitor and, unlike L-NOHA, is neither a substrate nor inhibitor of NOS (Tenu et al., 1999). As L-arginine supplementation in the presence of nor-NOHA improved the response to ACh, it is possible that nor-NOHA may in fact inhibit or compete with NOS in contrast to previous findings (Tenu et al., 1999). When used at the same concentration as L-NOHA, DFMO that has been reported to inhibit arginase with a similar potency (Selamnia et al., 1998) or the competitive and non-competitive arginase inhibitors, L-valine and nor-valine, did not prevent tolerance to ACh. Taken together, our results suggest that in the vasculature, caution should be used when assessing the effect of arginase by the use of these inhibitors alone, and that other methods should be used to verify the results obtained.
As arginase has been identified in both endothelial and vascular smooth muscle cells (Berkowitz et al., 2003; Buchwalow et al., 2004; Johnson et al., 2005), we examined the direct effect of the inhibitors as vascular relaxants. L-NOHA, nor-NOHA and BEC all caused concentration-dependent vasorelaxation in the aorta and L-NOHA and nor-NOHA in small mesenteric arteries, which was not endothelium-dependent. The relaxant effect of all three of these compounds is cGMP-dependent, because treatment with the sGC inhibitor, ODQ, in denuded aortic vessels abolished the responses. The guanididium groups of L-NOHA and nor-NOHA are thought to bind to arginase by displacing the metal-bridging hydroxide ion of the native enzyme and asymmetrically joining to the binuclear manganese cluster (Cox et al., 2001). Thus, it is possible that rather than binding to the hydroxyl group of arginase, the guanididium group of L-NOHA and nor-NOHA may bind to other active enzymes. Certainly, as an intermediate of NO production, this is not the first time that L-NOHA has been demonstrated to cause both endothelium-dependent (NOS- and sGC-dependent) and independent relaxation (proposed to be via an NO-dependent but NOS-independent mechanism) (Wallace et al., 1991; Abdul-Hussain et al., 1996; Vetrovsky et al., 2002). However, this is the first report of BEC inducing endothelium-independent vasorelaxation, as a previous report (Berkowitz et al., 2003) suggested it was endothelium-dependent (NOS- and sGC-dependent) in aortas from Wistar Kyoto rats. The vasorelaxation response was sensitive to ODQ but not L-NAME, suggesting BEC may be able to directly activate sGC in an NOS-independent manner. Future studies utilizing purified sGC may be able to identify its mechanism of action. While arginase activity was not measured in the current study, BEC has been shown to effectively decrease arginase activity (Kim et al., 2001; Berkowitz et al., 2003; Steppan et al., 2006). Interestingly, the NOS inhibitor L-NAME blunted the dilatory responses of L-NOHA in both intact and denuded vessels suggesting that the effects of L-NOHA are, in part, dependent on smooth muscle NOS.
In summary, the current study demonstrates that the commonly used arginase inhibitors differ in their potency in the vasculature when assessed by their ability to prevent tolerance to ACh. BEC and L-NOHA appear to be effective inhibitors of endothelial arginase in the aorta but also have direct, non-endothelium-dependent, cGMP-sensitive and vasorelaxant actions in aorta. In both aorta and mesenteric arteries, nor-NOHA may in fact compete for NOS. The amino acids, DFMO, L-valine and nor-valine were ineffective at preventing tolerance to ACh, suggesting they did not sufficiently increase L-arginine levels to an adequate level to have an effect functionally. Caution should thus be exercised in the interpretation and use of these antagonists in vascular tissue, without verification with other methods.
Acknowledgments
This study was supported by a National Health and Medical Research Council of Australia Program grant (JFP Chin-Dusting). NN Huynh is a recipient of a Monash Graduate Scholarship, and KL Andrews is a NHMRC Peter Doherty Fellow.
Glossary
Abbreviations
- BEC
(S)-(2-boronethyl)-L-cysteine HCl
- DFMO, D,L
α-difluoromethylornithine
- L-NAME
NG-nitro-L-arginine-methyl ester
- L-NOHA
NG-hydroxy-L-arginine
- nor-NOHA
Nω-hydroxy-nor-L-arginine
- ODQ
1H-[1,2,4]-oxadiazolol[4,3-1]quinoxaline-1-one
Conflict of interest
None.
References
- Abdul-Hussain MN, Jia YL, Hussain SNA. Mechanisms mediating the vasodilatory effects of N-hydroxy-arginine in coronary arteries. Eur J Pharmacol. 1996;305:155–161. doi: 10.1016/0014-2999(96)00163-x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachetti T, Comini L, Francolini G, Bastianon D, Valetti B, Cadei M, et al. Arginase pathway in human endothelial cells in pathophysiological conditions. J Mol Cell Cardiol. 2004;37:515–523. doi: 10.1016/j.yjmcc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Bagnost T, Berthelot A, Bouhaddi M, Laurant P, Andre C, Guillaume Y, et al. Treatment with the arginase inhibitor N(omega)-hydroxy-nor-L-arginine improves vascular function and lowers blood pressure in adult spontaneously hypertensive rat. J Hypertens. 2008;26:1110–1118. doi: 10.1097/HJH.0b013e3282fcc357. [DOI] [PubMed] [Google Scholar]
- Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- Boucher JL, Custot J, Vadon S, Delaforge M, Lepoivre M, Tenu JP, et al. N[omega]-Hydroxy-L-Arginine, an intermediate in the L-arginine to Nitric Oxide pathway, is a strong inhibitor of liver and macrophage arginase. Biochem Biophys Res Commun. 1994;203:1614–1621. doi: 10.1006/bbrc.1994.2371. [DOI] [PubMed] [Google Scholar]
- Buchwalow IB, Podzuweit T, Samoilova VE, Wellner M, Haller H, Grote S, et al. An in situ evidence for autocrine function of NO in the vasculature. Nitric Oxide. 2004;10:203–212. doi: 10.1016/j.niox.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Buga GM, Singh R, Pervin S, Rogers NE, Schmitz DA, Jenkinson CP, et al. Arginase activity in endothelial cells: inhibition by NG-hydroxy-L-arginine during high-output NO production. Am J Physiol. 1996;271:H1988–98. doi: 10.1152/ajpheart.1996.271.5.H1988. [DOI] [PubMed] [Google Scholar]
- Cama E, Colleluori DM, Emig FA, Shin H, Kim SW, Kim NN, et al. Human arginase II: crystal structure and physiological role in male and female sexual arousal. Biochemistry. 2003a;42:8445–8451. doi: 10.1021/bi034340j. [DOI] [PubMed] [Google Scholar]
- Cama E, Emig FA, Ash DE, Christianson DW. Structural and functional importance of first-shell metal ligands in the binuclear manganese cluster of arginase I. Biochemistry. 2003b;42:7748–7758. doi: 10.1021/bi030074y. [DOI] [PubMed] [Google Scholar]
- Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, Iyer RK. Arginases I and II: do their functions overlap? Mol Genet Metab. 2004;81:38–44. doi: 10.1016/j.ymgme.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Chataigneau T, Feletou M, Huang PL, Fishman MC, Duhault J, Vanhoutte PM. Acetylcholine-induced relaxation in blood vessels from endothelial nitric oxide synthase knockout mice. Br J Pharmacol. 1999;126:219–226. doi: 10.1038/sj.bjp.0702300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GF, Suzuki H. Calcium dependency of the endothelium-dependent hyperpolarization in smooth muscle cells of the rabbit carotid artery. J Physiol. 1990;421:521–534. doi: 10.1113/jphysiol.1990.sp017959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L60–L68. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- Colleluori DM, Ash DE. Classical and slow-binding inhibitors of human type II arginase. Biochemistry. 2001;40:9356–9362. doi: 10.1021/bi010783g. [DOI] [PubMed] [Google Scholar]
- Cox JD, Cama E, Colleluori DM, Pethe S, Boucher JL, Mansuy D, et al. Mechanistic and metabolic inferences from the binding of substrate analogues and products to arginase. Biochemistry. 2001;40:2689–2701. doi: 10.1021/bi002318+. [DOI] [PubMed] [Google Scholar]
- Custot J, Moali C, Brollo M, Boucher JL, Delaforge M, Mansuy D, et al. The new alpha-amino acid Nw- hydorxy-nor-L-arginine: a high -affinity inhibitor of arginase well adapted to bind to its manganese cluster. J Am Chem Soc. 1997;119:4086–4087. [Google Scholar]
- Daghigh F, Fukuto JM, Ash DE. Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications for the regulation of nitric oxide biosynthesis by arginase. Biochem Biophys Res Commun. 1994;202:174–180. doi: 10.1006/bbrc.1994.1909. [DOI] [PubMed] [Google Scholar]
- Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens. 2005;23:971–978. doi: 10.1097/01.hjh.0000166837.78559.93. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gold ME, Bush PA, Ignarro LJ. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989;164:714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- Hogan M, O'malley KD, Healy J, O'brien S, Bund SJ. Implications for repetitive application of acetylcholine in the determination of the mechanisms of endothelium-dependent relaxation. Vascul Pharmacol. 2005;43:227–233. doi: 10.1016/j.vph.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Holan V, Pindjakova J, Krulova M, Neuwirth A, Fric J, Zajicova A. Production of nitric oxide during graft rejection is regulated by the Th1/Th2 balance, the arginase activity, and L-arginine metabolism. Transplantation. 2006;81:1708–1715. doi: 10.1097/01.tp.0000226067.89690.2b. [DOI] [PubMed] [Google Scholar]
- Hunter A, Downs DE. The inhibition of arginase by amino acids. J Biol Chem. 1945;157:427–446. [Google Scholar]
- Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1057–R1062. doi: 10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- Khangulov SV, Pessiki PJ, Barynin VV, Ash DE, Dismukes GC. Determination of the metal ion separation and energies of the three lowest electronic states of dimanganese (II,II) complexes and enzymes: catalase and liver arginase. Biochemistry. 1995;34:2015–2025. doi: 10.1021/bi00006a023. [DOI] [PubMed] [Google Scholar]
- Kim NN, Cox JD, Baggio RF, Emig FA, Mistry SK, Harper SL, et al. Probing erectile function: S-(2-boronoethyl)-L-cysteine binds to arginase as a transition state analogue and enhances smooth muscle relaxation in human penile corpus cavernosum. Biochemistry. 2001;40:2678–2688. doi: 10.1021/bi002317h. [DOI] [PubMed] [Google Scholar]
- Kimura M, Jefferis AM, Watanabe H, Chin-Dusting J. Insulin inhibits acetylcholine responses in rat isolated mesenteric arteries via a non-nitric oxide nonprostanoid pathway. Hypertension. 2002;39:35–40. doi: 10.1161/hy1201.097198. [DOI] [PubMed] [Google Scholar]
- Lewis C, Zhu W, Pavkov ML, Kinney CM, Dicorleto PE, Kashyap VS. Arginase blockade lessens endothelial dysfunction after thrombosis. J Vasc Surg. 2008;48:441–446. doi: 10.1016/j.jvs.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TV, Dart AM, Chin-Dusting JP. Non-specific inhibition by human lipoproteins of endothelium dependent relaxation in rat aorta may be attributed to lipoprotein phospholipids. Cardiovasc Res. 1997;34:590–596. doi: 10.1016/s0008-6363(97)00061-8. [DOI] [PubMed] [Google Scholar]
- Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, et al. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway. Implications for atherosclerotic endothelial dysfunction. Circ. 2004;110:3307–3314. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- Morris CR, Vichinsky EP, Van Warmerdam J, Machado L, Kepka-Lenhart D, Morris SM, et al. Hydroxyurea and arginine therapy: impact on nitric oxide production in sickle cell disease. J Pediatr Hematol Oncol. 2003;25:629–634. doi: 10.1097/00043426-200308000-00008. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Schleiffer R, Raul F, Richert L, Berthelot A. How could aortic arginase activity enhancement be involved in DOCA-salt hypertension? Clin Exp Hypertens. 2004;26:1–12. doi: 10.1081/ceh-120027327. [DOI] [PubMed] [Google Scholar]
- Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, et al. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Masuda H, Kihara K, Kurosaki E, Yamauchi Y, Azuma H. Involvement of increased arginase activity in impaired cavernous relaxation with aging in the rabbit. J Urol. 2004;172:369–373. doi: 10.1097/01.ju.0000121691.06417.40. [DOI] [PubMed] [Google Scholar]
- Santhanam L, Lim HK, Miriel V, Brown T, Patel M, Balanson S, et al. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- Selamnia M, Mayeur C, Robert V, Blachier F. alpha]-Difluoromethylornithine (DFMO) as a potent arginase activity inhibitor in human colon carcinoma cells. Biochem Pharmacol. 1998;55:1241–1245. doi: 10.1016/s0006-2952(97)00572-8. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, et al. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci USA. 2006;103:4759–4764. doi: 10.1073/pnas.0506589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenu J-P, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher J-L. Effects of the new arginase inhibitor N[omega]-hydroxy-nor-arginine on NO synthase activity in murine macrophages. Nitric Oxide. 1999;3:427–438. doi: 10.1006/niox.1999.0255. [DOI] [PubMed] [Google Scholar]
- Thuraisingham RC, Roberts NB, Wilkes M, New DI, Mendes-Ribeiro AC, Dodd SM, et al. Altered L-arginine metabolism results in increased nitric oxide release from uraemic endothelial cells. Clin Sci (Lond) 2002;103:31–41. doi: 10.1042/cs1030031. [DOI] [PubMed] [Google Scholar]
- Vetrovsky P, Boucher JL, Schott C, Beranova P, Chalupsky K, Callizot N, et al. Involvement of NO in the endothelium-independent relaxing effects of N(omega)-hydroxy-L-arginine and other compounds bearing a C=NOH function in the rat aorta. J Pharmacol Exp Ther. 2002;303:823–830. doi: 10.1124/jpet.102.038612. [DOI] [PubMed] [Google Scholar]
- Wallace GC, Gulati P, Fukuto JM. N[omega]-Hydroxy-L-arginine: a novel arginine analog capable of causing vasorelaxation in bovine intrapulmonary artery. Biochem Biophys Res Commun. 1991;176:528–534. doi: 10.1016/0006-291x(91)90957-9. [DOI] [PubMed] [Google Scholar]
- Wu CC, Chen SJ, Yen MH. Different responses to acetylcholine in the presence of nitric oxide inhibitor in rat aortae and mesenteric arteries. Clin Exp Pharmacol Physiol. 1993;20:405–412. doi: 10.1111/j.1440-1681.1993.tb01717.x. [DOI] [PubMed] [Google Scholar]
- Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J. 2001;15:1264–1266. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, Mcdonald MM, et al. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–943. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]


