Abstract
Background and purpose:
Animal studies show that histamine plays a role in cognitive functioning and that histamine H3-receptor antagonists, which increase histaminergic function through presynaptic receptors, improve cognitive performance in models of clinical cognitive deficits. In order to test such new drugs in humans, a model for cognitive impairments induced by low histaminergic functions would be useful. Studies with histamine H1-receptor antagonists have shown limitations as a model. Here we evaluated whether depletion of L-histidine, the precursor of histamine, was effective in altering measures associated with histamine in humans and the behavioural and electrophysiological (event-related-potentials) effects.
Experimental approach:
Seventeen healthy volunteers completed a three-way, double-blind, crossover study with L-histidine depletion, L-tyrosine/L-phenylalanine depletion (active control) and placebo as treatments. Interactions with task manipulations in a choice reaction time task were studied. Task demands were increased using visual stimulus degradation and increased response complexity. In addition, subjective and objective measures of sedation and critical tracking task performance were assessed.
Key results:
Measures of sedation and critical tracking task performance were not affected by treatment. L-histidine depletion was effective and enlarged the effect of response complexity as measured with the response-locked lateralized readiness potential onset latency.
Conclusions and implications:
L-histidine depletion affected response- but not stimulus-related processes, in contrast to the effects of H1-receptor antagonists which were previously found to affect primarily stimulus-related processes. L-histidine depletion is promising as a model for histamine-based cognitive impairment. However, these effects need to be confirmed by further studies.
Keywords: sensorimotor performance, L-histidine depletion, L-tyrosine/L-phenylalanine depletion, evoked potentials, additive factor method, reaction time, sedation, sensory, motor
Introduction
Histamine has been associated with a number of clinical disorders in which cognitive performance is impaired (Onodera et al., 1994). Animal models for cognitive deficits in disorders like attention deficit hyperactivity disorder, Parkinson's disease, schizophrenia and sleep disorders have been used to test the effects of histamine H3-receptor antagonists as possible therapeutic agents (Leurs et al., 1998; Vohora, 2004; Esbenshade et al., 2006). Histamine H3-receptor antagonists increase histaminergic function through presynaptic receptors. These studies have shown that performance of a plethora of tasks is improved and have therefore attracted the attention of many pharmaceutical companies (Leurs et al., 1998; Esbenshade et al., 2006; Wijtmans et al., 2007).
To study the role of histamine in cognition, histaminergic functions can be manipulated. The main observation in such studies is that increasing histaminergic activity leads to improved cognitive performance and prolonged wakefulness, while decreasing histaminergic activity leads to diminished performance and increased sleepiness. More specifically, the role of histamine in human cognition has mostly been studied by looking at the effects of histamine H1-receptor antagonists. Impairment of sensorimotor performance in humans by centrally acting histamine H1-receptor antagonists has been well established and has often been attributed to their sedative effects (O'Hanlon and Ramaekers, 1995; Hindmarch and Shamsi, 1999; Kay, 2000; van Ruitenbeek et al., 2008). These effects are believed to reflect low levels of histaminergic functions in the brain.
Apart from histamine H1-receptor blockade, another way to decrease histaminergic function is by depleting the brain of the amino acid L-histidine, from which histamine is synthesized by decarboxylation (Cho et al., 1984). Whether L-histidine itself can be synthesized by humans is a matter of controversy. In a standard reference work on dietary requirements (Institute of Medicine, 2006), histidine is listed as an indispensable (essential) amino acid. However, this has been challenged in long-term depletion studies (Kriengsinyos et al., 2002). Depletion of L-histidine may cause a decrease in brain histamine, comparable to the decrease in dopamine levels following depletion of L-tyrosine/L-phenylalanine. In rodents, histamine metabolism can be altered more by changes in precursor availability than any other neurotransmitter, such as 5-HT, catecholamines or acetylcholine (Young, 1996). Additional support for the effects of low levels of histamine on cognitive performance comes from animal studies. Decreasing histamine synthesis in animals using the histidine decarboxylase (HDC) inhibitor, α-fluormethylhistidine or HDC knockout mice affects on cognitive performance (Kamei et al., 1993; Sakai et al., 1998; Chen et al., 1999; Acevedo et al., 2006). If the effects of low levels of histamine are H1-receptor mediated, L-histidine depletion should exert the same effects as H1-receptor antagonists. The aim of the present study was to assess the effects of L-histidine depletion on sensory- and motor-related cognitive processes in healthy human volunteers using a behavioural and psychophysiological approach, as in a previous study using an histamine H1-receptor antagonist (van Ruitenbeek et al., 2009). To the best of our knowledge this is the first study to assess the effects of L-histidine depletion on cognitive performance, that is, sensorimotor performance.
The behavioural approach aims to find interactions between treatments and task manipulations in sensorimotor task performance. Sensorimotor functioning can be regarded as a result of information processing through serial stages from perception, through decision making to action. Treatments interacting with task manipulations specifically affecting individual stages consequently affect at least the same stage as the task manipulation (Sternberg, 1969). It has been shown that H1-receptor blockade increases the effect of the visual degradation of stimuli and therefore affects sensory stages of information processing (Gaillard et al., 1988; van Ruitenbeek et al., 2009).
With the psychophysiological approach, the effects of treatments on separate processes in cognitive functioning can be measured using event-related potentials (ERPs). The time interval between a peak and a stimulus or response is indicative of the duration of processes occurring during that interval. For example, the duration of the interval between a stimulus and the P300 peak latency is indicative of the duration of sensory processes (Riedel et al., 2006; Polich, 2007). The lateralized readiness potential (LRP) is associated with motor programming and the interval between the onset of the LRP and the response is indicative of the duration of motor-related processes (Miller and Hackley, 1992; Hackley and Miller, 1995). An earlier study has shown that H1-receptor blockade increases the duration of the P300 peak latency, but does not affect the interval between the LRP onset and the response (van Ruitenbeek et al., 2009). Thus, H1-blockade affects sensory, but not motor processes and we expected L-histidine depletion to have similar effects.
As the behavioural effects of L-histidine depletion have never been assessed before, an active control treatment was needed to demonstrate sensitivity of the tests and procedures. Brain levels of another neurotransmitter dopamine can be decreased by depleting its precursors L-tyrosine and L-phenylalanine (McTavish et al., 1999). A decrease in dopamine levels was observed after L-tyrosine/L-phenylalanine depletion (Montgomery et al., 2003) and impaired cognitive performance (Harmer et al., 2001), especially in tasks assessing working memory (Harrison et al., 2004). In addition, an increase in dopamine levels by administration of L-dopa has shown to have selective effects on sensory stages of information processing, as it interacted with stimulus intensity and not with stimulus-response mapping or stimulus foreperiod duration (Rihet et al., 2002). Therefore, L-tyrosine/L-phenylalanine depletion was used in our study as an active control.
If L-histidine depletion decreased brain histamine levels and the effects of H1-receptor antagonists do reflect low levels of histaminergic fucntion, then the behavioural effects of L-histidine depletion should be similar to those of H1-receptor antagonists, such as impaired psychomotor performance and increased sedation. Moreover, if L-histidine depletion affects sensorimotor performance the effects may be stage specific. The results of this study show that L-histidine depletion was effective in reducing L-histidine levels, measured in plasma, and that L-histidine depletion affected motor-related processes, in contrast to the effects of H1-receptor blockade (van Ruitenbeek et al., 2009).
Methods
Subjects
This study was approved by the ethics committee of Maastricht University and University Hospital Maastricht and carried out in accordance with the World Medical Association Declaration of Helsinki and its amendments. Twenty-two healthy volunteers (6 male) between 18 and 35 years (mean ± SD: 21 ± 2) were recruited by means of posters placed at Maastricht University locations. They were paid for their participation. Five subjects, all female, withdrew from the study due to side effects of the treatments (nausea and vomiting) after drinking the amino acid mixture. Data from another subject were lost due to a technical error. The remaining 16 subjects out of the intended 18 had a mean (±SD) age of 21 (±2) years.
The general health of the subjects was screened using a medical history questionnaire. Exclusion criteria were a significant history or presence of any mental or physical disorder: gastrointestinal, hepatic, renal, cardiovascular or neurological. Also, drug abuse, a body mass index outside the limits of 18.5 and 30.0 kg·m−2 and drinking more than 20 standard alcoholic consumptions per week or five beverages containing caffeine per day were regarded as exclusion criteria. For women, pregnancy and lactation were also regarded as exclusion criteria. No drugs or medication, except oral contraceptives, aspirin and paracetamol, were allowed to be taken from a week before the first test day until the end of the study. Smoking and the use of caffeine were prohibited on test days, the use of alcohol from 24 h before and during each test day and the use of psychoactive drugs 2 weeks prior to the first test day. On each test day subjects received a low-protein, carbohydrate rich diet, which was similar to that given by Riedel et al. (1999) and Sambeth et al. (2009).
All subjects received written information about the study procedures and signed an informed consent form prior to enrolment.
Study design and treatments
The study was conducted according to a double-blind, placebo-controlled, three-way, crossover design. The treatments were solutions of amino acids (Table 1), taken orally, in 200-mL tap water. The balanced drink (BAL) contained the entire range of amino acids (total of 104.4 g); the L-histidine free drink (HID) consisted of the same mixture of amino acids without the amino acid L-histidine (total of 101.2 g); the L-tyrosine/L-phenylalanine free drink (TYD) was the same mixture as in BAL but without the amino acids L-tyrosine and L-phenylalanine, with a total of 91.8 g of amino acids (all mixtures from Basic Pharma Manufacturing BV, Roermond, The Netherlands). Female subjects received 85% of the drink that male subjects received to adjust for differences in body weight.
Table 1.
Content of the amino acid mixtures (g)
| Balanced drink (BAL) | Histidine depletion (HID) | Tyrosine depletion (TYD) | |
|---|---|---|---|
| Isoleucine | 8.0 | 8.0 | 8.0 |
| Leucine | 13.5 | 13.5 | 13.5 |
| Lysine | 11.0 | 11.0 | 11.0 |
| Methionine | 3.0 | 3.0 | 3.0 |
| Valine | 8.9 | 8.9 | 8.9 |
| Threonine | 6.5 | 6.5 | 6.5 |
| Tryptophan | 2.3 | 2.3 | 2.3 |
| Alanine | 5.5 | 5.5 | 5.5 |
| Arginine | 4.9 | 4.9 | 4.9 |
| Cysteine | 2.7 | 2.7 | 2.7 |
| Glycine | 3.2 | 3.2 | 3.2 |
| Serine | 6.9 | 6.9 | 6.9 |
| Proline | 12.2 | 12.2 | 12.2 |
| Histidine | 3.2 | x | 3.2 |
| Tyrosine | 6.9 | 6.9 | x |
| Phenylalanine | 5.7 | 5.7 | x |
The mixture of amino acids to induce L-histidine depletion (HID) is lacking in the histidine constituent and that to induce L-tyrosine and L-phenylalanine depletion (TYD) lacks these two amino acids
Treatment days were spaced apart by a washout period of at least 7 days. The order of treatments and sequence of task conditions were balanced between subjects, although this was not complete, due to the dropout of six subjects.
Procedure
All subjects were trained to perform the tasks at a plateau level within 2 weeks prior to the first test day. On treatment days, subjects arrived well rested at the test facility at 9:00 am after refraining from eating from 9:00 pm the night before. One cup of tea or coffee (without milk or sugar) was allowed before 8:00 am (6 h before testing) to prevent possible caffeine withdrawal effects. Consumption of tea of coffee was held constant over the treatments days within subjects.
After arrival, subjects completed visual analogue scales measuring subjective mood. A catheter was inserted into the inner bend of the elbow to take blood samples before and at 2, 4, 6 and 7 h after treatment (T0, T2, T4, T6 and T7 respectively). At 9:15 am subjects were given the amino acid drink, which had to be consumed within 15 min. Three hours after treatment, subjects received a small, low-protein meal. After treatment, subjects could watch television and play board games in a specially equipped room. EEG electrodes were attached to the head of the subject 4 h after treatment and cognitive assessments started 5 h after treatment (14:15 pm) and lasted for 45 min. Assessments included a baseline EEG recording (2 min eyes open and 2 min eyes closed), followed by a critical tracking task (CTT). Thereafter, a choice reaction time task and a cued simple reaction time task were performed during which EEG was recorded. Finally, subjects completed visual analogue scales assessing subjective alertness.
Behavioural assessments
Choice reaction time task (CRT)
The CRT task used in this study was based on Smulders et al. (1995) and was the same as to the task used by van Ruitenbeek et al. (2009). The speed of information processing of the input and output stages are assessed by manipulating the quality of the visual stimuli and complexity of the motor responses respectively. Smulders et al. (1995) and van Ruitenbeek et al. (2009) found selective effects of stimulus quality (SQ) on the interval between the stimulus and P300 peak latency and selective effects of response complexity (RC) on the interval between the LRP onset and the response.
The task consisted of a repeated presentation of the numbers 2 and 5 in random order on a computer screen. Stimuli consisted of small squares surrounded by a frame of squares. The squares consisted of grids of 6 by 6 pixels. Stimulus presentation time was 200 ms, and the time between offset of a stimulus and the presentation of the next stimulus was varied between 1500 and 2200 ms. Subjects had to respond as fast as possible by pressing a left or right hand button with their left or right index finger when a 2 or a 5 appeared respectively. The task consisted of four blocks of 112 trials which each lasted approximately 4 min. In all blocks half of the stimuli were visually degraded and half of the stimuli were intact. Degradation was achieved by placing 20 dots (42%) from the frame at random positions in the field within the frame not occupied by the 26 squares of the digit. There were seven degraded versions of each digit to prevent subjects from responding to learned features of the stimulus instead of recognizing the digit.
In two blocks the complexity of the response was increased by asking the subjects to press three buttons instead of one in the following sequence: index, ring and middle finger. The pressing of the first button indicated the reaction time. The time between the first button press and the third was also recorded as ‘motor time’. Blocks requiring three button presses are indicated as complex (C) blocks and blocks requiring a single button press are indicated as simple (S) blocks. The blocks were presented in the orders SCCS and CSSC. Half of the subjects were presented with the SCCS order and the other half with the CSSC order.
The primary performance variables in this task were the average reaction times (ms) of the correct responses for the four different task conditions, that is, intact-simple, degraded-simple, intact-complex and degraded-complex. Accuracy scores (%) were logarithmically transformed due to the non-linear nature of a decrease in accuracy (Dickman and Meyer, 1988).
Critical tracking task
The CTT measures the ability to control an unstable error signal in a first-order compensatory tracking task (Jex et al., 1966). The task has been shown to be sensitive to effects of histamine manipulations (van Ruitenbeek et al., 2008). Error is displayed as a horizontal deviation of a yellow triangle from the midpoint on a horizontal scale. Compensatory movements correct the error by returning the triangle to the centre. The frequency of the error gradually increases until the subject loses control. The frequency at which control is lost is the critical frequency or lambda-c (rad·s−1). The CTT includes five trials of which the highest and lowest scores are removed. The average of the three remaining scores is the final score.
Cued simple reaction time task
A pre-cued simple reaction time task was used to assess treatment effects on the contingent negative variation (CNV) (Walter et al., 1964). The CNV is a motor process-related slow potential which develops when there is a clear temporal relationship between a warning and an imperative signal (Rizzo et al., 1985). In this task subjects started by focusing on a fixation cross presented in the centre of the screen for 1 s. Then a warning signal (red circle) appeared in the centre of the screen, which was followed by the imperative stimulus (green circle) after exactly 2 s. Subjects were asked to respond as fast as possible by pressing a button on a response box with their right index finger. The average amplitude (µV) of a 500 ms time window of the CNV is a measure of response preparation and was a dependent variable in this study. A decrease in average amplitude indicates a decreased supply of preparatory potentiality (Rockstroh et al., 1991). Also, reaction time was measured as an indication of response slowing.
Subjective alertness scale
Subjects' mood was assessed using a series of 16 analogue scales of 100 mm. These provide three factor analytically defined summary scores for ‘alertness’, ‘contentedness’ and ‘calmness’ (Bond and Lader, 1974) of which alertness was of main interest. The scores on the nine items associated with the factor alertness were added and taken as dependent measure.
Baseline EEG
Subjects sat still with their eyes open for a 2-min period during which baseline EEG was recorded. Thereafter, baseline EEG was again recorded, but now subjects had their eyes closed. The power (µV2) of the delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz) and beta (12–30 Hz) frequency bands were taken as objective indicators of sedation and cortical activity.
Plasma samples
After taking a blood sample (5 mL) the catheter was cleaned in order to keep it open during the test day. The blood sample was centrifuged for 5 min at 4°C at 2522× g and plasma (1 mL) was frozen and stored at −80°C until it was analysed quantitatively for amino acids by high-performance liquid chromatography (van Eijk et al., 1993).
For the L-tyrosine/L-phenylalanine depletion we assessed changes in total L-tyrosine and L-phenylalanine levels from baseline at T7 (i.e. around testing). L-tyrosine and L-phenylalanine are transported across the blood-brain barrier by a transport system that is active towards all the large neutral amino acids (LNAA). As there is competition between the various LNAA for transport into the brain, the brain concentration is best approximated by the ratio of the plasma concentration of an amino acid to the sum of the plasma concentration of all other LNAA (ΣLNAA). Therefore, we also calculated the L-tyrosine and L-phenylalanine/ΣLNAA ratio for these time points. Although L-histidine is weakly basic it is transported into the brain by the same transport system as the LNAA (Oldendorf and Szabo, 1976; Pardridge, 1983) Therefore, for the L-histidine depletion, we assessed the change from baseline in total L-histidine and in L-histidine/ΣLNAA ratio for T2, T4, T6 and T7. The T2, T4 and T6 measurements were added due to the unknown decrease in L-histidine levels over time.
Event-related potentials
EEG recordings
During performance on the cued simple reaction time task and CRT task EEG was recorded to measure the P300, LRP and CNV ERPs. Dependent variables were the stimulus locked (S-locked) and response locked (R-locked) P300 amplitudes and peak latencies, S-locked and R-locked LRP onsets and the surface under the CNV. EEG activity was recorded by means of an electrocap from an array of 32 electrodes from the standard 10–20 system (Jasper, 1957). All electrodes were filled with electrode-gel and were line referenced to the right mastoid electrode. Off-line they were referenced to both left and right mastoids. The FPz electrode was used as ground electrode.
The horizontal electrooculogram (EOG) was recorded using electrodes attached to the outer canthi of the eyes and vertical EOG was recorded from electrodes attached above and below the left or right eye and in line with the pupil.
All electrode impedances were kept below 5 kΩ. Signals were amplified using Neuroscan Synamps amplifiers and collected using Neuroscan software. All signals were sampled at a 1000 Hz and filtered online using a 100 Hz low-pass filter and a 0.05 Hz high-pass filter.
Signal analysis
Continuous signals obtained during the performance on the choice reaction time task were filtered off-line using a 1 Hz high-pass filter after which EEG was corrected for vertical and horizontal eye movements according to a procedure by Semlitsch et al. (1986). Dependent variables from the choice reaction time task were duration of the interval (ms) between stimulus and P300 peak amplitude (S-locked P300) and between the response and the P300 peak amplitude (R-locked P300), and the interval between the stimulus onset and LRP onset (S-locked LRP) and the interval between the response and LRP onset (R-locked LRP). In addition, the amplitude of the S-locked and R-locked P300 was determined as a measure of resource availability for stimulus processing. The S-locked data were epoched in 1100 ms sweeps starting 100 ms before stimulus presentation and the interval between sweep onset and stimulus served as baseline. The R-locked data were epoched from 475 ms before to 625 ms after the response. For the analysis of the P300 all sampled EEG and EOG epochs were low pass filtered using a 3.6 Hz low-pass filter (Smulders et al., 1995) and for the analysis of the LRP the data were filtered using a 11.1 Hz low-pass filter (Miller and Hackley, 1992). Sweeps containing artefacts exceeding +75 or −75 µV on the Fz, Cz, Pz, Oz, C3 or C4 electrodes were rejected. This resulted in an average acceptance of 93% of the epochs.
The length of the S-locked and R-locked intervals of the P300 was determined at the Cz electrode sites. The S-locked P300 latencies were determined as the time between onset of the stimulus and the latency of the largest local maximum using the Jackknife method (Miller et al., 1998; Ulrich and Miller, 2001). The amplitude was determined as the amplitude of that local maximum. The R-locked P300 intervals were determined as the time between the largest local maximum of the P300 component and the given response, also using the Jackknife method. The amplitude was determined as the amplitude of that local maximum.
lateralized readiness potentials were computed by subtracting C4 from C3, point by point, for right and left hand trials and subtracting left hand from right hand trials. The onset latencies of the S-locked and R-locked LRP waveforms were determined using the Jackknife scoring method with a 1 µV absolute criterion (Miller et al., 1998; Ulrich and Miller, 2001).
For the cued simple reaction time task, the CNV was determined in a window of 500 ms as a measure of response preparation. The data were first epoched and then filtered using a 30 Hz low–pass filter and 0.05 high-–pass filter. The area report 500 ms before the beginning of the imperative stimulus was the dependent variable.
The power of the delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz) and beta frequency (13–30 Hz) bands were calculated by means of a fast Fourier transformation of the means of the midline electrodes (Fz, Cz and Pz).
Statistical analysis
All dependent variables were screened for normality of their distributions and no deviations from normality were detected.
Baseline differences in blood-plasma levels of L-histidine, L-histidine/ΣLNAA ratio, L-tyrosine/L-phenylalanine and L-tyrosine + L-phenylalanine/ΣLNAA ratio were analysed using anova for repeated measures with Treatment as within subject factor (BAL, HID, TYD). The plasma levels at T7 were further analysed as changes from baseline (ΔT = T7 − T0) with Treatment as within subject factor with three levels (BAL, HID, TYD). All significant (P < 0.05) Treatment effects were followed by two treatment-placebo comparisons. Additionally, due to the unknown pattern of decrease in plasma levels over time, the L-histidine levels and the L-histidine/ΣLNAA ratio in the HID condition were analysed with Time as a within subject factor with five levels (T0, T2, T4, T6 and T7). Significant (P < 0.05) effect of Time was followed by four simple comparisons with baseline (T0).
Within subject factors in the CRT were SQ (intact, degraded) and RC (simple, complex). Performance and ERP data from the CRT were analysed for main effects of Treatment (BAL, HID, TYD) and interactions of Treatment with SQ and RC using a 3 × 2 × 2 within subjects factorial model. F-values for differences in S-locked and R-locked LRP onset latencies were divided by (n− 1)2 to correct for the reduction of variance induced by the Jackknife method (Ulrich and Miller, 2001). If overall multivariate F-tests indicated a significant main effect or interaction (P < 0.05), data were further analysed using two univariate drug-placebo comparisons.
Performance on the CTT, CNV surface area, subjective drowsiness scores and plasma levels were analysed for Treatment (three levels) effects using repeated measures anova. The power of each frequency band was analysed separately for effects of the treatments using a 3 × 3 within subject model with Treatment and Electrode (Fz, Cz and Pz) as factors when subjects held their eyes open and when they held their eyes closed. All data were analysed using SPSS for Windows (version 12.0.1).
Results
Missing data
One subject was removed from the CNV data set due to incomplete data collection. The analysis was done on complete data sets from 15 subjects. Due to difficulties during blood sampling, several blood samples could not be drawn, resulting in 14 complete data sets for the TYD condition. Therefore, Treatment effects were analysed using 14 data sets. Data sets for the BAL and HID conditions contained 16 subjects. The changes from baseline in the HID condition were analysed using 16 data sets.
Plasma levels of amino acids
Table 2 displays the average absolute values of L-histidine and L-tyrosine/L-phenylalanine plasma levels and L-histidine/ΣLNAA and L-tyrosine + L-phenylalanine/ΣLNAA ratio over time and their changes from baseline.
Table 2.
Effects of treatments on amino acid levels (µmol·L−1)
| Treatment |
Baseline (T0) |
ΔT (T7–T0) |
||||
|---|---|---|---|---|---|---|
| BAL | HID | TYD | BAL | HID | TYD | |
| L-histidine | 93.8 (7.1) | 85.7 (7.8) | 92.1 (8.0) | 2.5 (5.2) | −17.1 (5.5)* | 8.6 (9.7) |
| L-histidine/ΣLNAA ratio | 0.18 (0.009) | 0.17 (0.014) | 0.18 (0.016) | −0.021 (0.013) | −0.083 (0.011)* | −0.003 (0.021) |
| L-tyrosine/L-phenylalanine | 107.3 (6.0) | 112.2 (10.3) | 111.7 (9.2) | 59.8 (12.7) | 71.1 (10.6) | −61.0 (5.3)* |
| L-tyrosine + L-phenylalanine/ ΣLNAA ratio | 0.28 (0.018) | 0.28 (0.008) | 0.27 (0.013) | 0.120 (0.021) | 0.088 (0.019) | −0.159 (0.015)* |
Means (±SEM) of absolute values of L-histidine levels, L-histidine/ΣLNAA ratio, L-tyrosine/L-phenylalanine levels and L-tyrosine + L-phenylalanine/ΣLNAA ratio at baseline (T0) and the change from baseline after 7 h (ΔT) after the balanced mixture (BAL), L-histidine depleted mixture (HID) and L-tyrosine/L-phenylalanine depleted mixture (TYD).
Indicates significant (P < 0.05) change from baseline as compared with placebo.
Plasma levels of L-histidine, L-histidine/ΣLNAA ratio, L-tyrosine/L-phenylalanine, L-tyrosine + L-phenylalanine/ΣLNAA ratio did not differ in the three conditions at baseline [F(2,12) = 2.0, P= 0.181 and Fs < 1 respectively].
Treatment had a marginally significant main effect on the change from baseline in absolute L-histidine levels (F(2,12) = 3.5, P= 0.063). Treatment-placebo comparisons showed that decrease from baseline of L-histidine levels at T7 in the HID condition (20%) was larger than the change in the BAL condition [F(1,13) = 7.6, P= 0.017]. L-histidine levels did not decrease after treatment in the TYD condition (F < 1), compared with the BAL condition. Treatment also had a main effect on the L-histidine/ΣLNAA ratio in blood [F(2,12) = 19.0, P < 0.001]. The decrease from baseline in the HID condition (48%) was larger than the decrease in the BAL condition (12%) [F(1,13) = 33.3, P < 0.001]. There was no significant difference in L-histidine/ΣLNAA ratio change from baseline between the TYD and BAL conditions (F < 1).
A significant main effect of Time in the HID condition indicated successful reductions in plasma L-histidine levels [F(4,12) = 9.0, P < 0.001]. Levels were decreased at T4 [22%: F(1,15) = 15.1, P < 0.001], T6 [26%: F(1,15) = 21.5, P < 0.001] and T7 [20%: F(1,15) = 11.0, P < 0.005]. L-histidine levels were not significantly reduced at T2 as compared with T0 [F(1,15) = 1.9, P= 0.186]. A main effect of Time also indicated a reduction of the L-histidine/ΣLNAA ratio [F(4,12) = 45.0, P < 0.001]. The ratio was reduced at T2 [58%: F(1,15) = 70.1, P < 0.001], at T4 [59%: F(1,15) = 62.0, P < 0.001], at T6 [46%: F(1,15) = 37.0, P < 0.001] and at T7 [36%: F(1,15) = 14.4, P= 0.002] (Figure 1).
Figure 1.
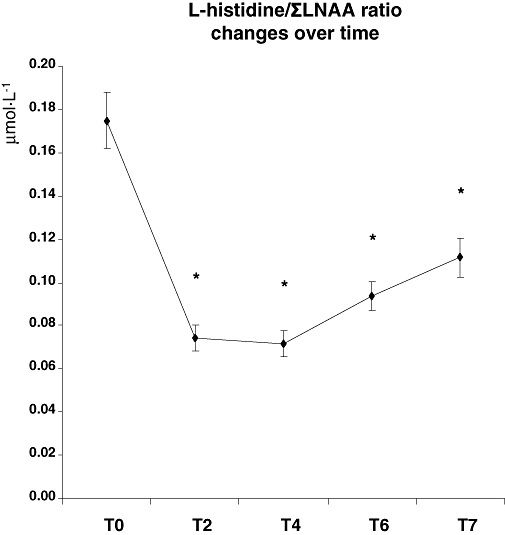
L-histidine/ΣLNAA ratio in plasma over time as a measure of L-histidine depletion. *indicates significant changes from baseline (T0) as tested with simple comparison analyses using a 0.05 significance level.
Treatment had a significant main effect on the change from baseline of absolute L-tyrosine/L-phenylalanine levels [F(2,12) = 62.4, P < 0.001]. Treatment-placebo contrasts showed that L-tyrosine/L-phenylalanine levels decreased (55%) after treatment in the TYD condition as compared with the BAL condition [F(1,13) = 96.5, P < 0.001]. The change from baseline in L-tyrosine/L-phenylalanine levels did not differ between the HID and BAL conditions (F < 1). Treatment also affected the change from baseline in L-tyrosine/L-phenylalanine/ΣLNAA ratio [F(2,12) = 58.7, P < 0.001]. Treatment-placebo comparisons showed that there was a decrease of the ratio in the TYD condition (59%) at T7, compared with the change after BAL treatment [F(1,13) = 125.6, P < 0.001]. The change from baseline of the L-tyrosine/L-phenylalanine/ΣLNAA ratio did not significantly differ between the HID and BAL treatments [F(1,13) = 2.2, P= 0.163].
CRT task
Table 3 shows means and standard errors of performance measures and ERPs from the CRT task.
Table 3.
Summary of the data derived from the Choice Reaction Time Task after treatment with HID (L-histidine depletion), treatment with TYD (L-tyrosine/L-phenylalanine depletion) or treatment with BAL (the full amino acid mixture)
| Choice reaction time | Main effect treatment | SQ×treatment | RC×treatment |
Treatment |
|||||
|---|---|---|---|---|---|---|---|---|---|
| BAL Mean (±SEM) | HID Mean (±SEM) | TYD Mean (±SEM) | |||||||
| P | P | P | Intact | Degraded | Intact | Degraded | Intact | Degraded | |
| Reaction time (ms) | 0.786 | 0.953 | 0.395 | ||||||
| Simple | 414 (9.4) | 458 (13.2) | 414 (9.8) | 461 (12.9) | 414 (9.0) | 460 (13.5) | |||
| Complex | 443 (12.9) | 488 (15.1) | 448 (14.2) | 489 (13.6) | 439 (13.5) | 483 (16.7) | |||
| Accuracy (log%) | 0.242 | 0.649 | 0.222 | ||||||
| Simple | −0.05 (0.025) | −0.07 (0.026) | −0.01 (0.002) | −0.04 (0.009) | −0.01 (0.002) | −0.03 (0.006) | |||
| Complex | −0.02 (0.005) | −0.04 (0.008) | −0.02 (0.004) | −0.04 (0.008) | −0.01 (0.002) | −0.06 (0.006) | |||
| S-locked P300 | |||||||||
| Latency (ms) | 0.213 | 0.427 | 0.199 | ||||||
| Simple | 382 (0.4) | 394 (0.6) | 391 (0.5) | 400 (0.6) | 391 (0.3) | 402 (0.6) | |||
| Complex | 372 (0.4) | 389 (0.6) | 379 (0.5) | 389 (0.7) | 381 (0.4) | 398 (0.5) | |||
| Amplitude (µV) | 0.504 | 0.643 | 0.063 | ||||||
| Simple | 6.7 (0.07) | 5.8 (0.07) | 6.8 (0.05) | 6.3 (0.04) | 6.2 (0.06) | 6.0 (0.06) | |||
| Complex | 4.4 (0.08) | 5.2 (0.07) | 4.1 (0.06)* | 3.7 (0.05)* | 4.4 (0.06) | 3.9 (0.05) | |||
| R-locked P300 | |||||||||
| Latency (ms) | 0.181 | 0.610 | 0.641 | ||||||
| Simple | −19 (0.7) | −29 (0.6) | −15 (0.8) | −34 (0.9) | −10 (0.7) | −20 (0.7) | |||
| Complex | −59 (−1.9) | −61 (1.4) | −42 (1.1) | −73 (3.5) | −34 (1.3) | −43 (2.4) | |||
| Amplitude (µV) | 0.965 | 0.025 | 0.862 | ||||||
| Simple | 5.0 (0.06) | 4.5 (0.07) | 5.2 (0.04) | 3.9 (0.05) | 4.6 (0.05) | 4.8 (0.06) | |||
| Complex | 2.6 (0.06) | 2.1 (0.06) | 2.6 (0.06) | 2.1 (0.05) | 2.7 (0.06) | 2.2 (0.06) | |||
| S-locked LRP | |||||||||
| Onset (ms) | 0.336 | 0.218 | 0.286 | ||||||
| Simple | 252 (0.5) | 269 (0.8) | 253 (1.0) | 344 (3.5) | 270 (0.5) | 305 (2.0) | |||
| Complex | 247 (0.7) | 303 (0.7) | 279 (0.7) | 309 (1.2) | 271 (0.5) | 272 (0.5) | |||
| R-locked LRP | |||||||||
| Onset (ms) | 0.103 | 0.880 | 0.068 | ||||||
| Simple | −152 (0.9) | −152 (1.5) | −131 (0.7) | −118 (0.8) | −122 (0.7) | −124 (0.5) | |||
| Complex | −167 (1.2) | −172 (1.0) | −176 (1.2)* | −183 (1.6)* | −146 (0.5) | −152 (0.8) | |||
Significant or near significant main effects or interaction are indicated with bold P value. Significant (P < 0.05) differences in effects of response complexity (RC) after HID or TYD as compared with BAL are indicated with
SEMs of the electrophysiological measures are uncorrected for applying the Jackknife method.
Behavioural performance
Both SQ and RC affected response times [F(1,15) = 53.8, P < 0.001 and F(1,15) = 5.9, P= 0.028 respectively], but did not interact with each other (F < 1). Treatment did not affect reaction time and did not interact with SQ or RC (Fs < 1).
Accuracy of the responses was significantly decreased by stimulus degradation [F(1,15) = 10.5, P= 0.006], but RC had no effect [F(1,15) = 1.6, P= 0.230]. Effects of SQ and RC did not interact [F(1, 16) = 2.6, P= 0.130]. Treatment did not affect accuracy [F(2, 14) = 1.8, P= 0.242] and did not interact with SQ (F < 1) or RC [F(2, 14) = 1.7, P= 0.222].
P300
Stimulus quality significantly delayed the S-locked P300 peak latency [F(1,15) = 8.0, P= 0.013], whereas it tended to be shortened by RC [F(1,15) = 4.1, P= 0.061]. Treatment did not affect the P300 peak latency [F(2,14) = 1.7, P= 0.213] and did not interact with SQ or RC (Fs < 1). SQ, RC and Treatment did not affect the R-locked P300 peak latency [F(1,15) = 2.3, P= 0.154, F(1,15) = 2.0, P= 0.176 and F(2,14) = 1.9, P= 0.181 respectively].
S-locked P300 peak amplitude was significantly suppressed by RC [F(1,15) = 25.2, P < 0.001]. Treatment tended to interact with RC [F(2,14) = 3.4, P < 0.063], which was due to an enhanced amplitude suppressing effect of RC after HID [F(1,15) = 6.8, P < 0.002]. SQ and Treatment did not have main effects on the peak amplitude [F(1,15) = 1.7, P= 0.213 and F < 1, respectively] and did not interact (F < 1). The R-locked P300 amplitude was significantly suppressed by SQ and RC [F(1,15) = 4.7, P= 0.046 and F(1,15) = 16.2, P < 0.001 respectively], but Treatment did not decrease the amplitude (F < 1). However, Treatment interacted with SQ [F(2,14) = 4.9, P= 0.025]. Drug-placebo comparisons did not reveal any differences with BAL [HID: F(1,15) = 1.2, P= 0.285, TYD: F(1,15) = 1.7, P= 0.210].
LRP
The interval between the stimulus and onset of the LRP (i.e. S-locked LRP onset latency) was delayed by decreased SQ [F(2,14) = 7.9, P < 0.013]. Treatment and RC had no effect [F(2,14) = 1.2, P= 0.336 and F < 1 respectively] and Treatment did not interact with SQ [F(2,14) = 1.7, P= 0.218] or RC [F(2,14) = 1.4, P= 0.286]. SQ and RC also did not interact (F < 1).
The interval between the response and LRP onset (i.e. R-locked LRP onset) was increased by increased RC [F(1,15) = 13.8, P= 0.003]. Treatment tended to interact with RC [F(2,14) = 3.3, P= 0.068]; HID enhanced the effect of RC [F(1,15) = 6.8, P= 0.020] by increasing the interval between the LRP onset and the response in case of a complex response (Figure 2). Treatment [F(2,14) = 2.68, P= 0.103] did not have a main effect on the R-locked LRP onset. Nevertheless, drug-placebo comparisons showed that TYD decreased the interval between the R-locked LRP onset and the response [F(1,15) = 5.1, P= 0.039]. SQ had no effect on the R-locked LRP onset and did not interact with Treatment (Fs < 1).
Figure 2.
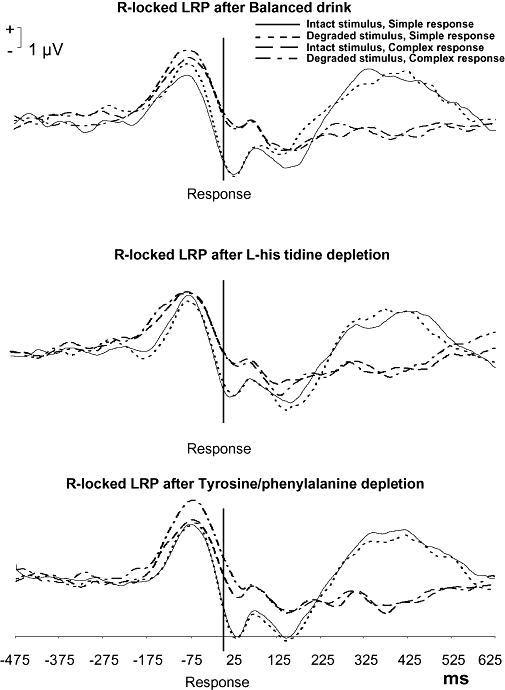
Effects of the treatments on the R-locked LRP onset latency as assessed 5 h after different amino acid treatments (BAL, HID, TYD). Treatment with TYD to induce L-tyrosine/L-phenylalanine depletion decreased the interval between the R-locked LRP and response as indicated by the drug-placebo comparison (P= 0.039). Treatment tended to interact with RC (P= 0.068). Drug-placebo comparisons indicated that L-histidine depletion (treatment with HID) increased the RC prolonging effect on interval (P= 0.020).
Cued simple reaction time task
Simple reaction time and associated area under the CNV did not differ between treatments [F < 1 and F(2,13) = 1.3, P= 0.314 respectively] (Table 4).
Table 4.
Mean (±SEM) performance scores on electrophysiological and behavioural measures
|
Treatment |
|||
|---|---|---|---|
| BAL | HID | TYD | |
| Simple reaction time task | |||
| CNV surface area (µV2) | 4.4 (1.1) | 3.3 (1.6) | 5.2 (1.1) |
| Reaction time (ms) | 260 (13.6) | 272 (68.3) | 250 (42.6) |
| Critical tracking task | |||
| Lambda (rad·s−1) | 3.7 (0.1) | 3.8 (0.2) | 3.6 (0.2) |
| Visual analogue scale | |||
| Alertness (mm) | 608 (39) | 593 (41) | 610 (38) |
Simple reaction time task and Critical Tracking Task performance, and subjective alertness after the balanced drink (BAL), L-histidine depletion (HID) and L-tyrosine/L-phenylalanine depletion (TYD). There were no significant differences between the treatments.
CTT
Treatment had no significant main effect on the average tracking performance [F(2,14) = 2.5, P= 0.115] (Table 4).
Drowsiness
Visual analogue scale
Treatment had no significant effect on subjective alertness (F < 1) (Table 4).
Frequency band power
Treatment did not affect the power of the delta [F(2,14) = 1.9, P= 0.184], theta and alpha (Fs < 1) and beta [F(2,14) = 2.9, P= 0.091] frequency bands when subjects held their eyes open. When subjects closed their eyes, Treatment also did not affect the power of the delta, theta, alpha (Fs < 1) and beta [F(2,14) = 2.9, P= 0.092] frequency bands (Table 5).
Table 5.
Mean (±SEM) frequency band power (µV2)
|
Eyes open |
Eyes closed |
||||||
|---|---|---|---|---|---|---|---|
| BAL | HID | TYD | BAL | HID | TYD | ||
| Delta power | Fz | 12.2 (0.90) | 12.3 (1.01) | 14.1 (1.41) | 18.6 (2.52) | 17.0 (1.80) | 20.3 (2.83) |
| Cz | 12.2 (0.78) | 12.8 (0.99) | 13.9 (1.02) | 17.2 (2.05) | 16.8 (1.61) | 19.0 (1.74) | |
| Pz | 12.0 (0.93) | 12.8 (1.17) | 13.6 (1.15) | 15.9 (2.40) | 14.7 (1.45) | 16.6 (1.73) | |
| Theta power | Fz | 10.6 (1.07) | 9.8 (1.14) | 10.7 (1.40) | 12.4 (0.90) | 11.1 (1.13) | 11.8 (1.35) |
| Cz | 9.5 (0.56) | 9.8 (0.80) | 10.0 (0.88) | 12.9 (1.18) | 11.8 (1.21) | 12.7 (1.52) | |
| Pz | 7.7 (0.55) | 7.8 (0.63) | 7.9 (0.69) | 11.1 (1.38) | 10.2 (1.11) | 10.8 (1.39) | |
| Alpha power | Fz | 6.8 (0.95) | 6.7 (1.22) | 7.2 (1.29) | 15.2 (2.66) | 14.1 (2.99) | 13.3 (2.40) |
| Cz | 8.2 (1.50) | 8.0 (1.52) | 8.5 (1.73) | 19.4 (3.94) | 18.6 (4.09) | 17.0 (3.20) | |
| Pz | 11.0 (2.14) | 10.5 (2.48) | 11.2 (2.56) | 29.8 (6.71) | 29.4 (7.51) | 27.5 (5.88) | |
| Beta power | Fz | 1.3 (0.12) | 1.2 (0.12) | 1.4 (0.18) | 1.7 (0.18) | 1.5 (0.16) | 1.7 (0.21) |
| Cz | 1.3 (0.13) | 1.3 (0.12) | 1.5 (0.18) | 1.8 (0.19) | 1.7 (0.17) | 2.0 (0.21) | |
| Pz | 1.5 (0.16) | 1.3 (0.18) | 1.5 (0.20) | 2.3 (0.33) | 2.1 (0.29) | 2.2 (0.24) | |
Power for Delta (1–4 Hz), Theta (4–8 Hz), Alpha (8–12 Hz) and Beta (12–30 Hz) frequency bands at the Fz, Cz and Pz electrodes in eyes open closed conditions were not affected by L-histidine depletion (HID) and L-tyrosine/L-phenylalanine depletion (TYD), compared with the values after the balanced drink (BAL).
Discussion
The aim of the present study was to decrease histamine levels in the brain of healthy volunteers by depleting subjects of its precursor amino acid, L-histidine. It is the first study that used this method in human volunteers in order to study the role of histamine in cognitive performance. The importance of using different methods to study the involvement of histamine in cognitive deficits needs to be stressed. Moreover, to study the involvement of histamine in cognition, the effects of L-histidine depletion are at least as important as studying the effects of histamine H1-receptor blockade on cognitive performance. In this study we determined if treatment with HID to induce depletion of L-histidine would exert effects that were comparable to those seen in other methods of reduced histaminergic activity (e.g. H1-receptor blockade).
In summary, results indicated that HID treatment affected response-related processes as shown with the significant interaction with RC as measured with the R-locked LRP onset latency and enhanced the amplitude suppressing effect of RC on the S-locked P300. However, L-histidine depletion did not significantly affect performance as measured with speed and accuracy in the CRT task, simple RT task or the CTT. In addition, HID treatment did not significantly affect subjective and objective measures of alertness and arousal, such as mood and EEG power spectrum.
The few significant effects on sensitive measures of psychomotor performance and sedation in this study call into question whether L-histidine depletion has effectively decreased histamine levels in the brain in this study. To evaluate the hypothesized behavioural effects, the neurophysiological consequences of precursor depletion need to be considered. This can only be done on the basis of observations concerning peripherally measured effects of the treatments. The analyses of the blood samples taken between T4 and T7 showed that after HID treatment, L-histidine levels in the plasma decreased by more than 20%. In comparison, the effects of TYD treatment on L-tyrosine/L-phenylalanine level were more pronounced as shown by a reduction of approximately 55%. However, brain concentration of L-histidine is best approximated by the L-histidine/ΣLNAA ratio as various LNAA are competitively transported across the blood-brain barrier. Analyses showed that the maximal reduction in L-histidine/ΣLNAA was 59%, which was similar to the maximal decrease in L-tyrosine + L-phenylalanine/ΣLNAA ratio. Compared with reductions in L-tyrosine/L-phenylalanine found in previous studies showing functional impairments (70–80%), reductions in L-histidine concentrations in the present study were only slightly lower (McTavish et al., 1999; Leyton et al., 2000; Montgomery et al., 2003). The relationship between the decreased L-histidine availability in the brain and the decrease in histamine levels are yet unknown. It may be speculated that histamine stores were not yet fully depleted at time of testing and that the stored histamine was used during testing. Depletion of the stored histamine and the effect on behavioural measures may have occurred later. We suggest that, in future studies, behavioural performance should be assessed over a longer period of time to establish the time of maximal behavioural impairment.
The question of whether L-histidine is an essential amino acid may be raised here. In the amino acid depletion technique the amino acid mixture induces protein synthesis and the level of the missing amino acid declines as it is incorporated into protein (Moja et al., 1991). The level of the amino acid will fall only if it is not synthesized at a rate similar to the rate it is incorporated into protein. As reductions in amino acid levels were observed in this study, even if histidine were synthesized by humans, it was not being synthesized at a rate that was sufficient to replace the histidine incorporated into protein.
Behaviourally, HID treatment increased the duration of the interval between the LRP onset and response indicating increased time needed for more complex response programming. In addition, HID treatment increased the effects of complex responses on the P300 amplitude indicating that increased response demands reduces resources available for stimulus processing (Kok, 1990; Beauducel et al., 2006). These effects were unexpected as a previous study (van Ruitenbeek et al., 2009) showed that the H1-receptor antagonist, dexchlorpheniramine, enhanced the effect of stimulus degradation on S-locked P300 peak latency, whereas no effects on motor processes were found. The effects on processing of visual stimuli are in line with the observation that the visual cortex and other visual regions, such as the lateral geniculate nucleus and the superior colliculus, are densely innervated by histaminergic fibres (Manning et al., 1996). In contrast, less histamine H1-receptors are located in motor structures like the caudate putamen and globus pallidus of the basal ganglia of the guinea-pig brain (Bouthenet et al., 1988). However, we did not observe effects of HID treatment on stimulus-related processes in this study, suggesting that blocking the histamine H1-receptor may have qualitatively different effects for those of decreasing histamine availability and hence histamine release. One obvious explanation is that decreased release of histamine following L-histidine depletion would affect the action of histamine on all its receptors (H1, H2, H3 and H4), as opposed to affecting only H1-receptors with the appropriate antagonist.
The involvement of histamine in motor processes is supported by animal studies in which the histamine synthesizing enzyme HDC is depleted. For example, Brabant et al. (2007) found that cocaine-induced hyperactivity is reduced in HDC knockout mice. In contrast, other authors found that methamphetamine-induced locomotor hyperactivity was facilitated in HDC knockout mice (Kubota et al., 2002; Iwabuchi et al., 2004) and after administration of a H1-receptor antagonist (Ito et al., 1997). As methamphetamine and cocaine increase dopamine activity it may be that histamine interacts with dopamine. Several mechanisms of interaction have been suggested, such as interactions with the GABA neurotransmitter system (Kubota et al., 2002), and the dopamine transporter (Matsunaga et al., 1998). However, a study assessing the latter hypothesis could not confirm this (Theunissen et al., 2006). Taken together, it is suggested that histamine in the brain plays a role in motor processes, which may be mediated by dopamine. However, the exact mechanism is not clear.
It may be argued that the effects found in this study are due to a non-specific effect of amino acid imbalance but we believe this is unlikely. A similar question was raised concerning tryptophan depletion, but acute lysine depletion has no effect on mood and cognition in humans, unlike tryptophan depletion (Klaassen et al., 1999). In addition, in rodents, histamine metabolism can be altered more by changes in precursor availability than any other neurotransmitter (Young, 1996). Therefore, the effects of HID treatment found in this study may be attributed to small changes in histamine release. To account for the limited effect of HID treatment that we found, it may be argued that a decrease in histamine release may not occur in all brain areas. Neurotransmitter release can be regulated differentially in different brain areas and small changes in histamine synthesis may lead to an inhomogeneous decrease of histamine release.
Surprisingly, treatment with TYD, that is, depletion of dopamine, decreased the interval between the LRP and the response, indicating increased motor processing capacity. However, TYD treatment did not affect the CNV and performance on the CTT and the CRT as measured with reaction time. Other studies of the effects of L-tyrosine/L-phenylalanine depletion yield inconsistent results. Some studies showed impaired performance (Harmer et al., 2001; Harrison et al., 2004) and altered mood (Leyton et al., 2000), while others did not observe such effects (McLean et al., 2004; Lythe et al., 2005). It has been suggested that these inconsistencies are due to large variability between subjects in brain dopamine availability. Mehta et al. (2005) showed that subjects who performed worse on a delayed response task and a planning task also showed the greatest reductions in dopamine levels in the striatum. It may be that there were many non-sensitive subjects in this study.
To conclude, contrary to our expectations, HID treatment had an impairing effect on motor-related processes, suggesting that histamine is involved in more than sensory processing, which is affected by histamine H1-receptor blockade. However, HID treatment did not impair psychomotor performance as measured by the CTT and reaction times and did not cause sedation as indicated by subjective and objective measures. The absence of these effects may be explained by small changes in histamine release.
Acknowledgments
The authors acknowledge D. Mendelsohn, MSc, J. Wolters, MSc and P. Swerts, MSc for their efforts in collecting the data and C. van Leeuwen, MD for the medical supervision.
Glossary
Abbreviations:
- BAL
balanced amino acid dink
- CRT
choice reaction time
- CTT
critical tracking task
- EOG
electrooculogram
- ERP
event-related potential
- HDC
histidine decarboxylase
- HID
L-histidine depleted drink
- LNAA
large neutral amino acids
- LRP
lateralized readiness potential
- RC
response complexity
- R-locked
response locked
- S-locked
stimulus locked
- SQ
stimulus quality
- TYD
L-tyrosine/L-phenylalanine depleted drink
Conflicts of interest
The study was paid by, carried out at and only reported within Maastricht University. A.V. has received grants from GlaxoSmithKline. A.S. received support from the Netherlands Organisation of Scientific Research (VENI grant 451-07-011). At times during the study, W.J.R. was employed by Hoffman-LaRoche R&D, Basel, Switzerland while remaining affiliated to Maastricht University. In the authors opinion this causes no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Supplement file 1: Histamine H1-receptor blockade predominantly impairs sensory processes in human sensorimotor performance.
Supplement file 2: Memory impairments in humans after acute tryptophan depletion using a novel gelatin-based protein drink.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Acevedo SF, Ohtsu H, Benice TS, Rizk-Jackson A, Raber J. Age-dependent measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc(−/−)) mice. Brain Res. 2006;1071:113–123. doi: 10.1016/j.brainres.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Beauducel A, Brocke B, Leue A. Energetical bases of extraversion: effort, arousal, EEG, and performance. Int J Psychophysiol. 2006;62:212–223. doi: 10.1016/j.ijpsycho.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- Bouthenet ML, Ruat M, Sales N, Garbarg M, Schwartz JC. A detailed mapping of histamine H1-receptors in guinea-pig central nervous system established by autoradiography with [125I]iodobolpyramine. Neuroscience. 1988;26:553–600. doi: 10.1016/0306-4522(88)90167-4. [DOI] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Anaclet C, Lin JS, Ohtsu H, Tirelli E. The psychostimulant and rewarding effects of cocaine in histidine decarboxylase knockout mice do not support the hypothesis of an inhibitory function of histamine on reward. Psychopharmacology (Berl) 2007;190:251–263. doi: 10.1007/s00213-006-0603-0. [DOI] [PubMed] [Google Scholar]
- Chen Z, Sugimoto Y, Kamei C. Effects of intracerebroventricular injection of alpha-fluoromethylhistidine on radial maze performance in rats. Pharmacol Biochem Behav. 1999;64:513–518. doi: 10.1016/s0091-3057(99)00128-8. [DOI] [PubMed] [Google Scholar]
- Cho ES, Anderson HL, Wixom RL, Hanson KC, Krause GF. Long-term effects of low histidine intake on men. J Nutr. 1984;114:369–384. doi: 10.1093/jn/114.2.369. [DOI] [PubMed] [Google Scholar]
- Dickman SJ, Meyer DE. Impulsivity and speed-accuracy tradeoffs in information processing. J Pers Soc Psychol. 1988;54:274–290. doi: 10.1037//0022-3514.54.2.274. [DOI] [PubMed] [Google Scholar]
- van Eijk HM, Rooyakkers DR, Deutz NE. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2–3 microns Spherisorb ODS II column. J Chromatogr. 1993;620:143–148. doi: 10.1016/0378-4347(93)80062-9. [DOI] [PubMed] [Google Scholar]
- Esbenshade TA, Fox GB, Cowart MD. Histamine H3 receptor antagonists: preclinical promise for treating obesity and cognitive disorders. Mol Interv. 2006;6:77–88. 59. doi: 10.1124/mi.6.2.5. [DOI] [PubMed] [Google Scholar]
- Gaillard AWK, Gruisen A, De Jong R. The influence of antihistamines on human performance. Eur J Clin Pharmacol. 1988;35:249–253. doi: 10.1007/BF00558261. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Miller J. Response complexity and precue interval effects on the lateralized readiness potential. Psychophysiology. 1995;32:230–241. doi: 10.1111/j.1469-8986.1995.tb02952.x. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, McTavish SF, Clark L, Goodwin GM, Cowen PJ. Tyrosine depletion attenuates dopamine function in healthy volunteers. Psychopharmacology (Berl) 2001;154:105–111. doi: 10.1007/s002130000613. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Olver JS, Norman TR, Burrows GD, Wesnes KA, Nathan PJ. Selective effects of acute serotonin and catecholamine depletion on memory in healthy women. J Psychopharmacol. 2004;18:32–40. doi: 10.1177/0269881104040225. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Shamsi Z. Antihistamines: models to assess sedative properties, assessment of sedation, safety and other side-effects. Clin Exp Allergy. 1999;29(Suppl.)(3):133–142. doi: 10.1046/j.1365-2222.1999.0290s3133.x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academic Press; 2006. [Google Scholar]
- Ito C, Onodera K, Watanabe T, Sato M. Effects of histamine agents on methamphetamine-induced stereotyped behavior and behavioral sensitization in rats. Psychopharmacology (Berl) 1997;130:362–367. doi: 10.1007/s002130050251. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Kubota Y, Ito C, Watanabe T, Watanabe T, Yanai K. Methamphetamine and brain histamine: a study using histamine-related gene knockout mice. Ann N Y Acad Sci. 2004;1025:129–134. doi: 10.1196/annals.1316.016. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol. 1957;10:371–375. [PubMed] [Google Scholar]
- Jex HR, McDonnell JD, Phatak AV. A ‘critical’ tracking task for man-machine research related to the operator's effective delay time. I. Theory and experiments with a first-order divergent controlled element. NASA CR-616. NASA Contract Rep NASA CR. 1966:1–105. [PubMed] [Google Scholar]
- Kamei C, Okumura Y, Tasaka K. Influence of histamine depletion on learning and memory recollection in rats. Psychopharmacology (Berl) 1993;111:376–382. doi: 10.1007/BF02244955. [DOI] [PubMed] [Google Scholar]
- Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol. 2000;105(6):S622–S627. doi: 10.1067/mai.2000.106153. Pt 2. [DOI] [PubMed] [Google Scholar]
- Klaassen T, Riedel WJ, Deutz NE, van Someren A, van Praag HM. Specificity of the tryptophan depletion method. Psychopharmacology (Berl) 1999;141:279–286. doi: 10.1007/s002130050835. [DOI] [PubMed] [Google Scholar]
- Kok A. Internal and external control: a two-factor model of amplitude change of event-related potentials. Acta Psychol (Amst) 1990;74:203–236. [PubMed] [Google Scholar]
- Kriengsinyos W, Rafii M, Wykes LJ, Ball RO, Pencharz PB. Long-term effects of histidine depletion on whole-body protein metabolism in healthy adults. J Nutr. 2002;132:3340–3348. doi: 10.1093/jn/132.11.3340. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Ito C, Sakurai E, Sakurai E, Watanabe T, Ohtsu H. Increased methamphetamine-induced locomotor activity and behavioral sensitization in histamine-deficient mice. J Neurochem. 2002;83:837–845. doi: 10.1046/j.1471-4159.2002.01189.x. [DOI] [PubMed] [Google Scholar]
- Leurs R, Blandina P, Tedford C, Timmerman H. Therapeutic potential of histamine H3 receptor agonists and antagonists. Trends Pharmacol Sci. 1998;19:177–183. doi: 10.1016/s0165-6147(98)01201-2. [DOI] [PubMed] [Google Scholar]
- Leyton M, Young SN, Pihl RO, Etezadi S, Lauze C, Blier P, et al. Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacology. 2000;22:52–63. doi: 10.1016/S0893-133X(99)00086-X. [DOI] [PubMed] [Google Scholar]
- Lythe KE, Anderson IM, Deakin JF, Elliott R, Strickland PL. Lack of behavioural effects after acute tyrosine depletion in healthy volunteers. J Psychopharmacol. 2005;19:5–11. doi: 10.1177/0269881105048886. [DOI] [PubMed] [Google Scholar]
- McLean A, Rubinsztein JS, Robbins TW, Sahakian BJ. The effects of tyrosine depletion in normal healthy volunteers: implications for unipolar depression. Psychopharmacology (Berl) 2004;171:286–297. doi: 10.1007/s00213-003-1586-8. [DOI] [PubMed] [Google Scholar]
- McTavish SF, Cowen PJ, Sharp T. Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology (Berl) 1999;141:182–188. doi: 10.1007/s002130050823. [DOI] [PubMed] [Google Scholar]
- Manning KA, Wilson JR, Uhlrich DJ. Histamine-immunoreactive neurons and their innervation of visual regions in the cortex, tectum, and thalamus in the primate Macaca mulatta. J Comp Neurol. 1996;373:271–282. doi: 10.1002/(SICI)1096-9861(19960916)373:2<271::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Sato T, Shuto H, Tsuruta Y, Suemaru K, Gomita Y, et al. Inhibition of neuronal dopamine uptake by some antiallergic drugs. Eur J Pharmacol. 1998;350:165–169. doi: 10.1016/s0014-2999(98)00253-2. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gumaste D, Montgomery AJ, McTavish SF, Grasby PM. The effects of acute tyrosine and phenylalanine depletion on spatial working memory and planning in healthy volunteers are predicted by changes in striatal dopamine levels. Psychopharmacology (Berl) 2005;180:654–663. doi: 10.1007/s00213-004-2128-8. [DOI] [PubMed] [Google Scholar]
- Miller J, Hackley SA. Electrophysiological evidence for temporal overlap among contingent mental processes. J Exp Psychol Gen. 1992;121:195–209. doi: 10.1037//0096-3445.121.2.195. [DOI] [PubMed] [Google Scholar]
- Miller J, Patterson T, Ulrich R. Jackknife-based method for measuring LRP onset latency differences. Psychophysiology. 1998;35:99–115. [PubMed] [Google Scholar]
- Moja EA, Restani P, Corsini E, Stacchezzini MC, Assereto R, Galli CL. Cycloheximide blocks the fall of plasma and tissue tryptophan levels after tryptophan-free amino acid mixtures. Life Sci. 1991;49:1121–1128. doi: 10.1016/0024-3205(91)90600-g. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, McTavish SF, Cowen PJ, Grasby PM. Reduction of brain dopamine concentration with dietary tyrosine plus phenylalanine depletion: an [11C]raclopride PET study. Am J Psychiatry. 2003;160:1887–1889. doi: 10.1176/appi.ajp.160.10.1887. [DOI] [PubMed] [Google Scholar]
- O'Hanlon JF, Ramaekers JG. Antihistamine effects on actual driving performance in a standard test: a summary of Dutch experience, 1989–94. Allergy. 1995;50:234–242. doi: 10.1111/j.1398-9995.1995.tb01140.x. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Szabo J. Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am J Physiol. 1976;230:94–98. doi: 10.1152/ajplegacy.1976.230.1.94. [DOI] [PubMed] [Google Scholar]
- Onodera K, Yamatodani A, Watanabe T, Wada H. Neuropharmacology of the histaminergic neuron system in the brain and its relationship with behavioral disorders. Prog Neurobiol. 1994;42:685–702. doi: 10.1016/0301-0082(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983;63:1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel WJ, Klaassen T, Deutz NE, van Someren A, van Praag HM. Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology (Berl) 1999;141:362–369. doi: 10.1007/s002130050845. [DOI] [PubMed] [Google Scholar]
- Riedel WJ, Mehta M, Unema P. Human cognition assessment in drug research. Curr Pharm Des. 2006;12:1–15. doi: 10.2174/138161206777698882. [DOI] [PubMed] [Google Scholar]
- Rihet P, Possamai CA, Micallef-Roll J, Blin O, Hasbroucq T. Dopamine and human information processing: a reaction-time analysis of the effect of levodopa in healthy subjects. Psychopharmacology (Berl) 2002;163:62–67. doi: 10.1007/s00213-002-1127-x. [DOI] [PubMed] [Google Scholar]
- Rizzo PA, Pierelli F, Pozzessere G, Fattapposta F, Sanarelli L, Morocutti C. Pain, anxiety, and contingent negative variation: a clinical and pharmacological study. Biol Psychiatry. 1985;20:1297–1302. doi: 10.1016/0006-3223(85)90114-3. [DOI] [PubMed] [Google Scholar]
- Rockstroh B, Elbert T, Lutzenberger W, Altenmuller E. Effects of the anticonvulsant benzodiazepine clonazepam on event-related brain potentials in humans. Electroencephalogr Clin Neurophysiol. 1991;78:142–149. doi: 10.1016/0013-4694(91)90114-j. [DOI] [PubMed] [Google Scholar]
- van Ruitenbeek P, Vermeeren A, Riedel W. Histamine H1-receptor blockade in humans affects psychomotor performance but not memory. J Psychopharmacol. 2008;22:663–672. doi: 10.1177/0269881107081526. [DOI] [PubMed] [Google Scholar]
- van Ruitenbeek P, Vermeeren A, Smulders FTY, Sambeth A, Riedel WJ. Histamine H1 receptor blockade predominantly impairs sensory processes in human sensorimotor performance. Br J Pharmacol. 2009;157:76–85. doi: 10.1111/j.1476-5381.2008.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Sakurai E, Sakurai E, Yanai K, Mirua Y, Watanabe T. Depletion of brain histamine induced by alpha-fluoromethylhistidine enhances radial maze performance in rats with modulation of brain amino acid levels. Life Sci. 1998;62:989–994. doi: 10.1016/s0024-3205(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Riedel WJ, Tillie DE, Blokland A, Postma A, Schmitt JA. Memory impairments in humans after acute tryptophan depletion using a novel gelatin-based protein drink. J Psychopharmacol. 2009;23:56–64. doi: 10.1177/0269881108089577. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smulders FTY, Kok A, Kenemans JL, Bashore TR. The temporal selectivity of additive factor effects on the reaction process revealed in ERP component latencies. Acta Psychol (Amst) 1995;90:97–109. doi: 10.1016/0001-6918(95)00032-p. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: extensions of donders' method. Acta Psychol (Amst) 1969;30:276–315. [Google Scholar]
- Theunissen EL, van Kroonenburgh MJ, van Deursen JA, Blom-Coenjaerts C, Ramaekers JG. Stimulating effects of the antihistamine fexofenadine: testing the dopamine transporter hypothesis. Psychopharmacology (Berl) 2006;187:95–102. doi: 10.1007/s00213-006-0406-3. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Miller J. Using the jackknife-based scoring method for measuring LRP onset effects in factorial designs. Psychophysiology. 2001;38:816–827. [PubMed] [Google Scholar]
- Vohora D. Histamine-selective H3 receptor ligands and cognitive functions: an overview. IDrugs. 2004;7:667–673. [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wijtmans M, Leurs R, de Esch I. Histamine H3 receptor ligands break ground in a remarkable plethora of therapeutic areas. Expert Opin Investig Drugs. 2007;16:967–985. doi: 10.1517/13543784.16.7.967. [DOI] [PubMed] [Google Scholar]
- Young SN. Behavioral effects of dietary neurotransmitter precursors: basic and clinical aspects. Neurosci Biobehav Rev. 1996;20:313–323. doi: 10.1016/0149-7634(95)00022-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


